Phytotoxicity Evaluation of Leaf Extracts and Isolation of Phytotoxic Compounds from Trewia nudiflora Linn. for Natural Weed Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species Collection
2.2. Methods of T. nudiflora Leaf Extraction
2.3. Different Purification Steps for the Isolation of Phytotoxic Compounds
2.4. Compound Efficacy Evaluation Through Growth Bioassay
2.5. Analysis
3. Results
3.1. Phytotoxicity of T. nudiflora Extracts on Cress and Italian Ryegrass
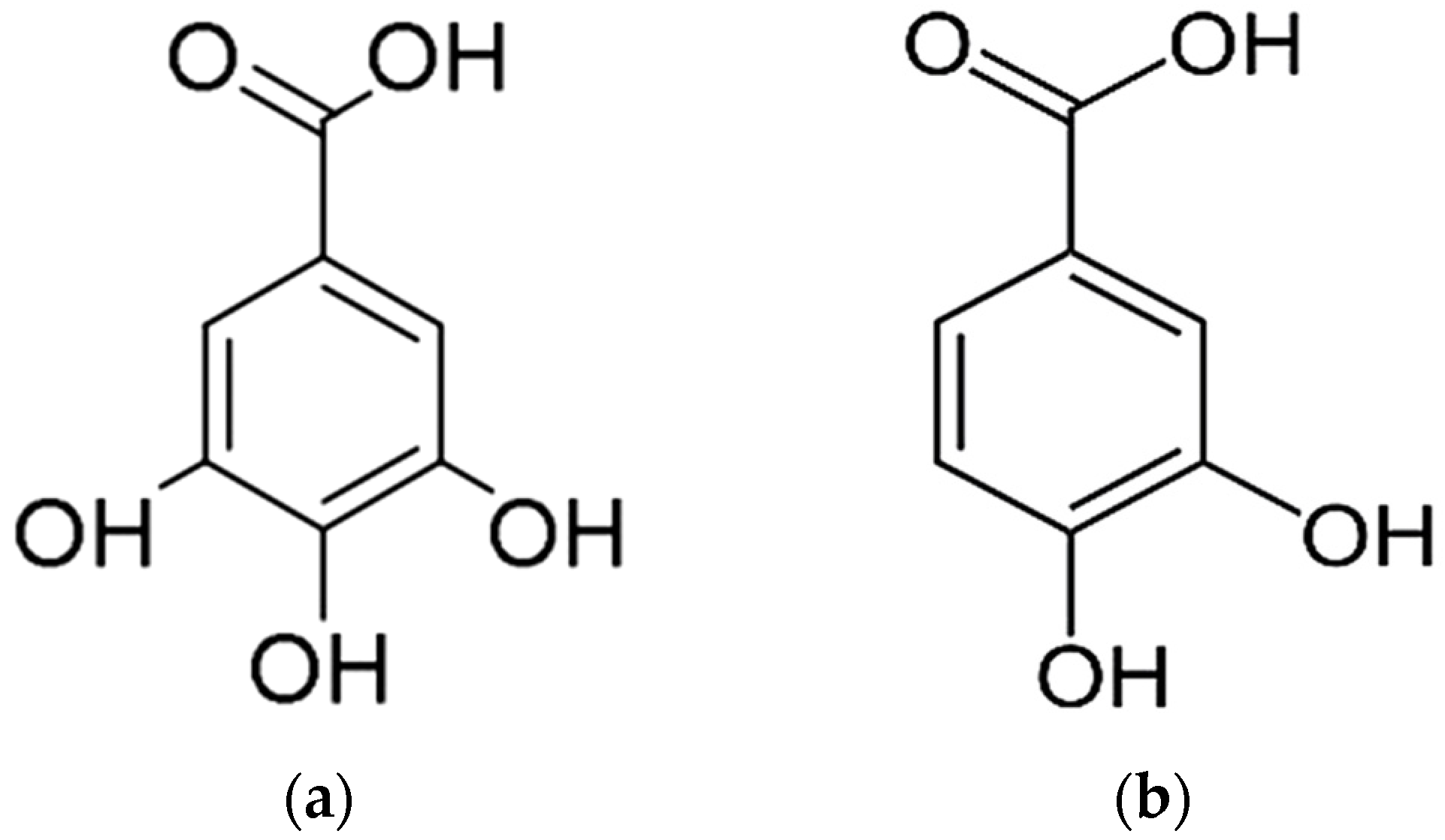
3.2. Identification of the T. nudiflora-Derived Isolated Compounds
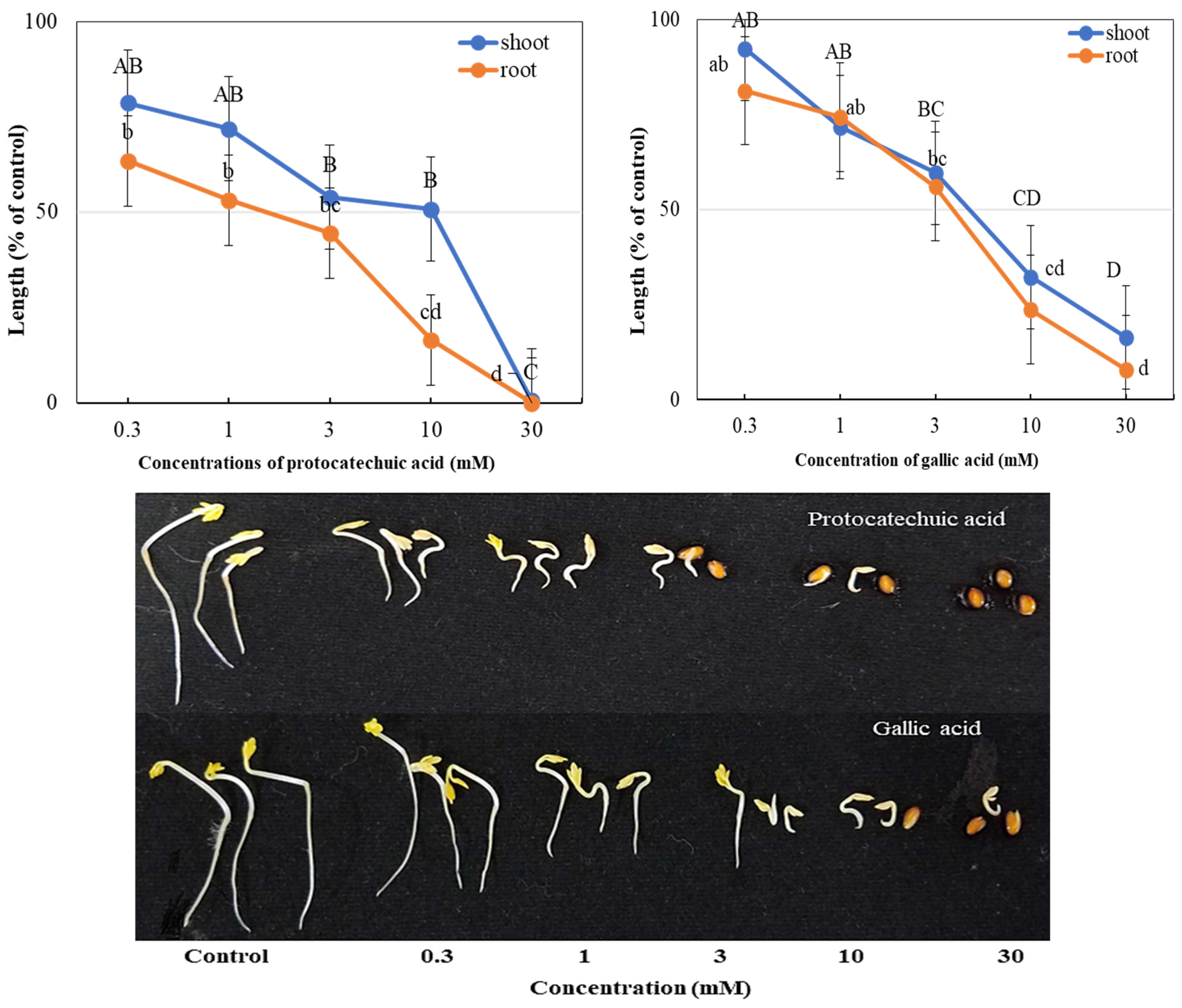
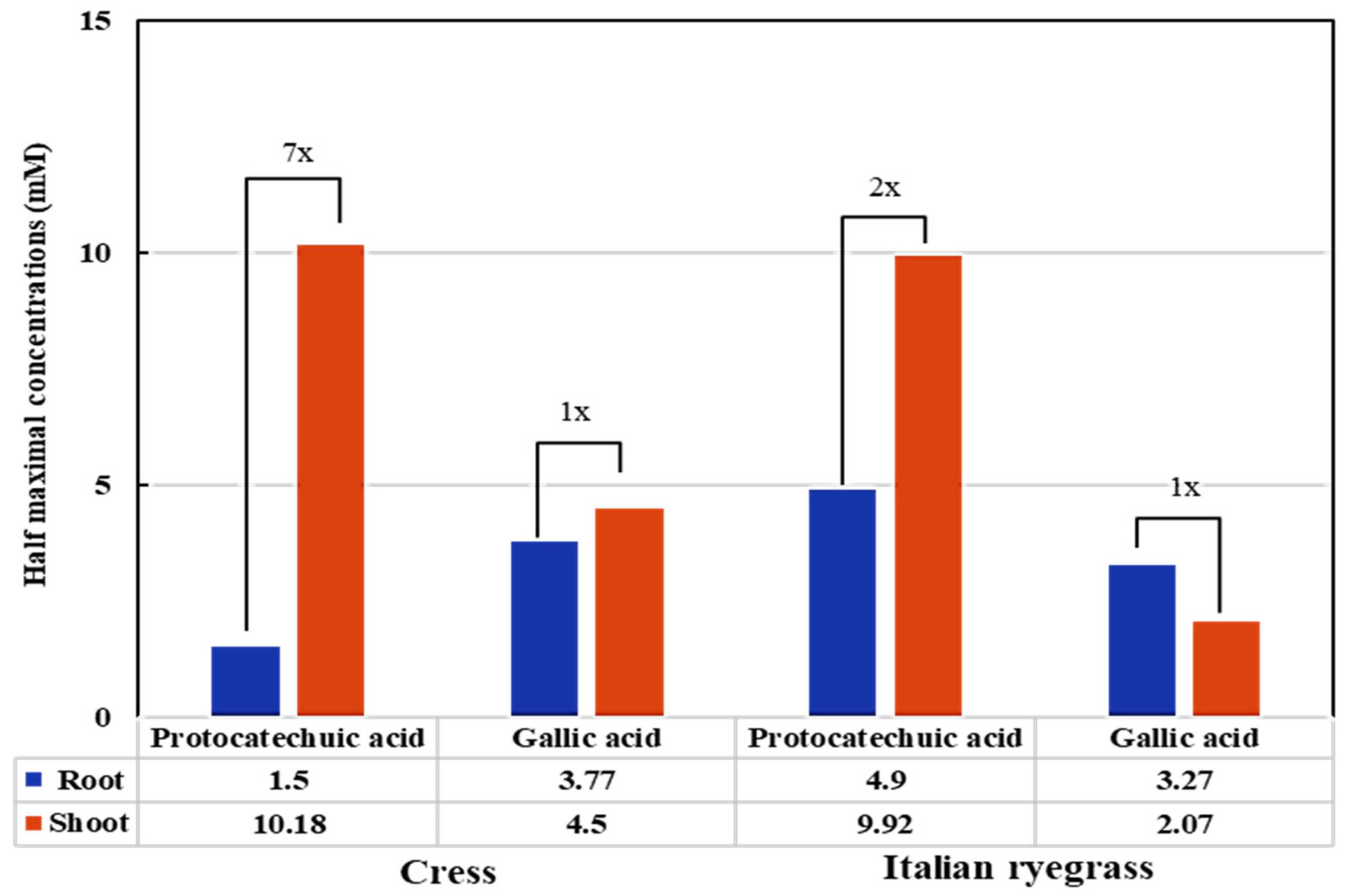
3.3. The Phytotoxicity of Protocatechuic and Gallic Acid Against Test Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Kumar, S.; Bhowmick, M.K.; Ray, P. Weeds as alternate and alternative hosts of crop pests. Indian J. Weed Sci. 2021, 53, 14–29. [Google Scholar] [CrossRef]
- Mada, D.; Duniya, N.; Adams, I.G. Effect of continuous application of herbicide on soil and environment with crop protection machinery in Southern Adamawa state. Int. Ref. J. Eng. Sci. 2013, 2, 4–9. Available online: www.irjes.comwww.irjes.com (accessed on 5 June 2020).
- Isenring, R. Pesticides and the Loss of Biodiversity; Pesticide Action Network Europe: London, UK, 2013; p. 26. Available online: https://www.researchgate.net/publication/327656372_Pesticides_and_the_loss_of_biodiversity_How_intensive_pesticide_use_affects_wildlife_populations_and_species_diversity (accessed on 31 January 2010).
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 4 June 2022).
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Heidarzadeh, A.; Pirdashti, H.; Esmaeili, M. Quantification of allelopathic substances and inhibitory potential in root exudates of rice (Oryza sativa) varieties on barnyard grass (Echinochloa crus-galli L.). Plant Omics 2010, 3, 204–209. Available online: https://search.informit.org/doi/abs/10.3316/informit.559679539296574 (accessed on 30 November 2010).
- Bhadoria, P.B.S. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Devasinghe, D.A.U.D.; Premarathne, K.P.; Sangakkara, U.R. Weed management by rice straw mulching in direct seeded lowland rice (Oryza sativa L.). Trop. Agric. Res. 2011, 22, 263–272. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Ghai, K.; Bhatt, N.; Bhushan, B.; Nayak, A. Phenological Studies on Trewia nudiflora. Int. J. Innov. Technol. Explor. Eng. (IJITEE) 2019, 8, 29–33. [Google Scholar] [CrossRef]
- Kulju, K.K.M.; Sierra, S.E.C.; Van Welzen, P.C. Re-shaping Mallotus [part 2]: Inclusion of Neotrewia, Octospermum and Trewia in Mallotus ss (Euphorbiaceae ss). Blumea—Biodivers. Evol. Biogeogr. Plants 2007, 52, 115–136. [Google Scholar] [CrossRef]
- Trewia nudiflora. Flora of China. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242352644 (accessed on 2 June 2021).
- Sultana, R.; Milon, M.M.M.; Kader, M.A.; Parvin, S.; Masud, G.M. Trewia nudiflora: A potential source of new drugs. J. Phytopharm. 2022, 11, 421–424. [Google Scholar] [CrossRef]
- Kumar, K.P.; Sastry, V.G. Protective of Trewia nudiflora against ischemic stroke in experimental rats. Int. J. Pharmacother. 2012, 2, 7–12. [Google Scholar]
- Balakrishnan, N.; Srivastava, M.; Tiwari, P. Preliminary phytochemical analysis and DPPH free radical scavenging activity of Trewia nudiflora Linn. roots and leaves. Pak. J. Biol. Sci. 2013, 16, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshimi, V.; Chaithanya Kumar, S.; Rajeswary, P.; Madhuri, A.; Chandrasekhar, U. Anti-Ulcerogenic activities of Trewia nudiflora in different experimental models. Int. J. Phytopharm. Res. 2012, 3, 68–71. [Google Scholar]
- Ripa, F.; Hossain, M.; Munira, M.; Roy, A.; Riya, F.; Alam, F.; Feda, F.; Taslim, U.; Nesa, M.; Rashid, M.; et al. Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects. Open Chem. 2022, 20, 1304–1312. [Google Scholar] [CrossRef]
- Khatun, R.; Kato-Noguchi, H. Piper longum L. leaf extracts, a candidate allelopathic plant that suppressed the growth of six test plants, could be a source of potent phytotoxic compounds. Res. Crop. 2022, 23, 874–880. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 16.0; IBM Corp.: Armonk, NY, USA, 2007. [Google Scholar]
- Islam, A.M.; Kato-Noguchi, H. Phytotoxic activity of Ocimum tenuiflorum extracts on germination and seedling growth of different plant species. Sci. World J. 2014, 1, 676242. [Google Scholar] [CrossRef]
- Gutzeit, D.; Wray, V.; Winterhalter, P.; Jerz, G. Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophae rhamnoides L. ssp. rhamnoides) by high-speed counter-current chromatography. Chromatographia 2007, 65, 1–7. [Google Scholar] [CrossRef]
- Braca, A.; Politi, M.; Sanogo, R.; Sanou, H.; Morelli, I.; Pizza, C.; De Tommasi, N. Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J. Agric. Food Chem. 2003, 51, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Kway, E.; Hisashi, K.N. Allelopathic potential of Acacia pennata (L.) Willd. leaf extracts against the seedling growth of six test plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1534–1542. [Google Scholar] [CrossRef]
- Khatun, M.R.; Hisashi, K.N. Growth inhibition of six test plants by Stephania japonica (Thunb.) Miers leaf extracts is an indication of allelopathic activity. Bulg. J. Agric. Sci. 2021, 27, 1093–1099. [Google Scholar]
- El-Rokiek, K.G.; El-Masry, R.R.; Messiha, N.K.; Ahmed, S.A. The allelopathic effect of mango leaves on the growth and propagative capacity of purple nut-sedge (Cyperus rotundus L.). J. Am. Sci. 2010, 6, 151–159. [Google Scholar]
- Saleem, K.; Perveen, S.; Sarwar, N.; Latif, F.; Akhtar, K.P.; Arshad, H.M.I. Identification of phenolics in mango leaves extract and their allelopathic effect on canary grass and wheat. Pak. J. Bot. 2013, 45, 1527–1535. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelopathic substances of mango (Mangifera indica L.). Weed Biol. Manag. 2020, 20, 131–138. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Yasir, M.; Sultana, B.; Amicucci, M. Biological activities of phenolic compounds extracted from Amaranthaceae plants and their LC/ESI-MS/MS profiling. J. Funct. Foods 2016, 26, 645–656. [Google Scholar] [CrossRef]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Simultaneous determination and anti-inflammatory effects of four phenolic compounds in Dendrobii herba. Nat. Prod. Res. 2017, 31, 2923–2926. [Google Scholar] [CrossRef]
- Karunakaran, T.; Ismail, I.S.; Ee, G.C.L.; Nor, S.M.M.; Palachandran, K.; Santhanam, R.K. Nitric oxide inhibitory and anti-Bacillus activity of phenolic compounds and plant extracts from Mesua species. Rev. Bras. Farmacogn. 2018, 28, 231–234. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. Daru-J. Pharm. Sci. 2019, 27, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. The allelopathic effects of Trewia nudiflora leaf extracts and its identified substances. Plants 2023, 12, 1375. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Trewia nudiflora Linn, a Medicinal Plant: Allelopathic Potential and Characterization of Bioactive Compounds from Its Leaf Extracts. Horticulturae 2023, 9, 897. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Hong, N.H.; Khanh, T.D.; Min, C.I. Assessment of phytotoxic action of Ageratum conyzoides L. (billy goat weed) on weeds. Crop Prot. 2004, 23, 915–922. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, X.C.; Pedrol, N. Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Hiroyuki, T.; Mitsuhiro, M.; Khanh, T.D.; Chung, I.M. Evaluation on phytotoxicity of neem (Azadirachta indica. A. Juss) to crops and weeds. Crop Prot. 2004, 23, 335–345. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Huang, Q. Allelopathic effects of gallic acid from Aegiceras corniculatum on Cyclotella caspia. J. Environ. Sci. 2013, 25, 776–784. [Google Scholar] [CrossRef]
- Fu, Y.H.; Quan, W.X.; Li, C.C.; Qian, C.Y.; Tang, F.H.; Chen, X.J. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in Rhododendron delavayi Franch. Photosynthetica 2019, 57, 377–387. [Google Scholar] [CrossRef]
- Hua-Qin, H.E.; Wen-Xiong, L.I.N. Studies on allelopathic physiobiochemical characteristics of rice. Chin. J. Eco-Agric. 2001, 9, 56–57. [Google Scholar]
- Wu, L.; Guo, X.; Harivandi, M.A. Allelopathic effects of phenolic acids detected in buffalograss (Buchloe dactyloides) clippings on growth of annual bluegrass (Poa annua) and buffalograss seedlings. Environ. Exp. Bot. 1998, 39, 159–167. [Google Scholar] [CrossRef]
- Bubna, G.A.; Lima, R.B.; Zanardo, D.Y.L.; Dos Santos, W.D.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Huang, C.Z.; Lei, X.U.; Jin-jing, S.; Zhang, Z.H.; Fu, M.L.; Teng, H.Y.; Yi, K.K. Allelochemical p-hydroxybenzoic acid inhibits root growth via regulating ROS accumulation in cucumber (Cucumis sativus L.). J. Integr. Agric. 2020, 19, 518–527. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and ap-plication of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Maffei, M.; Bertea, C.M.; Garneri, F.; Scannerini, S. Effect of benzoic acid hydroxy-and methoxy-ring substituents during cucumber (Cucumis sativus L.) germination. I. Isocitrate lyase and catalase activity. Plant Sci. 1999, 141, 139–147. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolicacids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlík, J.; Damme, P.V. Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]


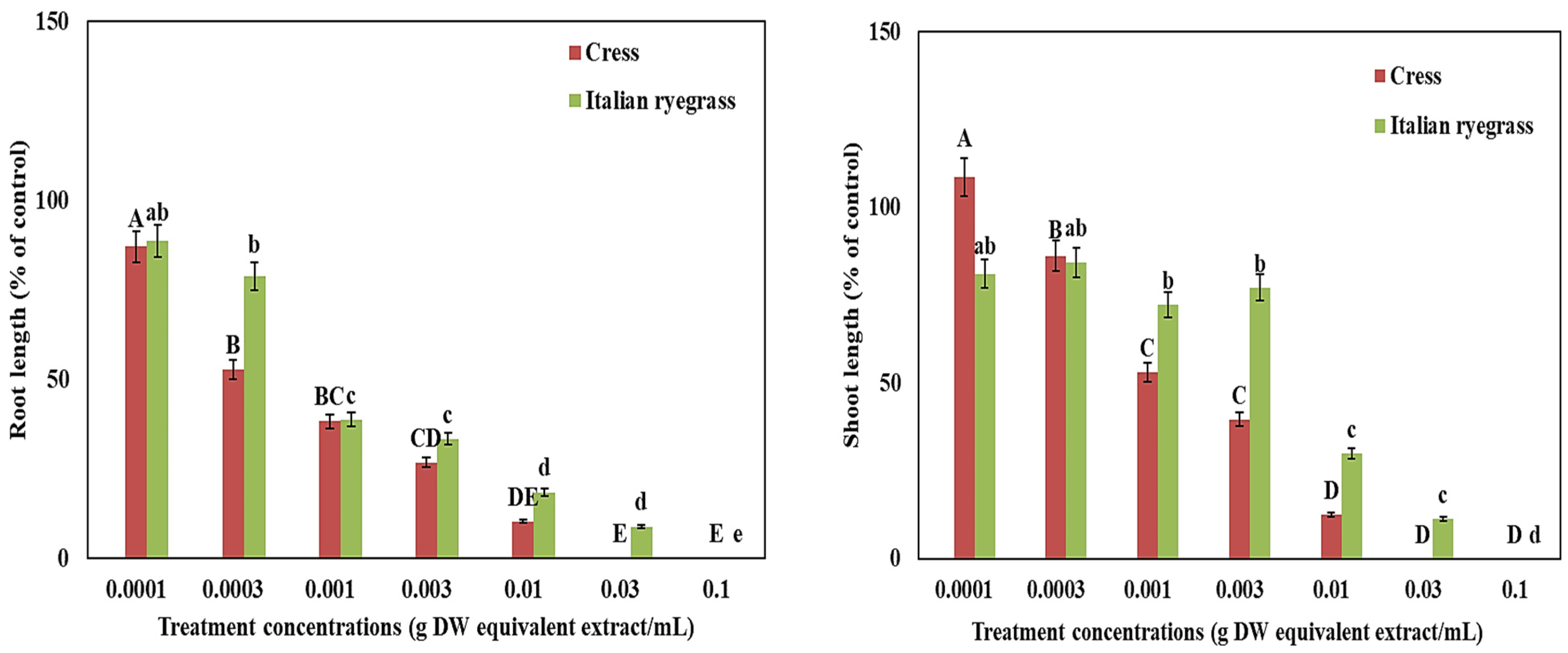

| Test Plant Species | I50 Value (g DW Equivalent T. nudiflora Leaf Extract in mL−1) | ||
|---|---|---|---|
| Dicots Monocots | root | shoot | |
| Cress | 0.00037 b | 0.00129 b | |
| Italian ryegrass | 0.00071 b | 0.00600 a | |
| Test Plants | I50 Value (mM) | ||
|---|---|---|---|
| Cress | Protocatechuic acid | Gallic acid | |
| Shoot | 10.18 a | 4.50 d | |
| Root | 1.50 h | 3.77 e | |
| Italian ryegrass | Shoot | 9.92 b | 2.07 g |
| Root | 4.90 c | 3.27 f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatun, M.R.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Phytotoxicity Evaluation of Leaf Extracts and Isolation of Phytotoxic Compounds from Trewia nudiflora Linn. for Natural Weed Control. Processes 2025, 13, 3691. https://doi.org/10.3390/pr13113691
Khatun MR, Tojo S, Teruya T, Kato-Noguchi H. Phytotoxicity Evaluation of Leaf Extracts and Isolation of Phytotoxic Compounds from Trewia nudiflora Linn. for Natural Weed Control. Processes. 2025; 13(11):3691. https://doi.org/10.3390/pr13113691
Chicago/Turabian StyleKhatun, Mst. Rokeya, Shunya Tojo, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2025. "Phytotoxicity Evaluation of Leaf Extracts and Isolation of Phytotoxic Compounds from Trewia nudiflora Linn. for Natural Weed Control" Processes 13, no. 11: 3691. https://doi.org/10.3390/pr13113691
APA StyleKhatun, M. R., Tojo, S., Teruya, T., & Kato-Noguchi, H. (2025). Phytotoxicity Evaluation of Leaf Extracts and Isolation of Phytotoxic Compounds from Trewia nudiflora Linn. for Natural Weed Control. Processes, 13(11), 3691. https://doi.org/10.3390/pr13113691







