Selective Removal of Arsenic and Antimony by Alkaline Leaching with Sodium Sulfide: Remediation of Metalloids-Contaminated Concentrates from Zimapán, Hidalgo, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaching Test
2.3. Kinetic Parameters: Reaction Orders (α, β) and Apparent Activation Energy (Ea)
| Rate Controlling Stage | Kinetic Equation (as a Function of the Conversion XAs,Sb) | Experimental Rate Constant, kexp | Symbol |
|---|---|---|---|
| Transport in the fluid film | kff = experimental rate constant in the fluid film in min−1 | ||
| b = stoichiometric a | |||
| D = molecular diffusion coefficient in m2 min−1 | |||
| [A] = fluid reagent concentration in mol m−3a | |||
| = molar density in mol m−3a | |||
| r0 = initial radius of the particle in m | |||
| Chemical reaction | kcr = experimental rate constant in the chemical reaction in min−1, | ||
| k0′ = chemical rate constant in (mol m−3)1−n min−1 n = reaction order |
2.4. Statistical Analysis and Predictive Modeling
3. Results and Discussion
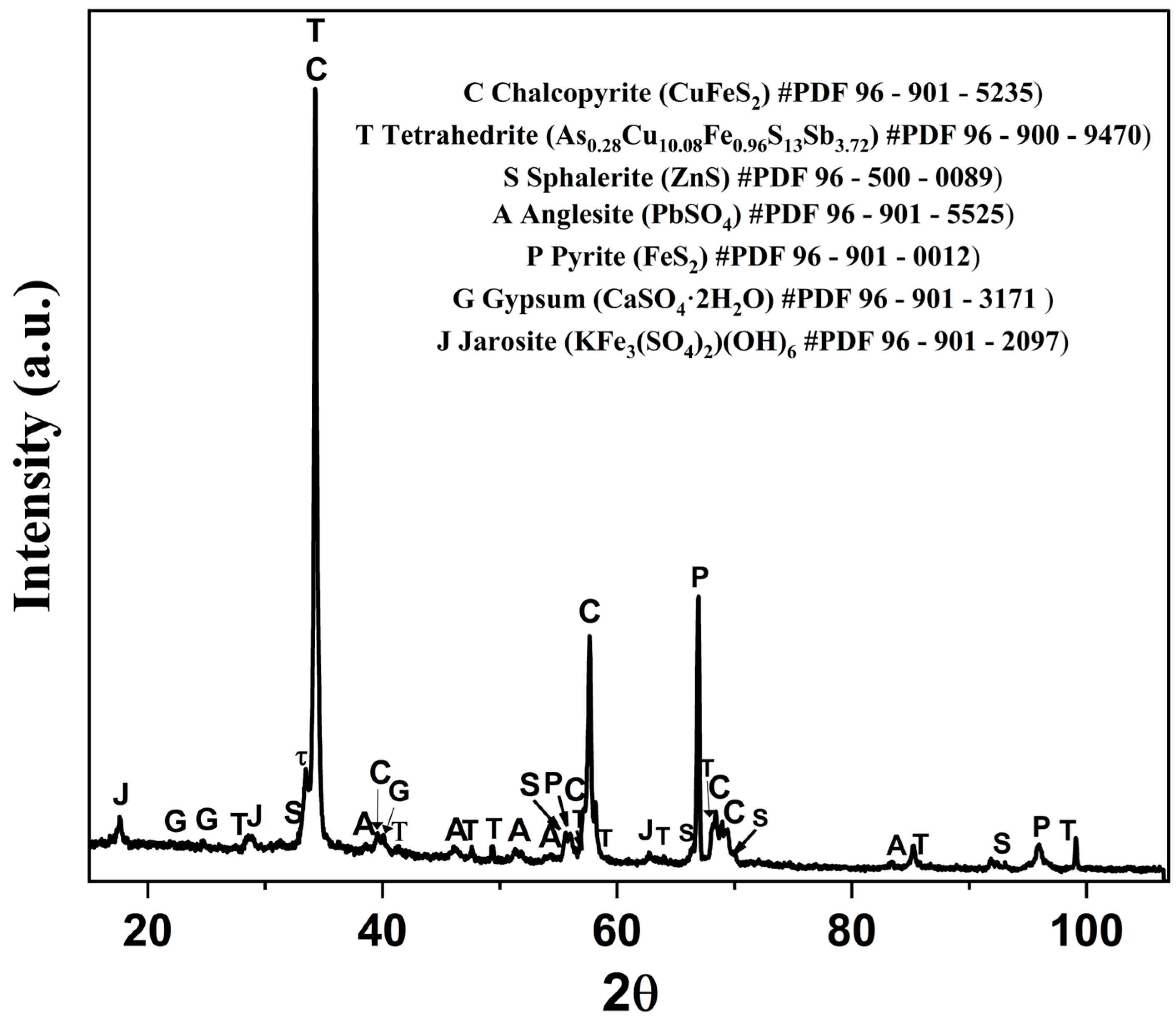
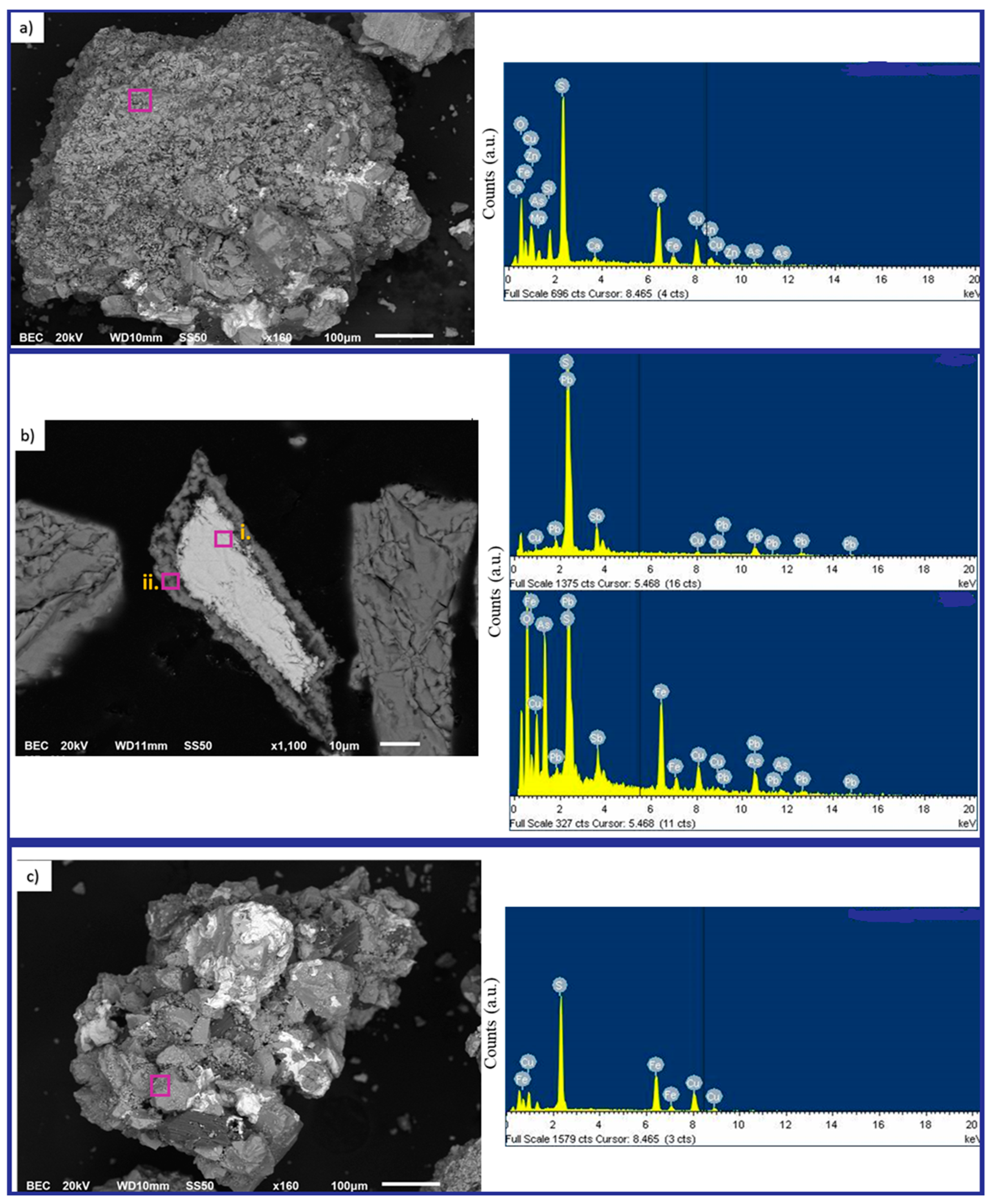
3.1. Characterization Details
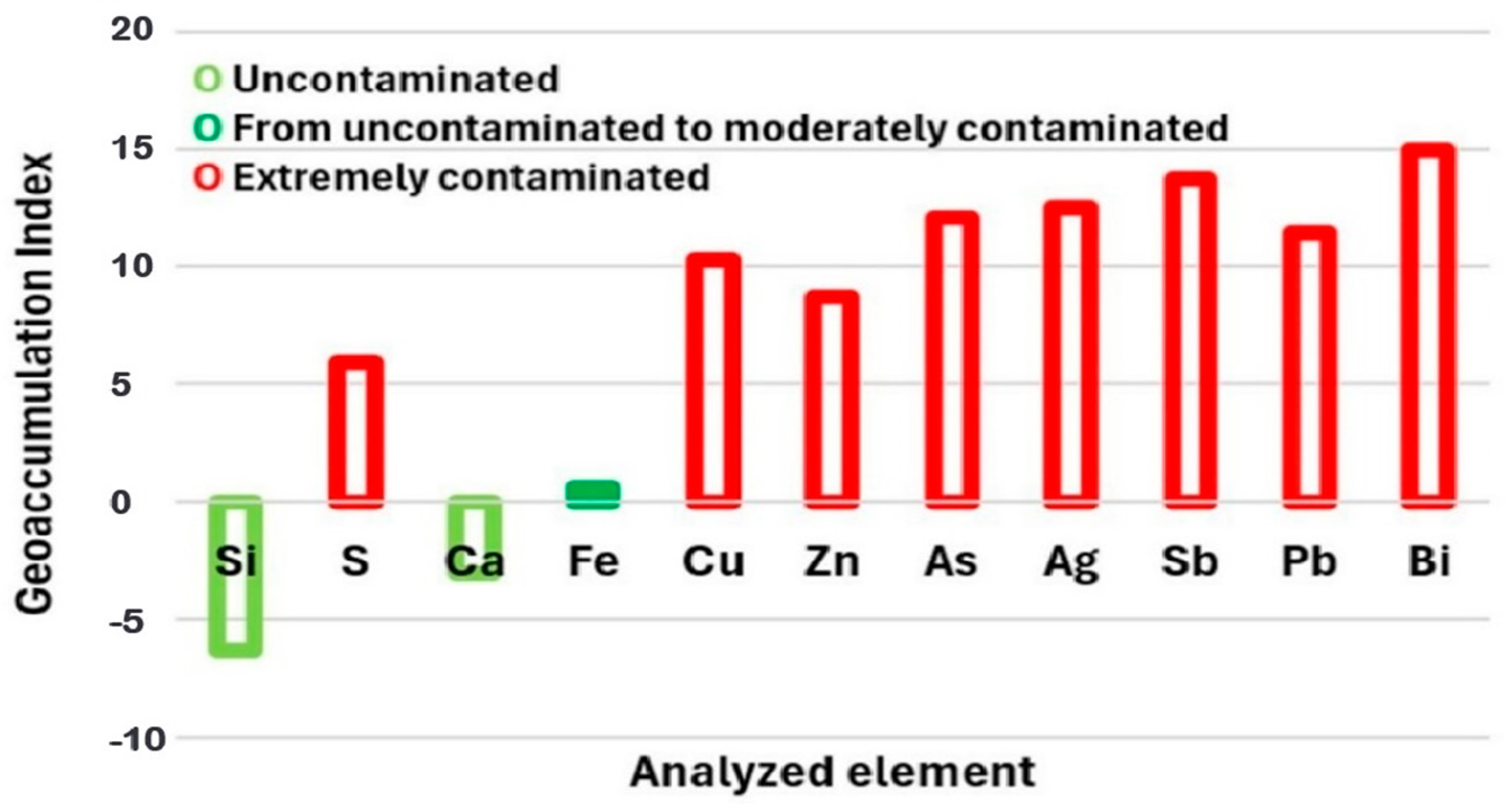
3.2. Geoaccumulation Index and Enrichment Factors
3.3. Extraction Profiles of As and Sb
- Influence of Na2S Concentration on the Extraction of As and Sb
- 2.
- Influence of NaOH concentration on the extraction of As and Sb
- 3.
- Influence of Temperature on the Extraction of As and Sb
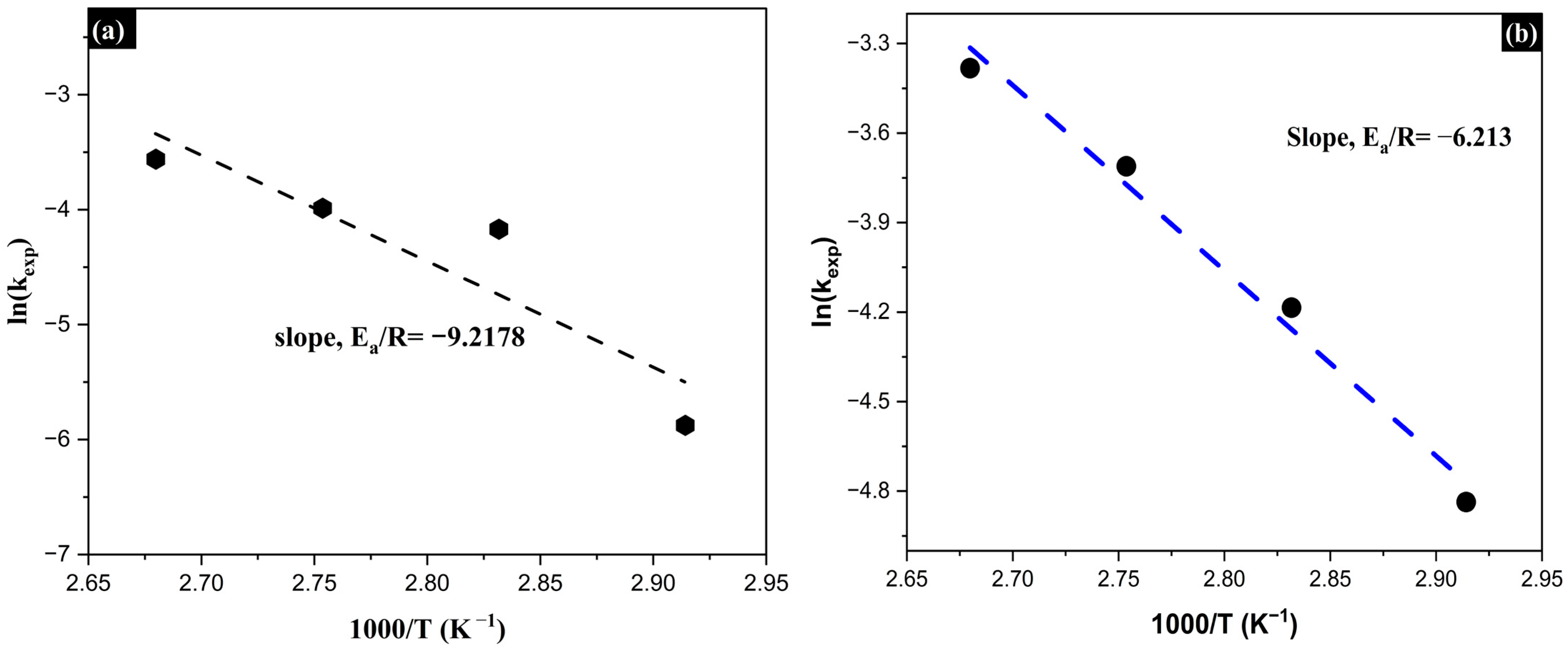
3.4. Reaction Orders (α, β) and Activation Energy (Ea)
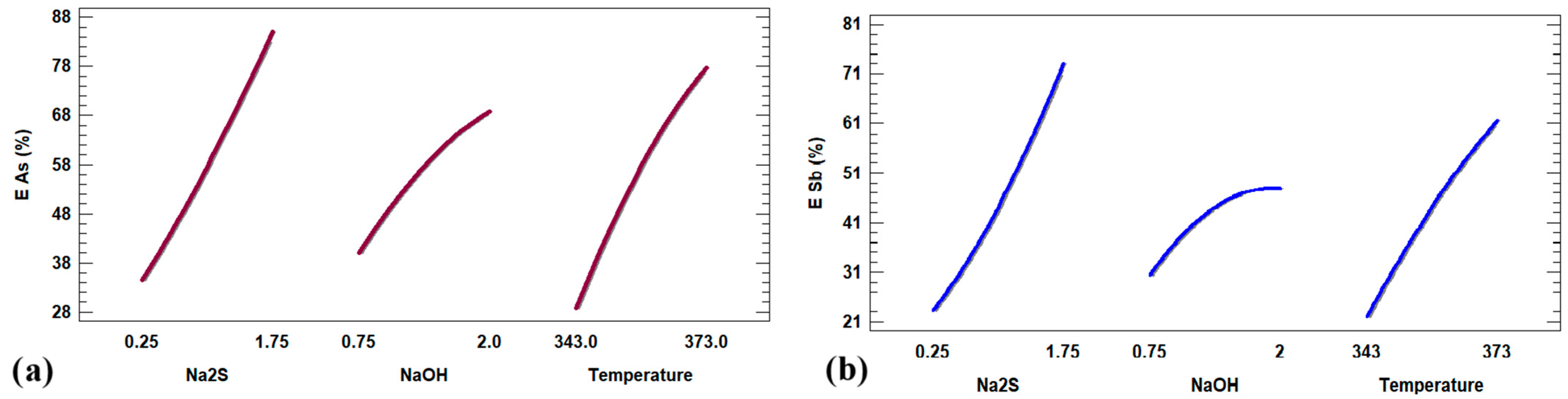
3.5. Exploratory Quadratic Modeling of As and Sb Extraction
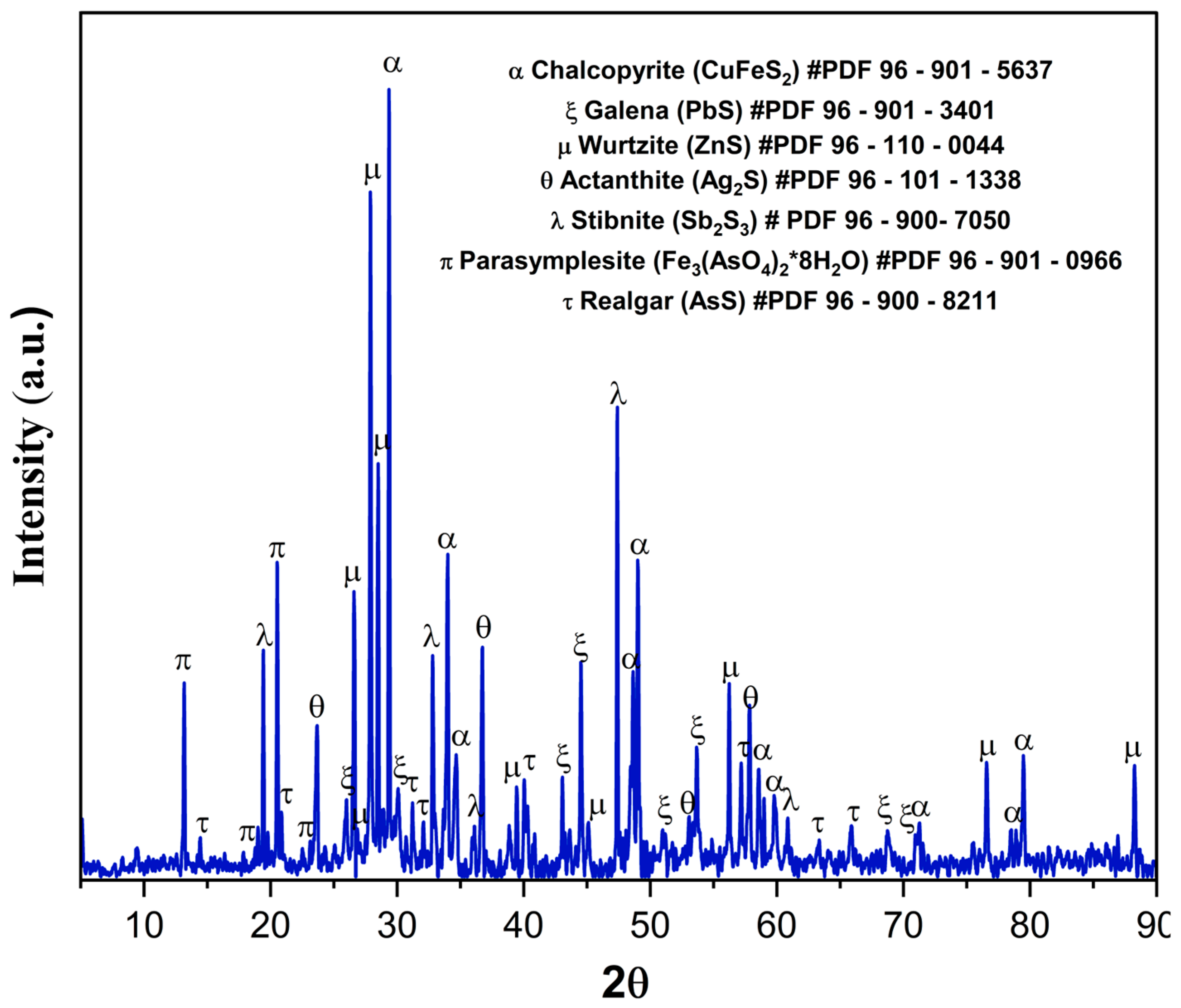
3.6. Solid Residue Characterization by XRD
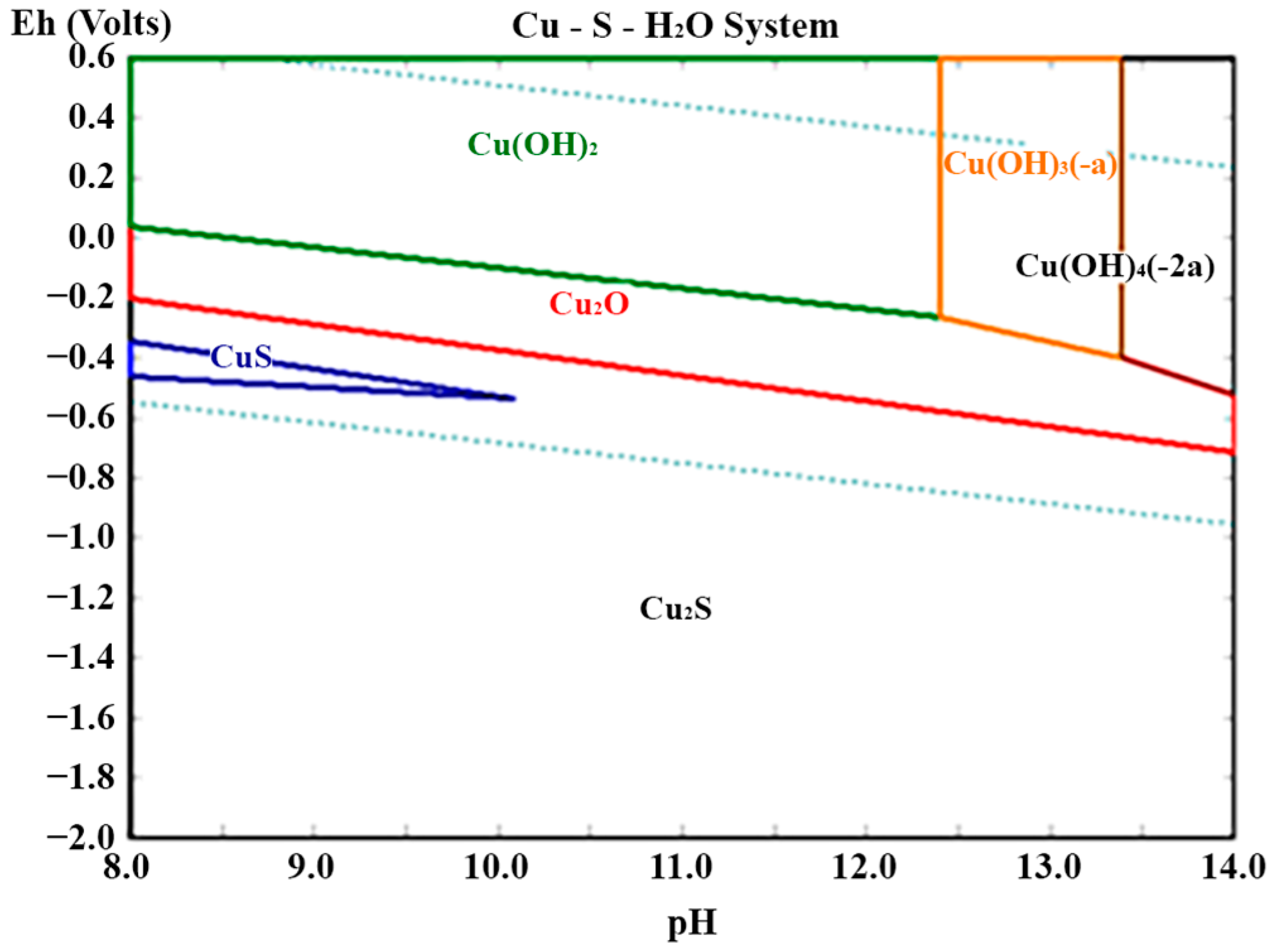
3.7. Feasibility and Scale-Up Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez, K.; Toro, N.; Gálvez, E.; Robles, P.; Wilson, R.; Navarra, A. Environmental, economic and technological factors affecting Chilean copper smelters—A critical review. J. Mater. Res. Technol. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Moats, M.; Alagha, L.; Awuah-Offei, K. Towards resilient and sustainable supply of critical elements from the copper supply chain: A review. J. Clean. Prod. 2021, 307, 127207. [Google Scholar] [CrossRef]
- Ruiz, A.T.; Juárez-Tapia, J.C.; Cisneros-Flores, G.; Martínez-Soto, J.I.; Reyes-Pérez, M.; Reyes-Domínguez, I.A.; Ortiz, H.G.; Flores Guerrero, U.M. Characterization of Solid Mining Waste in the Urbanized Area of Zimapan, Hidalgo, for the Identification of Economically Valuable Elements and Trace Elements. In Proceedings of the TMS 153rd Annual Meeting & Exhibition, Orlando, FL, USA, 3–7 May 2024; pp. 1876–1885. [Google Scholar]
- Prieto García, F.; Acevedo Sandoval, O.A.; Pérez Moreno, F.; Prieto Méndez, J.; Canales Flores, R.A. Arsenic contamination in groundwater in Zimapan, Hidalgo, Mexico. Desalination Water Treat. 2016, 57, 13038–13047. [Google Scholar] [CrossRef]
- Espinosa, E.; Armienta, M.A.; Cruz, O.; Aguayo, A.; Ceniceros, N. Geochemical distribution of arsenic, cadmium, lead and zinc in river sediments affected by tailings in Zimapán, a historical polymetalic mining zone of México. Environ. Geol. 2009, 58, 1467. [Google Scholar] [CrossRef]
- Chango-Cañola, A.P.; González-Sandoval Mdel, R.; Alarcón-Herrera, M.T.; García-Villanueva, L.A.; Fernández-Villagómez, G. Health and environmental risk due to acid mine drainage generating tailings in Zimapán, Mexico. Environ. Res. Commun. 2025, 7, 25015. [Google Scholar] [CrossRef]
- Angeles, L.; Reyes, M.; Perez, M.; Palacios, E.; Patiño, F.; Reyes, I.; Flores, M. Chemical and Mineralogical Characterization of a Mixed Sulphide Ore at Zimapan, Hidalgo, Mexico. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2017; pp. 607–615. [Google Scholar]
- Teja Ruiz, A.M.; Juárez Tapia, J.C.; Reyes Domínguez, I.A.; Hernández Cruz, L.E.; Reyes Pérez, M.; Patiño Cardona, F.; Flores Guerrero, M.U. Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32−-O2-NaOH System. Metals 2017, 7, 411. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- de Koning, A.; Kleijn, R.; Huppes, G.; Sprecher, B.; van Engelen, G.; Tukker, A. Metal supply constraints for a low-carbon economy? Resour. Conserv. Recycl. 2018, 129, 202–208. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Faris, N.; Pownceby, M.I.; Bruckard, W.J.; Chen, M. The Direct Leaching of Nickel Sulfide Flotation Concentrates—A Historic and State-of-the-Art Review Part I: Piloted Processes and Commercial Operations. Miner. Process. Extr. Metall. Rev. 2023, 44, 407–435. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Wang, H.; Dong, K.; Zhu, R.; Jiang, Z.; Wei, G. Mechanism of arsenic distribution and migration during iron extraction by copper slag-steel slag combined reforming: A potential solution to refractory wastes. J. Clean. Prod. 2023, 420, 138399. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; He, Y.; Xu, S.; Hu, B.; Cao, H.; Zhou, J.; Zheng, G. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Min. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Zheng, Q.; Che, X.; Cui, X.; Wei, S.; Li, H.; Shi, X. Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag. Sustainability 2023, 15, 13947. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Q.; Wang, Y.; Long, T.; Deng, S. Effects of pyrite, quartz and sodium sulfite on roasting of a refractory sulfide concentrate and gold, silver, copper leaching during cyanidation. Hydrometallurgy 2024, 226, 106306. [Google Scholar] [CrossRef]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony Recovery from End-of-Life Products and Industrial Process Residues: A Critical Review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Armienta, M.A.; Mugica, V.; Reséndiz, I.; Arzaluz, M.G. Arsenic and metals mobility in soils impacted by tailings at Zimapán, México. J. Soils Sediments 2016, 16, 1267–1278. [Google Scholar] [CrossRef]
- Armienta, M.A.; Villaseñor, G.; Cruz, O.; Ceniceros, N.; Aguayo, A.; Morton, O. Geochemical processes and mobilization of toxic metals and metalloids in an As-rich base metal waste pile in Zimapán, Central Mexico. Appl. Geochem. 2012, 27, 2225–2237. [Google Scholar] [CrossRef]
- Zou, H.; Ren, B. Analyzing topsoil heavy metal pollution sources and ecological risks around antimony mine waste sites by a joint methodology. Ecol. Indic. 2023, 154, 110761. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Corona Sánchez, J.E.; González Chávez Mdel, C.A.; Carrillo González, R.; Scheckel, K.; Tapia Maruri, D.; García Cue, J.L. Metal(loid) bioaccessibility of atmospheric particulate matter from mine tailings at Zimapan, Mexico. Environ. Sci. Pollut. Res. 2021, 28, 19458–19472. [Google Scholar] [CrossRef]
- Zhuang, F.; Huang, J.; Li, H.; Peng, X.; Xia, L.; Zhou, L.; Zhang, T.; Liu, Z.; He, Q.; Luo, F.; et al. Biogeochemical behavior and pollution control of arsenic in mining areas: A review. Front. Microbiol. 2023, 14, 1043024. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Yang, Z.; Wu, P.; Zhang, K.; Chen, S. Study on antimony and arsenic cycling, transformation and contrasting mobility in river-type reservoir. Appl. Geochem. 2022, 136, 105132. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef] [PubMed]
- Radková, A.B.; Jamieson, H.E.; Campbell, K.M.; Hudson-Edwards, K.A. Antimony in Mine Wastes: Geochemistry, Mineralogy, and Microbiology. Econ. Geol. 2023, 118, 621–637. [Google Scholar] [CrossRef]
- Patel, K.S.; Pandey, P.K.; Martín-Ramos, P.; Corns, W.T.; Varol, S.; Bhattacharya, P.; Zhu, Y. A review on arsenic in the environment: Contamination, mobility, sources, and exposure. RSC Adv. 2023, 13, 8803–8821. [Google Scholar] [CrossRef]
- Monteiro De Oliveira, E.C.; Caixeta, E.S.; Santos, V.S.V.; Pereira, B.B. Arsenic exposure from groundwater: Environmental contamination, human health effects, and sustainable solutions. J. Toxicol. Environ. Health Part B 2021, 24, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.G.; Bennett, W.W.; Doriean, N.; Hockmann, K.; Karimian, N.; Burton, E.D. Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Sci. Total Environ. 2020, 710, 136354. [Google Scholar] [CrossRef]
- Escot-Espinoza, V.M.; Rodríguez-Márquez, S.; Briseño-Bugarín, J.; López-Luna, M.A.; Flores de la Torre, J.A. Presence of Potentially Toxic Elements in Historical Mining Areas in the North-Center of Mexico and Possible Bioremediation Strategies. Toxics 2024, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Drahota, P.; Venhauerova, P.; Strnad, L. Speciation and mobility of arsenic and antimony in soils and mining wastes from an abandoned Sb–Au mining area. Appl. Geochem. 2023, 152, 105665. [Google Scholar] [CrossRef]
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Delgado Quezada, V.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New findings on source, mobilization and mobility in human environments in 20 countries based on decadal research 2010–2020. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1727–1865. [Google Scholar] [CrossRef]
- Briseño-Bugarín, J.; Araujo-Padilla, X.; Escot-Espinoza, V.M.; Cardoso-Ortiz, J.; Flores de la Torre, J.A.; López-Luna, A. Lead (Pb) Pollution in Soil: A Systematic Review and Meta-Analysis of Contamination Grade and Health Risk in Mexico. Environments 2024, 11, 43. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wang, X. Concentration and speciation of antimony and arsenic in soil profiles around the world’s largest antimony metallurgical area in China. Environ. Geochem. Health 2015, 37, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Antimony Toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tan, D.; Deng, S.; Lei, M. Pollution and ecological risk assessment of antimony and other heavy metals in soils from the world’s largest antimony mine area, China. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Min. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Ye, L.; Ouyang, Z.; Chen, Y.; Chen, Y. Ferric chloride leaching of antimony from stibnite. Hydrometallurgy 2019, 186, 210–217. [Google Scholar] [CrossRef]
- Ling, H.; Malfliet, A.; Blanpain, B.; Guo, M. Selective removal of arsenic from crude antimony trioxide by leaching with nitric acid. Sep. Purif. Technol. 2022, 281, 119976. [Google Scholar] [CrossRef]
- Hernández, M.C.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Technological trends, emerging applications and metallurgical strategies in antimony recovery from stibnite. Min. Eng. 2022, 175, 107304. [Google Scholar] [CrossRef]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandström, Å. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Min. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Liang, K.; Ma, B.; An, Y.; Zhang, Y.; Chen, Y.; Wang, C. A Summary of Smelting and Secondary Recovery Process of Antimony. JOM 2025, 77, 2666–2683. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M. Selective leaching of antimony and arsenic from mechanically activated tetrahedrite, jamesonite and enargite. Int. J. Min. Process 2006, 81, 44–50. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandström, Å. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Min. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M. The Novel Lixiviants for Maximizing Antimony Extraction from Tetrahedrite-Rich Concentrate: Mechanism and Kinetic Studies. Miner. Process. Extr. Metall. Rev. 2022, 43, 798–812. [Google Scholar] [CrossRef]
- Ji, Y.; Feng, Y.; Wu, J.; Zhu, T.; Bai, Z.; Duan, C. Using geoaccumulation index to study source profiles of soil dust in China. J. Environ. Sci. 2008, 20, 571–578. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Y.; Zheng, Y.; Jin, H.; Huang, W.; Liu, Q.; Shou, L.; Zeng, J.; Chen, Q.; Chen, J. Elemental geochemical evidence for the river-derived sources of trace metals in surface sediments from Hangzhou Bay, East China Sea. Environ. Res. 2024, 250, 118588. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Meng, T.; He, L.; Zhang, S. Biogeochemical behavior, health risk assessment and source identification of antimony and arsenic in soil from a legacy antimony smelter in Gansu, Northwest China. Environ. Sci. Eur. 2023, 35, 114. [Google Scholar] [CrossRef]
- Kim, B.S.M.; Angeli, J.L.F.; Ferreira, P.A.L.; de Mahiques, M.M.; Figueira, R.C.L. Critical evaluation of different methods to calculate the Geoaccumulation Index for environmental studies: A new approach for Baixada Santista—Southeastern Brazil. Mar. Pollut. Bull. 2018, 127, 548–552. [Google Scholar] [CrossRef]
- Ballester, A.; Verdeja, L.F.; Sancho, J. Metalurgia Extractiva Fundamentos; Síntesis S.A.: Madrid, Spain, 2000; Volume 1. [Google Scholar]
- Levenspiel, O. Ingeniería de las Reacciones Químicas; Reverté: Barcelona, Spain, 2010. [Google Scholar]
- Ruiz, M.C.; Daroch, F.; Padilla, R. Digestion kinetics of arsenic removal from enargite–tennantite concentrates. Min. Eng. 2015, 79, 47–53. [Google Scholar] [CrossRef]
- Parada, F.; Jeffrey, M.I.; Asselin, E. Leaching kinetics of enargite in alkaline sodium sulphide solutions. Hydrometallurgy 2014, 146, 48–58. [Google Scholar] [CrossRef]
- Blanco-Vino, W.; Zamora, G.; Ordóñez, J.I. Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies. Mining 2024, 4, 284–301. [Google Scholar] [CrossRef]
- Labastida, I.; Armienta, M.A.; Lara, R.H.; Briones, R.; González, I.; Romero, F. Kinetic approach for the appropriate selection of indigenous limestones for acid mine drainage treatment with passive systems. Sci. Total Environ. 2019, 677, 404–417. [Google Scholar] [CrossRef]
- Duarte Zaragoza, V.M.; Gutiérrez Castorena, E.V.; del Carmen Gutiérrez Castorena, M.; Carrillo González, R.; Ortiz Solorio, C.A.; Trinidad Santos, A. Heavy metals contamination in soils around tailing heaps with various degrees of weathering in Zimapán, Mexico. Int. J. Environ. Stud. 2015, 72, 24–40. [Google Scholar] [CrossRef]
- Calla-Choque, D.; Nava-Alonso, F.; Fuentes-Aceituno, J.C. Acid decomposition and thiourea leaching of silver from hazardous jarosite residues: Effect of some cations on the stability of the thiourea system. J. Hazard. Mater. 2016, 317, 440–448. [Google Scholar] [CrossRef]
- Navarro Flores, A.; Martínez Sola, F. Evaluation of Metal Attenuation from Mine Tailings in SE Spain (Sierra Almagrera): A Soil-Leaching Column Study. Mine Water Environ. 2010, 29, 53–67. [Google Scholar] [CrossRef]
- Qiao, W.; Wang, Y.; He, P.; Yin, X.; Zhang, D.; Bai, G.; Sun, W.; Luo, Z.; Wei, X.; Lan, J.; et al. Groundwater arsenic and antimony mobility from an antimony mining area: Controls of sulfide oxidation, carbonate and silicate weathering, and secondary mineral precipitation. Water Res. 2025, 273, 123086. [Google Scholar] [CrossRef]
- Barago, N.; Mastroianni, C.; Pavoni, E.; Floreani, F.; Parisi, F.; Lenaz, D.; Covelli, S. Environmental impact of potentially toxic elements on soils, sediments, waters, and air nearby an abandoned Hg-rich fahlore mine (Mt. Avanza, Carnic Alps, NE Italy). Environ. Sci. Pollut. Res. 2023, 30, 63754–63775. [Google Scholar] [CrossRef]
- Tongggiroh, A.; Imran, A.M.; Haerany, S. Environmental Geochemistry of Heavy Metals and Plagioclase Background Enrichment Factor in Coastal Sediments at Lumpue-Parepare, South Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 370, 12002. [Google Scholar] [CrossRef]
- Looi, L.J.; Aris, A.Z.; Yusoff FMd Isa, N.M.; Haris, H. Application of enrichment factor, geoaccumulation index, and ecological risk index in assessing the elemental pollution status of surface sediments. Environ. Geochem. Health 2019, 41, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Mahvi, A.H.; Hashemi, S.Y.; Arfaeinia, H.; Pasalari, H.; Zarei, A.; Changani, F. Spatial distribution, enrichment and geo-accumulation of heavy metals in surface sediments near urban and industrial areas in the Persian Gulf. Desalination Water Treat. 2019, 158, 130–139. [Google Scholar] [CrossRef]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Castro, L.N.; Rendina, A.E.; Orgeira, M.J. Assessment of toxic metal contamination using a regional lithogenic geochemical background, Pampean area river basin, Argentina. Sci. Total Environ. 2018, 627, 125–133. [Google Scholar] [CrossRef]
- Teja-Ruiz, A.M.; Juárez-Tapia, J.C.; Hernández-Cruz, L.E.; Reyes-Pérez, M.; Patiño-Cardona, F.; Reyes-Dominguez, I.A.; Flores-Guerrero, M.U.; Palacios-Beas, E.G. Influence of Temperature on the Formation of Ag Complexed in a S2O32−–O2 System. Minerals 2017, 7, 16. [Google Scholar] [CrossRef]
- Teja-Ruiz, A.; Juárez-Tapia, J.; Hernández-Ortiz, O.; Reyes-Domínguez, I.; Cisneros-Flores, G.; Martínez-Soto, J.; Flores-Guerrero, U.; Beas, E.P. Alkaline-reductive pretreatment proposal for the removal of economically burdened semimetals from silver sulfosalts. Sep. Purif. Technol. 2025, 361, 131243. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Tian, Q.; Qin, H. Selective removal of arsenic from high arsenic dust in the NaOH-S system and leaching behavior of lead, antimony, zinc and tin. Hydrometallurgy 2021, 202, 105607. [Google Scholar] [CrossRef]
- Cuevas, J.; Bruckard, W.J.; Pownceby, M.I.; Sparrow, G.J.; Torpy, A. Alkaline sulphide leaching of tennantite in copper flotation concentrates to selectively dissolve arsenic. Miner. Process. Extr. Metall. 2022, 131, 229–238. [Google Scholar] [CrossRef]
- Delfini, M.; Ferrini, M.; Manni, A.; Massacci, P.; Piga, L. Arsenic leaching by Na2S to decontaminate tailings coming from colemanite processing. Min. Eng. 2003, 16, 45–50. [Google Scholar] [CrossRef]
- He, H.; Wang, J.; Li, Y.; Song, Z.; Sivaprasada, M. Thermodynamic analysis of hot water leaching of sulphur from desulphurisation slag by Eh–pH diagrams of the Ca-S-H2O system. Miner. Process. Extr. Metall. 2019, 128, 161–167. [Google Scholar] [CrossRef]
- Sparrow, G.J.; Woodcock, J.T. Cyanide and Other Lixiviant Leaching Systems for Gold with Some Practical Applications. Miner. Process. Extr. Metall. Rev. 1995, 14, 193–247. [Google Scholar] [CrossRef]
- Han, C.; Wang, W.; Xie, F.; Zhang, T. Mechanism and kinetics of mercuric sulfide leaching with cuprous-thiosulfate solutions. Sep. Purif. Technol. 2017, 177, 223–232. [Google Scholar] [CrossRef]
- Anderson, C.G.; Twidwell, L.G. The Alkaline Sulfide Hydrometallurgical Separation, Recovery and Fixation of Tin, Arsenic, Antimony, Mercury and Gold. In Proceedings of Lead & Zinc 2008; SAIMM: Johannesburg, South Africa, 2008; pp. 121–132. Available online: https://scholar.google.com/citations?user=zS4bYV8AAAAJ&hl=en&oi=ao (accessed on 10 October 2025).
- Suess, E.; Planer-Friedrich, B. Thioarsenate formation upon dissolution of orpiment and arsenopyrite. Chemosphere 2012, 89, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.G. The Application and Economics of Industrial Alkaline Leaching of Copper Enargite Concentrates. In Proceedings of the Copper Cobalt Africa Conference, Livingstone, Zambia, 6–8 July 2015. [Google Scholar]
- Faraji, F.; Alizadeh, A.; Rashchi, F.; Mostoufi, N. Kinetics of leaching: A review. Rev. Chem. Eng. 2022, 38, 113–148. [Google Scholar] [CrossRef]
- Nolasco, M.C.; Rodríguez, I.; Vilasó, J.E.; Flores, M.U.; Pandiyan, T.; Gutiérrez, E.J.; Aguilar, J.; Reyes, M.; Reyes, I.A. Selective extraction of silver from jarosite residues produced in the zinc hydrometallurgical process using thiourea under acidic conditions: Kinetic analysis and leaching optimization. Hydrometallurgy 2025, 231, 106396. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Baba, A.A. Dissolution kinetics studies of kaolin ore as raw material for mesoporous silica by acid leaching. Can. Metall. Q. 2024, 63, 203–212. [Google Scholar] [CrossRef]
- Tunç Parlak, T.; Yildiz, K. Investigation of the effect of mechanical activation on nickel and cobalt extraction from laterite at atmospheric pressure by kinetics and leach residue analysis. Can. Metall. Q. 2025, 64, 680–697. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Jing, T.; Zhang, T. Effect of mechanical activation on leaching of zinc and indium from indium-bearing zinc ferrite with sulphur dioxide as leachant and reductant. Can. Metall. Q. 2021, 60, 150–159. [Google Scholar] [CrossRef]
- Đorđević, T.; Kolitsch, U.; Serafimovski, T.; Tasev, G.; Tepe, N.; Stöger-Pollach, M.; Hofmann, T.; Boev, B. Mineralogy and Weathering of Realgar-Rich Tailings At a Former As-Sb-Cr Mine At Lojane, North Macedonia. Can. Mineral. 2019, 57, 403–423. [Google Scholar] [CrossRef]
- Intratec. Sodium Sulfides Price; Intratec Chemical Data: Houston, TX, USA; Available online: https://www.intratec.us/ (accessed on 14 October 2025).
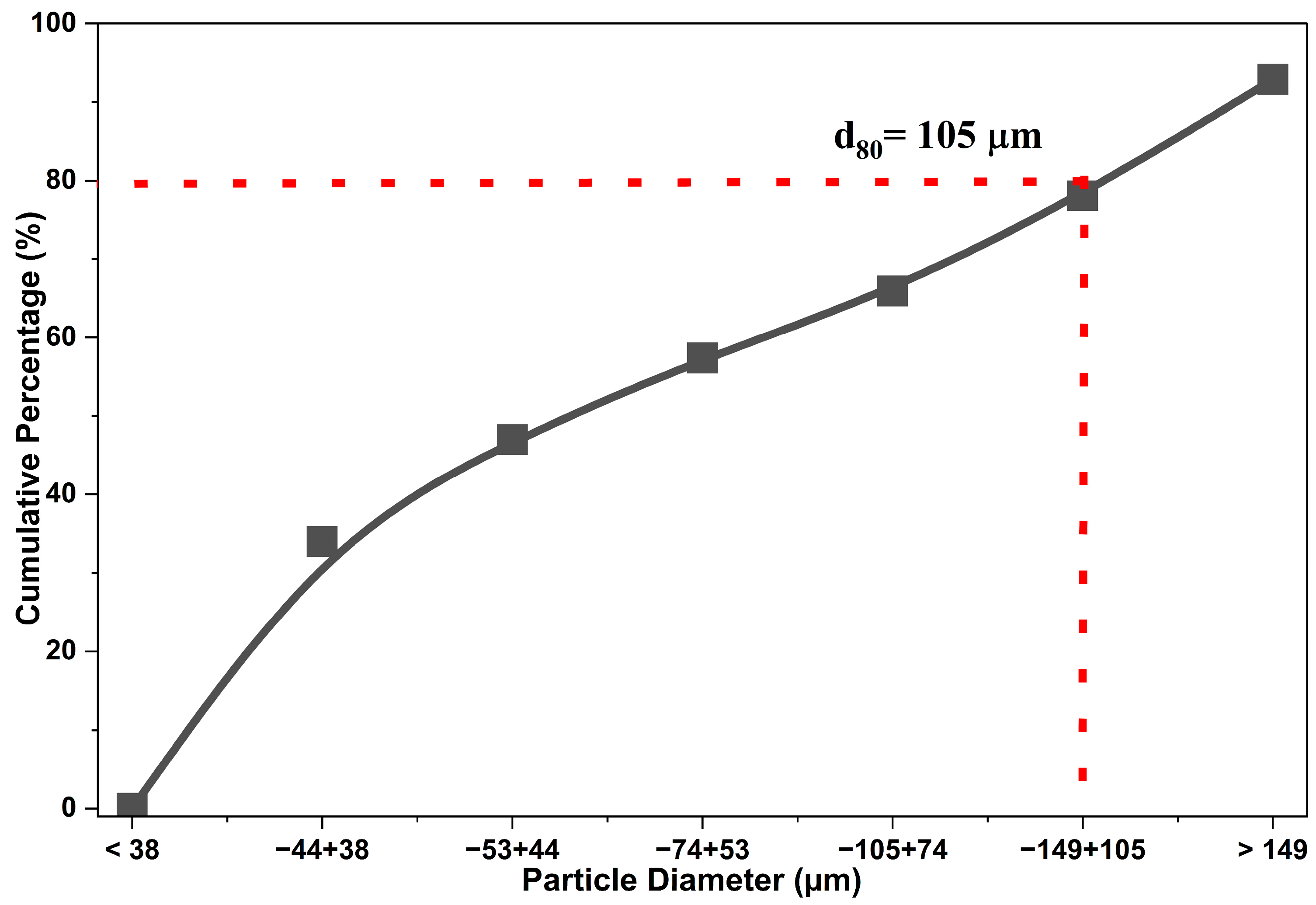

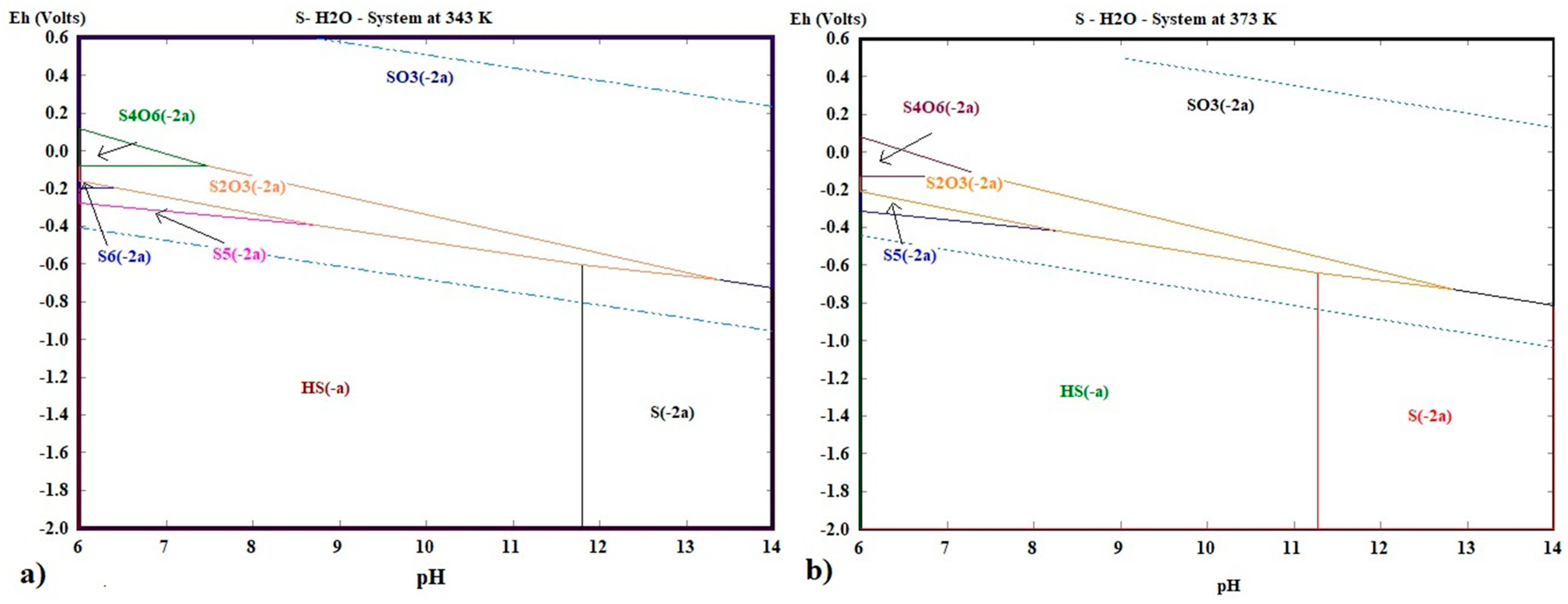
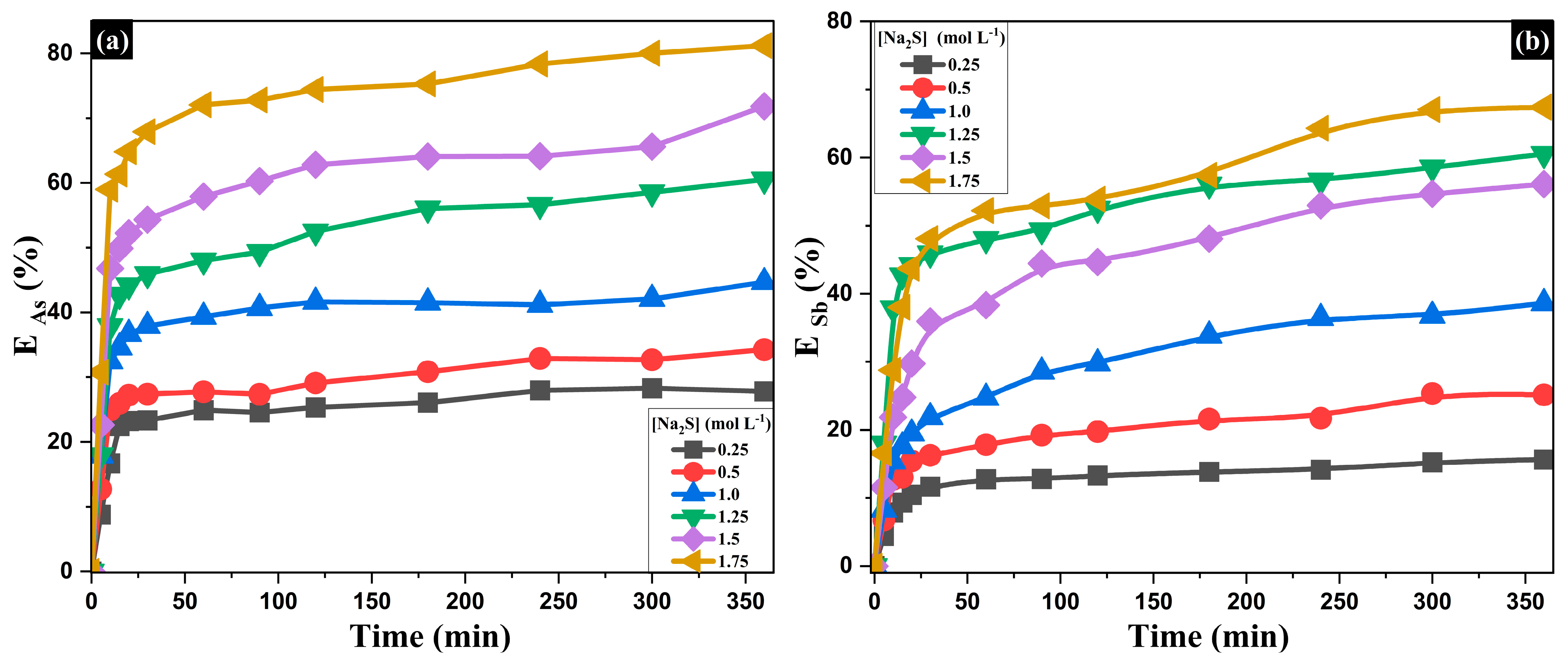

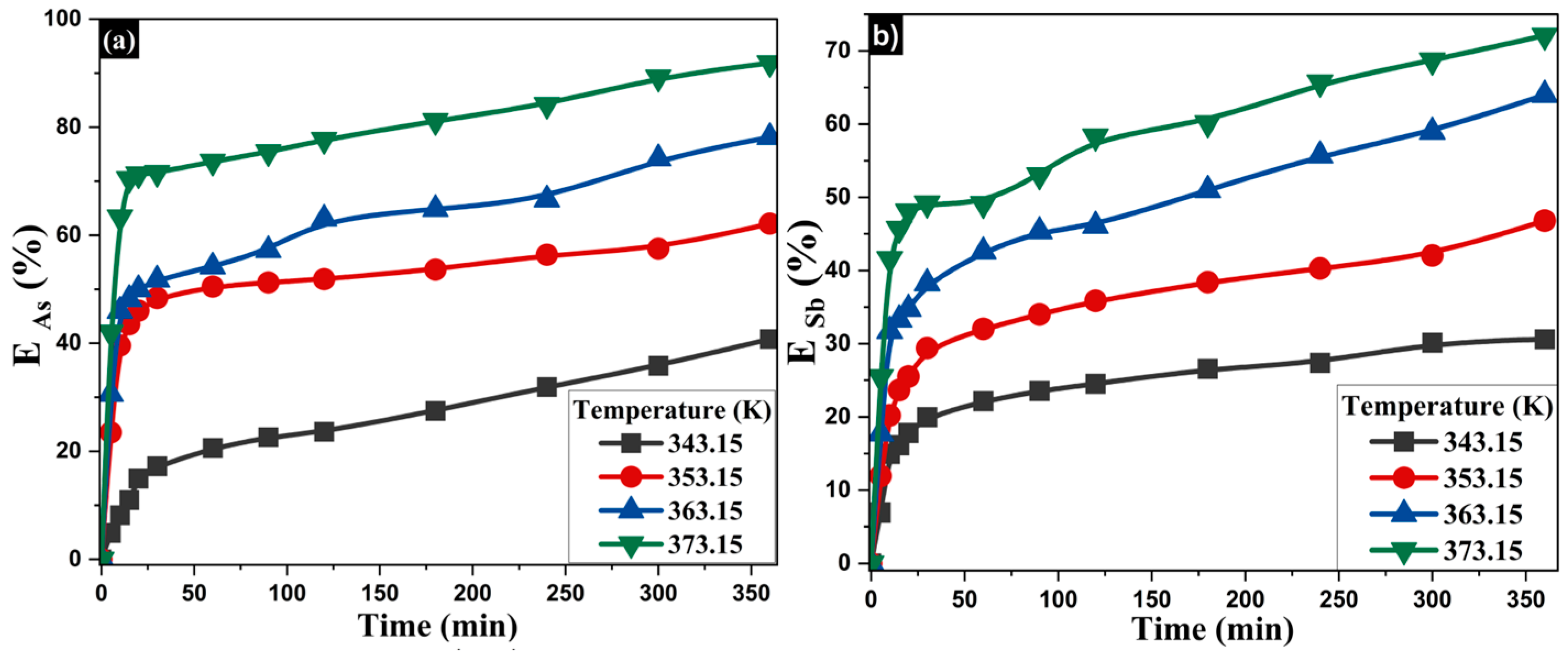

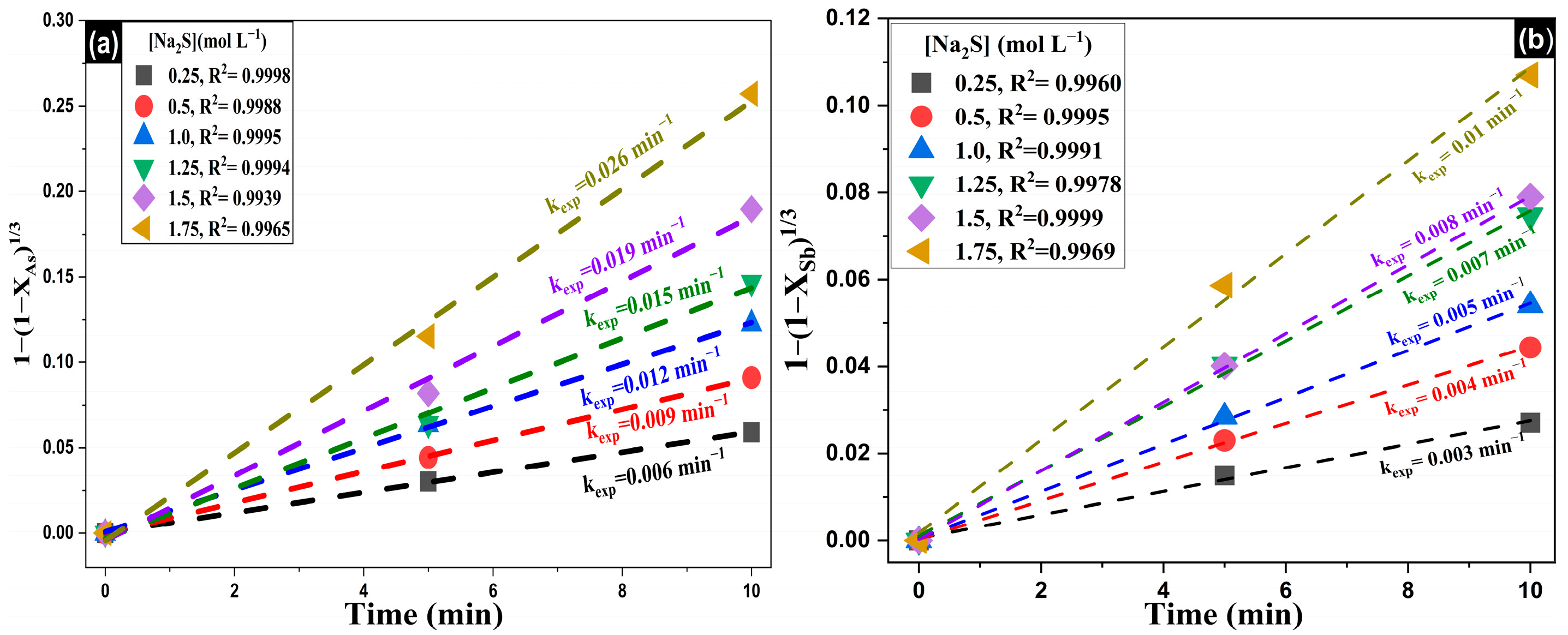
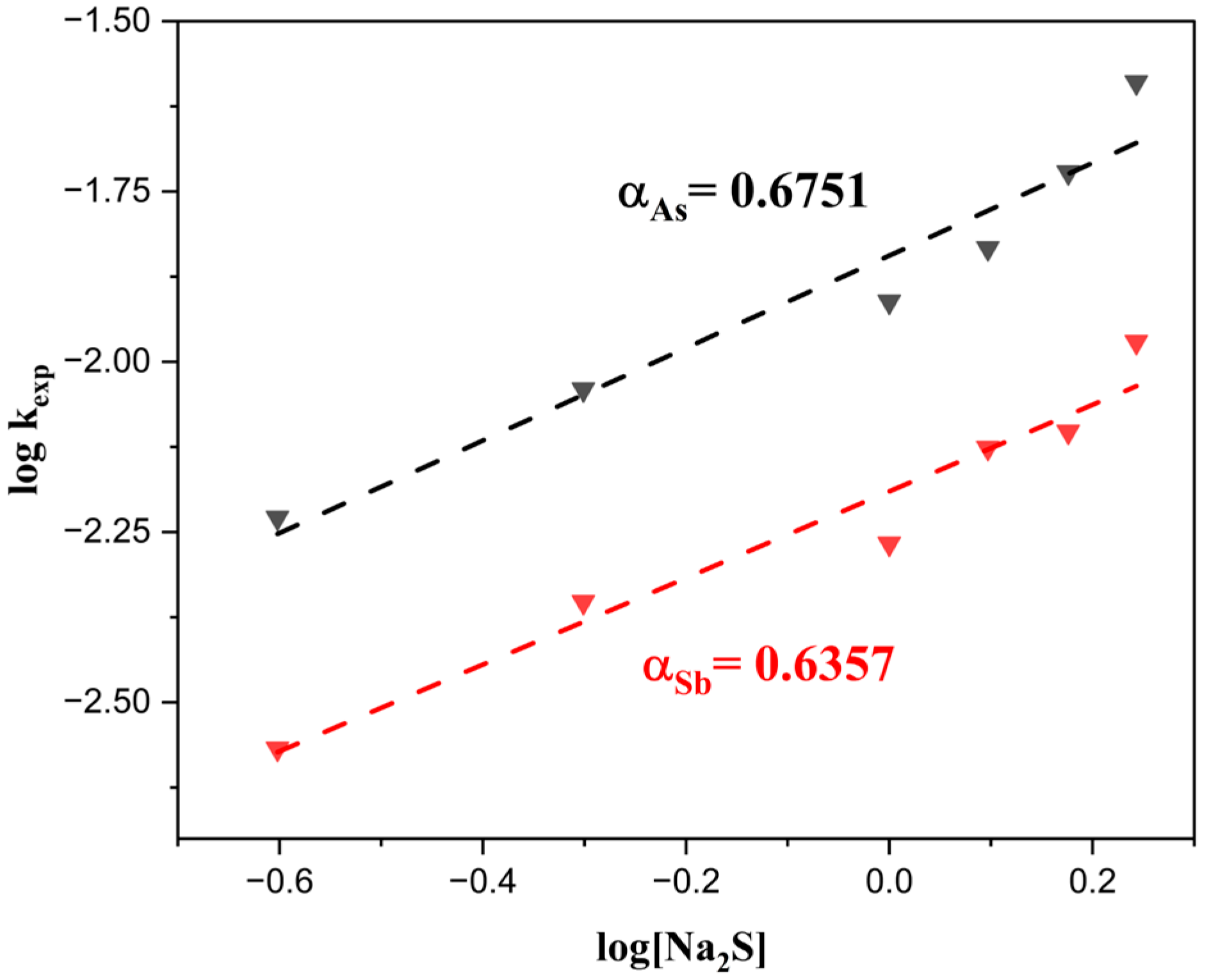
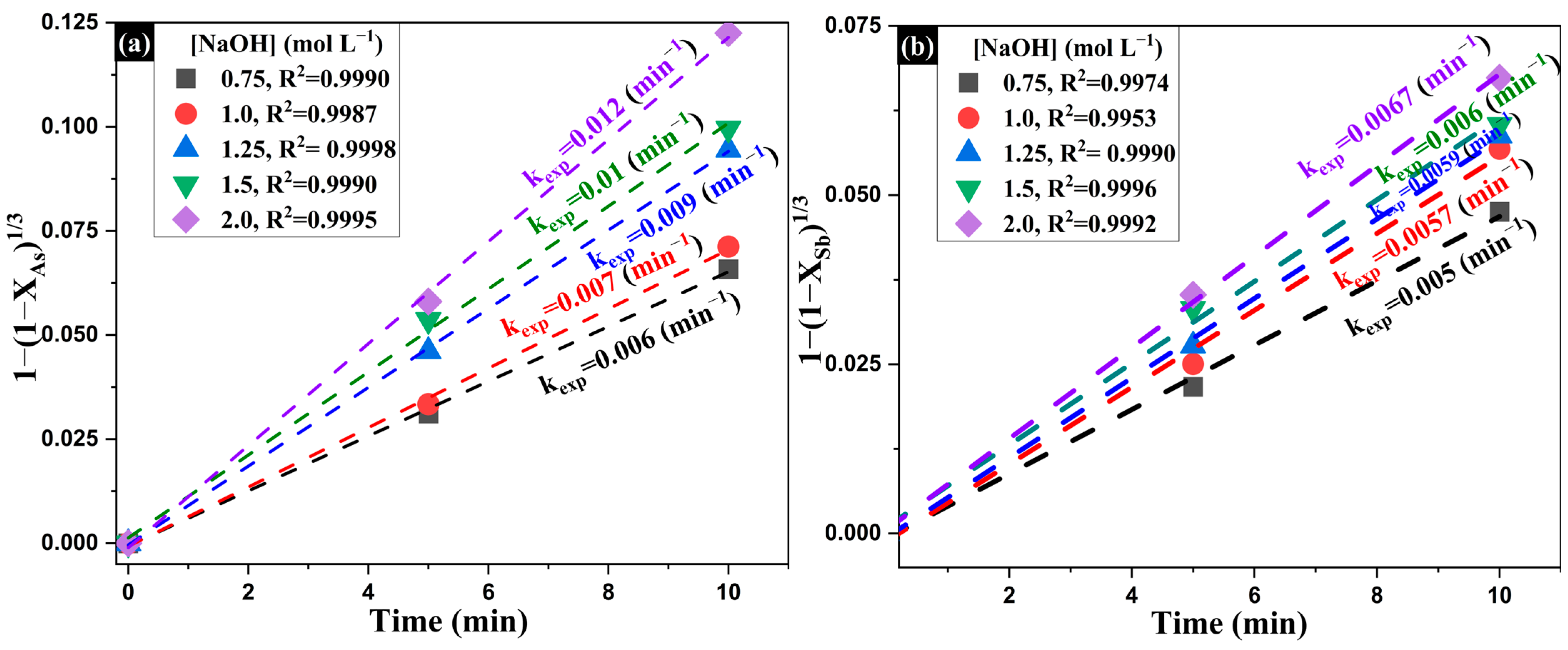
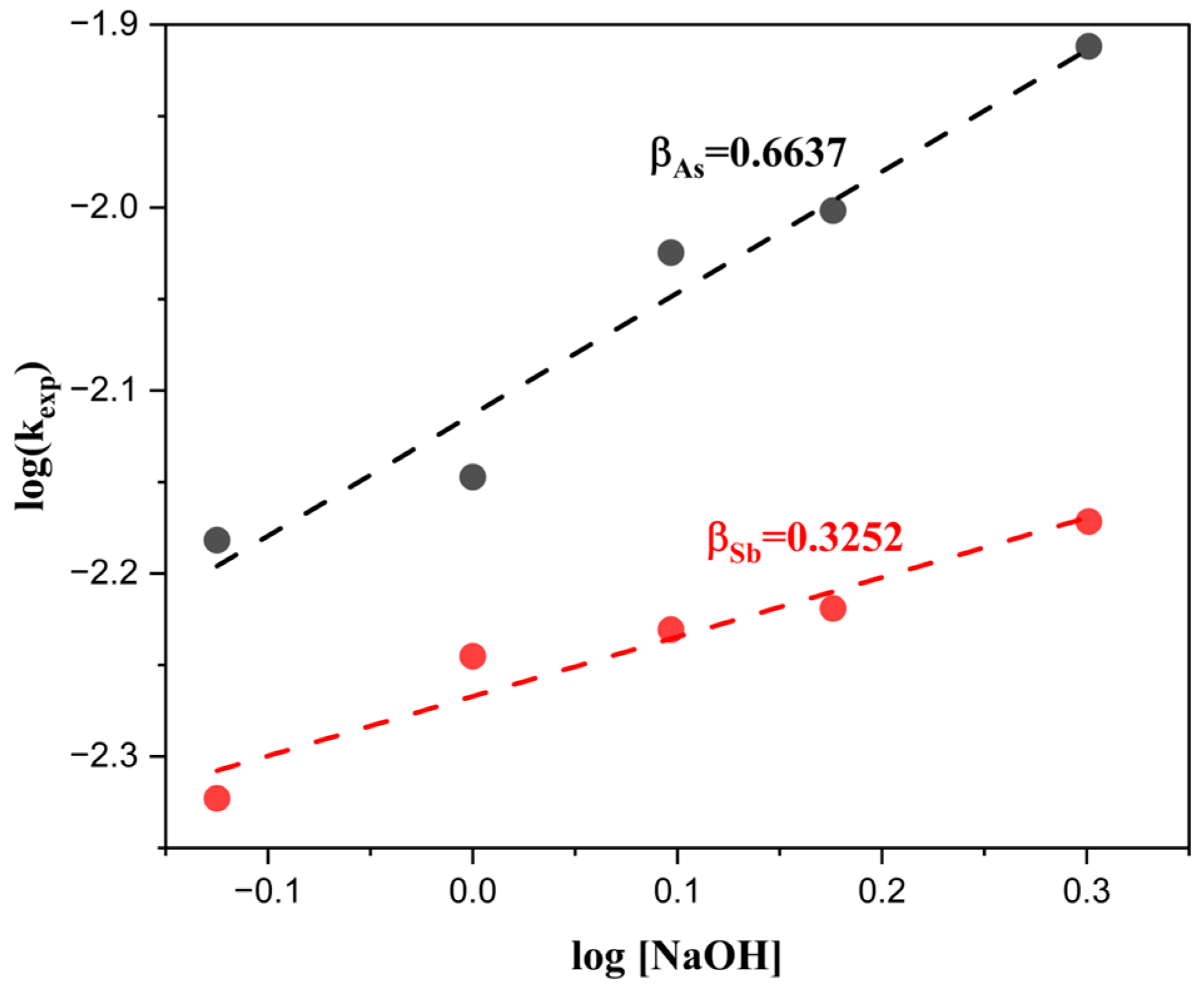
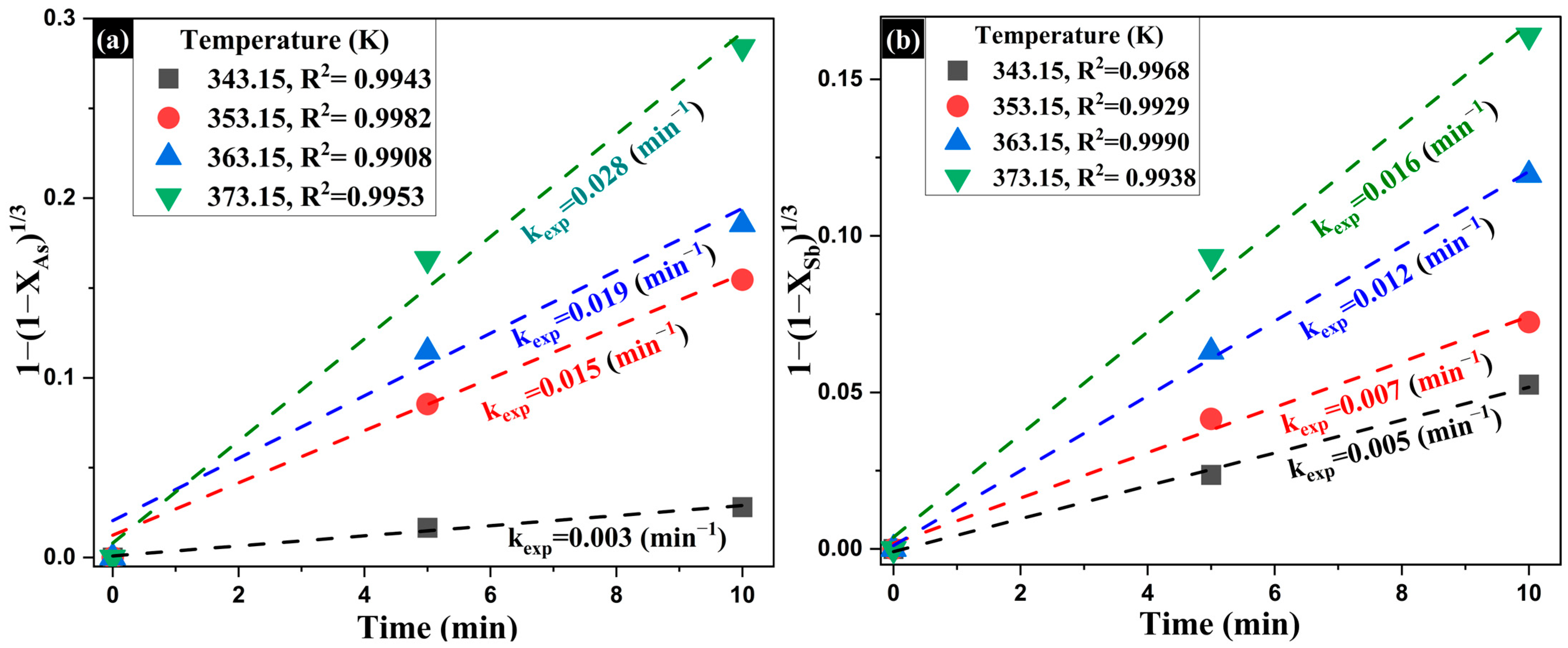
| Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mesh | (μm) | Ag (gr ton−1) | Cu | Fe | Zn | Pb | As | Sb | Ca |
| >100 | >149 | 41.67 | 21.17 | 11.42 | 7.37 | 0.89 | 1.19 | 0.34 | 2.18 |
| −100 + 140 | −149 + 105 | 144.33 | 22.33 | 11.02 | 7.60 | 3.32 | 1.82 | 0.51 | 0.65 |
| −140 + 200 | −105 + 74 | 75.61 | 23.09 | 12.21 | 8.11 | 1.60 | 1.72 | 0.44 | 0.67 |
| −200 + 270 | −74 + 53 | 111.83 | 24.24 | 13.84 | 8.39 | 2.10 | 1.61 | 0.34 | 0.56 |
| −270 + 325 | −53 + 44 | 68.06 | 21.43 | 12.37 | 1.00 | 0.69 | 0.96 | 0.32 | 2.71 |
| −325 + 400 | −44 + 38 | 60.33 | 22.25 | 12.98 | 8.06 | 0.95 | 0.78 | 0.35 | 2.26 |
| <−400 | <38 | 73.50 | 23.97 | 14.47 | 7.79 | 2.01 | 0.96 | 0.39 | 1.26 |
| Na2S (mol·L−1) | NaOH (mol·L−1) | Temperature (K) | EAs (%) | kexp (min−1) | ESb (%) | kexp (min−1) |
|---|---|---|---|---|---|---|
| 0.25 | 1.5 | 353 | 28.29 | 0.0059 | 15.67 | 0.0027 |
| 0.5 | 1.5 | 353 | 34.27 | 0.0091 | 25.36 | 0.0044 |
| 1 | 1.5 | 353 | 44.66 | 0.0123 | 38.61 | 0.0054 |
| 1.25 | 1.5 | 353 | 60.54 | 0.0147 | 47.00 | 0.0075 |
| 1.5 | 1.5 | 353 | 71.85 | 0.0190 | 56.09 | 0.0079 |
| 1.75 | 1.5 | 353 | 81.22 | 0.0257 | 67.39 | 0.0107 |
| 1.25 | 0.75 | 353 | 39.11 | 0.0066 | 33.04 | 0.0048 |
| 1.25 | 1 | 353 | 40.15 | 0.0071 | 40.02 | 0.0057 |
| 1.25 | 1.25 | 353 | 46.20 | 0.0095 | 46.20 | 0.0059 |
| 1.25 | 1.5 | 353 | 60.58 | 0.0100 | 48.49 | 0.0060 |
| 1.25 | 2 | 353 | 67.82 | 0.0123 | 51.72 | 0.0067 |
| 1.25 | 1.5 | 343 | 40.78 | 0.0028 | 30.61 | 0.0053 |
| 1.25 | 1.5 | 353 | 62.13 | 0.0145 | 46.80 | 0.0072 |
| 1.25 | 1.5 | 363 | 78.14 | 0.0186 | 63.99 | 0.0119 |
| 1.25 | 1.5 | 373 | 91.87 | 0.0284 | 72.09 | 0.0164 |
| Source | DF | Sum of Squares | Adj MS | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| As | Na2S | 1 | 41.58 | 41.58 | 484.34 | 0.0021 |
| NaOH | 1 | 66.16 | 66.16 | 770.61 | 0.0013 | |
| Temperature | 1 | 13.91 | 13.91 | 162.07 | 0.0061 | |
| Na2S·Na2S | 1 | 8.97 | 8.97 | 104.54 | 0.0094 | |
| NaOH·NaOH | 1 | 22.01 | 22.01 | 256.39 | 0.0039 | |
| T·T | 1 | 10.32 | 10.32 | 120.24 | 0.0082 | |
| Error | 2 | 0.1717 | 0.08585 | |||
| Total | 8 | 3660.42 | ||||
| Sb | Na2S | 1 | 41.78 | 41.78 | 49.31 | 0.0197 |
| NaOH | 1 | 40.53 | 40.53 | 47.83 | 0.0203 | |
| Temperature | 1 | 12.78 | 12.78 | 15.08 | 0.0604 | |
| Na2S·Na2S | 1 | 8.31 | 8.31 | 9.82 | 0.0885 | |
| NaOH·NaOH | 1 | 17.88 | 17.88 | 21.11 | 0.0442 | |
| T·T | 1 | 10.1 | 10.1 | 11.92 | 0.0746 | |
| Error | 2 | 1.69 | 0.8473 | |||
| Total | 8 | 2430.42 |
| [Na2S] (mol·L−1) | [NaOH] (mol·L−1) | T (K) | %As | %Sb |
|---|---|---|---|---|
| 1 | 1.375 | 358 | 57.74 | 44.16 |
| 0.25 | 0.75 | 358 | 15.57 | 8.56 |
| 1.75 | 0.75 | 358 | 68.49 | 60.28 |
| 0.25 | 2 | 358 | 44.29 | 26.03 |
| 1.75 | 2 | 358 | 97.21 | 77.75 |
| 0.25 | 1.375 | 343 | 5.54 | 4.75 |
| 1.75 | 1.375 | 343 | 58.46 | 48.97 |
| 1 | 1.375 | 358 | 57.74 | 44.16 |
| 0.25 | 1.375 | 373 | 56.84 | 38.73 |
| 1.75 | 1.375 | 373 | 99.00 | 90.45 |
| 1 | 0.75 | 343 | 9.85 | 7.25 |
| 1 | 2 | 343 | 38.57 | 24.72 |
| 1 | 0.75 | 373 | 61.15 | 48.73 |
| 1 | 2 | 373 | 89.87 | 66.20 |
| 1 | 1.375 | 358 | 57.74 | 44.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisneros, G.; Juárez, J.C.; Reyes, I.A.; Reyes, M.; Urbano, G.; Martínez, J.I.; Teja, A.M.; Flores, M.U. Selective Removal of Arsenic and Antimony by Alkaline Leaching with Sodium Sulfide: Remediation of Metalloids-Contaminated Concentrates from Zimapán, Hidalgo, Mexico. Processes 2025, 13, 3347. https://doi.org/10.3390/pr13103347
Cisneros G, Juárez JC, Reyes IA, Reyes M, Urbano G, Martínez JI, Teja AM, Flores MU. Selective Removal of Arsenic and Antimony by Alkaline Leaching with Sodium Sulfide: Remediation of Metalloids-Contaminated Concentrates from Zimapán, Hidalgo, Mexico. Processes. 2025; 13(10):3347. https://doi.org/10.3390/pr13103347
Chicago/Turabian StyleCisneros, Gabriel, Julio C. Juárez, Iván A. Reyes, Martín Reyes, Gustavo Urbano, Jesús I. Martínez, Aislinn M. Teja, and Mizraim U. Flores. 2025. "Selective Removal of Arsenic and Antimony by Alkaline Leaching with Sodium Sulfide: Remediation of Metalloids-Contaminated Concentrates from Zimapán, Hidalgo, Mexico" Processes 13, no. 10: 3347. https://doi.org/10.3390/pr13103347
APA StyleCisneros, G., Juárez, J. C., Reyes, I. A., Reyes, M., Urbano, G., Martínez, J. I., Teja, A. M., & Flores, M. U. (2025). Selective Removal of Arsenic and Antimony by Alkaline Leaching with Sodium Sulfide: Remediation of Metalloids-Contaminated Concentrates from Zimapán, Hidalgo, Mexico. Processes, 13(10), 3347. https://doi.org/10.3390/pr13103347






