1. Introduction
Catalytic cracking is an important part of refining that turns heavy hydrocarbon feedstocks like vacuum residue (VR) into lighter, more useful fractions like gasoline, diesel, and kerosene. This change makes oil refineries more cost-effective by cutting down on waste and increasing fuel yield. The type and design of the catalyst employed have a big effect on how well and selectively this process works. Zeolites, alumina, and silica are traditional catalysts that have been widely used in industry because they are very acidic, stable at high temperatures, and have large surface areas that make it easier to break carbon–carbon bonds in complex hydrocarbons [
1,
2]. Zeolites have demonstrated exceptional catalytic efficacy due to their well-defined microporous architectures and superior hydrothermal stability.
Nevertheless, constraints in the diffusion of substantial molecules via micropores have driven researchers to investigate alternate materials that exhibit improved mesoporosity and redox stability. Kaolinite that has been treated with acid, especially metakaolinite that has been heated to 800 °C (MKA800), is a good support material because it is more acidic on the surface, can withstand heat better, and is cheaper. Recent improvements have concentrated on altering MKA800 with transition and rare-earth metals, such as cerium (Ce), to augment its catalytic properties. The Ce
3+/Ce
4+ couple in cerium oxide (CeO
2) gives it unique redox behavior, which allows it to store and release oxygen during cracking. This, in turn, promotes hydrogen transfer, stops coke formation, and makes the catalyst more stable under harsh conditions [
3]. The primary catalytic uses of cerium are categorized into four major groups.
Figure 1 summarizes the principal catalytic applications of ceria into four main categories.
Figure 1.
Main applications of ceria-based catalysts [
4].
Figure 1.
Main applications of ceria-based catalysts [
4].
Cerium-modified catalysts have been studied in analogous hydroprocessing domains; however, their utilization in VR catalytic cracking, especially over MKA800, is still restricted. Razmgar et al. [
4] underscored the adaptability of ceria in hydrogenation reactions.
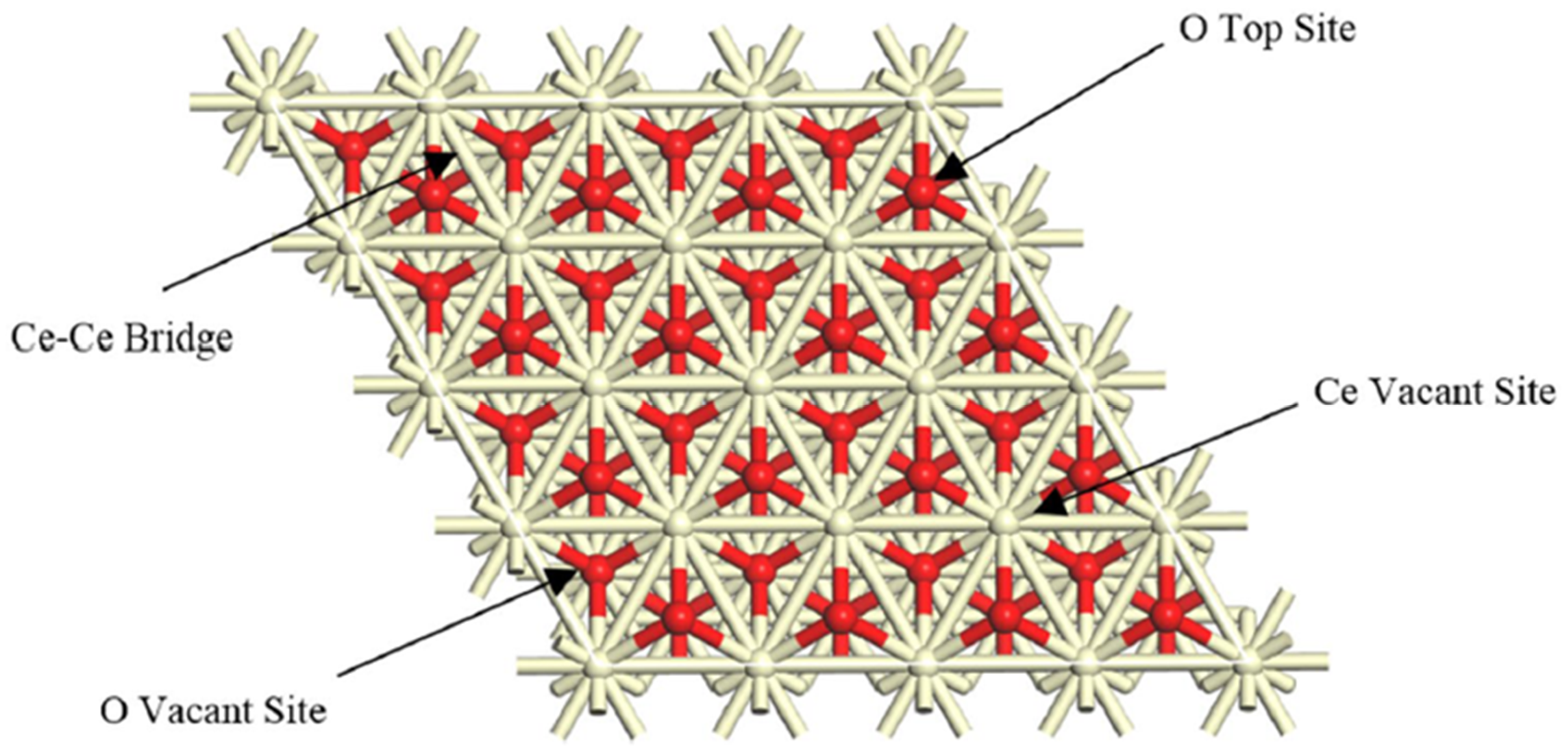
Figure 2 illustrates the adsorption sites for hydrogenation on the ceria surface as observed from a top–down perspective.
Figure 2.
Adsorption sites for hydrogenation over the ceria surface from a top view [
4].
Figure 2.
Adsorption sites for hydrogenation over the ceria surface from a top view [
4].
Whilst Wang et al. [
5] demonstrated the high effectiveness of Fe–Mn mixed metal oxide oxygen carriers in chemical looping processes for upgrading vacuum residue. Moreover, the capacity of ceria to augment asphaltene conversion by promoting hydrogen donation has been delineated in various recent investigations [
3,
6]. These characteristics render cerium an attractive option for enhancing cracking performance when incorporated into kaolinite-based supports.
Kinetic modeling is essential for comprehending the mechanistic facets of catalytic cracking. Lumped kinetic models, such as four-lump and eight-lump frameworks, are commonly employed to streamline the intricate mixing of heavy oils and forecast product distributions across diverse operating conditions [
7,
8]. These kinds of models make it possible to determine reaction rate constants, activation energies, and product selectivity, which are important for designing reactors and testing catalysts.
A summary of past kinetic modeling studies in
Table 1 shows the many different catalytic systems and methods that have been used to crack VR and heavy oil. Consequently, this study seeks to formulate and test an eight-lump kinetic model to elucidate the cracking behavior of VR over cerium-modified MKA800 under atmospheric circumstances while simultaneously investigating its thermodynamic properties.
Table 1.
Summary of the previous work concerning kinetic modeling of VR cracking.
Table 1.
Summary of the previous work concerning kinetic modeling of VR cracking.
| Catalyst/System | Feedstock | Reactor | Temp. (°C) | Pressure | Highlights | Reference |
|---|
| Silica sand | Vacuum residue (VR) | Fluidized bed | 600–750 | N/A | High alkene/olefin yield | [9] |
| CeZr, FeCoCeZr | Oil sand bitumen | Fixed bed | 470 | N/A | ↑ Gas oil, ↓ coke | [10] |
| CoMo/γ-Al2O3 | THAI heavy crude | Microwave | 425 | N/A | ↑ API, ↓ S, ↓ viscosity | [11] |
| Mn-based catalysts | Asalacha heavy oil | DSC | 30–600 | N/A | ↓ Activation energy | [12] |
| CoMoP, NiMoP/γ-Al2O3 | Mexican heavy crude | Batch (autoclave) | 380 | 10.6 MPa | ↑ API, ↓ viscosity | [13] |
| Fe–Mn oxide | VR | Fixed bed | 550 | 1 atm | ↑ Gasoline/diesel yield | [14] |
| Ni-Mo/Al2O3 | VR | Ebullated bed | 401–412 | 18–20 MPa | High conversion | [3] |
| ZrO2 | VR | Batch | 470 | N/A | ↑ Liquid yield | [15] |
| Fe naphthenate | Residual oil | Batch (aquathermolysis) | 340 | 3 MPa | ↓ Viscosity/sulfur | [16] |
| Ionic liquids (IL) | Heavy crude oil | Batch (aquathermolysis) | 175 | N/A | ↓ Viscosity, ↓ asphaltenes | [17] |
| MoS2 | Cold lake VR | Semi-batch | 415 | 5.5 MPa | ↑ Hydroconversion | [18] |
| Fe2O3–ZrO2–Al2O3 | Atmospheric residue (AR) | Fixed bed | 475 | 1 atm | ↑ Reforming/cracking | [19] |
These works (which are present in
Table 1) provide valuable insight into mass transfer limitations and reaction pathways relevant to the present VR catalytic cracking system.
4. Analysis of Kinetic Modeling
The following are earlier investigations pertaining to kinetic modeling for vacuum and atmospheric residue cracking:
Singh et al. [
43] created a kinetic model for the thermal cracking of vacuum residue that included five lumps and ten reactions. According to estimates from Indian refineries, two leftover feedstocks were of Middle Eastern and Indian origin. Four distinct temperatures between 400 and 430 °C and a residence period ranging from 3 to 15 min were examined. The reaction route was identified using Delplot analysis.
Martinez and Ancheyta [
44] examined five-lump kinetic models for the hydrocracking of heavy oil in a CSTR reactor within a temperature range of 380 to 420 °C. The findings indicate that lighter lumps exhibit greater sensitivity to temperature variations, whereas heavier lumps undergo hydrocracking with greater ease. The reaction pathway of naphtha to gases was determined to be the least favored step in hydrocracking. The mean absolute error of the developed kinetic model was below 5%.
Gao et al. [
45] formulated an eight-lump kinetic model for the catalytic cracking of vacuum residue, comprising the components CP, CN, CA, HCO, LPG, dry gas, light oil, and coke. This model incorporates twenty-one kinetic parameters: twenty associated with reactions and one pertaining to catalyst deactivation. Experimental data were collected from a fixed-bed reactor and a pilot plant across four distinct temperatures (460, 480, 500, and 520 °C). The model demonstrates a high degree of simulation accuracy, with predicted yields closely aligning with the experimental findings. The eight-lump kinetic model is illustrated in
Figure 11.
Based on information estimated from commercial scale residue hydrockracking units (RHC), Manek and Haydary [
3] constructed a kinetic model of VR hydrocracking. The datasets reflect temperature, flow, and product yields. The temperature and pressure under study were between 401 and 412 °C and 18 and 20 MPa, respectively. VR, vacuum distillate, gas oil, kerosene, naphtha, and gas are the six fractions and eight reaction stages that make up the kinetic model. The model offers a range of potential product yield compositions and can be integrated into refinery production planning systems for mass balance computation.
Zheng et al. [
46] employed a lumping strategy in the kinetic modeling of vacuum pyrolysis of POA within a pilot-scale semi-batch reactor. A five-lump kinetic model was proposed, and the Arrhenius kinetic parameters were estimated. The pyrolysis of biomass POA into biofuels was conducted at varying temperatures of 410, 430, and 450 °C, alongside different reaction durations of 10, 15, 20, 25, 40, 50, and 60 min.
Navarro and Almao [
47] employed a Ni/K catalyst in the catalytic steam cracking of vacuum residue at operating temperatures ranging from 435 to 445 °C and a pressure of 300 psig. The evaluation of the lumped kinetic model encompassed the generation of asphaltenes. The selection of operating conditions was determined to be critically significant for ensuring catalytic activity in the steam cracking of vacuum residue. The alteration in the mechanism was noted under high selectivity from a kinetic perspective. The HVGO and LGVO fractions were found to exhibit significantly greater reactivity under the operational conditions of catalytic steam cracking.
Figure 12 illustrates the reaction pathway for the kinetic model.
Kaminski and Hussein [
23] employed three distinct schemes of a five-lump model, which encompasses gas, coke, asphaltene, maltene, and distillate fractions, in the thermal cracking of VR and AR. The procedure was conducted in a batch reactor at temperatures ranging from 400 to 420 °C and a pressure of 3 to 5 kPa. The kinetic parameters were derived through a comparative analysis of experimental and predicted outcomes. The observed compositional differences between AR and VR regarding asphaltene characteristics and hydrogen donor nature and content likely contributed to the swift deactivation of the catalyst and the subsequent loss of the selective cracking mechanism noted during AR cracking.
Table 9 shows the summary of previous researchers who used kinetic models.
This study utilized a first-order simplified kinetic model, as depicted in
Figure 11, comprising eight lumps (vacuum residue, heavy vacuum gas oil, light vacuum gas oil, heavy gas oil, light gas oils, heavy naphtha, and light naphtha) and twenty-eight reactions. The model was based on several assumptions: (1) uniform concentration and temperature, (2) a power law kinetic model, and (3) the neglect of gaseous products and pressure effects [
10].
5. Current Study Kinetic Model
This section details this study’s assumptions, kinetic model equations, and evaluation criteria.
5.1. Assumptions
A first-order kinetic model is used in this study. The model is first simplified using some reasonable assumptions.
- (1)
Uniform concentration.
- (2)
Uniform temperature.
- (3)
Power law kinetic model.
- (4)
Neglecting gaseous products.
- (5)
Neglecting the effect of pressure.
5.2. Kinetic Model Equations
The developed kinetic model contains eight-lump and twenty-eight reactions; these lumps re as follows: heavy vacuum gas oil, light vacuum gas oil, heavy naphtha, light naphtha, kerosene, atmospheric gas oil, vacuum reduced crude (VRC) and coke. The gaseous product is neglected due to its smaller weights comparing to other liquid products. Each heavy lump undergoes cracking reaction to lighter fractions (lumps). The developed kinetic model applied in this study is presented in
Figure 13.
A mass balance conservation equation should be developed for each individual lump. The mass balance equations for each lump are represented in Equations (1)–(8);
In which;
where A
o is the pre-exponential factor (mole/gm at.min), E is the activation energy (j/mol), R is the universal gas constant (8.314 j/mol K) and T is the absolute temperature (K).
5.3. Evaluation Criteria
Two statistical criteria were used in order to evaluate the effectiveness of developed kinetic model in this study; they are mean relative error and mean absolute error. The mean absolute error (MAE) and mean relative error (MRE) were calculated using Equations (9) and (10), respectively. [Note that MAE is the absolute difference between experimental and predicted values; MAE is dimensionless (reported simply as a number, not %). MRE is relative to the experimental value; it is usually expressed as a percentage (%)].
where N, M,
and
are the components’ number, dataset number, experimental weight fraction values and predicted weight fraction values, respectively.
The percentage VR conversion was calculated using Equation (11), and percentage yield in weight fraction for cracking products was calculated using Equation (12).
Average molecular weights were used to convert the reactant and products weights to moles and vice versa.
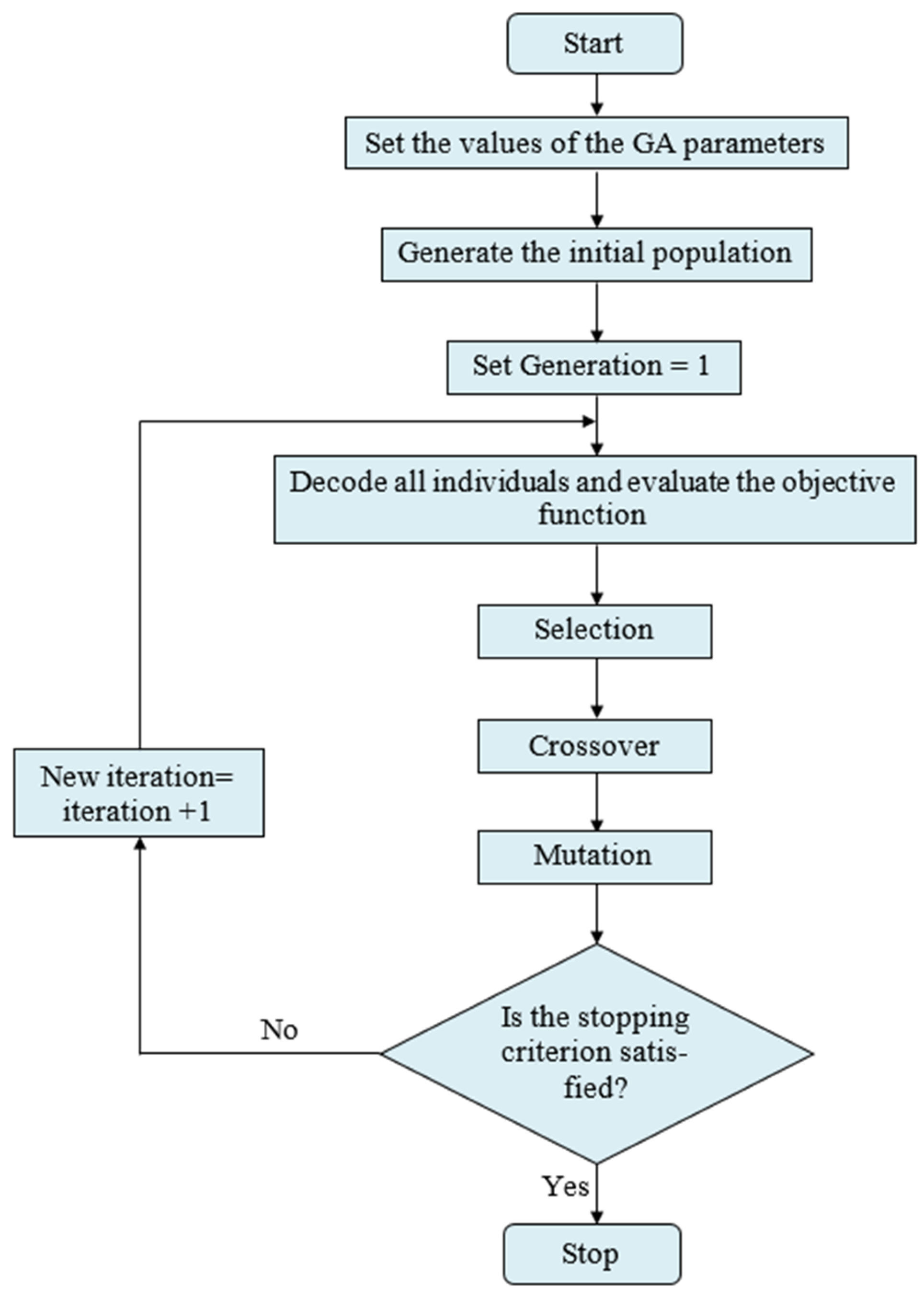
6. Kinetic Parameters Prediction by Genetic Algorithm (GA)
To broaden and deepen the search space of choice variables, stochastic optimization algorithms use probabilistic (i.e., random) components. And when looking for a global optimum, the added randomness could be just what you need to push past a local solution. Several stochastic optimization approaches fall into this category. These include evolutionary algorithms (such as genetic algorithms, differential evolution), particle swarm optimization, simulated annealing, harmony search, and PSO.
A genetic algorithm (GA) is a stochastic method that mimics natural evolution within the solution space of optimization problems. It functions on a set of possible solutions (i.e., individuals) during each iteration (i.e., generation). Through the integration of select individuals from the existing population based on established procedures, a subsequent generation emerges, featuring individuals of enhanced quality. Initially developed by John Holland in the 1960s, genetic algorithms have undergone extensive study, experimentation, and application across various domains within the engineering sector.
The initial phase of the genetic algorithm involves the random generation of an initial population comprising Npop solutions inside the viable region. Genetic algorithms operate on this population by employing crossover and mutation to alter certain chromosomes according to defined genetic operations, so producing a new population with enhanced attributes. Reproductive candidates are chosen according to their objective function values and the Darwinian concept of survival of the fittest [
49]. The algorithm of the genetic algorithm stochastic optimization method is illustrated in
Figure 14.
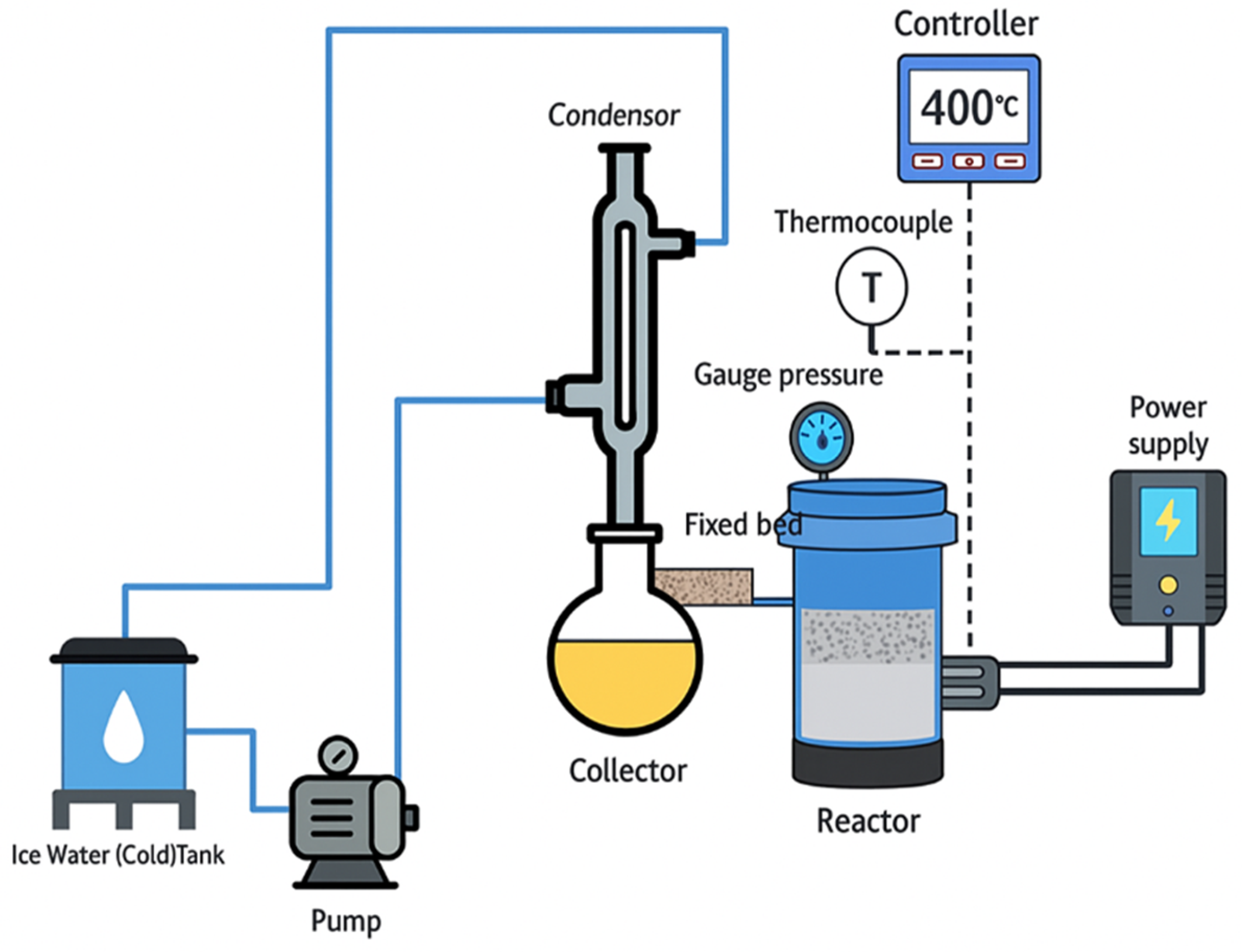
The results of the vacuum residue’s steam catalytic cracking were demonstrated using kinetic modeling. There were twenty-eight reactions in the recently created kinetic model, which was divided into eight groups: heavy vacuum gas oil, light vacuum gas oil, kerosene, light naphtha, heavy naphtha, atmospheric gas oil, vacuum reduced crude (VRC) and coke. These eight lumps all react in their own unique way. The model results were predicted using the fourth-order Runge–Kutta integration method, and the ideal kinetic parameters were estimated using the genetic algorithm optimization approach. The code for each program was written in MATLAB 2015a with the ga command used for stochastic optimization and the ode45 utility for numerical integration.
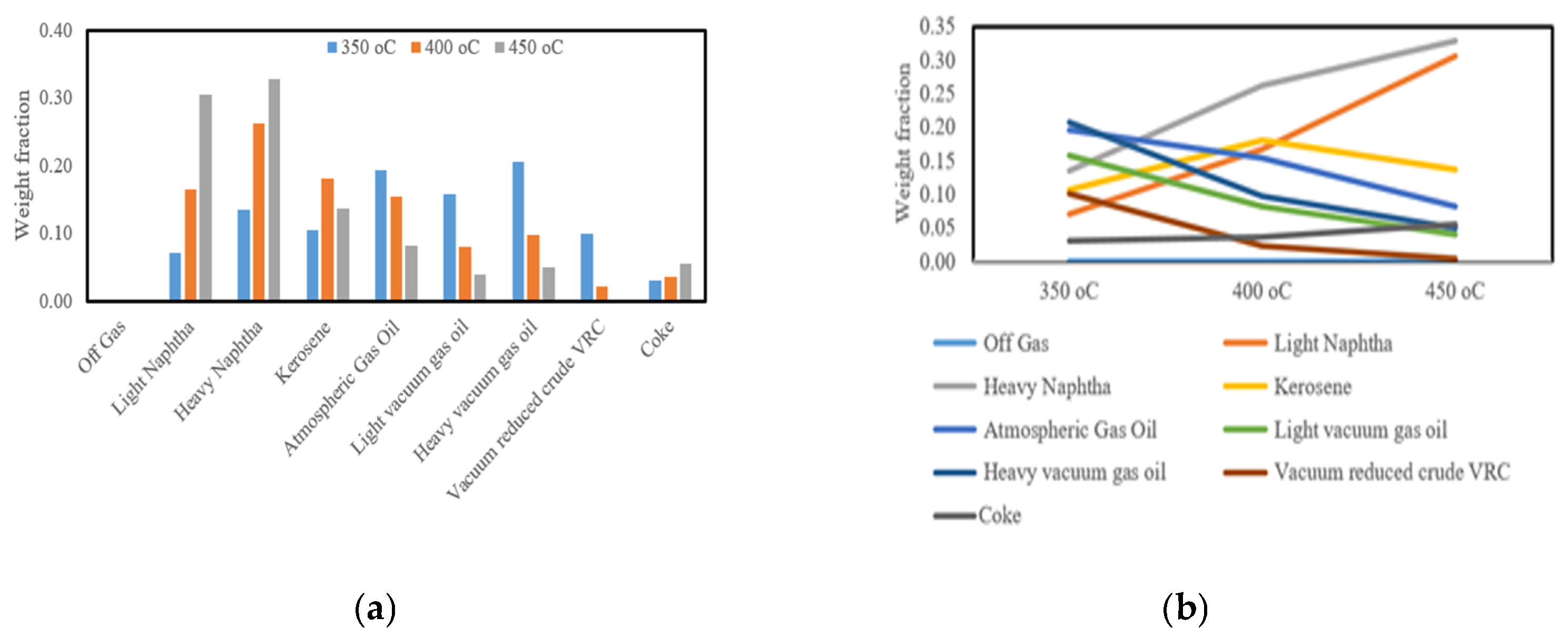
Figure 15 illustrates the influence of temperature on product distribution during vacuum residue cracking. As the reaction temperature increases from 350 °C to 450 °C, the formation of light products such as light and heavy naphtha becomes more pronounced, while kerosene and atmospheric gas oil fractions gradually decline. At higher temperatures, off-gas yields also rise significantly, reflecting deeper molecular scission and enhanced conversion of heavier hydrocarbons into lighter fragments. Coke yield, although relatively small, shows a slight increase at 450 °C, indicating the growing role of condensation and polyaromatic stability under severe conditions. These results highlight the balance between promoting liquid fuel generation and avoiding over-cracking into gas and coke, a behavior consistent with recent findings on residue pyrolysis and cracking kinetics [
50,
51,
52].
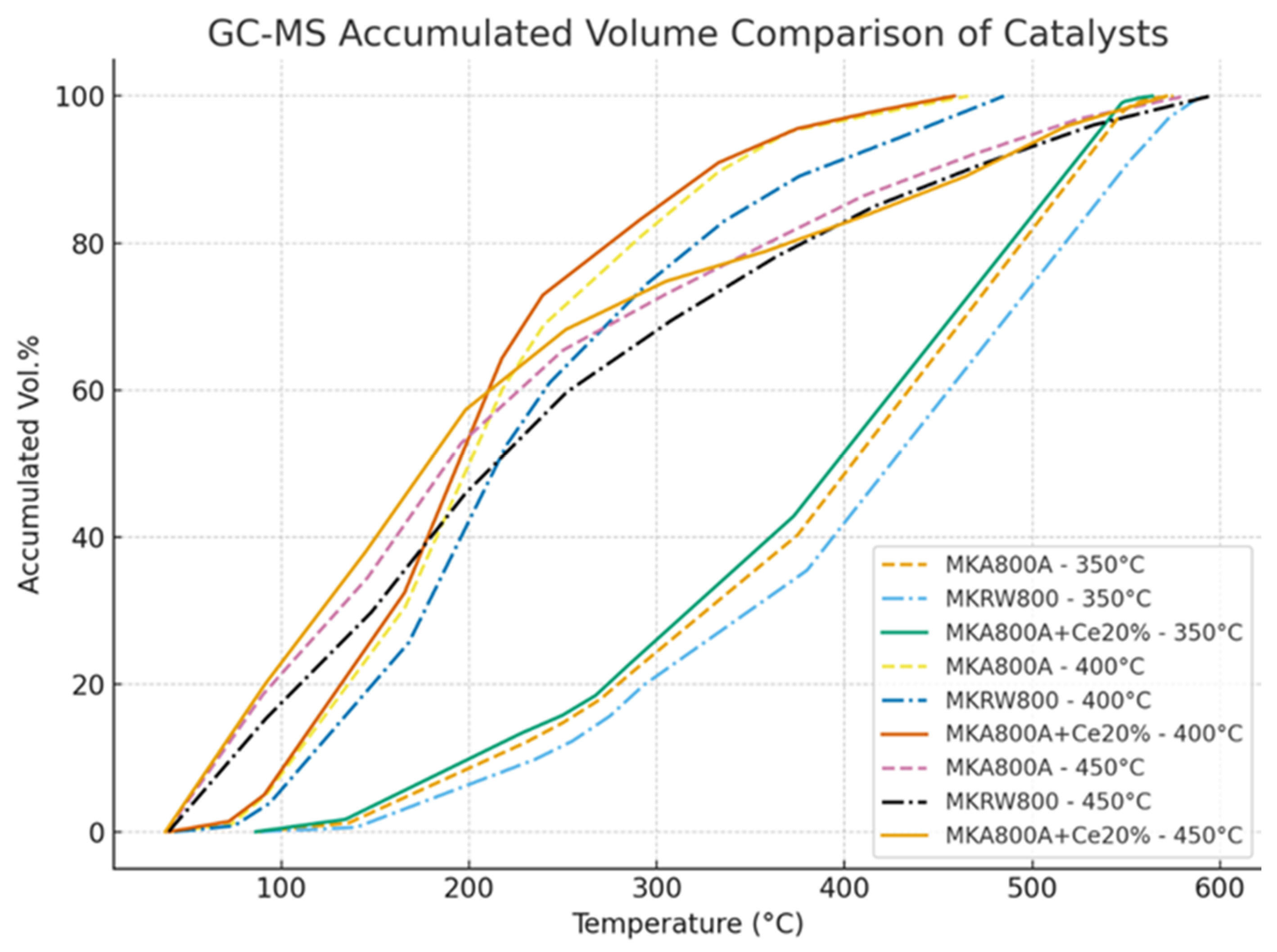
Figure 16 shows a comparison of the experimental and anticipated weight percentages of cracking products, while
Table 10 shows the comparison between experimental and predicted weight percent of cracking products at different temperatures. The kinetic model shows an adequate depiction of the experimental results. The input data covered a wide range of operating variables, which led to a moderate mean relative error (MRE) of 9.64% and mean absolute error (MAE) of 0.015052, as in Table 12 below.
As shown in
Table 11, the reaction kinetic parameters were predicted using eight separate tests, ranging from five to twelve, which effectively covered all model variables.
The kinetic trends reflected in
Table 11 capture the core mechanics of vacuum residue cracking: reactions with lower activation energy (Ea) proceed readily under mild conditions, favoring liquid fuel formation, whereas higher Ea pathways require severe conditions and lead to gaseous products. The pre-exponential factor (A) further shapes this behavior: a high A combined with high Ea accelerates gas yield once enough heat is applied; moderate A paired with lower Ea supports selective liquid production. Meanwhile, coke formation typically involves low Ea and low A, pointing to slow yet thermodynamically driven accumulation of stable polyaromatics. These patterns are supported by recent experimental findings: Pyrolysis of vacuum residue via Friedman and distributed activation energy modeling (DAEM) showed an average Ea of ~180 kJ/mol, rising with conversion, and revealed significant interactions among SARA fractions (SARA is a standard method of classifying crude oil or heavy fractions (like vacuum residue) into four chemical groups: Saturates (S), Aromatics (A), Resins (R), Asphaltenes (A)) that modulate Ea values (one-parallel DAEM: 227.6 kJ/mol; four-parallel: 204.6 kJ/mol) [
53]. Thermogravimetric analysis with a nanocatalyst likewise demonstrated a reduction in Ea—from ~91.54 kJ/mol to ~89.68 kJ/mol—highlighting the catalyst’s role in accelerating decomposition and potentially reducing coke formation [
54].
As shown in
Table 12, the kinetic model achieved a mean relative error (MRE) of 9.64% and a mean absolute error (MAE) of 0.01505. In modeling studies, such low error values are generally taken as evidence that the predictions reproduce the experimental data with reasonable accuracy. Several recent works confirm that smaller MRE and MAE values reflect a closer match between the predicted and observed results, supporting the reliability of the developed model in describing product distributions during residue cracking [
55,
56]. This research advances sustainability by recycling waste materials into alternative fuels and useful materials while valorizing local raw materials like Iraqi kaolin to develop innovative geopolymeric systems and reduce reliance on imports [
57,
58,
59,
60,
61,
62].
7. Conclusions
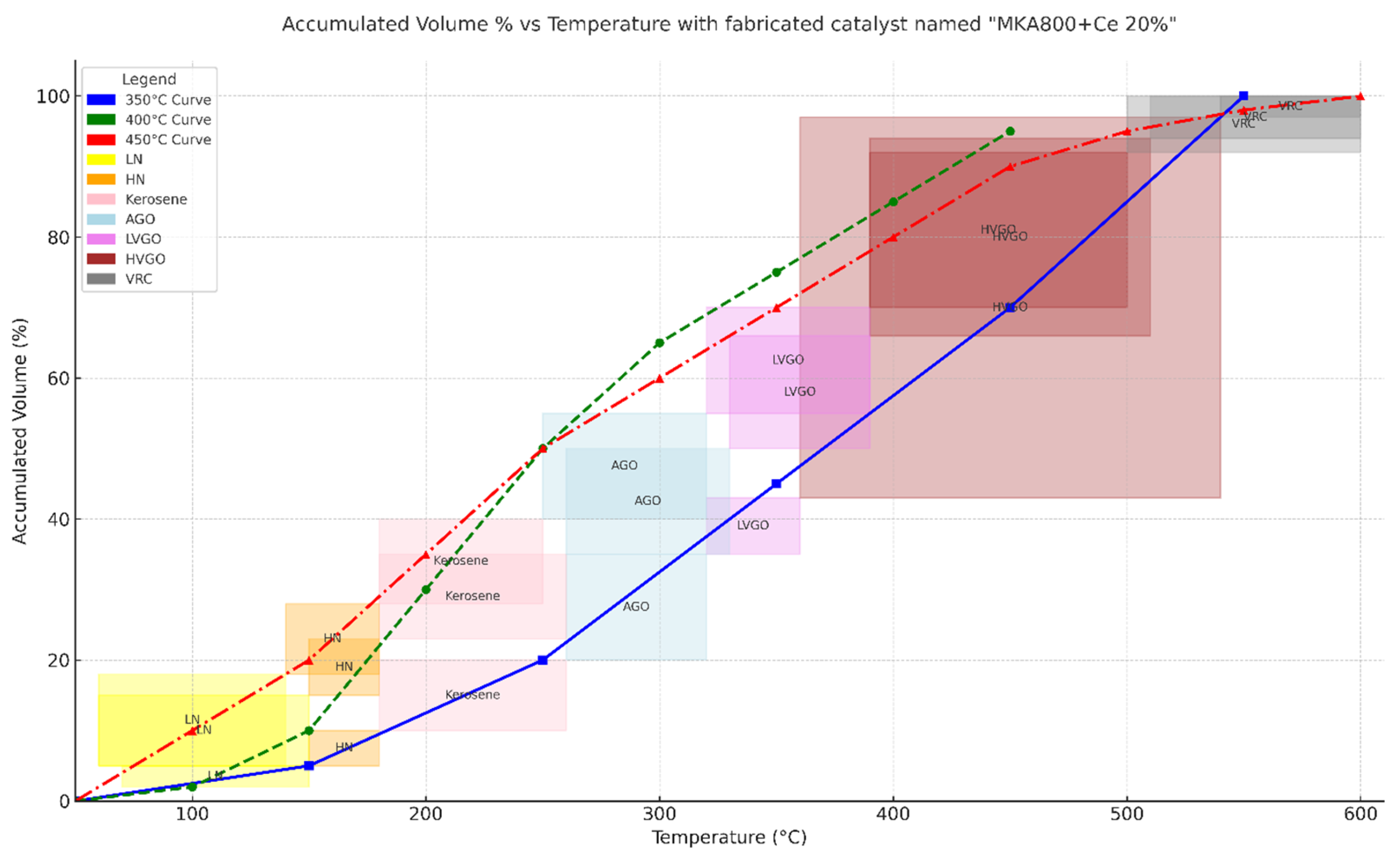
The experimental evaluation of the MKA800@Ce20% catalyst for VR cracking has shown promising results across varying temperatures. At 350 °C, the cracking was minimal, with the majority of the product consisting of unconverted VR and heavy fractions (VRC, HVGO). At 400 °C, moderate catalytic activity was observed, leading to an increased yield of middle distillates, including light vacuum gas oil (LVGO) and atmospheric gas oil (AGO). The best catalytic performance was obtained at 450 °C, where the total liquid yield reached 11.72 g per 20 g of VR feedstock (61% conversion to liquid), with coke formation limited to 3.81 g (19.1%) and gases making up the remainder. GC-MS confirmed that the major fractions in the liquid were light hydrocarbons, including LN, HN, and kerosene. The high yield and selectivity at elevated temperatures were attributed to the synergistic effect of acid-leached metakaolin and cerium impregnation, which enhanced redox cycling (Ce4+/Ce3+) and reduced coke formation. These results demonstrate that MKA800@Ce20% is a cost-effective and thermally robust catalyst suitable for heavy residue upgrading under atmospheric conditions. The eight-lump kinetic model that was created was tested and found to be correct for both product distribution and reaction kinetics because it had low MAE and MRE values. Genetic algorithms were also used to find valid kinetic constants for 28 different cracking reactions. The evaluation of the catalyst indicated that cerium was effectively added, which improved the surface characteristics and increased the thermal stability.