Multiphysical Coupling Analysis of Sealing Performance of Underground Lined Caverns for Hydrogen Storage
Abstract
1. Introduction
2. Governing Equations
2.1. Gas Thermodynamics Within the Cavern
2.2. Gas Leakage and the Effect of Temperature on Permeable Property
2.3. Heat Transfer Around the Cavern
2.4. Thermomechanical Deformation of the Sealing Layer
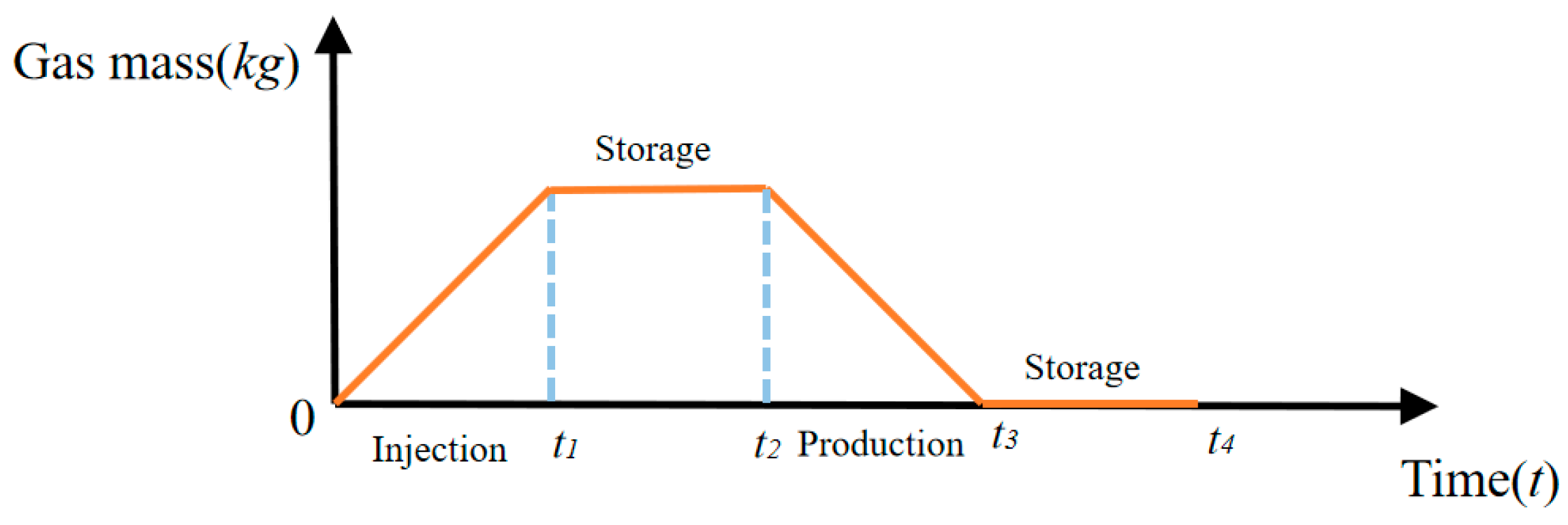
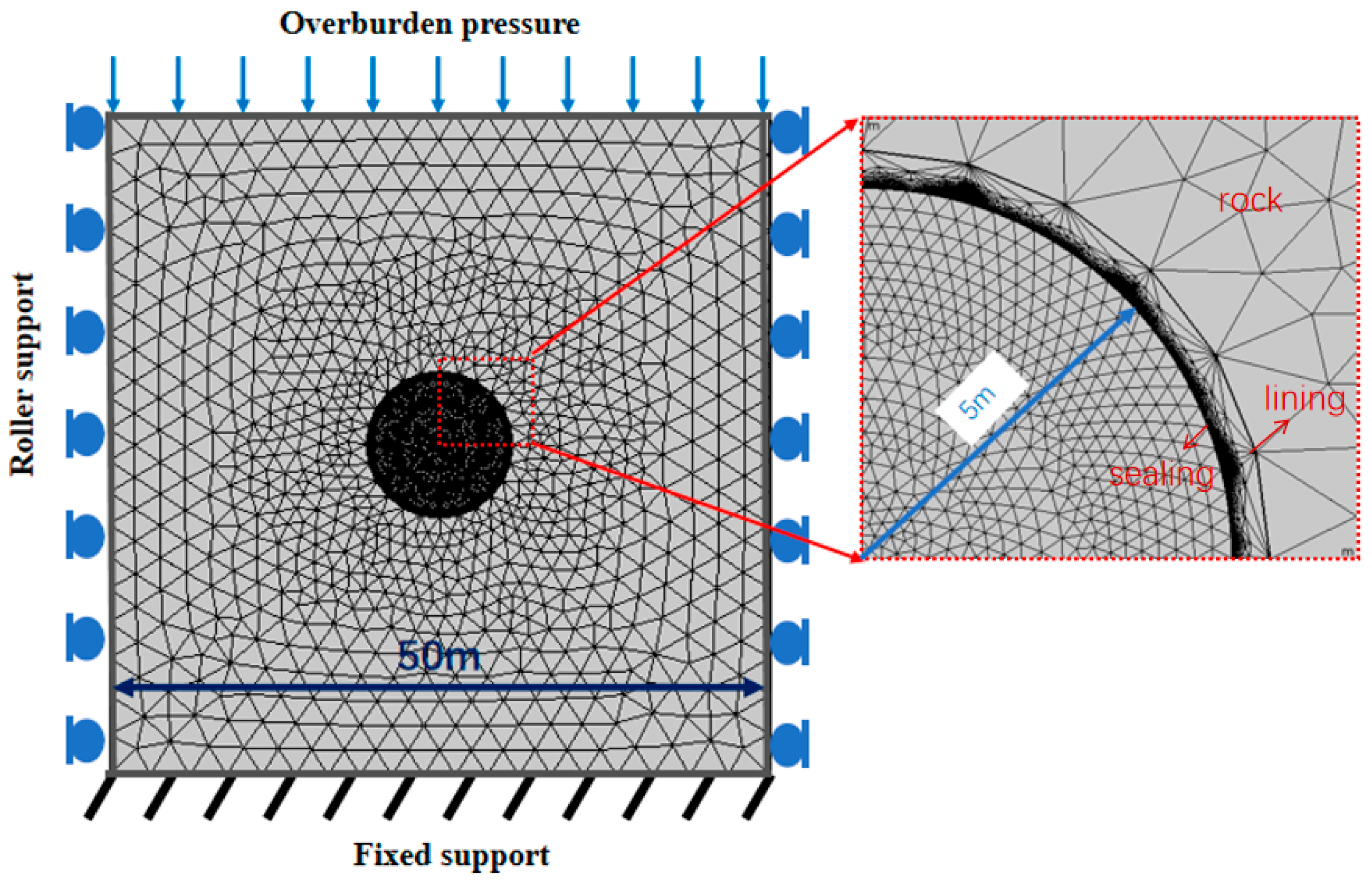
3. Model Validation
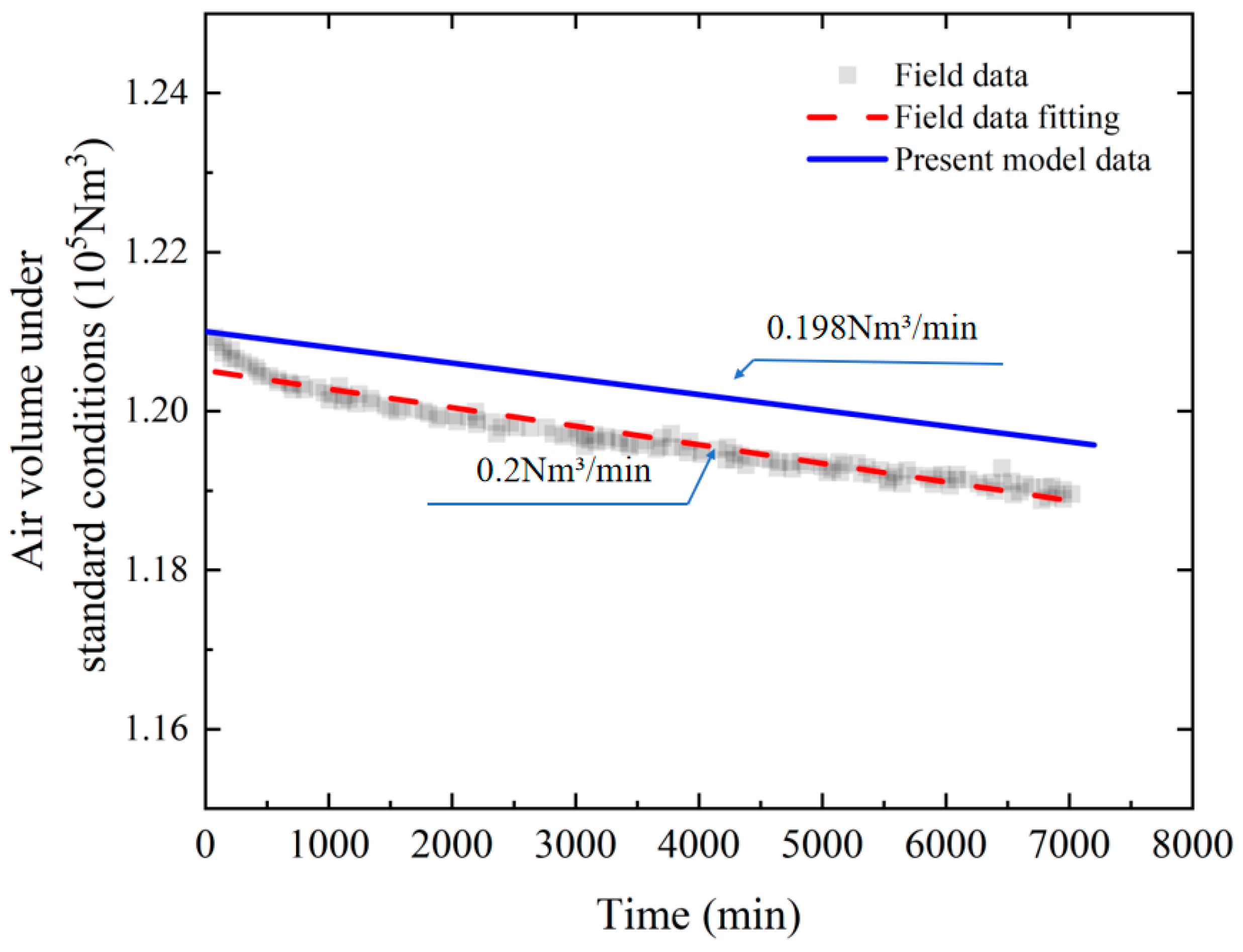
3.1. Validation Against Analytical Solutions
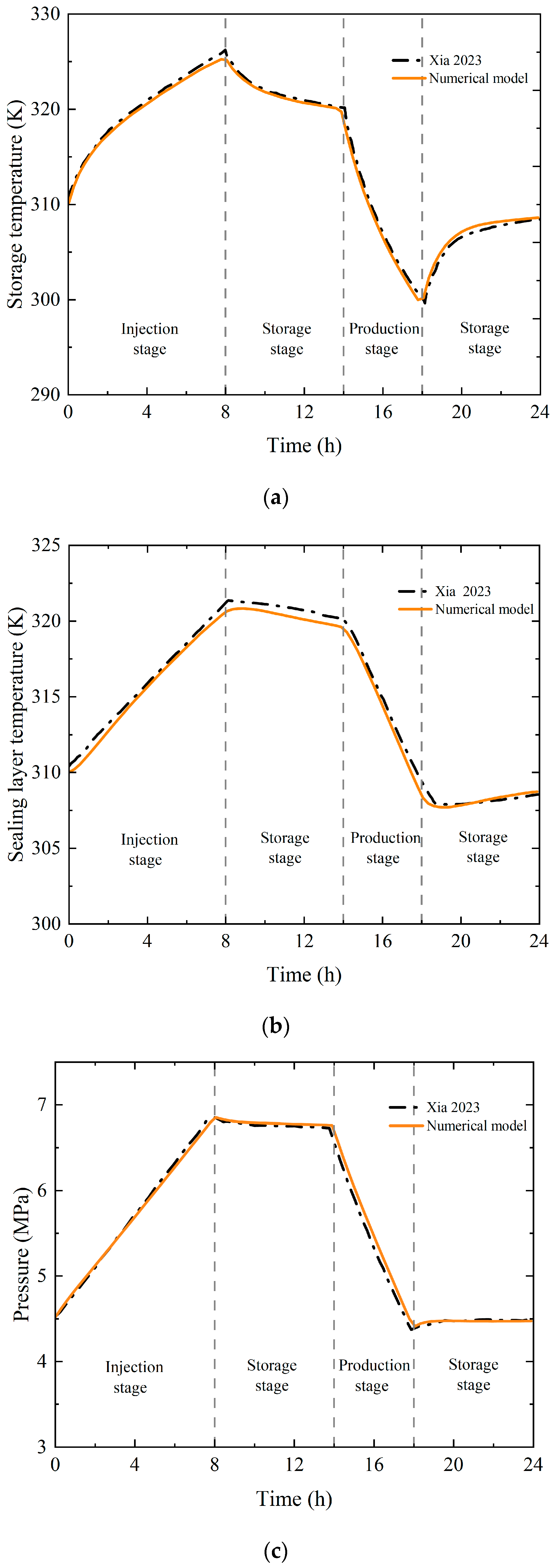
3.2. Validation Against Field Operational Data
4. Results and Discussion
4.1. Sealing Performance and Mechanical Characteristics of Lined Cavern Materials
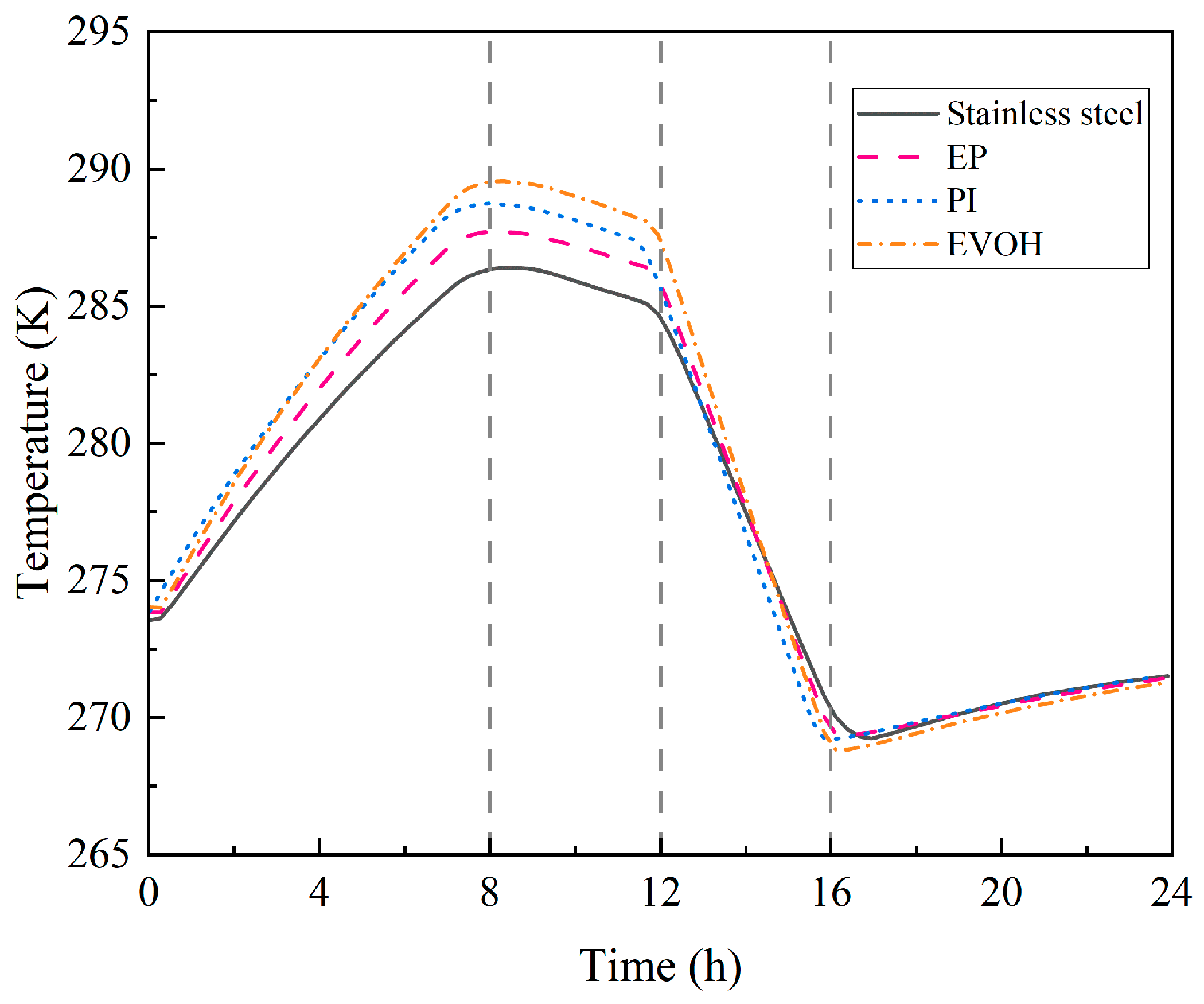
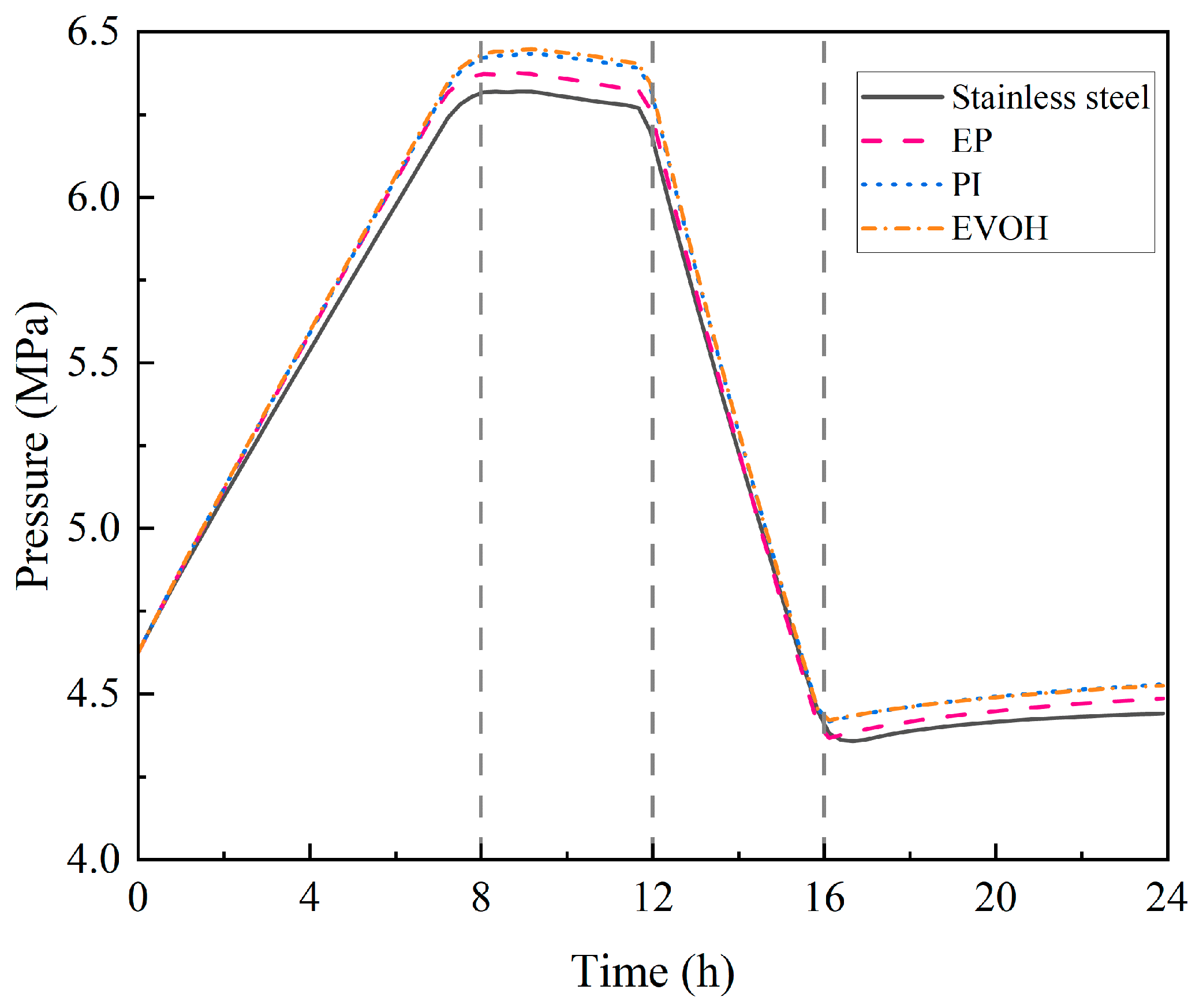
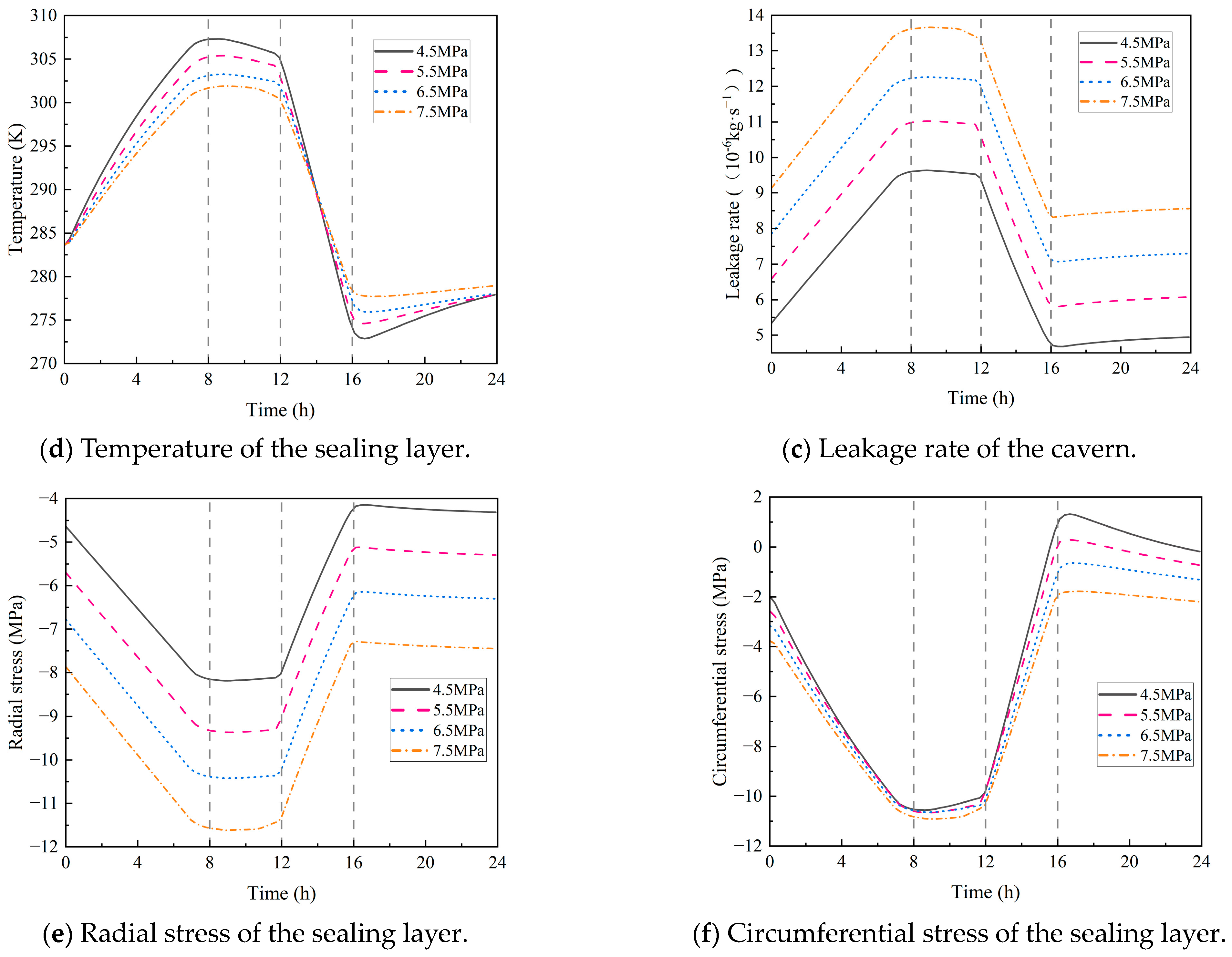
4.1.1. Temperature and Pressure Evolution Within the Cavern
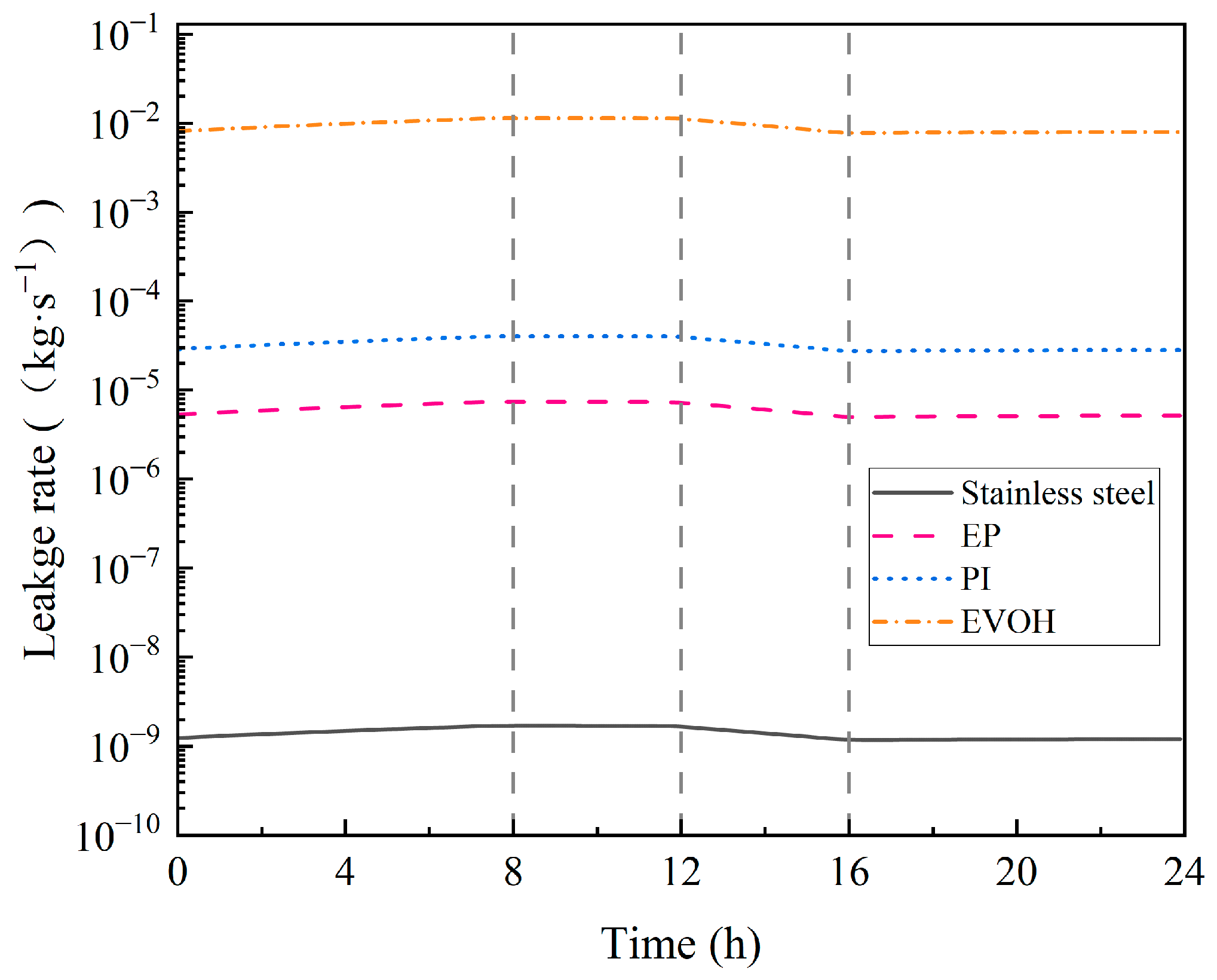
4.1.2. Hydrogen Leakage Dynamics in Cavern
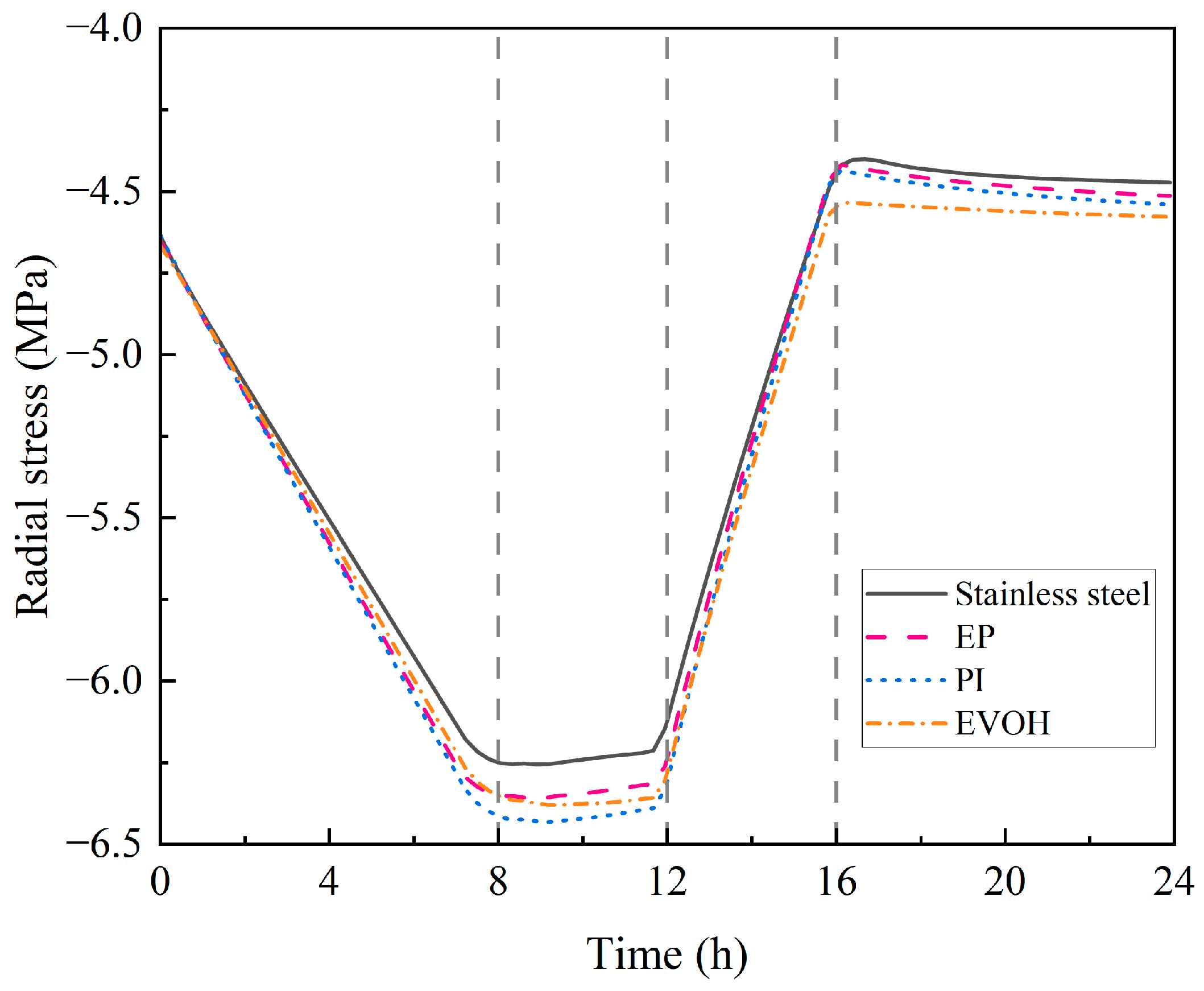
4.1.3. Mechanical Characteristics of Sealing Layers
4.2. Impact of Operating Conditions
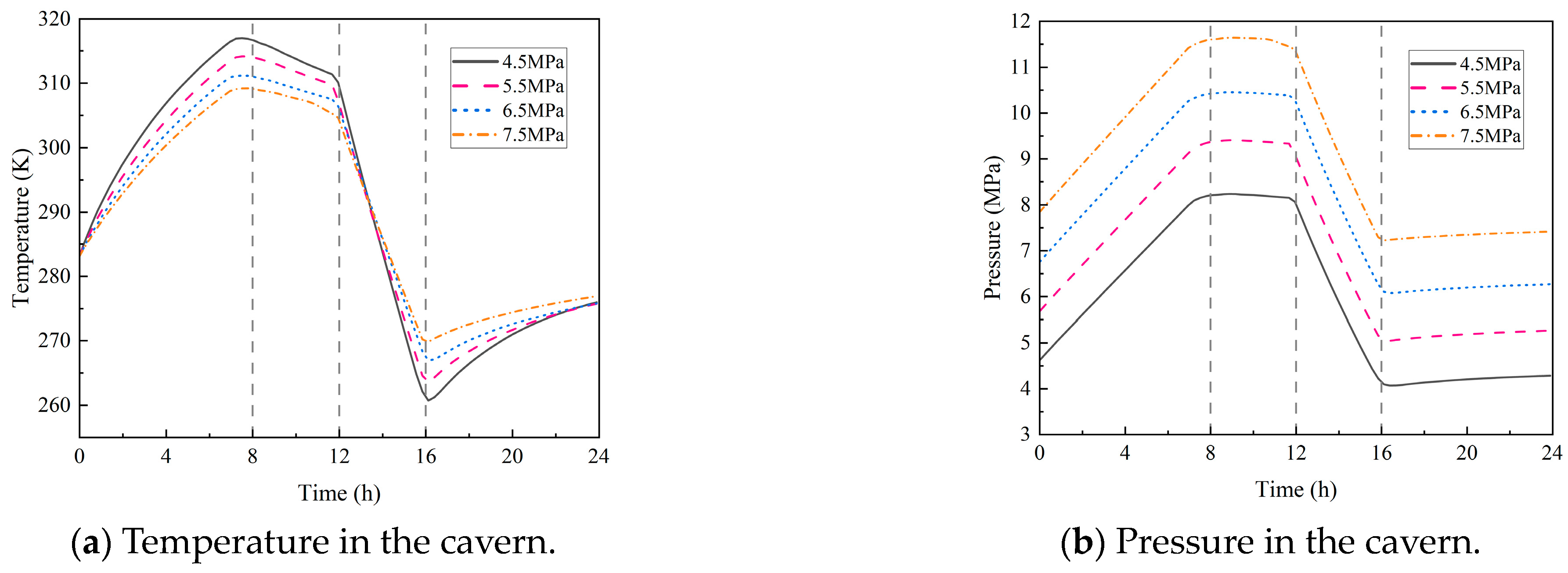
4.2.1. Initial Pressure in the Cavern
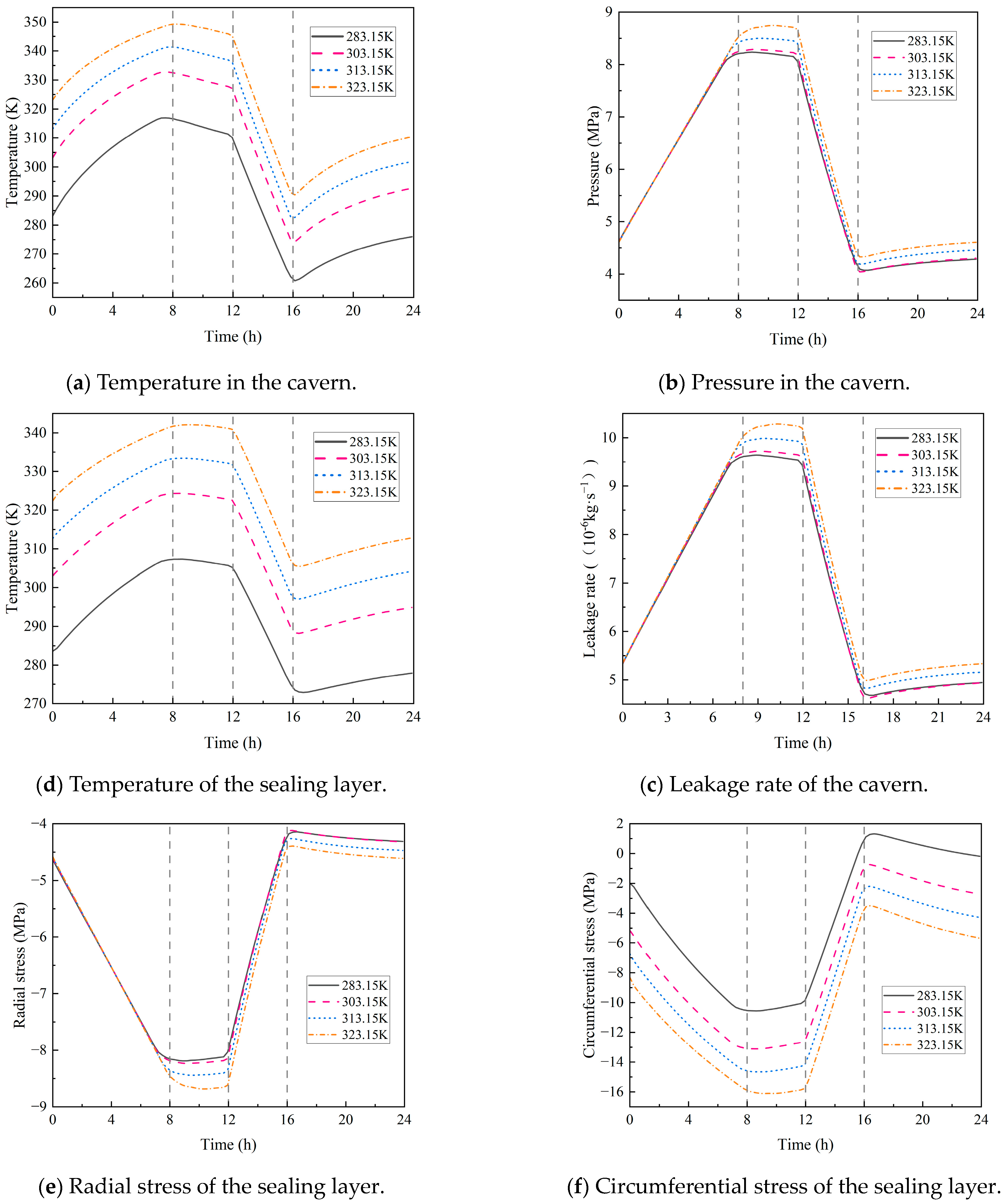
4.2.2. Initial Temperature in the Cavern
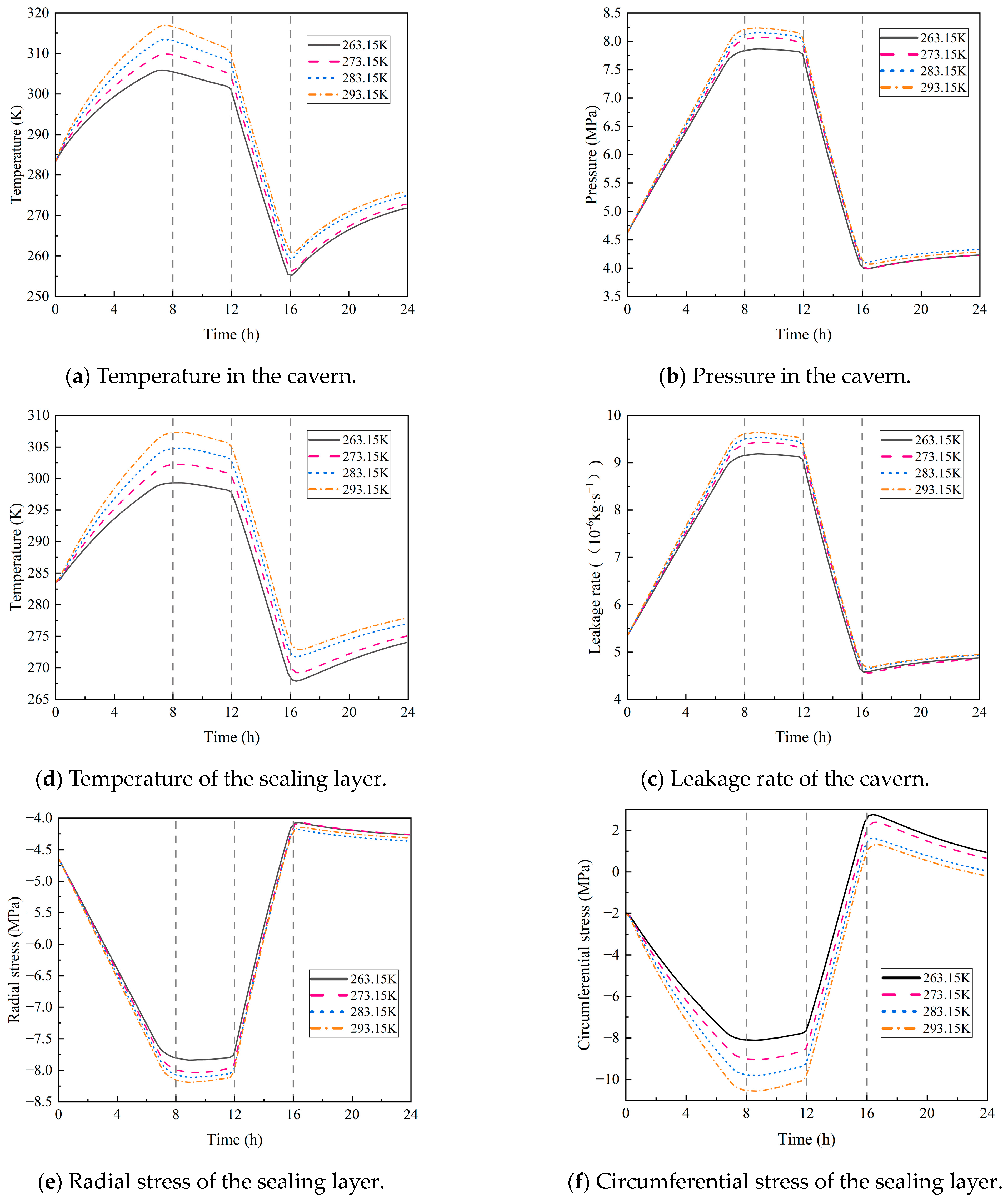
4.2.3. Hydrogen Injection Temperature
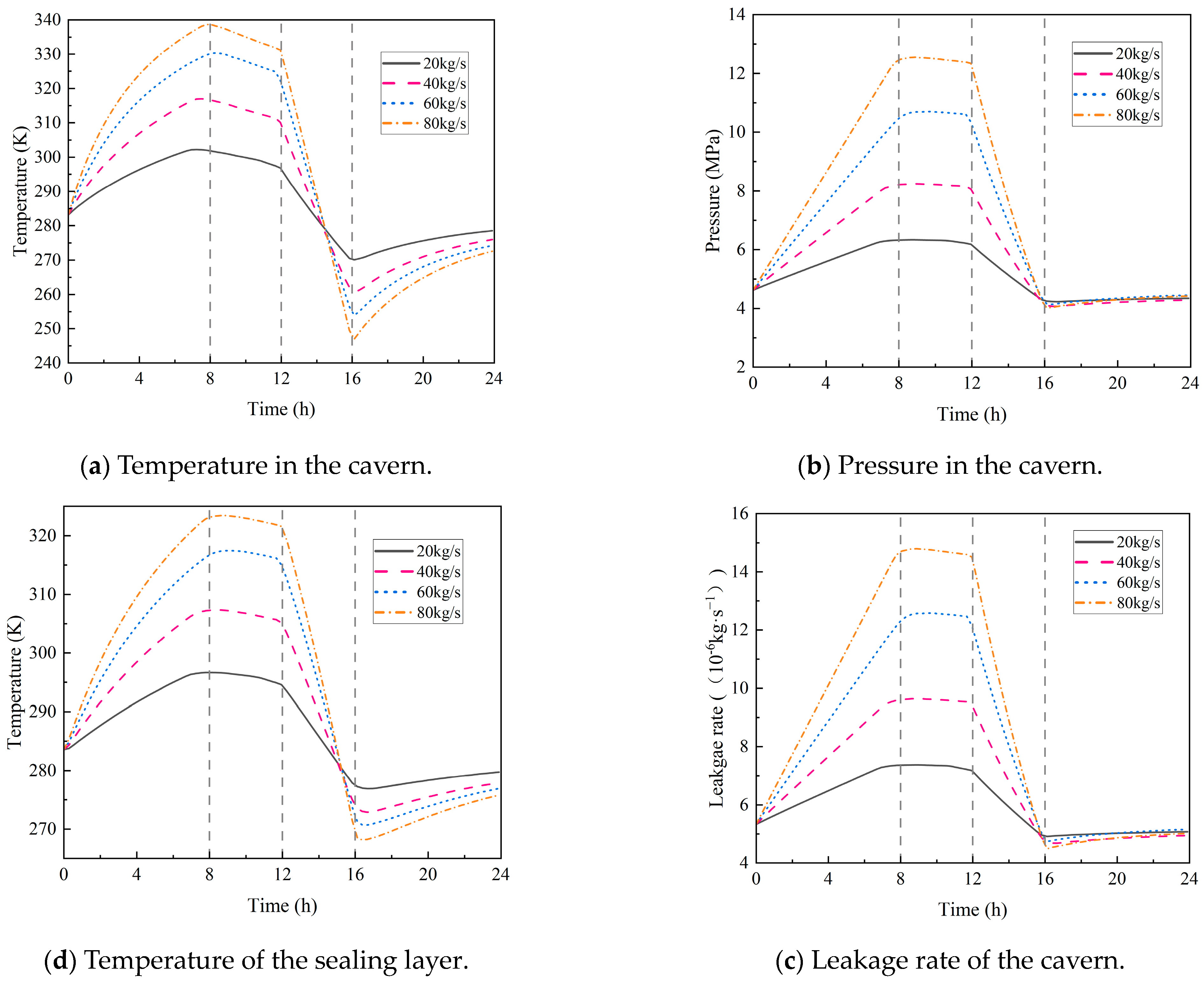
4.2.4. Hydrogen Injection and Production Rate
5. Conclusions
- (1)
- During the injection phase (0–8 h), continuous hydrogen injection synchronously raises cavern pressure and temperature, and the pressure drives a slow upward leakage rate. In the first storage phase (8–12 h), pressure and temperature stabilize, and the leakage rate peaks with small fluctuations. In the production phase (12–16 h), hydrogen discharge lowers cavern pressure and temperature significantly, reducing the leakage rate slowly. Although the sealing layer may develop local tensile stress (due to the thermal expansion coefficient difference with the support structure), leakage stays controlled. In the second storage phase (16–24 h), cavern temperature and pressure rise slowly with tiny variations, and the leakage rate remains stable;
- (2)
- Stainless steel has the best sealing performance, but it has drawbacks such as its high cost and susceptibility to hydrogen embrittlement (which may cause the deterioration of mechanical properties during long-term service). Epoxy resin (EP) offers excellent sealing, temperature resistance, cost-effectiveness, and no hydrogen embrittlement, making it ideal as a stainless steel alternative under medium–low-pressure and cost-sensitive conditions. Polyimide (PI) excels in anti-aging, high-temperature resistance, and sealing reliability, although it is costly. Ethylene–vinyl alcohol copolymer (EVOH) has poor hydrogen barrier performance and sensitivity to temperature/humidity, making it suitable only for stable environments. For anti-aging, PI is best, followed by EP, with EVOH being relatively weaker;
- (3)
- Both the initial temperature of the storage and the hydrogen injection temperature have a significant impact on the circumferential stress of the sealing layer. For every 10 K increase in the initial temperature, the circumferential stress rises by 11%. For every 10 K increase in the injection temperature, the circumferential stress increases by 10%, with little impact on the leakage rate. In addition, the initial pressure of the storage and the hydrogen injection rate exert a considerable influence on airtightness. Each 1 MPa increase in the initial pressure causes the leakage rate to rise by 11%, and every 20 kg/s increase in the injection rate leads to a 12% increase in the leakage rate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cavern volume | Diffusive flux | ||
| Density of hydrogen within the cavern | Diffusion coefficient | ||
| Dimensionless periodic function for gas injection | Concentration gradient | ||
| Dimensionless periodic function for gas production | Hydrogen concentration | ||
| Gas mass flow rate of the compressor | Thickness of the sealing layer | ||
| Hydrogen mass leakage rate from the cavern | Solubility coefficient | ||
| Constant-volume specific heat capacity of the gas | Hydrogen partial pressures at the inner boundaries of the sealing layer | ||
| Temperature of the gas within the cavern | Hydrogen partial pressures at the outer boundaries of the sealing layer | ||
| Enthalpy of the injected gas | Gas pressure on the outer side of the sealing layer | ||
| the enthalpy of the gas | Permeability coefficient of stainless steel | ||
| Gas compressibility factor | Molar mass of hydrogen | ||
| Gas constant | Permeability of the polymeric material, | ||
| Specific internal energy of the gas | Density of air at under standard atmospheric pressure | ||
| Heat transfer rate at the cavern wall surface | Activation energy | ||
| Ratio of injected gas mass flow rate to produced gas mass flow rate | Density of the -th layer medium | ||
| Constant-pressure specific heat capacity of the gas | Constant-pressure specific heat capacity of the -th layer medium | ||
| Temperature of the injected gas | Thermal conductivity of the -th layer medium | ||
| Partial derivative of the gas compressibility factor with respect to temperature at the initial state | Temperature of the -th layer medium | ||
| Pressure within the cavern | Cauchy stress tensor | ||
| Gas pressure at the critical state | Body force vector per unit volume | ||
| Gas temperature at the critical state | Fourth-order elasticity tensor | ||
| Convective heat transfer coefficient | : | Double-dot product (tensor contraction) | |
| Cavern surface area | Infinitesimal strain tensor | ||
| Temperature at the cavern wall surface | Coefficient of thermal expansion | ||
| Radius of the cavern |
References
- Shu, K.Y.; Guan, B.; Zhuang, Z.Q.; Chen, J.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Dang, H.; et al. Reshaping the energy landscape: Explorations and strategic perspectives on hydrogen energy preparation, efficient storage, safe transportation and wide applications. Int. J. Hydrogen Energy 2025, 97, 160–213. [Google Scholar] [CrossRef]
- Mwakipunda, G.C.; Kouassi, A.K.F.; Ayimadu, E.T.; Komba, N.A.; Nadege, M.N.; Mgimba, M.M.; Ngata, M.R.; Yu, L. Underground hydrogen storage in geological formations: A review. J. Rock Mech. Geotech. Eng. 2025, 17, 6704–6741. [Google Scholar] [CrossRef]
- Leng, G.; Yan, W.; Chen, Z.; Li, Z.; Liu, B.; Deng, P.; Zhang, C.; Liu, W.; Qi, H. Technical challenges and opportunities of hydrogen storage: A comprehensive review on different types of underground storage. J. Energy Storage 2025, 114, 115900. [Google Scholar] [CrossRef]
- Ho, T.A.; Dang, S.T.; Dasgupta, N.; Choudhary, A.; Rai, C.S.; Wang, Y. Nuclear magnetic resonance and molecular simulation study of H2 and CH4 adsorption onto shale and sandstone for hydrogen geological storage. Int. J. Hydrogen Energy 2024, 51, 158–166. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Rep. 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Sari, M.; Sarout, J.; Esteban, L.; Mandal, P.P.; Nguyen, D.; Mallants, D. Hydrogen geo-storage in rock salt formations: Petrophysical characterisation and geomechanical testing of a potential host formation in Western Australia. In Proceedings of the Australian Hydrogen Research Conference, Canberra, Australia, 8–10 February 2023. [Google Scholar]
- Michelsen, J.; Hagemann, B.; Ganzer, L.; Hujer, W. Measurement of hydrogen diffusion through caprock samples. In Proceedings of the Sixth International Conference on Fault and Top Seals, Vienna, Austria, 26–28 September 2022; Volume 2022, pp. 1–5. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, M.B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E.; Iglauer, S. Hydrogen storage in depleted gas reservoirs: A comprehensive review. Fuel 2023, 337, 127032. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Karellas, S.; Tzouganatos, N. Comparison of the performance of compressed-air and hydrogen energy storage systems: Karpathos island case study. Renew. Sustain. Energy Rev. 2014, 29, 865–882. [Google Scholar] [CrossRef]
- Kim, H.M.; Rutqvist, J.; Jeong, J.H.; Choi, B.-H.; Ryu, D.-W.; Song, W.-K. Characterizing excavation damaged zone and stability of pressurized lined rock caverns for underground compressed air energy storage. Rock Mech. Rock Eng. 2013, 46, 1113–1124. [Google Scholar] [CrossRef]
- Allen, R.D.; Doherty, T.J.; Kannberg, L.D. Summary of Selected Compressed Air Energy Storage Studies; Pacific Northwest Laboratory: Richland, WA, USA, 1985; PNL-5091. [Google Scholar]
- Wu, D.; Wang, J.G.; Hu, B.; Yang, S.-Q. A coupled thermo-hydro-mechanical model for evaluating air leakage from an unlined compressed air energy storage cavern. Renew. Energy 2020, 146, 907–920. [Google Scholar] [CrossRef]
- Rutqvist, J.; Kim, H.M.; Ryu, D.W.; Synn, J.-H.; Song, W.-K. Modeling of coupled thermodynamic and geomechanical performance of underground compressed air energy storage in lined rock caverns. Int. J. Rock Mech. Min. Sci. 2012, 52, 71–81. [Google Scholar] [CrossRef]
- Qin, S.K.; Xia, C.C.; Zhou, S.W. Air tightness of compressed air storage energy caverns with polymer sealing layer subjected to various air pressures. J. Rock Mech. Geotech. Eng. 2023, 15, 2105–2116. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, C.C.; Zhou, S.W.; Zhang, P. Air tightness and mechanical properties of polymer sealing layers in compressed air energy storage lined caverns. Chin. J. Rock Mech. Eng. 2018, 37, 2685–2696. [Google Scholar]
- Ahmed, I.H.; Alakoul, K.A.; Al-Manea, A.; Wetaify, A.R.H.; Saleh, K.; Al-Rbaihat, R.; Alahmer, A. An overview of hydrogen production techniques: Challenges and limiting factors in achieving wide-scale productivity. AIP Conf. Proc. 2024, 3051, 050003. [Google Scholar] [CrossRef]
- Hu, B.W.; Yu, Y.L.; Mi, Z.X.; Xu, F.; Li, S.; Li, W.; Wei, C.; Zhang, T. Comparative analysis of thermodynamic and mechanical responses between underground hydrogen storage and compressed air energy storage in lined rock caverns. Int. J. Min. Sci. Technol. 2024, 34, 531–543. [Google Scholar] [CrossRef]
- Bachand, A.; Doyon, B.; Raymond, J. Thermo-physical numerical model for hydrogen storage in underground tanks and caverns. Int. J. Hydrogen Energy 2024, 66, 66–80. [Google Scholar] [CrossRef]
- Wei, L.Y.; Zhen, M.X.; Hu, B.W.; Li, S.; Liu, R.; Ye, J. Three-dimensional thermo-hydro-mechanical coupling model of lined rock cavern hydrogen storage and stability analysis of cavern group operation. J. China Univ. Min. Technol. 2024, 53, 1099–1116. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, P.; Wang, T.; Tang, X.; Wang, Z.; Liu, L.; Luo, J.; Tang, J. Thermodynamic performance of hydrogen in lined rock caverns for large-scale underground storage: Short-term and long-term analyses. J. Energy Storage 2025, 117, 116149. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, L.; Li, X.; Hou, B.; Wu, X.; Gui, X.; Cao, D.; Liu, J.; Li, J.; Duan, J.; et al. Robust, high-temperature-eesistant polyimide separators with vertically aligned uniform nanochannels for high-performance lithium-ion batteries. ACS Nano 2024, 18, 32162–32174. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, F.; Fager, A.; Crouse, B. Numerical analysis of hydrogen storage in lined rock cavern under high pressure and its implications to hydrogen embrittlement. Int. J. Hydrogen Energy 2024, 80, 1021–1029. [Google Scholar] [CrossRef]
- Gajda, D. Epoxy resin for sealing the underground hydrogen storage reservoirs. In Proceedings of the 5th International Conference on Energy Harvesting, Storage and Transfer (EHST’21), Virtual Conference, 21–23 May 2021. [Google Scholar] [CrossRef]
- Kushnir, R.; Dayan, A.; Ullmann, A. Temperature and pressure variations within compressed air energy storage caverns. Int. J. Heat Mass Transf. 2021, 55, 5616–5630. [Google Scholar] [CrossRef]
- Kushnir, R.; Ullmann, A.; Dayan, A. Thermodynamic models for the temperature and pressure variations within adiabatic caverns of compressed air energy storage plants. J. Energy Resour. Technol. 2012, 132, 021901. [Google Scholar] [CrossRef]
- Díaz, A.; Alegre, J.M.; Cuesta, I.I. A review on diffusion modelling in hydrogen related failures of metals. Eng. Fail. Anal. 2016, 66, 577–595. [Google Scholar] [CrossRef]
- Liu, M. Corrosion and mechanical behavior of metal materials. Materials 2023, 16, 973. [Google Scholar] [CrossRef] [PubMed]
- Flaconnèche, B.; Martin, J.; Klopffer, M.H. Transport properties of gases in polymers: Experimental methods. Oil Gas Sci. Technol. 2001, 56, 245–259. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, C.N.; Chi, Y.H.; Lin, Y.L. Permeation characteristics of hydrogen through palladium membranes in binary and ternary gas mixtures. Int. J. Energy Res. 2017, 41, 1579–1595. [Google Scholar] [CrossRef]
- Xia, C.C.; Zhou, Y.; Zhou, S.W.; Wang, X.J.; Mei, S.H.; Zhou, S.W. Key Technologies of Underground Rock-Lined Caverns for Compressed Air Energy Storage; Tongji University Press: Shanghai, China, 2023; pp. 45–56. [Google Scholar]
- Hori, M.; Goda, Y.; Onishi, H. Mechanical behaviour of surrounding rock mass and new lining structure of air-tight pressure cavern. In Proceedings of the ISRM Congress, Sandton, South Africa, 8–12 September 2003. ISRM-10CONGRESS-088. [Google Scholar]
- Zhou, Y.; Xia, C.C.; Zhao, H.B.; Wang, X.J.; Mei, S.H.; Zhou, S.W. A method for estimating air leakage through inner seals and mechanical responses of the surrounding rock of lined rock caverns for compressed air energy storage. Chin. J. Rock Mech. Eng. 2017, 36, 297–309. [Google Scholar] [CrossRef]
- Li, S.; He, D.; Liu, X.P.; Zhang, C.; Wang, S.-M.; Yu, Q.-H.; Qiu, H.-C.; Jiang, L.-J. Hydrogen permeation properties of alumina coating on 316L stainless steel. J. Inorg. Mater. 2013, 28, 775–779. [Google Scholar]
- Gajda, D.; Lutyński, M. Hydrogen permeability of epoxy composites as liners in lined rock caverns—Experimental study. Appl. Sci. 2021, 11, 3885. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, L.; Li, H.; Qi, G.; Gao, X.; Yang, W. Molecular simulation study on the hydrogen permeation behavior and mechanism of common polymers. Polymers 2024, 16, 953. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Mano, H.; Sakurai, M.; Notomi, M. Evaluation of hydrogen permeability of polymer membranes. J. Soc. Mater. Sci. Jpn. 2022, 71, 903–909. [Google Scholar] [CrossRef]
- QX/T 357-2016; Technical Specification for Meteorological Hydrogen Operation Safety. Chinese Meteorological Administration: Beijing, China, 2016.



| Parameters | Definitions | Values | Units |
|---|---|---|---|
| Cavern volume | 3 × 105 | ||
| Cavern radius | 8 | ||
| Sealing layer thickness | 0.1 | ||
| Lining layer thickness | 0.5 | ||
| Initial air temperature | 310 | ||
| Initial air pressure | 4.5 | ||
| Injection air temperature | 322.4 |
| Position | Density/ | Young’s Modulus/ | Poisson’s Ratio | Heat Transfer Coefficient/ | Specific Heat/ | Thermal Expansion Coefficient/ | |
|---|---|---|---|---|---|---|---|
| Analytical solution | Surrounding rock | 2700 | 30 | 0.3 | 3.5 | 1000 | 1.2 × 10−5 |
| Lining layer | 2500 | 30 | 0.3 | 1.4 | 837 | 1.2 × 10−5 | |
| Sealing layer | 7800 | 200 | 0.3 | 45 | 500 | 1.7 × 10−5 | |
| Hokkaido | Surrounding rock | 2600 | 2.4 | 0.3 | 1.714 | 1055 | 1.0 × 10−5 |
| Lining layer | 2400 | 32.5 | 0.2 | 2.94 | 960 | 1.0 × 10−5 | |
| Sealing layer | 920 | 0.0015 | 0.4995 | 0.0091 | 1940 | 4.8 × 10−4 |
| Parameters | Air | Hydrogen | Units |
|---|---|---|---|
| Specific heat at volume () | 0.718 × 103 | 9.934 × 103 | |
| Specific heat at pressure () | 1.005 × 103 | 14.05 × 103 | |
| Gas constant () | 0.287 × 103 | 4.124 × 103 | |
| Gas pressure in critical state () | 3.766 | 1.297 | |
| Gas temperature in critical state () | 132.65 | 33.3 |
| Parameters | Definitions | Values | Units |
|---|---|---|---|
| Cavern volume | 1611.6 | ||
| Cavern radius | 3 | ||
| Sealing layer thickness | 9 × 10−3 | ||
| Lining layer thickness | 0.3 | ||
| Initial air temperature | 301.65 | ||
| Initial air pressure | 0 | ||
| Injection air temperature | 294.65 |
| Density/ | Young’s Modulus/ | Poisson’s Ratio | Heat Transfer Coefficient/ | Specific Heat/ | Thermal Expansion Coefficient/ | Permeability/ | |
|---|---|---|---|---|---|---|---|
| Stainless steel | 8000 | 200 | 0.3 | 20 | 500 | 1.7 × 10−5 | |
| EP | 1100 | 3 | 0.4 | 0.3 | 1000 | 5.6 × 10−5 | 1.365 × 10−18 [36] |
| EVOH | 1140 | 3.6 | 0.42 | 0.15 | 1700 | 8 × 10−5 | 2.231 × 10−15 [37] |
| PI | 1350 | 3 | 0.35 | 0.2 | 1200 | 2 × 10−5 | 7.302 × 10−16 [38] |
| Surrounding rock | 2700 | 30 | 0.3 | 3.5 | 1000 | 1.2 × 10−5 | |
| Lining layer | 2500 | 30 | 0.3 | 1.4 | 837 | 1.2 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Li, Y.; Zou, J.; Chen, Y. Multiphysical Coupling Analysis of Sealing Performance of Underground Lined Caverns for Hydrogen Storage. Processes 2025, 13, 3716. https://doi.org/10.3390/pr13113716
Cui S, Li Y, Zou J, Chen Y. Multiphysical Coupling Analysis of Sealing Performance of Underground Lined Caverns for Hydrogen Storage. Processes. 2025; 13(11):3716. https://doi.org/10.3390/pr13113716
Chicago/Turabian StyleCui, Shaodong, Yin Li, Junwu Zou, and Yun Chen. 2025. "Multiphysical Coupling Analysis of Sealing Performance of Underground Lined Caverns for Hydrogen Storage" Processes 13, no. 11: 3716. https://doi.org/10.3390/pr13113716
APA StyleCui, S., Li, Y., Zou, J., & Chen, Y. (2025). Multiphysical Coupling Analysis of Sealing Performance of Underground Lined Caverns for Hydrogen Storage. Processes, 13(11), 3716. https://doi.org/10.3390/pr13113716





