Research on the Metallogenic Enrichment Model of Poly-Metallic Black Shales and Their Geological Significance: A Case Study of the Cambrian Niutitang Formation
Abstract
1. Introduction
2. Geological Settings

3. Sampling and Experiments
3.1. Samples
3.2. Experiments and Methods
4. Results
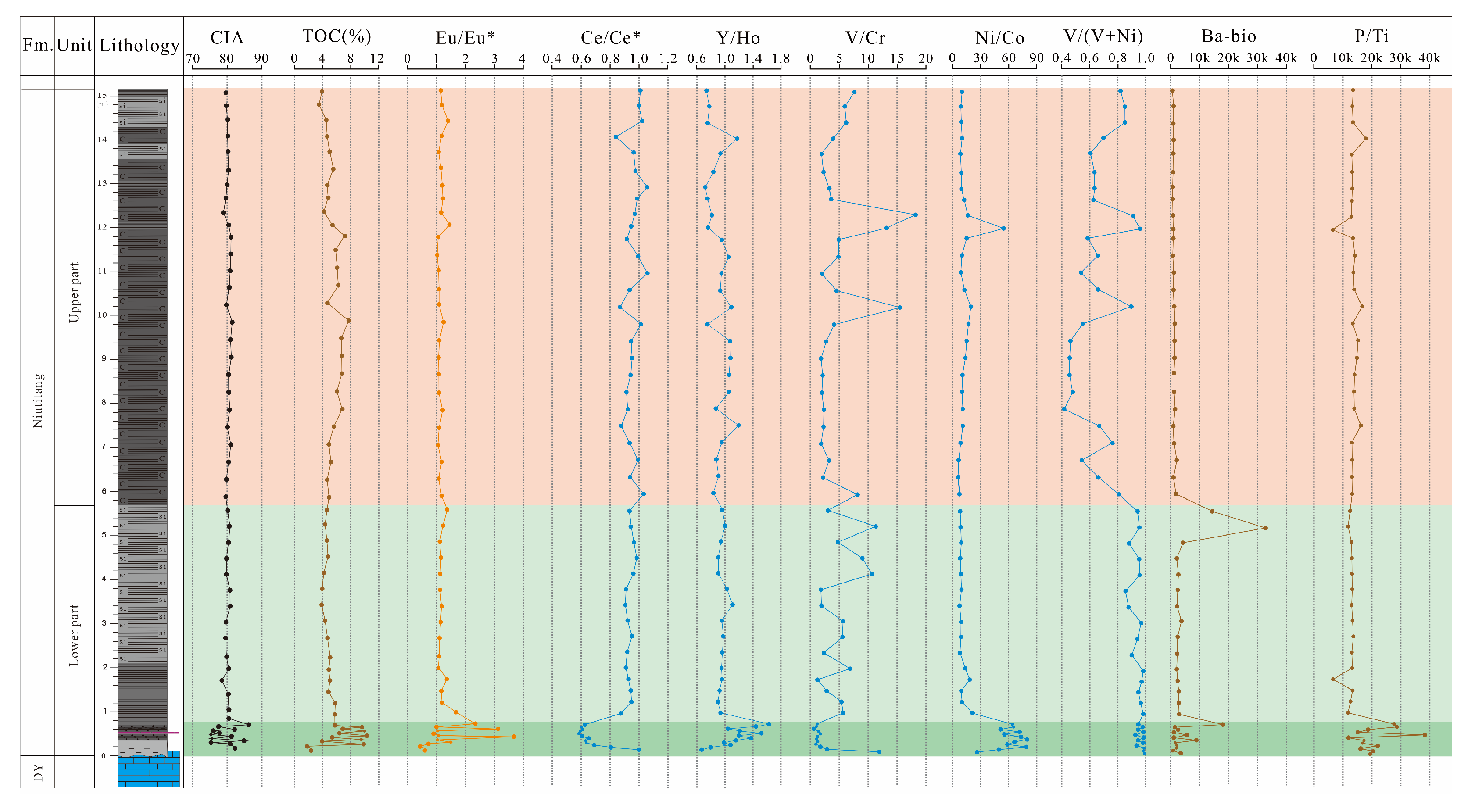
4.1. Total Carton and Major Element
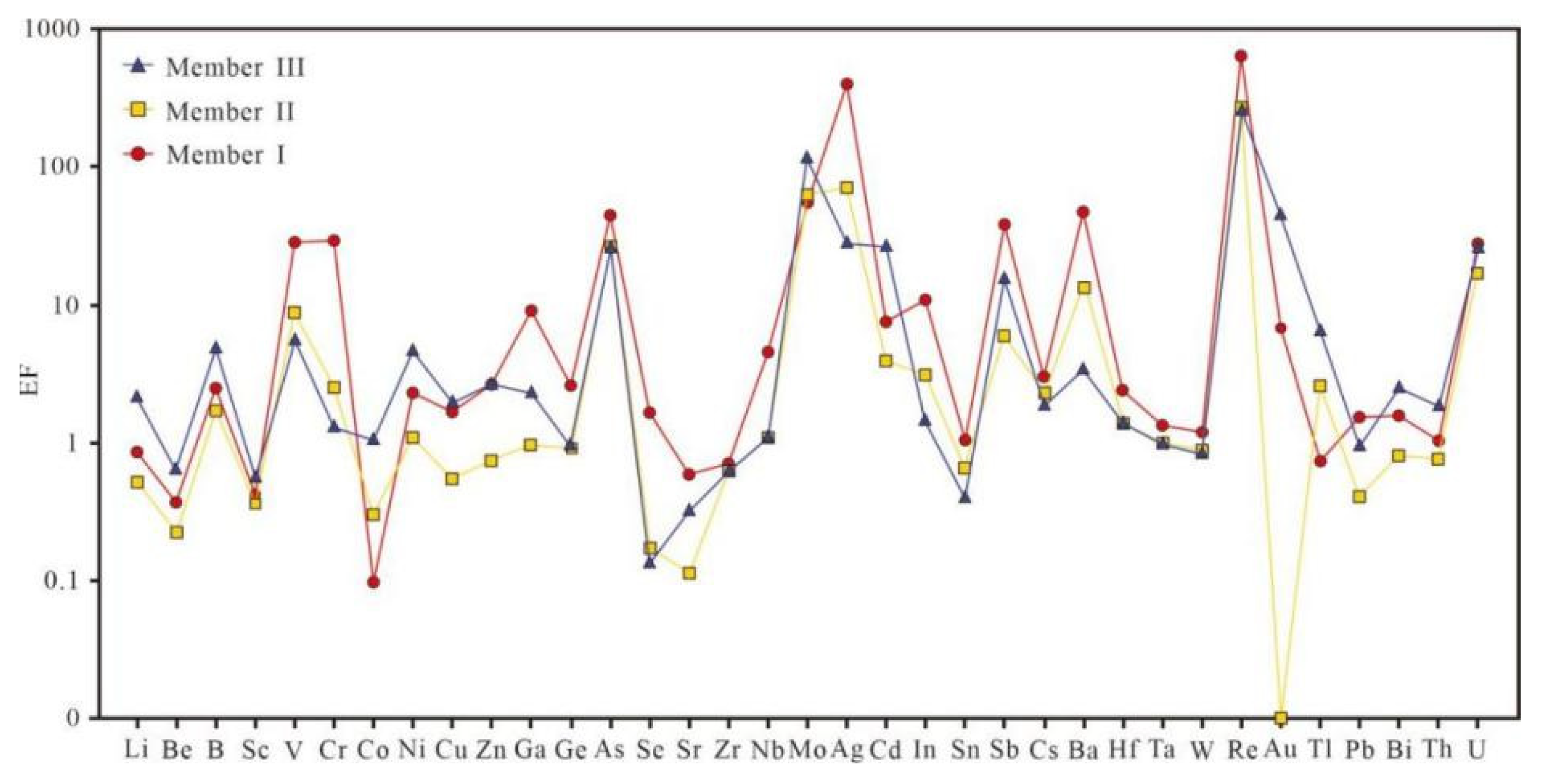
4.2. Trace Element
4.3. Rare Earth Element
5. Discussion
5.1. Detrital-Terrigenous Input
5.2. Hydrothermal Activity
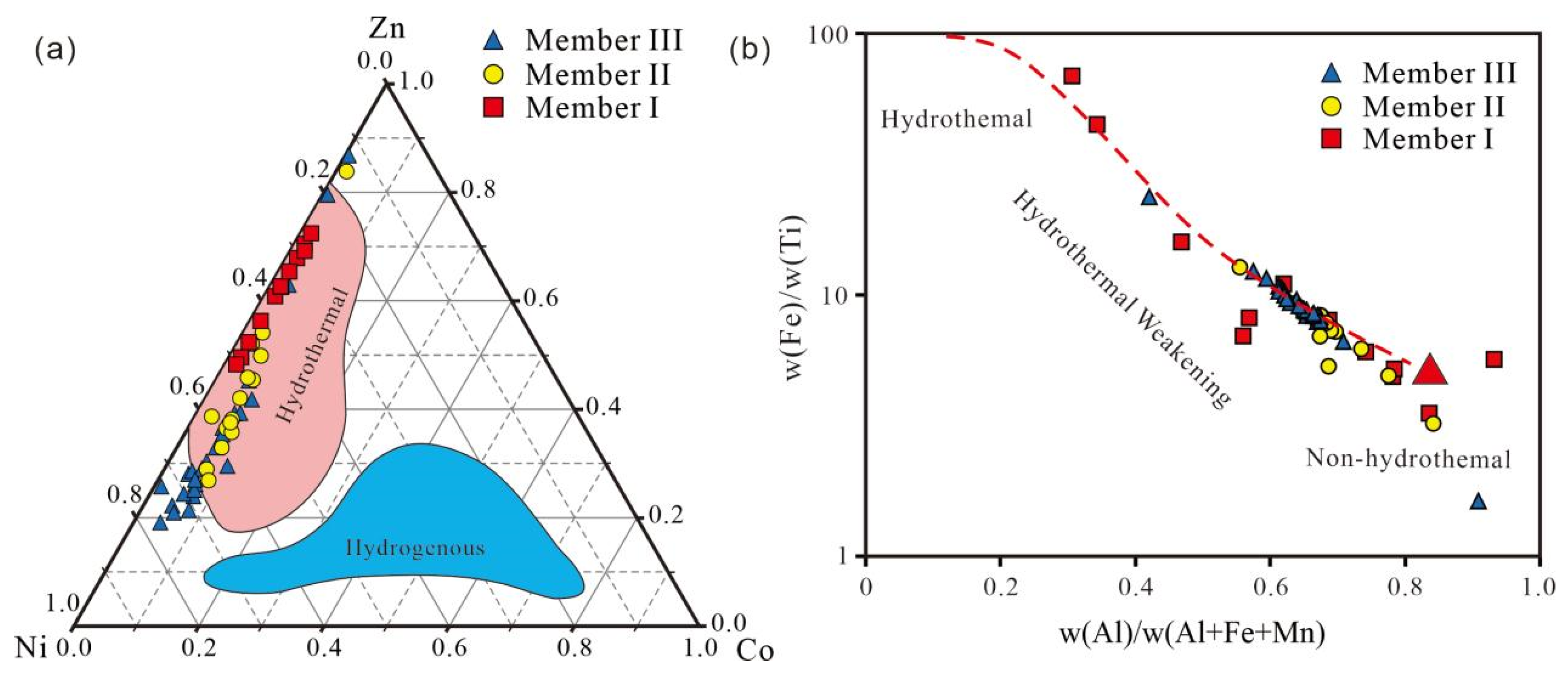

5.3. Redox Conditions
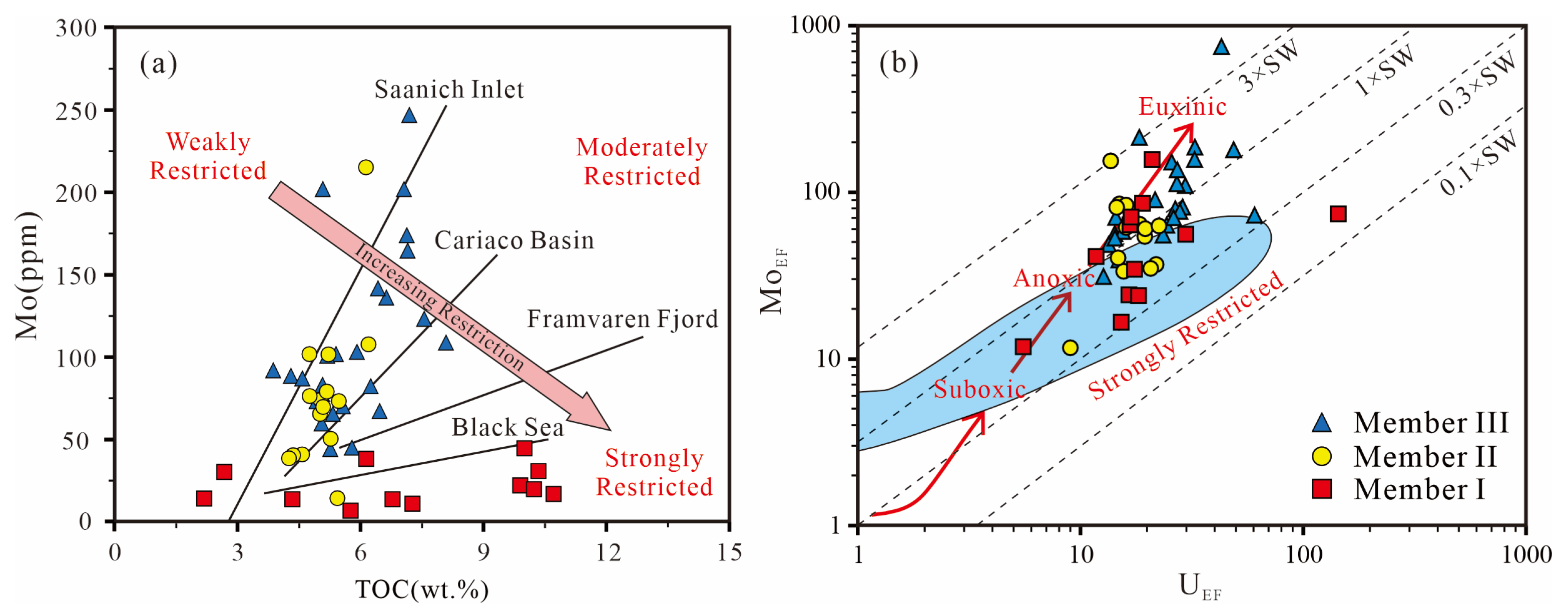
5.4. Organic Scavenging of Metals
5.5. Tectonic Settings and Hydrographic Restrictions
5.6. Metal Enrichment Patterns
6. Conclusions
- During deposition of Member I, intense submarine-hydrothermal activity governed metal enrichment. Elements such as As, Ba, Ag, Cr, V, Re, Cs, and Ga are extremely enriched, yet their abundances show no significant correlation with total organic carbon (TOC), indicating that hydrothermal input, rather than organic complexation, was the dominant factor. Highly restricted basin hydrology rapidly established euxinic bottom waters, promoting synchronous precipitation and sequestration of redox-sensitive metals (Mo, U, V) together with organic matter. Thus, the exceptional metal enrichment in Member I reflects a “hydrothermal dominance and sulfide preservation” synergy, with only minor influence from terrigenous fluxes.
- In Members II and III, waning hydrothermal activity led to a concomitant decline in metal concentrations. Relaxation of basin restriction and enhanced water-column ventilation shifted depositional conditions from euxinic to dysoxic–suboxic, lowering the enrichment of redox-sensitive elements such as Cr and V. In these intervals, TOC is positively correlated with metals (Ag, Re, Ga), implying that organic ligand complexation/adsorption became the principal enrichment mechanism. Although terrigenous input increased (rising Er/Nd ratios), it remained insufficient to dominate metal accumulation. Consequently, enrichment in Members II and III was controlled by the interplay between organic-matter abundance and redox-interface fluctuations.
- Overall, metal enrichment in the Niutitang Formation follows a stage-dependent, multi-source model: Member I is characterized by hydrothermal–euxinic cooperation, whereas Members II and III are dominated by organic-matter and redox-controlled processes.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, H. Interaction Mechanisms Between Organic Matter and Vanadium in Black Shales; China University of Petroleum: Beijing, China, 2022. [Google Scholar]
- Chen, L. Sedimentology and Geochemistry of Early Cambrian Black Shale Series in the Hunan–Guizhou Region; Graduate University of Chinese Academy of Sciences (Institute of Geochemistry): Guiyang, China, 2006. [Google Scholar]
- Wu, C.; Chen, Q.; Lei, J. The genesis factors and organic petrology of black shale series from the upper Sinian to the lower Cambrian, southwest of China. Acta Petrol. Sin. 1999, 15, 453–461. [Google Scholar]
- Shi, C.; Cao, J.; Hu, K.; Bian, L.; Han, S.; Yao, S. A review of origins of mineral deposits hosted in black rock series and the mineralizing functions of their sea water, hydrothermal fluid and bio-organics. Earth Sci. Front. 2013, 20, 19–31. [Google Scholar]
- Fan, D. Metalliferous black shale series and manganese deposits. Bull. Mineral. Petrol. Geochem. 1988, 2, 84–86. [Google Scholar]
- Fan, D.; Yang, X.; Wng, L.; Chen, N. Petrological and geochemical characteristics of Ni–Mo polymetallic black shale series of the Lower Cambrian. Geochimica 1973, 3, 143–164. [Google Scholar]
- Jin, Z.; Wang, X.; Wang, H.; Ye, Y.; Zhang, S. Organic carbon cycling and black shale deposition: An Earth System Science perspective. Natl. Sci. Rev. 2023, 10, 243. [Google Scholar] [CrossRef]
- Wen, H.; Zhou, Z.; Ma, W.; Zhu, Y. Research progresses and main scientific issues of strategically critical minerals in black rock series. Bull. Mineral. Petrol. Geochem. 2024, 43, 14–34. [Google Scholar] [CrossRef]
- Xu, L.; Lehmann, B.; Mao, J. Seawater contribution to polymetallic Ni–Mo–PGE–Au mineralization in Early Cambrian black shales of South China: Evidence from Mo isotope, PGE, trace element, and REE geochemistry. Ore Geol. Rev. 2012, 52, 66–84. [Google Scholar] [CrossRef]
- Lehmann, B.; Nägler, T.F.; Holland, H.D.; Wille, M.; Mao, J.; Pan, J.; Ma, D.; Dulski, P. Highly metalliferous carbonaceous shale and Early Cambrian seawater. Geology 2007, 35, 403–406. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, P.; Li, T.; Zhao, K.; Yan, H.; Li, J.; Zhou, L. Nitrogen geochemistry and abnormal mercury enrichment of shales from the lowermost Cambrian Niutitang Formation in South China: Implications for the marine redox conditions and hydrothermal activity. Glob. Planet. Change 2021, 199, 103449. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, S.; Yang, J.; Pi, D.; Ling, H.; Chen, Y. Rare earth element and Sr-Nd isotope geochemistry of phosphate nodules from the Lower Cambrian Niutitang Formation, NW Hunan Province, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 132–143. [Google Scholar] [CrossRef]
- Gao, P.; He, Z.; Li, S.; Lash, G.; Li, B.; Huang, B.; Yan, D. Volcanic and hydrothermal activities recorded in phosphate nodules from the Lower Cambrian Niutitang Formation black shales in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 505, 381–397. [Google Scholar] [CrossRef]
- Han, T.; Fan, H.; Zhu, X.; Wen, H.; Zhao, C.; Xiao, F. Submarine hydrothermal contribution for the extreme element accumulation during the early Cambrian, South China. Ore Geol. Rev. 2017, 86, 297–308. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, X.; Teng, G.; Xie, X.; Yin, L.; Bian, L.; Feng, Q.; Algeo, T. The lower Cambrian Niutitang Formation at Yangtiao (Guizhou, SW China): Organic matter enrichment, source rock potential, and hydrothermal influences. J. Pet. Geol. 2015, 38, 411–432. [Google Scholar] [CrossRef]
- Meert, J.G.; Lieberman, B.S. A palaeomagnetic and palaeobiogeographical perspective on latest neoproterozoic and early cambrian tectonic events. J. Geol. Soc. 2004, 161, 477–487. [Google Scholar] [CrossRef]
- Li, C.; Love, G.D.; Lyons, T.W.; Fike, D.A.; Sessions, A.L.; Chu, X. A stratified redox model for the Ediacaran Ocean. Science 2010, 328, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lehmann, B.; Du, A.; Zhang, G.; Ma, D.; Wang, Y.; Zeng, M.; Kerrich, R. Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in Lower Cambrian black shales of South China and its geologic significance. Econ. Geol. 2002, 97, 1051–1061. [Google Scholar] [CrossRef]
- Pi, D.; Liu, C.; Shields-Zhou, G.A.; Jiang, S. Trace and rare earth element geochemistry of black shale and kerogen in the early Cambrian Niutitang Formation in Guizhou Province, South China: Constraints for redox environments and origin of metal enrichments. Precambrian Res. 2013, 225, 218–229. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, L.; Li, C.; Qu, W.; Pei, H.; Qiao, W.; Shen, B. New Re-Os isotopic constrains on the formation of the metalliferous deposits of the Lower Cambrian Niutitang formation. J. Earth Sci. 2016, 27, 271–281. [Google Scholar] [CrossRef]
- Lehmann, B.; Frei, R.; Xu, L.; Mao, J. Early Cambrian Black Shale-Hosted Mo-Ni and V Mineralization on the Rifted Margin of the Yangtze Platform, China: Reconnaissance Chromium Isotope Data and a Refined Metallogenic Model. Econ. Geol. 2016, 111, 89–103. [Google Scholar] [CrossRef]
- Li, S. Tracing the Sources of Precious Metals in the Cambrian Basal Black Rock Series of Hunan-Guizhou, South China. Sci. Sin. (Terrae) 2000, 2, 169–174. [Google Scholar]
- Emsbo, P.; Hofstra, A.H.; Johnson, C.A.; Koenig, A.; Grauch, R.; Zhang, X.; Hu, R.; Su, W.; Pi, D. Lower Cambrian metallogenesis of south China: Interplay between diverse basinal hydrothermal fluids and marine chemistry. In Mineral Deposit Research: Meeting the Global Challenge, Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Springer: Berlin/Heidelberg, Germany, 2005; pp. 115–118. [Google Scholar]
- Hofstra, A.H.; Emsbo, P. Solubility of metals and nutrients in brines: Implications for ore deposits, bioproductivity, and anoxia in sedimentary basins. GSA Abstr. 2004, 36, 200. [Google Scholar]
- Jiang, S.; Chen, Y.; Ling, H.; Yang, J.; Feng, H.; Ni, P. Trace- and rare-earth element geochemistry and Pb–Pb dating of black shales and intercalated Ni–Mo–PGE–Au sulfide ores in Lower Cambrian Strata, Yangtze Platform, South China. Miner. Depos. 2006, 41, 453–467. [Google Scholar] [CrossRef]
- Shu, L.; Faure, M.; Yu, J.; Jahn, B.M. Geochronological and geochemical features of the Cathaysia block (South China): New evidence for the Neoproterozoic breakup of Rodinia. Precambrian Res. 2011, 187, 263–276. [Google Scholar] [CrossRef]
- Okada, Y.; Sawaki, Y.; Komiya, T.; Hirata, T.; Takahata, N.; Sano, Y.; Han, J.; Maruyama, S. New chronological constraints for Cryogenian to Cambrian rocks in the Three Gorges, Weng’an and Chengjiang areas, South China. Gondwana Res. 2014, 25, 1027–1044. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z. History of Neoproterozoic rift basins in South China: Implications for Rodinia break-up. Precambrian Res. 2003, 122, 141–158. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, X.; Shi, X.; Xiao, S.; Zhang, S.; Dong, J. The origin of decoupled carbonate and organic carbon isotope signatures in the early Cambrian (ca. 542–520 Ma) Yangtze platform. Earth Planet. Sci. Lett. 2012, 317–318, 96–110. [Google Scholar] [CrossRef]
- Wei, S.; Fu, Y.; Liang, H.; Ge, Z.; Zhou, W.; Wang, G. Re–Os geochronology of the Cambrian stage-2 and -3 boundary in Zhijin County, Guizhou Province, China. Acta Geochim. 2017, 37, 323–333. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Zhou, M. Expanded deepwater euxinia recorded in the Ediacaran–Cambrian boundary interval in South China. J. Asian Earth Sci. 2022, 230, 105192. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, Y.; Li, C.; Cai, X.; Gu, C. Sedimentary environment and mineralization of the black shale polymetallic layer in the Early Cambrian, SW China: Constraints from in situ La-ICP-Ms Analysis of pyrite. Acta Geol. Sin. (Engl. Ed.) 2024, 98, 416–429. [Google Scholar] [CrossRef]
- Jin, C.; Li, C.; Algeo, T.J.; Planavsky, N.J.; Cui, H.; Yang, X.; Zhao, Y.; Zhang, X.; Xie, S. A highly redox-heterogeneous ocean in South China during the early Cambrian (~529–514 Ma): Implications for biota-environment co-evolution. Earth Planet. Sci. Lett. 2016, 441, 38–51. [Google Scholar] [CrossRef]
- Deng, X.; Yang, K.; Liu, Y.; She, Z. Characteristics and tectonic evolution of Qianzhong Uplift. Earth Sci. Front. 2010, 17, 79–89. [Google Scholar]
- Lan, Z.; Li, X.; Chu, X.; Tang, G.; Yang, S.; Yang, H.; Liu, H.; Jiang, T.; Wang, T. SIMS U-Pb zircon ages and Ni-Mo-PGE geochemistry of the lower Cambrian Niutitang Formation in South China: Constraints on Ni-Mo-PGE mineralization and stratigraphic correlations. J. Asian Earth Sci. 2017, 137, 141–162. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, T.; Huff, W.D.; Liu, S. Prominent Lower Cambrian K-bentonites in South China: Distribution, mineralogy, and geochemistry. J. Sediment. Res. 2014, 84, 842–853. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, D.; Zhao, F.; Li, X.; Qiu, L.; Zhang, Y. Stratigraphic sequences, abundance anomalies and occurrences of As, Sb, Au, Ag in the Lower Cambrian Niutitang Formation in Kaiyang Phosphate Mine area. Acta Petrol. Sin. 2016, 32, 3252–3268. [Google Scholar]
- Li, Z.; Bogdanova, S.V.; Collins, A.S.; Davidson, A.; De Waele, B.; Ernst, R.E.; Fitzsimons, I.C.W.; Fuck, R.A.; Gladkochub, D.P.; Jacobs, J.; et al. Assembly, configuration, and break-up history of Rodinia: A synthesis. Precambrian Res. 2008, 160, 179–210. [Google Scholar] [CrossRef]
- Awan, R.S.; Liu, C.; Gong, H.; Dun, C.; Tong, C.; Chamssidini, L.G. Paleo-sedimentary environment in relation to enrichment of organic matter of Early Cambrian black rocks of Niutitang Formation from Xiangxi area China. Mar. Pet. Geol. 2020, 112, 104057. [Google Scholar] [CrossRef]
- GB/T 19145-2022; Determination for Total Organic Carbon in Sedimentary Rock. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 1–24. [Google Scholar] [CrossRef]
- Shields, G.; Stille, P. Diagenetic constraints on the use of cerium anomalies as palaeoseawater redox proxies: An isotopic and REE study of Cambrian Phosphorites. Chem. Geol. 2001, 175, 29–48. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks; influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 1989, 21, 169–200. [Google Scholar]
- Nesbitt, H.; Young, G. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Baudin, F.; Riboulleau, A. Analysis of marine environmental conditions based onmolybdenum–uranium covariation—Applications to Mesozoic paleoceanography. Chem. Geol. 2012, 324, 46–58. [Google Scholar] [CrossRef]
- Schoepfer, S.D.; Shen, J.; Wei, H.; Tyson, R.V.; Ingall, E.; Algeo, T.J. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth-Sci. Rev. 2015, 149, 23–52. [Google Scholar] [CrossRef]
- Editorial Committee of the Industrial Requirements for Mineral Resources Manual. Industrial Requirements for Mineral Resources Manual; Geological Publishing House: Beijing, China, 2010. [Google Scholar]
- Nozaki, Y. Rare earth elements and their isotopes in the Ocean. In Encyclopedia of Ocean Science; Academic Press: Cambridge, MA, USA, 2001; pp. 2354–2366. [Google Scholar]
- Zhao, L.; Chen, Z.; Algeo, T.; Chen, J.; Chen, Y.; Tong, J.; Gao, S.; Zhou, L.; Hu, Z.; Liu, Y. Rare-earth element patterns in Conodont Albid Crowns: Evidence for massive inputs of volcanic ash during the latest Permian Biocrisis? Glob. Planet. Change 2013, 105, 135–151. [Google Scholar] [CrossRef]
- Abedini, A.; Calagari, A.A. REEs geochemical characteristics of lower Cambrian phosphatic rocks in the Gorgan-Rasht Zone, northern Iran: Implications for diagenetic effects and depositional conditions. J. Afr. Earth Sci. 2017, 135, 115–124. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Y.; Liu, X.; Zhai, G.; Wang, Y.; Zhang, C. Depositional environment and organic matter enrichment of the lower Cambrian Niutitang shale in western Hubei Province, South China. Mar. Pet. Geol. 2019, 109, 381–393. [Google Scholar] [CrossRef]
- Korzhinsky, M.A.; Tkachenko, S.I.; Shmulovich, K.I.; Taran, Y.A.; Steinberg, G.S. Discovery of a pure rhenium mineral at Kudriavy volcano. Nature 1994, 369, 51–52. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Qing, H.; Yan, D.; Li, R. Hydrothermal venting activities in the Early Cambrian, South China: Petrological, geochronological and stable isotopic constraints. Chem. Geol. 2009, 258, 168–181. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Wang, D.; Yan, D.; Zhou, X.; Wang, Q. Petrology and geochemistry of chert on the marginal zone of Yangtze Platform, western Hunan, South China, during the Ediacaran–Cambrian transition. Sedimentology 2011, 59, 809–829. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, S.; Tian, H.; Chen, Y.; Feng, X.; Fan, Q.; Xu, M.; Du, W. Enrichment mechanisms of organic matter in the marine slope facies shales from the Niutitang Formation in the western Xuefeng Uplift. Pet. Sci. Bull. 2024, 9, 853–865. [Google Scholar]
- Han, S.; Hu, K.; Cao, J.; Pan, J.; Xia, F.; Wu, W. Origin of early Cambrian black-shale-hosted barite deposits in South China: Mineralogical and geochemical studies. J. Asian Earth Sci. 2015, 106, 79–94. [Google Scholar] [CrossRef]
- Pašava, J.; Kříbek, B.; Vymazalová, A.; Sýkorová, I.; Žák, K.; Orberger, B. Multiple sources of metals of mineralization in Lower Cambrian black shales of Couth China: Evidence from geochemical and petrographic study. Resour. Geol. 2008, 58, 25–42. [Google Scholar] [CrossRef]
- Wen, Z.; Hua, Y.; Liqin, X.; Yihua, Y. Lake-bottom hydrothermal activities and their influence on high-quality source rock development: A case from Chang 7 source rocks in Ordos Basin. Pet. Explor. Dev. 2010, 37, 424–429. [Google Scholar]
- Perner, M.; Hansen, M.; Seifert, R.; Strauss, H.; Koschinsky, A.; Petersen, S. Linking geology, fluid chemistry, and microbial activity of basalt- and ultramafic-hosted deep-sea hydrothermal vent environments. Geobiology 2013, 11, 340–355. [Google Scholar] [CrossRef]
- Zierenberg, R.A.; Adams, M.W.W.; Arp, A.J. Life in extreme environments: Hydrothermal vents. Proc. Natl. Acad. Sci. USA 2000, 97, 12961–12962. [Google Scholar] [CrossRef]
- Steiner, M.; Wallis, E.; Erdtmann, B.D.; Zhao, Y.; Yang, R. Submarine-hydrothermal exhalative ore layers in black shales from South China and associated fossils—insights into a Lower Cambrian facies and bio-evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 169, 165–191. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.; Zhang, S.; Xi, Z.; Yang, N.; Yang, G.; Li, L.; Li, Y. Paleo-environmental conditions of the Early Cambrian Niutitang Formation in the Fenggang area, the southwestern margin of the Yangtze Platform, southern China: Evidence from major elements, trace elements and other proxies. J. Asian Earth Sci. 2018, 159, 81–97. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Elsworth, D.; Pan, Z.; Sun, X.; Ao, W. Sedimentary characteristics of the Lower Cambrian Niutitang shale in the southeast margin of Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2016, 36, 1140–1150. [Google Scholar] [CrossRef]
- Varencov, I.M.; Grasselly, G.; Grasselly, G. Geology and Geochemistry of Manganese: Manganese Deposits on Continents; Akademiao Kiado: Budapest, Hungary, 1980; Volume 2. [Google Scholar]
- Choi, J.H.; Hariya, Y. Geochemistry and depositional environment of Mn oxide deposits in the Tokoro Belt, northeastern Hokkaido, Japan. Econ. Geol. 1992, 87, 1265–1274. [Google Scholar] [CrossRef]
- Yang, G.; Gao, J.; Yang, R.; Liu, Z.; Xu, H. The genesis and geological significance of the siliciclastic rocks of the Middle Permian Maokou Formation at Yingpan, Nayong, Guizhou. Geol. Rev. 2022, 68, 1621–1633. [Google Scholar]
- Ver Straeten, C.A.; Brett, C.E.; Sageman, B.B. Mudrock sequence stratigraphy: A multi-proxy (sedimentological, paleobiological and geochemical) approach, Devonian Appalachian Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 304, 54–73. [Google Scholar] [CrossRef]
- Yamamoto, K. Geochemical characteristics and depositional environments of cherts and associated rocks in the Franciscan and Shimanto Terranes. Sediment. Geol. 1987, 52, 65–108. [Google Scholar] [CrossRef]
- Adachi, M.; Yamamoto, K.; Sugisaki, R. Hydrothermal chert and associated siliceous rocks from the northern pacific their geological significance as indication od ocean ridge activity. Sediment. Geol. 1986, 47, 125–148. [Google Scholar] [CrossRef]
- Boström, K.; Kraemer, T.; Gartner, S. Provenance and accumulation rates of opaline silica, Al, Ti, Fe, Mn, Cu, Ni and Co in Pacific pelagic sediments. Chem. Geol. 1973, 11, 123–148. [Google Scholar] [CrossRef]
- Nance, W.B.; Taylor, S.R. Rare earth element patterns and crustal evolution—I. Australian post-Archean sedimentary rocks. Geochim. Cosmochim. Acta 1976, 40, 1539–1551. [Google Scholar] [CrossRef]
- Michard, A.; Albarède, F. The REE content of some hydrothermal fluids. Chem. Geol. 1986, 55, 51–60. [Google Scholar] [CrossRef]
- Olivarez, A.M.; Owen, R.M. The europium anomaly of seawater: Implications for fluvial versus hydrothermal REE inputs to the oceans. Chem. Geol. 1991, 92, 317–328. [Google Scholar] [CrossRef]
- Douville, E.; Bienvenu, P.; Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Appriou, P.; Gamo, T. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems. Geochim. Cosmochim. Acta 1999, 63, 627–643. [Google Scholar] [CrossRef]
- Owen, A.W.; Armstrong, H.A.; Floyd, J.D. Rare earth element geochemistry of upper Ordovician cherts from the Southern Uplands of Scotland. J. Geol. Soc. 1999, 156, 191–204. [Google Scholar] [CrossRef]
- Dulski, P. Interferences of oxide, hydroxide and chloride analyte species in the determination of rare earth elements in geological samples by inductively coupled plasma-mass spectrometry. Fresenius’ J. Anal. Chem. 1994, 350, 194–203. [Google Scholar] [CrossRef]
- He, T.; Li, W.; Yang, E.; Lu, S.; Pan, W.; Zhang, B.; Ying, J.; Zhu, P.; Wang, X. Coupled weathering-hydrothermal process and its geological significance during the Early Cambrian in the Tarim Basin. J. Northeast. Pet. Univ. 2022, 46, 47–61. [Google Scholar]
- Brumsack, H.J. Geochemistry of recent Toc-rich sediments from the Gulf of California and the Black Sea. Geol. Rundsch. 1989, 78, 851–882. [Google Scholar] [CrossRef]
- Scheffler, K.; Buehmann, D.; Schwark, L. Analysis of late Palaeozoic glacial to postglacial sedimentary successions in South Africa by geochemical proxies—Response to climate evolution and sedimentary environment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 184–203. [Google Scholar] [CrossRef]
- Calvert, S.E.; Pedersen, T.F. Chapter fourteen elemental proxies for palaeoclimatic and palaeoceanographic variability in marine sediments: Interpretation and application. Dev. Mar. Geol. 2007, 1, 567–644. [Google Scholar]
- Jones, B.; Manning, D.A.C. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Li, Y.; Fan, T.; Zhang, J.; Zhang, J.; Wei, X.; Hu, X.; Zeng, W.; Fu, W. Geochemical changes in the Early Cambrian interval of the Yangtze Platform, South China: Implications for hydrothermal influences and paleocean redox conditions. J. Asian Earth Sci. 2015, 109, 100–123. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Investigating the use of sedimentary geochemical proxies for paleoenvironment interpretation of thermally mature organic-rich strata: Examples from the Devonian–Mississippian shales, Western Canadian Sedimentary Basin. Chem. Geol. 2009, 260, 1–19. [Google Scholar] [CrossRef]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef]
- Feng, Z. Geochemistry of bioessential elements and the origin and evolution of life. Earth Environ. 1980, 7, 1–14. [Google Scholar]
- Canfield, D.E.; Jorgensen, B.B.; Fossing, H.; Glud, R.; Gundersen, J.; Ramsing, N.B.; Thamdrup, B.; Hansen, J.W.; Nielsen, L.P.; Hall, P.O. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 1993, 113, 27–40. [Google Scholar] [CrossRef]
- Grauch, R.I.; Lewan, M.D.; Lamothe, P.J. Element partitioning into generated petroleum and hydrothermal fluids as determined by hydrous pyrolysis of retort shale. GSA Abstr. 2004, 36, 200. [Google Scholar]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Pedersen, T.F.; Calvert, S.E. Anoxia vs. productivity: What controls the formation of organic-carbon-rich sediments and sedimentary rocks? AAPG Bull. 1990, 74, 454–466. [Google Scholar]
- Watson, A.J.; Bakker, D.C.E.; Ridgwell, A.J.; Boyd, P.W.; Law, C.S. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature 2000, 407, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Schoepfer, S.D.; Feng, Q.; Zhou, L.; Yu, J.; Song, H.; Wei, H.; Algeo, T.J. Marine productivity changes during the end-Permian crisis and Early Triassic recovery. Earth-Sci. Rev. 2014, 149, 136–162. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Yan, D.; Wei, H.; Xiang, L. Evolution from an anoxic to oxic deep ocean during the Ediacaran–Cambrian transition and implications for bioradiation. Chem. Geol. 2012, 306, 129–138. [Google Scholar] [CrossRef]
- Resing, J.A.; Sedwick, P.N.; German, C.R.; Jenkins, W.J.; Moffett, J.W.; Sohst, B.M.; Tagliabue, A. Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean. Nature 2015, 523, 200–203. [Google Scholar] [CrossRef]
- Coale, K.H.; Fitzwater, S.E.; Gordon, R.M.; Johnson, K.S.; Barber, R.T. Control of community growth and export production by upwelled iron in the equatorial Pacific Ocean. Nature 1996, 379, 621–624. [Google Scholar] [CrossRef]
- Aitchison, J.C.; Flood, P.G. Geochemical constraints on the depositional setting of Palaeozoic cherts from the New England orogen, NSW, eastern Australia. Mar. Geol. 1990, 94, 79–95. [Google Scholar] [CrossRef]
- Murray, R.W.; Jones, D.L.; Brink, M.R.B. Diagenetic formation of bedded chert: Evidence from chemistry of the chert-shale couplet. Geology 1992, 20, 271–274. [Google Scholar] [CrossRef]
- Murray, R.W. Chemical criteria to identify the depositional environment of chert: General principles and applications. Sediment. Geol. 1994, 90, 213–232. [Google Scholar] [CrossRef]
- Roser, B.P.; Korsch, R.J. Determination of Tectonic Setting of Sandstone-Mudstone Suites Using SiO2 content and K2O/Na2O ratio. J. Geol. 1986, 94, 635–650. [Google Scholar] [CrossRef]
- Li, C.; Shi, W.; Cheng, M.; Jin, C.; Algeo, T.J. The redox structure of Ediacaran and early Cambrian oceans and its controls. Sci. Bull. 2020, 65, 2141–2149. [Google Scholar] [CrossRef]
- Algeo, T.J.; Lyons, T.W. Mo–total organic carbon covariation in modern anoxic marine environments: Implications for analysis of paleoredox and paleohydrographic conditions. Paleoceanography 2006, 21, 1–23. [Google Scholar] [CrossRef]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Algeo, T.J.; Rowe, H. Paleoceanographic applications of trace-metal concentration data. Chem. Geol. 2012, 324, 6–18. [Google Scholar] [CrossRef]
- Zhang, Y. Palaeo-Sedimentary Environment Identification and Depositional Models of Marine Organic-Rich Shales; China University of Geosciences CUGB: Beijing, China, 2017. [Google Scholar]
- Ma, K.; Hu, S.; Wang, T.; Zhang, B.; Qin, S.; Shi, S.; Wang, K.; Qingyu, H. Sedimentary environments and mechanisms of organic matter enrichment in the Mesoproterozoic Hongshuizhuang Formation of northern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 475, 176–187. [Google Scholar] [CrossRef]
- Gao, L. Structural Characteristics of Organisms in the Cambrian Phosphorites of Zhijin, Guizhou, and Their Relationship to Phosphogenesis; Guizhou University: Guiyang, China, 2019. [Google Scholar]

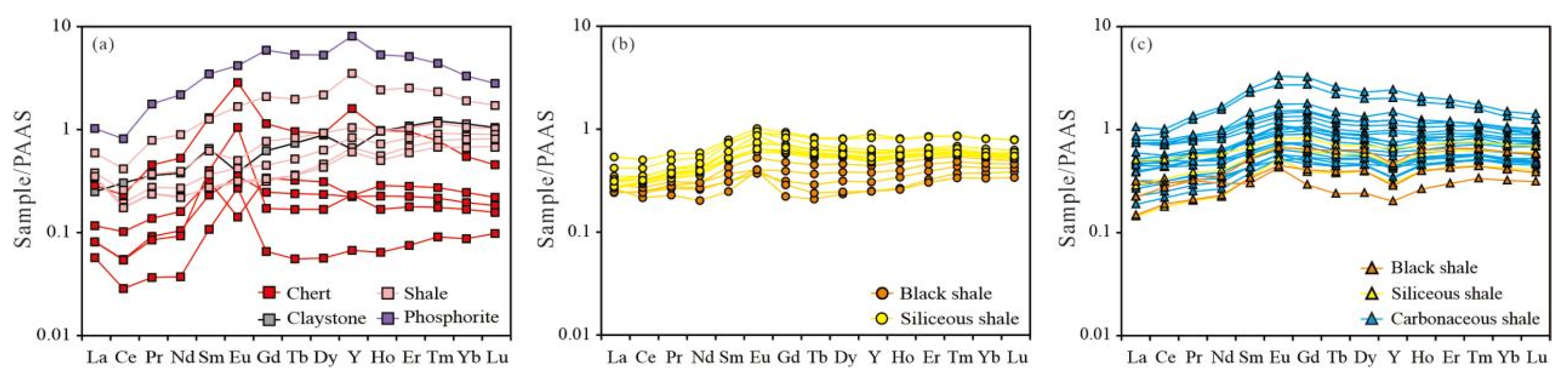



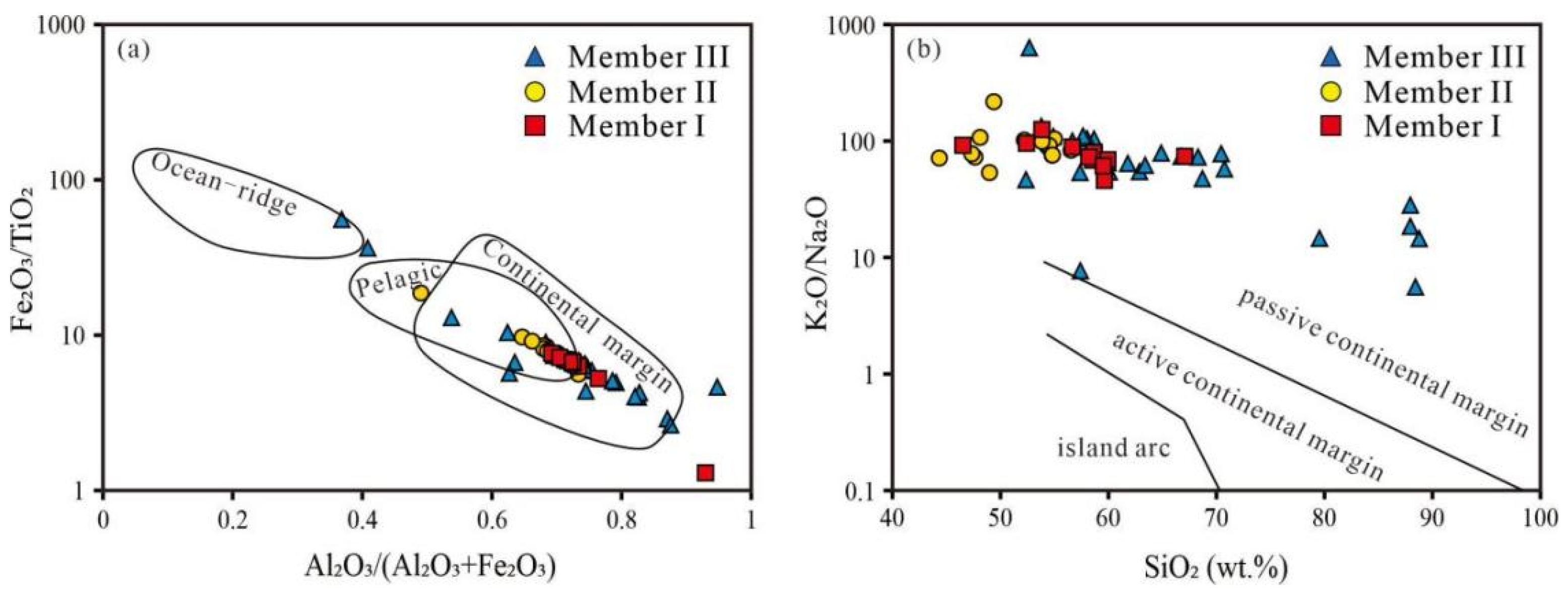

| Lithology | Samples | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | CIA | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mudstone | KY-1 | 52.67 | 0.31 | 25.51 | 1.41 | <0.001 | 2.02 | 0.06 | 0.008 | 4.97 | 0.37 | 12.00 | 82.53 | 2.28 |
| Chert | KY-2.1 | 87.95 | 0.06 | 1.95 | 3.35 | 0.001 | 0.20 | 0.02 | 0.014 | 0.39 | 0.10 | 5.23 | 80.77 | 1.79 |
| KY-2.3 | 88.77 | 0.06 | 1.56 | 2.26 | 0.001 | 0.11 | 0.07 | 0.015 | 0.21 | 0.14 | 6.11 | 84.74 | 3.95 | |
| KY-2.5 | 88.43 | 0.14 | 1.55 | 0.89 | <0.001 | 0.12 | 0.06 | 0.039 | 0.21 | 0.10 | 7.76 | 81.16 | 5.36 | |
| KY-2.9 | 87.95 | 0.09 | 1.68 | 0.78 | <0.001 | 0.13 | 0.06 | 0.016 | 0.29 | 0.09 | 8.18 | 82.06 | 6.87 | |
| KY-2.11 | 79.54 | 0.26 | 2.44 | 1.45 | <0.001 | 0.14 | 2.96 | 0.021 | 0.30 | 2.08 | 10.08 | 86.05 | 5.74 | |
| Organic-rich blackshales | KY-2.2 | 70.44 | 0.44 | 8.16 | 2.15 | 0.002 | 0.84 | 0.04 | 0.031 | 2.37 | 0.24 | 14.98 | 75.38 | 9.84 |
| KY-2.4 | 68.30 | 0.48 | 9.02 | 3.11 | 0.003 | 0.90 | 0.05 | 0.036 | 2.58 | 0.31 | 14.58 | 75.63 | 9.51 | |
| KY-2.6 | 68.70 | 0.52 | 10.03 | 1.49 | 0.002 | 1.02 | 0.03 | 0.062 | 2.89 | 0.11 | 14.56 | 75.31 | 10.32 | |
| KY-2.8 | 70.74 | 0.48 | 8.88 | 1.88 | 0.003 | 0.84 | 0.09 | 0.044 | 2.46 | 0.21 | 13.96 | 76.01 | 9.95 | |
| KY-2.10 | 62.87 | 0.40 | 8.01 | 1.67 | 0.001 | 0.66 | 5.48 | 0.038 | 2.04 | 4.01 | 14.80 | 77.47 | 9.61 | |
| Phosphorite | KY-2.7 | 57.40 | 0.12 | 1.80 | 1.55 | <0.001 | 0.18 | 15.78 | 0.045 | 0.34 | 11.14 | 10.90 | 77.73 | 6.39 |
| Black shale | KY-3 | 66.73 | 0.77 | 14.09 | 2.00 | 0.005 | 0.95 | 0.55 | 0.042 | 3.04 | 0.16 | 11.90 | 80.43 | 5.74 |
| KY-4 | 63.40 | 0.74 | 13.37 | 2.92 | 0.008 | 0.94 | 1.71 | 0.047 | 2.86 | 0.18 | 13.41 | 80.45 | 5.80 | |
| KY-5 | 61.79 | 0.66 | 12.23 | 4.42 | 0.026 | 1.40 | 2.87 | 0.042 | 2.65 | 0.19 | 13.25 | 80.28 | 4.83 | |
| KY-6 | 57.37 | 0.70 | 12.07 | 5.07 | 0.004 | 0.93 | 2.61 | 0.055 | 2.90 | 0.04 | 18.35 | 78.45 | 5.05 | |
| KY-7 | 64.88 | 0.68 | 12.52 | 3.41 | 0.006 | 0.84 | 2.05 | 0.035 | 2.71 | 0.20 | 12.12 | 80.42 | 4.88 | |
| KY-34 | 67.06 | 0.84 | 14.37 | 1.09 | 0.003 | 0.87 | 0.16 | 0.042 | 3.10 | 0.05 | 11.76 | 80.43 | 5.40 | |
| KY-37 | 58.68 | 0.76 | 13.29 | 4.82 | 0.033 | 1.09 | 2.81 | 0.037 | 2.96 | 0.22 | 15.30 | 79.99 | 4.68 | |
| KY-43 | 59.53 | 0.76 | 13.44 | 5.10 | 0.034 | 2.00 | 2.64 | 0.048 | 3.03 | 0.23 | 12.61 | 79.64 | 3.91 | |
| Siliceous | KY-8 | 58.66 | 0.63 | 11.73 | 4.25 | 0.041 | 2.56 | 3.73 | 0.026 | 2.66 | 0.18 | 15.01 | 79.83 | 5.08 |
| KY-9 | 59.96 | 0.64 | 11.59 | 3.91 | 0.036 | 2.15 | 3.96 | 0.041 | 2.62 | 0.20 | 14.59 | 79.60 | 4.70 | |
| KY-10 | 60.07 | 0.67 | 11.96 | 3.90 | 0.033 | 1.97 | 3.94 | 0.050 | 2.67 | 0.20 | 13.80 | 79.68 | 4.36 | |
| KY-11 | 54.89 | 0.63 | 11.07 | 4.73 | 0.057 | 3.37 | 5.53 | 0.022 | 2.36 | 0.17 | 16.43 | 80.81 | 3.86 | |
| KY-12 | 53.78 | 0.65 | 10.99 | 6.62 | 0.051 | 2.87 | 5.20 | 0.018 | 2.36 | 0.18 | 16.54 | 80.79 | 3.96 | |
| KY-13 | 58.01 | 0.70 | 12.22 | 4.12 | 0.038 | 2.57 | 4.14 | 0.027 | 2.77 | 0.20 | 15.23 | 79.83 | 4.18 | |
| KY-14 | 57.64 | 0.69 | 12.40 | 4.32 | 0.032 | 2.18 | 3.85 | 0.026 | 2.82 | 0.19 | 15.18 | 79.83 | 4.78 | |
| KY-15 | 56.66 | 0.70 | 12.13 | 4.49 | 0.037 | 2.61 | 4.33 | 0.027 | 2.65 | 0.19 | 16.53 | 80.39 | 4.62 | |
| KY-16 | 52.36 | 0.96 | 12.06 | 4.13 | 0.035 | 2.55 | 4.41 | 0.055 | 2.51 | 0.20 | 17.10 | 80.61 | 4.36 | |
| KY-17 | 54.36 | 0.79 | 12.11 | 4.40 | 0.034 | 2.49 | 4.25 | 0.030 | 2.68 | 0.19 | 16.54 | 80.14 | 4.63 | |
| KY-39 | 56.66 | 0.73 | 12.71 | 5.04 | 0.044 | 2.41 | 3.31 | 0.032 | 2.79 | 0.20 | 15.79 | 80.25 | 5.01 | |
| KY-41 | 59.94 | 0.76 | 13.08 | 5.48 | 0.025 | 1.49 | 2.19 | 0.041 | 2.87 | 0.23 | 13.01 | 80.11 | 4.54 | |
| KY-42 | 58.19 | 0.76 | 13.22 | 5.04 | 0.041 | 2.35 | 3.47 | 0.039 | 2.97 | 0.22 | 12.84 | 79.79 | 3.48 | |
| Carbonaceous shale | KY-18 | 56.54 | 0.69 | 12.43 | 4.51 | 0.034 | 2.19 | 4.23 | 0.034 | 2.84 | 0.20 | 15.73 | 79.62 | 4.93 |
| KY-19 | 55.00 | 0.69 | 12.38 | 5.25 | 0.038 | 2.75 | 4.04 | 0.027 | 2.81 | 0.19 | 16.78 | 79.80 | 4.66 | |
| KY-20 | 54.52 | 0.68 | 12.16 | 4.47 | 0.055 | 2.56 | 4.76 | 0.029 | 2.65 | 0.19 | 17.43 | 80.40 | 5.18 | |
| KY-21 | 49.39 | 0.62 | 11.20 | 11.64 | 0.038 | 1.92 | 3.43 | 0.011 | 2.39 | 0.17 | 18.78 | 81.02 | 4.87 | |
| KY-22 | 39.82 | 0.55 | 9.79 | 5.35 | 0.120 | 6.11 | 9.91 | 0.019 | 2.20 | 0.31 | 24.94 | 80.04 | 5.59 | |
| KY-23 | 52.21 | 0.67 | 11.89 | 5.45 | 0.048 | 2.88 | 4.71 | 0.025 | 2.55 | 0.23 | 18.76 | 80.72 | 6.80 | |
| KY-24 | 44.34 | 0.58 | 10.61 | 4.90 | 0.086 | 5.02 | 8.25 | 0.032 | 2.28 | 0.19 | 22.96 | 80.45 | 6.04 | |
| KY-25 | 47.63 | 0.62 | 11.11 | 5.25 | 0.066 | 4.15 | 6.64 | 0.033 | 2.40 | 0.21 | 21.34 | 80.42 | 6.76 | |
| KY-26 | 48.12 | 0.65 | 11.32 | 5.34 | 0.070 | 3.73 | 6.39 | 0.022 | 2.36 | 0.27 | 21.17 | 81.16 | 6.74 | |
| KY-27 | 47.35 | 0.62 | 11.11 | 5.67 | 0.066 | 3.80 | 6.49 | 0.030 | 2.33 | 0.28 | 21.62 | 80.94 | 6.67 | |
| KY-28 | 54.78 | 0.71 | 12.29 | 5.63 | 0.039 | 1.89 | 3.33 | 0.033 | 2.49 | 0.21 | 18.72 | 81.42 | 7.70 | |
| KY-29 | 48.99 | 0.68 | 11.51 | 4.52 | 0.087 | 4.21 | 6.98 | 0.048 | 2.54 | 0.43 | 19.17 | 79.83 | 4.69 | |
| KY-30 | 53.84 | 0.72 | 12.36 | 4.97 | 0.044 | 2.61 | 3.84 | 0.027 | 2.67 | 0.24 | 17.93 | 80.57 | 6.24 | |
| KY-31 | 54.81 | 0.72 | 12.25 | 4.51 | 0.059 | 2.89 | 4.46 | 0.034 | 2.58 | 0.23 | 17.90 | 80.84 | 6.07 | |
| KY-32 | 53.83 | 0.68 | 11.87 | 4.68 | 0.063 | 2.99 | 4.89 | 0.020 | 2.51 | 0.24 | 17.91 | 81.02 | 5.86 | |
| KY-33 | 52.41 | 0.68 | 11.45 | 5.03 | 0.066 | 3.20 | 4.79 | 0.025 | 2.40 | 0.21 | 18.99 | 81.07 | 7.17 | |
| KY-35 | 59.62 | 0.77 | 13.13 | 4.05 | 0.035 | 1.62 | 3.04 | 0.066 | 3.04 | 0.20 | 13.99 | 78.94 | 4.19 | |
| KY-36 | 58.54 | 0.77 | 13.36 | 5.40 | 0.041 | 1.46 | 2.54 | 0.044 | 3.02 | 0.21 | 14.66 | 79.67 | 4.79 | |
| KY-38 | 58.63 | 0.76 | 13.22 | 5.02 | 0.033 | 1.79 | 2.46 | 0.036 | 2.86 | 0.22 | 15.83 | 80.42 | 5.52 | |
| KY-40 | 46.52 | 0.63 | 10.80 | 4.80 | 0.108 | 5.07 | 8.34 | 0.026 | 2.39 | 0.53 | 20.06 | 80.16 | 4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Ni, Z.; Shao, G.; Zhang, W.; Cheng, N. Research on the Metallogenic Enrichment Model of Poly-Metallic Black Shales and Their Geological Significance: A Case Study of the Cambrian Niutitang Formation. Processes 2025, 13, 3537. https://doi.org/10.3390/pr13113537
Shi K, Ni Z, Shao G, Zhang W, Cheng N. Research on the Metallogenic Enrichment Model of Poly-Metallic Black Shales and Their Geological Significance: A Case Study of the Cambrian Niutitang Formation. Processes. 2025; 13(11):3537. https://doi.org/10.3390/pr13113537
Chicago/Turabian StyleShi, Kai, Zhiyong Ni, Ganggang Shao, Wen Zhang, and Nuo Cheng. 2025. "Research on the Metallogenic Enrichment Model of Poly-Metallic Black Shales and Their Geological Significance: A Case Study of the Cambrian Niutitang Formation" Processes 13, no. 11: 3537. https://doi.org/10.3390/pr13113537
APA StyleShi, K., Ni, Z., Shao, G., Zhang, W., & Cheng, N. (2025). Research on the Metallogenic Enrichment Model of Poly-Metallic Black Shales and Their Geological Significance: A Case Study of the Cambrian Niutitang Formation. Processes, 13(11), 3537. https://doi.org/10.3390/pr13113537






