Thermochemical Characteristics of Anaerobic Dairy Digestate and Its Pyrolysis Conversion for Producing Porous Carbon Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determinations for Thermochemical Properties of Digestate
2.3. Pyrolysis Experim Ents
2.4. Determinations for Pore and Chemical Characteristics of Digestate-Based Biochar Products
3. Results and Discussion
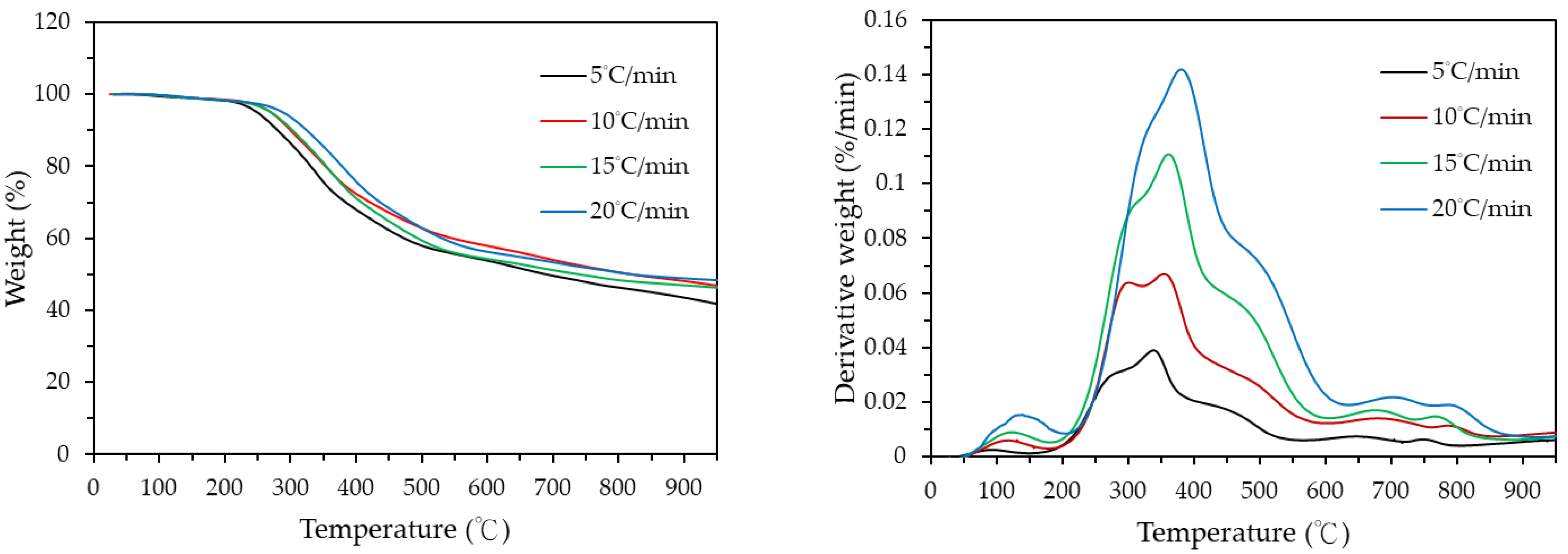
3.1. Thermochemical Characteristics of Digestate
3.2. Pore Properties of Digestate-Based Biochar Products
- As shown in Table 2, the pyrolysis temperature was a determining factor that influenced the pore properties of the digestate-based biochars. Biochar produced at 550 °C exhibited limited pore development, with a BET surface area of <20 m2/g. By contrast, biochar produced at 850 °C showed a pronounced increase in pore properties, achieving the maximum values with a BET surface area greater than 190 m2/g and a total pore volume exceeding 0.17 cm3/g. These results suggested that pore development became more pronounced at higher pyrolysis temperatures due to enhanced charring [30,35], resulting in more developed porous structures. However, the BET surface areas of the biochars produced at 650 °C and 750 °C did not follow a consistent increasing trend compared with those produced at 550 °C and 850 °C, which reflected the heterogeneity and non-uniform nature of the digestate precursor. In addition, micropore properties (micropore surface area and micropore volume) were positively correlated with the total pore properties (BET surface area and total pore volume). Except for BC-D-550, the ratio of micropore contribution ranged from 60% to 70%, indicating that the resulting materials were predominantly microporous carbons. It should be noted that the calculated average pore diameters, which ranged from 1.7 nm to 4.0 nm, were not fully consistent with the microporous nature of the materials. This discrepancy could have arisen from the calculation assumption of independent cylindrical pores, whereas the actual pores in digestate-based biochars may have been slit-shaped and cross-connected.
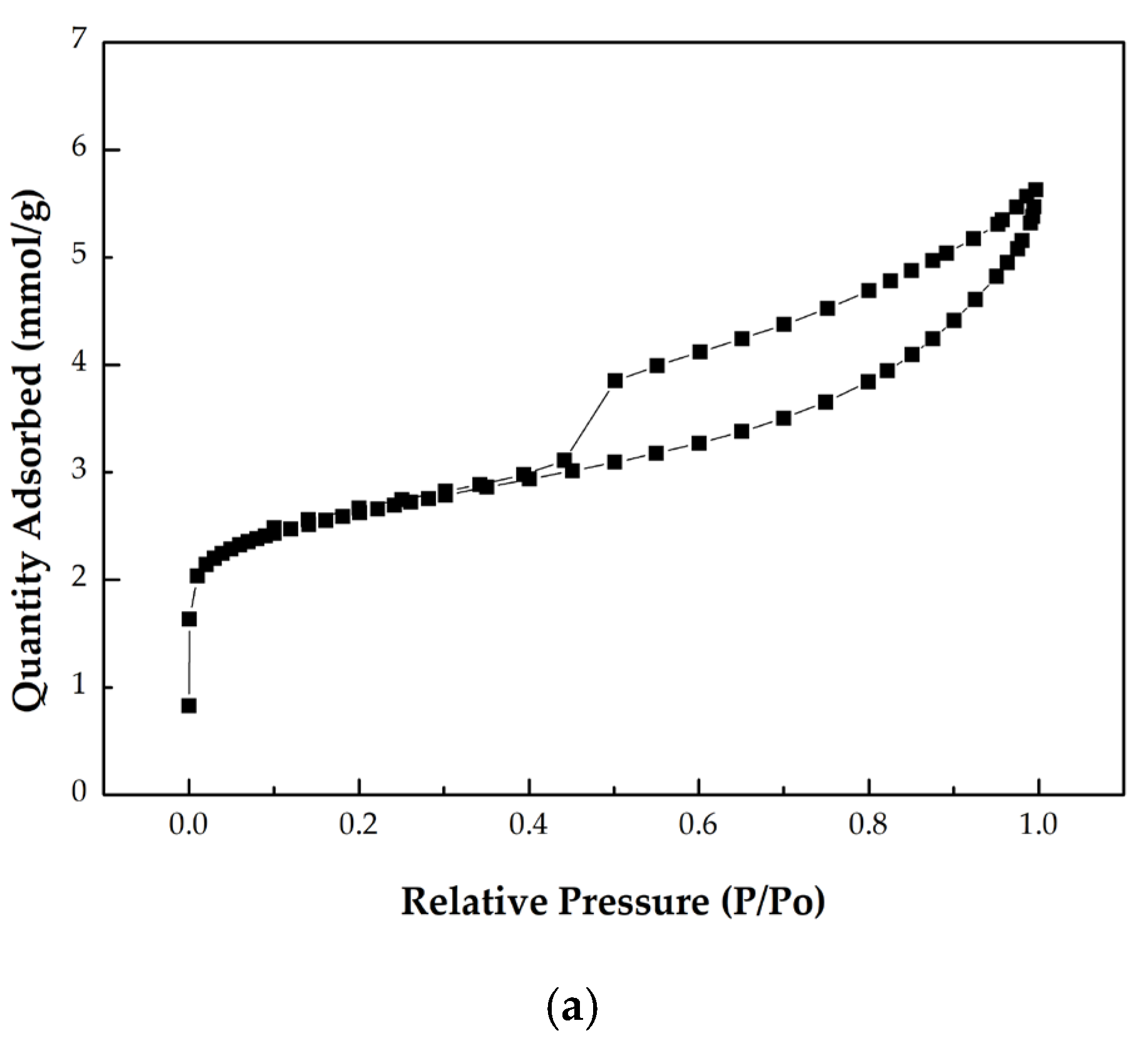
- According to the International Union of Pure and Applied Chemistry (IUPAC) classification of adsorption isotherms [36], microporous materials are typically associated with Type I isotherms, which exhibit high uptake in the low relative pressure region (P/P0 < 0.05). Conversely, mesoporous materials are characterized by the Type IV isotherms, which display a hysteresis loop due to capillary condensation during adsorption and a different desorption mechanism. The initial part of the Type IV isotherm is generally attributed to monolayer-multilayer adsorption at relative pressures of about 0.40. The optimal digestate-based biochar (BC-D-850) displayed a combination of microporous and mesoporous characteristics, as evident from its isotherm profile (Figure 3). Moreover, the observed hysteresis loop corresponded to the IUPAC Type H4 classification, typically associated with narrow slit-shaped pores [28].
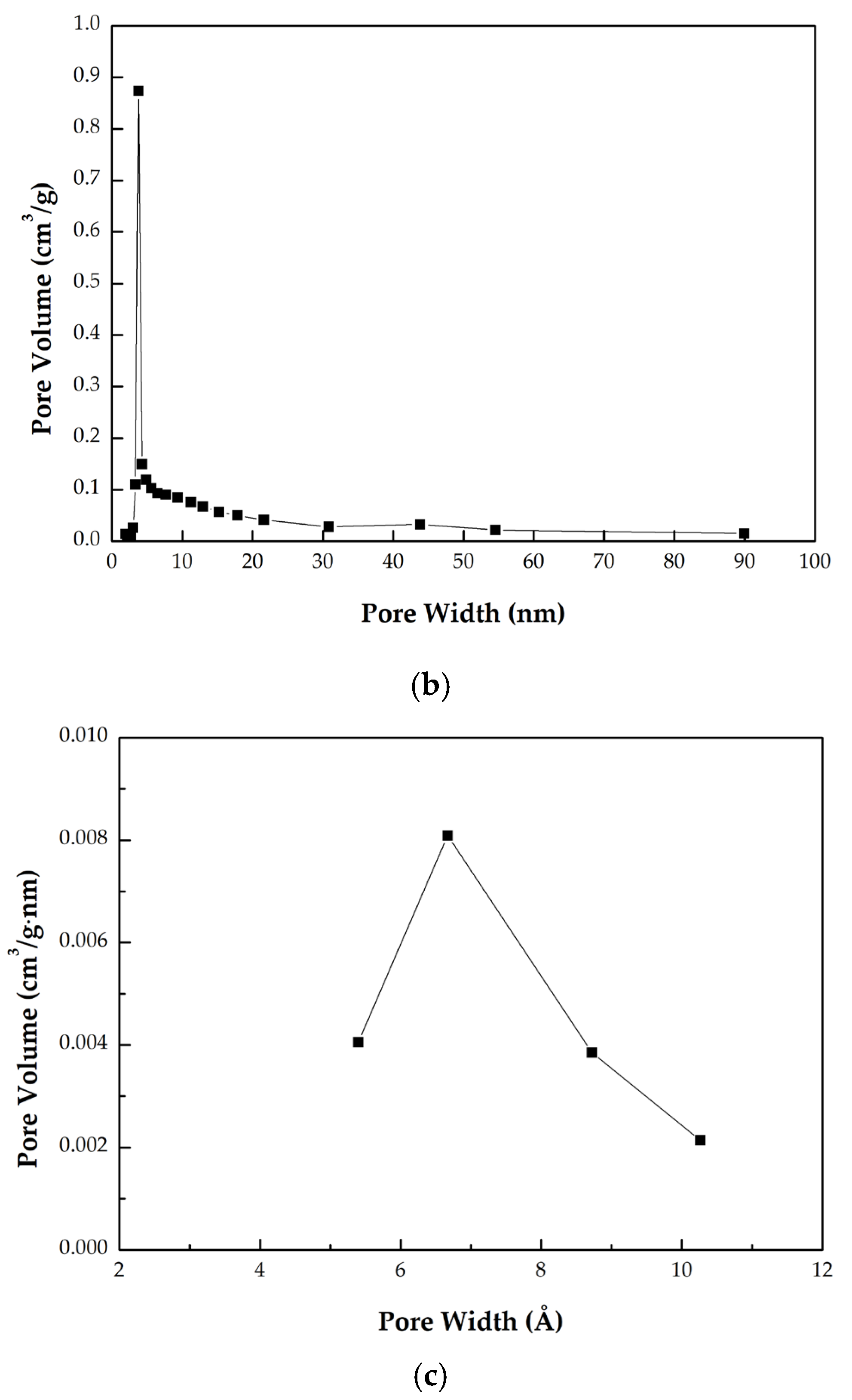
- The pore size distributions derived from the BJH and HK methods are presented in Figure 4 and Figure 5, respectively. As shown in Figure 4, a pronounced peak was observed at approximately 3.8 nm, indicating the presence of mesopores within the 2–50 nm range in the optimal biochar [28]. Additionally, significant microporosity within the 0.6–0.8 nm range was observed in Figure 5, consistent with the values reported in Table 2 and the isotherm profile in Figure 3.
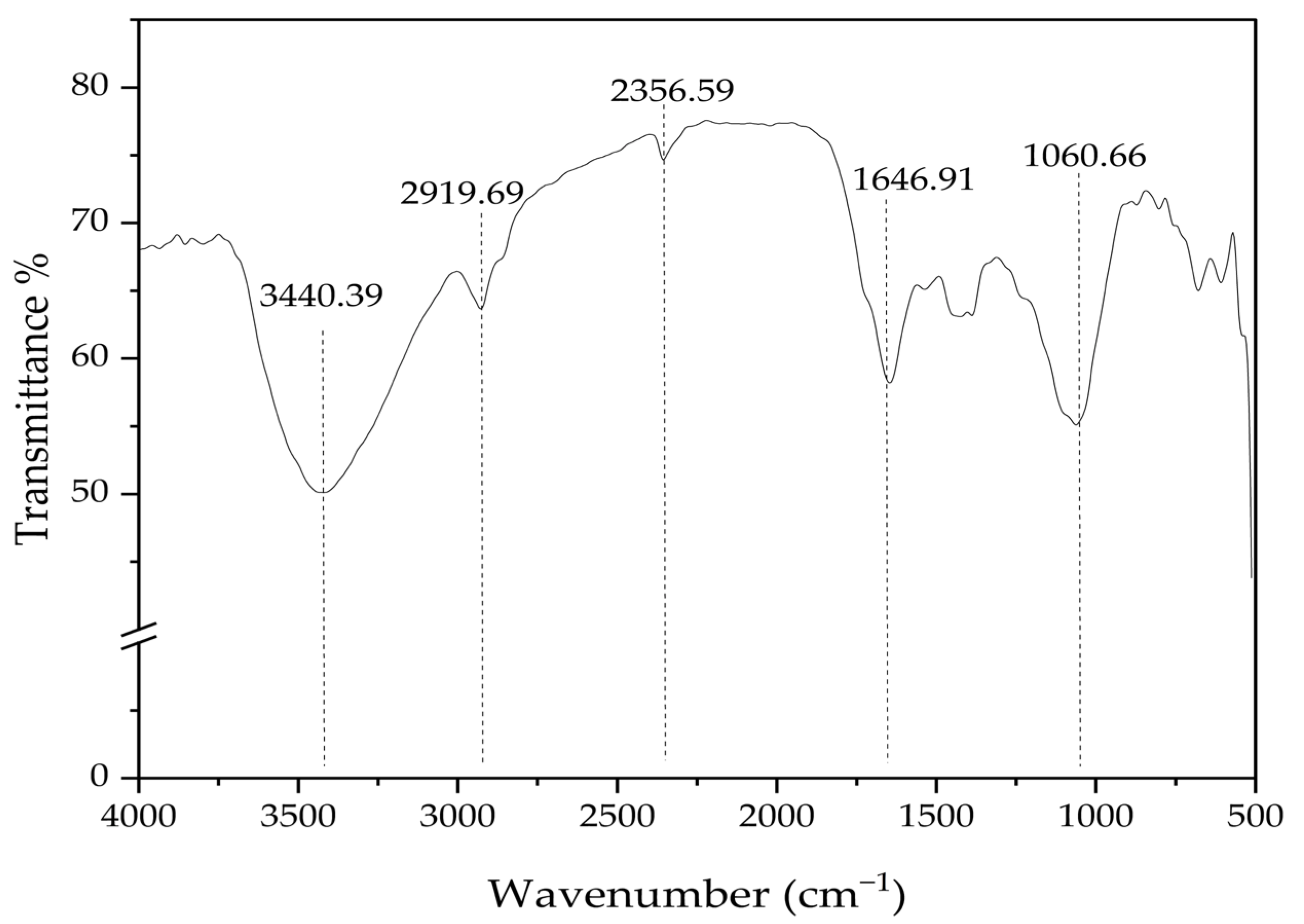
3.3. Textural and Chemical Characteristics of Digestate-Based Biochar Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baştabak, B.; Koçar, G. A review of the biogas digestate in agricultural framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Slepetiene, A.; Ceseviciene, J.; Amaleviciute-Volunge, K.; Mankeviciene, A.; Parasotas, I.; Skersiene, A.; Jurgutis, L.; Volungevicius, J.; Veteikis, D.; Mockeviciene, I. Solid and liquid phases of anaerobic digestate for sustainable use of agricultural soil. Sustainability 2023, 15, 1345. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K. Anaerobic digestate management for carbon neutrality and fertilizer use: A review of current practices and future opportunities. Biomass Bioenergy 2024, 180, 106991. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef] [PubMed]
- Tayibi, S.; Monlau, F.; Bargaz, A.; Jimenez, R.; Barakat, A. Synergy of anaerobic digestion and pyrolysis processes for sustainable waste management: A critical review and future perspectives. Renew. Sustain. Energy Rev. 2021, 152, 111603. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthigadevi, G.; Bharathiraja, B.; Kumar, R.P.; Abo, L.D.; Prabhu, S.V.; Balachandar, R.; Jayakumar, M. Current and prognostic overview on the strategic exploitation of anaerobic digestion and digestate: A review. Environ. Res. 2023, 216 Pt 2, 114526. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Bashkirov, V.N.; Bulygina, K.S. Thermochemical processing of digestate from biogas plant for recycling dairy manure and biomass. Biomass Conv. Bioref. 2023, 13, 685–695. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.S.; Lee, D.J. Anaerobic digestate valorization beyond agricultural application: Current status and prospects. Bioresour. Technol. 2023, 373, 128742. [Google Scholar] [CrossRef]
- Abdelfatah-Aldayyat, E.; González-Rojo, S.; Gómez, X. Reviewing digestate thermal valorization: Focusing on the energy demand and the treatment of process water. Environments 2024, 11, 239. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Yan, B.; Zhou, S.; Zhu, X.; Cheng, Z.; Chen, G. Thermochemical processing of digestate derived from anaerobic digestion of lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2024, 199, 114518. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, J.; Guan, D.; Wang, Y.; Xie, J.; Zhang, H.; Sun, Y.; Zhu, J.; Guo, L. A comprehensive review on the preparation of biochar from digestate sources and its application in environmental pollution remediation. Sci. Total Environ. 2024, 912, 168822. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Habchi, S.; Maraldi, M.; Valenti, F.; El Bari, H. Comprehensive review of technologies for separate digestate treatment and agricultural valorisation within circular and green economy. Bioresour. Technol. 2024, 409, 131252. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Limousy, L.; Drané, M.; Richard, C.; Juge, M.; Aemig, Q.; Trably, E.; Escudié, R.; Peyrelasse, C.; Bennici, S. Integration of Digestate-Derived Biochar into the anaerobic digestion process through circular economic and environmental approaches—A review. Materials 2024, 17, 3527. [Google Scholar] [CrossRef] [PubMed]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, H.; Zhao, Z.; Yang, Z.; Qiu, L.; Guo, X. Combined effects of liquid digestate recirculation and biochar on methane yield, enzyme activity, and microbial community during semi-continuous anaerobic digestion. Bioresour. Technol. 2022, 364, 128042. [Google Scholar] [CrossRef]
- Alghashm, S.; Song, L.; Liu, L.; Ouyang, C.; Zhou, J.L.; Li, X. Improvement of biogas production using biochar from digestate at different pyrolysis temperatures during OFMSW anaerobic digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- Basinas, P.; Chamrádová, K.; Vosnaki, O.; Rusín, J. Enhancement of biogas generation by utilizing raw and modified with HNO3 biochar obtained from pyrolysis of biomass and digestate. Detritus 2023, 24, 28–39. [Google Scholar] [CrossRef]
- Manga, M.; Aragón-Briceño, C.; Boutikos, P.; Semiyaga, S.; Olabinjo, O.; Muoghalu, C.C. Biochar and its potential application for the improvement of the anaerobic digestion process: A critical review. Energies 2023, 16, 4051. [Google Scholar] [CrossRef]
- Tariq, M.; Mehmood, A.; Abbas, Y.; Rukh, S.; Shah, F.A.; Hassan, A.; Gurmani, A.R.; Ahmed, Z.; Yun, S. Digestate quality and biogas enhancement with laterite mineral and biochar: Performance and mechanism in anaerobic digestion. Renew. Energy 2024, 220, 119703. [Google Scholar] [CrossRef]
- Silva, I.; Lapa, N.; Ribeiro, H.; Duarte, E. Pig slurry anaerobic digestion: The role of biochar as an additive towards biogas and digestate improvement. Appl. Sci. 2025, 15, 1037. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Liu, L.; Yan, Z.; Chui, C.; Yang, N.; Wang, C.; Shen, G.; Chen, Q. Enhancing methane production in anaerobic digestion of food waste using co-pyrolysis biochar derived from digestate and rice Straw. Molecules 2025, 30, 1766. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, S.; Quan, C.; Gao, N.; Johnston, C.; Wu, C. One-pot synthesis of digestate-derived biochar for carbon dioxide capture. Fuel 2020, 279, 118525. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Li, N.; Liu, W.; Yan, B.; Yu, Y.; Liang, L.; Chen, G.; Hou, L.; Wang, S. Tunable active sites on biogas digestate derived biochar for sulfanilamide degradation by peroxymonosulfate activation. J. Hazard. Mater. 2022, 421, 126794. [Google Scholar] [CrossRef] [PubMed]
- Basinas, P.; Rusín, J.; Chamrádová, K.; Kaldis, S.P. Pyrolysis of the anaerobic digestion solid by-product: Characterization of digestate decomposition and screening of the biochar use as soil amendment and as additive in anaerobic digestion. Energy Convers. Manag. 2023, 277, 116658. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Wang, F.; Yang, X.; Wang, X.; Chou, S.; Xu, P.; Yi, W. Comparison study on silicon forms and leaching characteristics of pyrolysis biochar and hydrochar derived from cow dung digestate. J. Anal. Appl. Pyrolysis 2024, 183, 106846. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tsai, W.T.; Chen, J.W.; Lin, Y.Q.; Chang, Y.M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Tsai, W.T.; Fang, Y.Y.; Cheng, P.H.; Lin, Y.Q. Characterization of mesoporous biochar produced from biogas digestate implemented in an anaerobic process of large-scale hog farm. Biomass Conv. Bioref. 2018, 8, 945–951. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, the Netherlands, 2006. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: London, UK, 2013. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Li, H.; Liu, Z.; Ma, W.; Liang, X. Thermal stability of oxygen-containing functional groups on activated carbon surfaces in a thermal oxidative environment. J. Chem. Eng. Jpn. 2004, 47, 21–27. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Gharehkhani, S.; Afifi, A.B.M. Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Johnston, C.T. Biochar analysis by Fourier-transform infra-red spectroscopy. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 199–228. [Google Scholar]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Chia, C.H.; Downie, A.; Munroe, P. Characteristics of biochar: Physical and structural properties. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 89–109. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Li, Q.; Zhang, X.; Mao, M.; Wang, X.; Shang, J. Carbon content determines the aggregation of biochar colloids from various feedstocks. Sci. Total Environ. 2023, 880, 163313. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M. Adsorption Engineering; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Kunhikrishnan, A.; Bibi, I.; Bolan, N.; Seshadri, B.; Choppala, G.; Niazi, N.K.; Kim, W.I.; Ok, Y.S. Biochar for inorganic contaminant management in waste and wastewater. In Biochar: Production, Characterization, and Applications; Ok, Y.S., Uchimiya, S.M., Chang, S.X., Bolan, N., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 167–219. [Google Scholar]
- Cordova, B.M.; Santa Cruz, J.P.; Ocampo, M.T.V.; Huamani-Palomino, R.G.; Baena- Moncada, A.M. Simultaneous adsorption of a ternary mixture of brilliant green, rhodamine B and methyl orange as artificial wastewater onto biochar from cocoa pod husk waste. Quantification of dyes using the derivative spectrophotometry method. New J. Chem. 2020, 44, 8303–8316. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface treatment of biochar—Methods, surface analysis and potential applications: A comprehensive review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
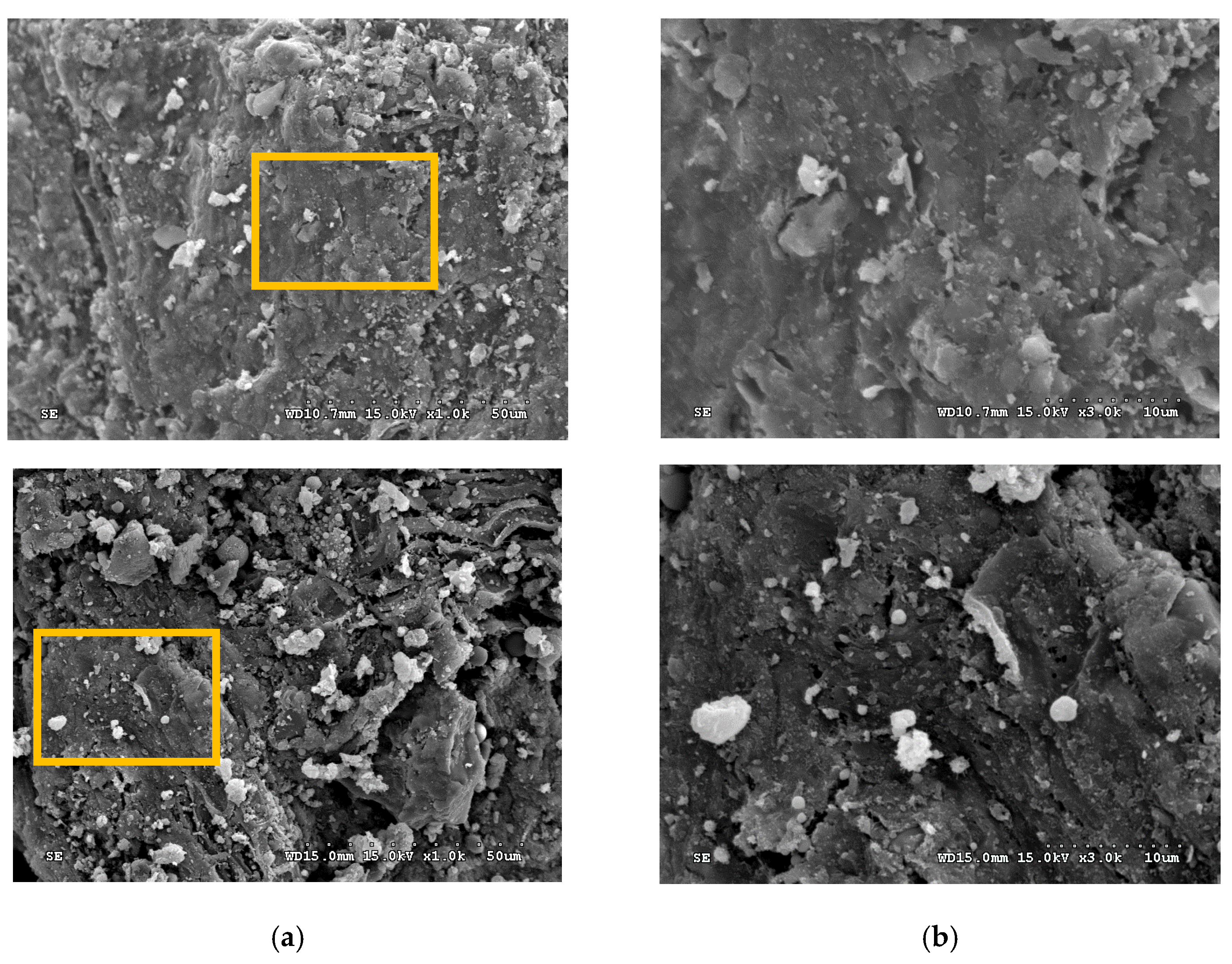
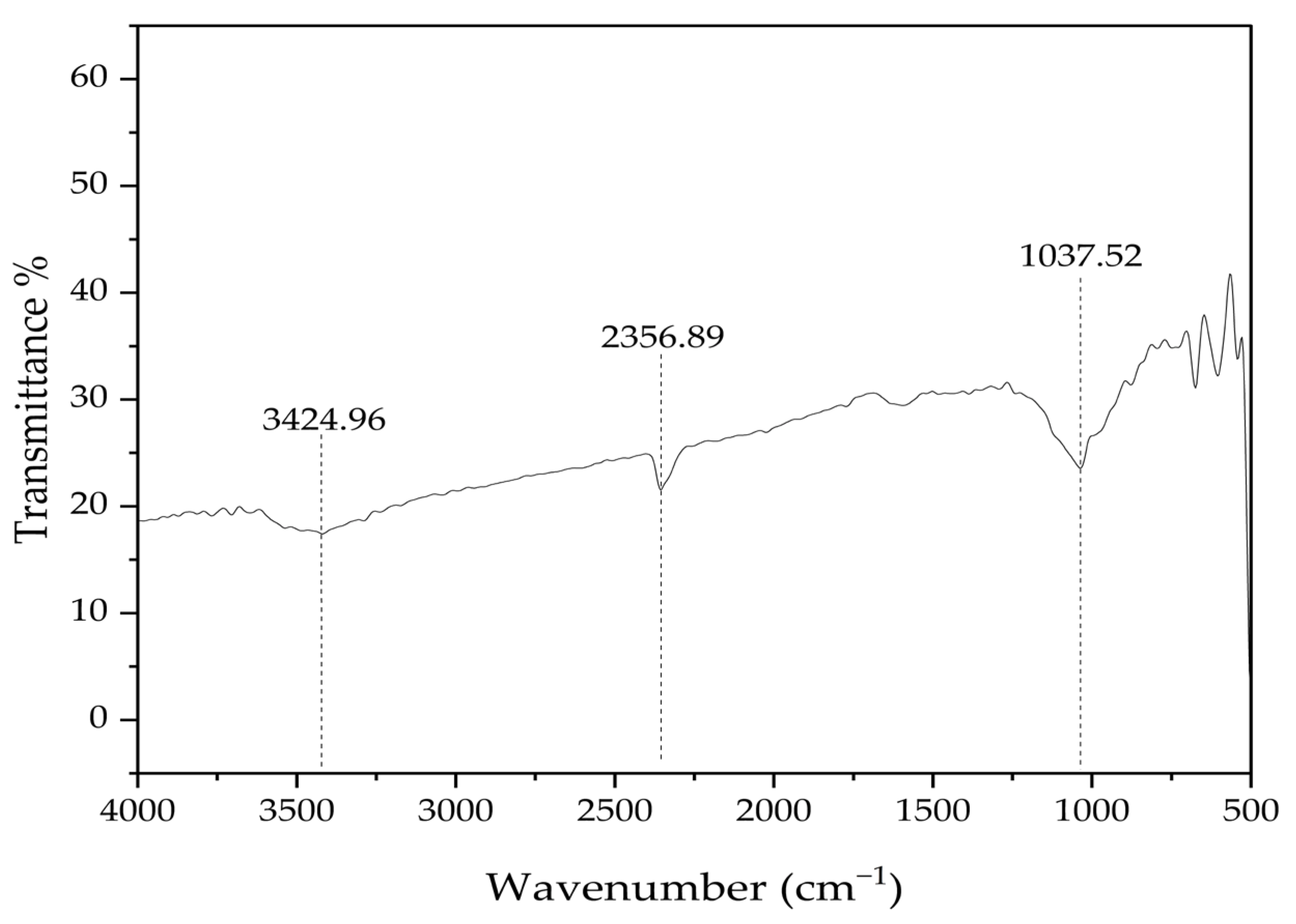
| Property | Value |
|---|---|
| Proximate analysis a,b | |
| Ash (wt%) | 28.03 ± 0.42 |
| Volatile matter (wt%) | 58.91 ± 056 |
| Fixed carbon c (wt%) | 13.06 |
| Elemental analysis b,d | |
| Carbon (wt%) | 40.93 |
| Oxygen (wt%) | 38.85 |
| Calcium (wt%) | 8.31 |
| Phosphorus (wt%) | 2.63 |
| Silicon (wt%) | 2.58 |
| Aluminum (wt%) | 1.89 |
| Sulfur (wt%) | 1.54 |
| Iron (wt%) | 1.17 |
| Sodium (wt%) | 0.81 |
| Magnesium (wt%) | 0.69 |
| Chlorine (wt%) | 0.60 |
| Calorific value (MJ/kg) a,b | 18.87 ± 0.25 |
| Biochar (BC-D) Product a | SBET b (m2/g) | Smicro c (m2/g) | Vt d (cm3/g) | Vmicro e (cm3/g) | Dave f (Å) |
|---|---|---|---|---|---|
| BC-D-550-I | 15.44 | 2.41 | 0.0258 | 0.0007 | 62.09 |
| BC-D-550-II | 10.10 | 2.58 | 0.0069 | 0.0008 | 93.39 |
| BC-D-650-I | 90.76 | 61.63 | 0.0443 | 0.0266 | 33.16 |
| BC-D-650-II | 154.88 | 109.53 | 0.0678 | 0.0471 | 29.74 |
| BC-D-750-I | 121.44 | 84.39 | 0.0563 | 0.0362 | 31.80 |
| BC-D-750-II | 74.05 | 45.98 | 0.0706 | 0.0198 | 35.39 |
| BC-D-850-I | 209.44 | 115.30 | 0.1948 | 0.0496 | 36.46 |
| BC-D-850-II | 190.72 | 115.44 | 0.1630 | 0.4961 | 32.94 |
| Elemental Composition | BC-D-550 | BC-D-650 | BC-D-750 | BC-D-850 |
|---|---|---|---|---|
| Carbon (wt%) | 26.82 | 30.85 | 44.14 | 52.95 |
| Oxygen (wt%) | 36.71 | 36.32 | 31.46 | 22.79 |
| Calcium (wt%) | 19.00 | 9.06 | 6.82 | 7.58 |
| Phosphorus (wt%) | 5.71 | 4.07 | 4.56 | 1.17 |
| Silicon (wt%) | 2.98 | 9.09 | 5.25 | 6.04 |
| Aluminum (wt%) | 2.08 | 3.90 | 3.04 | 2.49 |
| Other elements (wt%) | 6.70 | 6.71 | 4.73 | 6.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Morgan, H.M., Jr.; Tsai, W.-T. Thermochemical Characteristics of Anaerobic Dairy Digestate and Its Pyrolysis Conversion for Producing Porous Carbon Materials. Processes 2025, 13, 3380. https://doi.org/10.3390/pr13113380
Tsai C-H, Morgan HM Jr., Tsai W-T. Thermochemical Characteristics of Anaerobic Dairy Digestate and Its Pyrolysis Conversion for Producing Porous Carbon Materials. Processes. 2025; 13(11):3380. https://doi.org/10.3390/pr13113380
Chicago/Turabian StyleTsai, Chi-Hung, Hervan Marion Morgan, Jr., and Wen-Tien Tsai. 2025. "Thermochemical Characteristics of Anaerobic Dairy Digestate and Its Pyrolysis Conversion for Producing Porous Carbon Materials" Processes 13, no. 11: 3380. https://doi.org/10.3390/pr13113380
APA StyleTsai, C.-H., Morgan, H. M., Jr., & Tsai, W.-T. (2025). Thermochemical Characteristics of Anaerobic Dairy Digestate and Its Pyrolysis Conversion for Producing Porous Carbon Materials. Processes, 13(11), 3380. https://doi.org/10.3390/pr13113380







