Enhancing Tetradesmus sp. Biomass Recovery: The Influence of Culture Media on Surface Physicochemical Properties
Abstract
1. Introduction
2. Methodology
2.1. Microalgae Cultivation
2.2. Microbial Adhesion to Solvents Experiments (MATS)
2.3. Zeta Potential Measurements
2.4. Surface Contact Angle and Surface Free Energy
2.5. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR)
2.6. Statistical Analysis
3. Results and Discussion
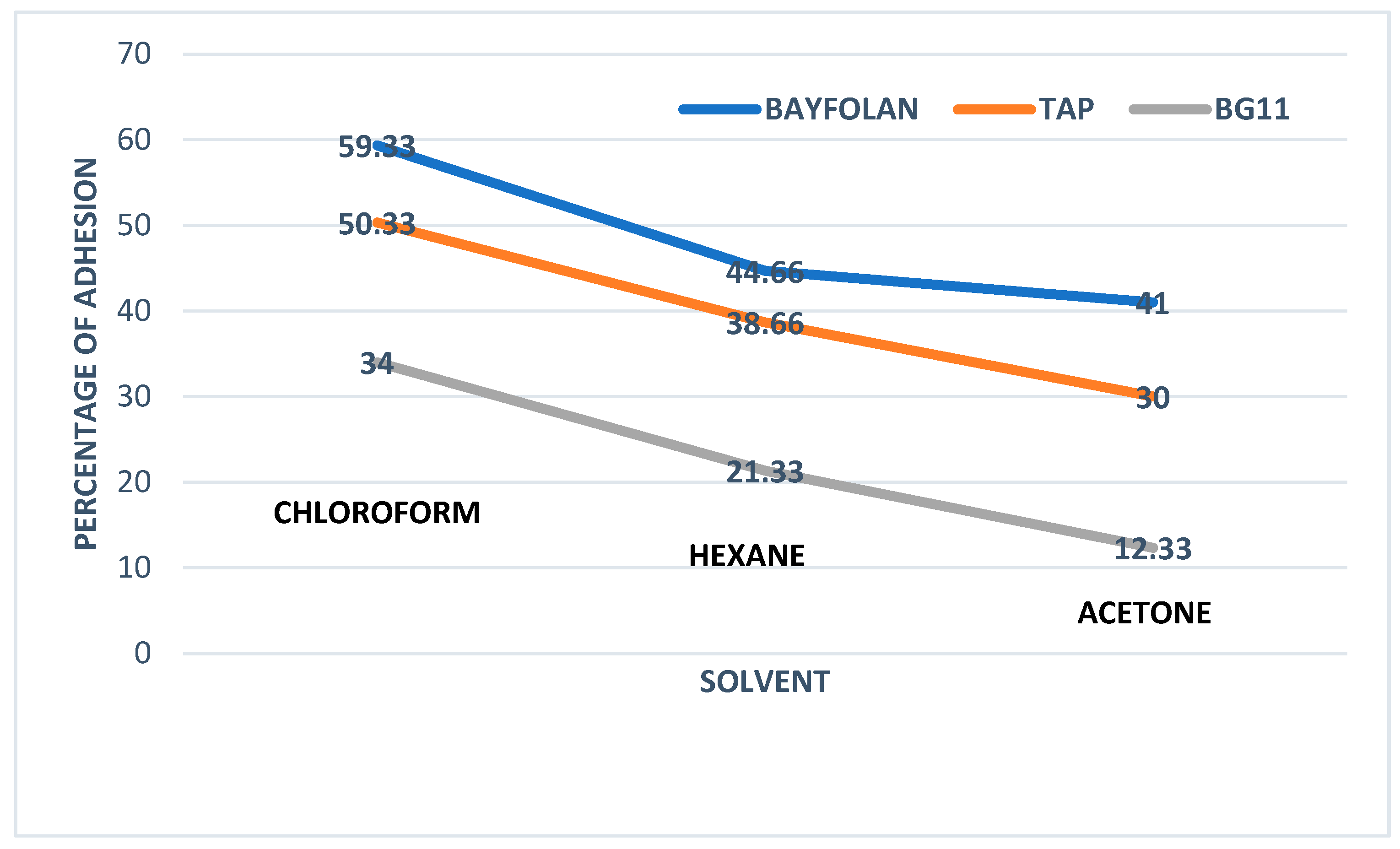
3.1. Hydrophobicity Assessment
3.2. Hydrophobicity in Microalgae: A Comparative and Mechanistic Discussion
3.3. Zeta Potential Determinations: A Comparative and Electrostatic Perspective
3.4. Functional Groups (ATR-FTIR Spectroscopy)
| Microalgae Species | Hydrophobicity Characteristics | Zeta Potential Characteristics | Relevant Culture Conditions (If Applicable) | Source |
|---|---|---|---|---|
| Tetradesmus sp. | Inherent hydrophobicity; Bayfolan enhances (ΔGsws: −26.36 MJm−2); Water contact angles 34.6–49.0° | Consistently negative (−10 to −14 mV); non-significant difference across media | BG11, TAP, Bayfolan®; stationary phase | This study |
| Chlorella vulgaris | Naturally hydrophilic; hydrophobicity enhanced by cationic surfactants (e.g., C16TAB) | Consistently negative; magnitude smaller than Anabaena variabilis at same pH | Various pH, C16TAB addition | [26] |
| Anabaena variabilis | Naturally hydrophobic | Consistently negative; larger magnitude than Chlorella vulgaris at same pH | Various pH | [33] |
| Green microalgae (general) | Can be hydrophilic (CA 30–58°) or slightly hydrophobic | Generally negative at physiological pH (4–8) | Natural pH | [32] |
| Species forming colonies | Distinctly hydrophobic surfaces | Not specified | Not specified | [27] |
| Chlorella vulgaris | Moderate hydrophobic surfaces; contact angle and adhesion increase under certain pH/light regimes | Negative; magnitude becomes less negative under stress or adsorbate presence | Varied pH; light stress; presence of ionic/charged particles | [40] |
| Chlorella sorokiniana | Hydrophobicity influenced by biomass fraction; whole-cell biomass less hydrophobic than protein-rich fractions; surface tension lowers under certain pH | Negative zeta; magnitude influenced by fraction and pH | Semi-pilot scale; fractions of biomass; pH 7 and others | [33] |
| Tribonema sp., Scenedesmus sp. | Tribonema highly hydrophobic; Scenedesmus moderately hydrophobic when compared under same medium | Zeta potentials negative; differences in harvesting efficiency tied to hydrophobicity | Same current and shear conditions in electro-flotation | [36] |
4. Conclusions
5. Recommendations for Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Briones-Baez, M.F.; Aguilera-Vazquez, L.; Rangel-Valdez, N.; Martinez-Salazar, A.L.; Zuñiga, C. Multi-Objective Optimization of Microalgae Metabolism: An Evolutive Algorithm Based on FBA. Metabolites 2022, 12, 603. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae biorefinery: An integrated route for the sustainable production of high-value-added products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’oSto, L. Biomass from microalgae: The potential of domestication towards sus-tainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, K.; Bastiaens, L.; Vandamme, D.; Gouveia, L. Harvesting of microalgae: Overview of process options and their strengths and drawbacks. In Microalgae-Based Biofuels and Bioproducts; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Danquah, M.K.; Gladman, B.; Moheimani, N.; Forde, G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009, 151, 73–78. [Google Scholar] [CrossRef]
- Barros, A.C.; Gonçalves, A.L.; Simões, M. Microalgal/cyanobacterial biofilm formation on selected surfaces: The effects of surface physicochemical properties and culture media composition. J. Appl. Phycol. 2019, 31, 375–387. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Ferreira, C.; Loureiro, J.A.; Pires, J.C.; Simões, M. Surface physicochemical properties of selected single and mixed cultures of microalgae and cyanobacteria and their relationship with sedimentation kinetics. Bioresour. Bioprocess. 2015, 2, 21. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae. Microb Cell Fact. 2023, 22, 239. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Pérez-Bravo, S.G.; Castañeda-Chávez, M.R.; Aguilera-Vázquez, L.; Gallardo-Rivas, N.V.; Morales-Rodríguez, M.L.; Páramo-García, U. Cultivation of Scenedesmus dimorphus with Bayfolan® supplementation under different photoperiods: Biomass, lipid profile and wastewater treatment potential. Resources 2023, 12, 140. [Google Scholar] [CrossRef]
- Hegewald, E.; Wolf, M.; Keller, A.; Friedl, T.; Krienitz, L. ITS2 sequence–structure phylogeny in the Scenedesmaceae with special reference to Coelastrum, Tetradesmus and Desmodesmus. Phycologia 2019, 49, 325–335. [Google Scholar] [CrossRef]
- Laraib, N.; Hussain, A.; Javid, A.; Bukhari, S.M.; Ali, W.; Manzoor, M.; Jabeen, F. Mixotrophic Cultivation of Scenedesmus dimorphus for Enhancing Biomass Productivity and Lipid Yield. Iran J. Sci. Technol. Trans. Sci. 2021, 45, 397–403. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.J.; Zhong, C.Q. Cultivating Scenedesmus dimorphus in lactic acid wastewater for cost-effective biodiesel production. Sci. Total Environ. 2021, 792, 148428. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, Y.; Chen, J. Cultivation of Scenedesmus dimorphus for C/N/P removal and lipid production. Electron. J. Biotechnol. 2015, 18, 46–50. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Growth and lipid accumulation of Scenedesmus obliquus in response to different nitrogen levels. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Xia, L.; Li, H.; Song, S. Cell surface characterization of some oleaginous green algae. J. Appl. Phycol. 2016, 28, 2323–2332. [Google Scholar] [CrossRef]
- Busscher, H.J.; Weerkamp, A.H.; van der Mei, H.C.; van Pelt, A.W.; de Jong, H.P.; Arends, J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 1984, 48, 980–983. [Google Scholar] [CrossRef] [PubMed]
- van Oss, C. Hydrophobicity of biosurfaces—Origin, quantitative determination and interaction energies. Colloids Surfaces B Biointerfaces 1995, 5, 91–110. [Google Scholar] [CrossRef]
- Ozkan, A.; Berberoglu, H. Physico-chemical surface properties of microalgae. Colloids Surf. B Biointerfaces 2013, 112, 287–293. [Google Scholar] [CrossRef]
- Hao, W.; Yanpeng, L.; Zhou, S.; Xiangying, R.; Wenjun, Z.; Jun, L. Surface characteristics of microalgae and their effects on harvesting performance by air flotation. Int. J. Agric. Biol. Eng. 2017, 10, 125–133. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.; Li, M.; Zhang, X.; Wang, G.; Chou, A.; Chen, L.; Yan, H.; Zuo, Y.Y. Rapid Spectrophotometric Method for Determining Surface Free Energy of Microalgal Cells. Anal. Chem. 2014, 86, 8751–8756. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Angelaalincy, M.; Senthilkumar, N.; Karpagam, R.; Kumar, G.G.; Ashokkumar, B.; Varalakshmi, P. Enhanced Extracellular Polysaccharide Production and Self-Sustainable Electricity Generation for PAMFCs by Scenedesmus sp. SB1. ACS Omega 2017, 2, 3754–3765. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Wang, L.; Schenk, P.M. Effective harvesting of low surface-hydrophobicity microalgae by froth flotation. Bioresour. Technol. 2014, 159, 437–441. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Chafai, D.E.; Nemogová, I.; Dráber, P.; Cifra, M. Zeta potential for cell surface-nanoenvironment interaction assessment Zeta potential for cell surface—Nanoenvironment interaction assessment. Int. J. Bioelectromagn. 2018, 20, 36–38. [Google Scholar] [CrossRef]
- Qi, S.; Chen, J.; Hu, Y.; Hu, Z.; Zhan, X.; Stengel, D.B. Low energy harvesting of hydrophobic microalgae (Tribonema sp.) by electro-flotation without coagulation. Sci. Total. Environ. 2022, 838, 155866. [Google Scholar] [CrossRef]
- Liu, J.; Tao, Y.; Wu, J.; Zhu, Y.; Gao, B.; Tang, Y.; Li, A.; Zhang, C.; Zhang, Y. Effective flocculation of target microalgae with self-flocculating microalgae induced by pH decrease. Bioresour. Technol. 2014, 167, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic charges on microalgae surface: Mechanism and applications. J. Environ. Chem. Eng. 2022, 10, 107516. [Google Scholar] [CrossRef]
- Lesniewska, N.; Duval, J.F.L.; Caillet, C.; Razafitianamaharavo, A.; Pinheiro, J.P.; Bihannic, I.; Gley, R.; Le Cordier, H.; Vyas, V.; Pagnout, C.; et al. Physicochemical surface properties of Chlorella vulgaris: A multiscale assessment, from electrokinetic and proton uptake descriptors to intermolecular adhesion forces. Nanoscale 2024, 16, 5149–5163. [Google Scholar] [CrossRef] [PubMed]
- Ciempiel, W.; Czemierska, M.; Szymańska-Chargot, M.; Zdunek, A.; Wiącek, D.; Jarosz-Wilkołazka, A.; Krzemińska, I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules 2022, 27, 7153. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by Fourier-Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef]
- Georgiou, D.; Charisis, A.; Theocharidou, A.; Ritzoulis, C.; Papapanagiotou, G.; Samara, C.; Chatzidoukas, C.; Kalogianni, E.P. Foaming Properties of Chlorella sorokiniana Microalgal Biomass. Colloids Interfaces 2024, 8, 66. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, P.; Sharma, H.; Gupta, R.K. Sustainable approaches in microalgal biomass harvesting: Current advances and future perspectives. Int. J. Agric. Biosci. 2024, III, 35–47. [Google Scholar] [CrossRef]

| Probe Liquids | γlLW | γl+ | γl− |
|---|---|---|---|
| Water | 21.8 | 25.5 | 25.5 |
| Glycol | 29.0 | 1.92 | 47.0 |
| Glycerol | 34.0 | 3.92 | 57.4 |
| Culture Media | Contact Angles (°) | ||
|---|---|---|---|
| water | glycol | glycerol | |
| Bayfolan® | 49.0 ± 0.9 | 20.0 ± 0.43 | 45.0 ± 1.1 |
| TAP | 37.1 ± 1.8 | 15.0 ± 0.22 | 38.0 ± 2.1 |
| BG11 | 34.6 ± 3.9 | 13.0 ± 0.18 | 36. 0 ± 3.3 |
| Culture Media | Surface Tension Parameters and Total Free Energy of Interaction (MJm−2) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|
| ΔGswsTOT | ||||||
| Bayfolan® | 8.6957 | 58.4589 | 49.2526 | 107.3173 | −26.3574 | −14.1 ± 1.2 |
| TAP | 97.5693 | 161.2983 | 88.1754 | 238.5166 | −132.8269 | −10.9 ± 0.6 |
| BG11 | 136.9969 | 197.7151 | 98.0634 | 278.5113 | −273.9238 | −10.3 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzures-Mendoza, A.C.; Páramo-García, U.; Gallardo-Rivas, N.V.; Aguilera-Vázquez, L.; Mendoza-Martínez, A.M. Enhancing Tetradesmus sp. Biomass Recovery: The Influence of Culture Media on Surface Physicochemical Properties. Processes 2025, 13, 3099. https://doi.org/10.3390/pr13103099
Anzures-Mendoza AC, Páramo-García U, Gallardo-Rivas NV, Aguilera-Vázquez L, Mendoza-Martínez AM. Enhancing Tetradesmus sp. Biomass Recovery: The Influence of Culture Media on Surface Physicochemical Properties. Processes. 2025; 13(10):3099. https://doi.org/10.3390/pr13103099
Chicago/Turabian StyleAnzures-Mendoza, Ana Carolina, Ulises Páramo-García, Nohra Violeta Gallardo-Rivas, Luciano Aguilera-Vázquez, and Ana María Mendoza-Martínez. 2025. "Enhancing Tetradesmus sp. Biomass Recovery: The Influence of Culture Media on Surface Physicochemical Properties" Processes 13, no. 10: 3099. https://doi.org/10.3390/pr13103099
APA StyleAnzures-Mendoza, A. C., Páramo-García, U., Gallardo-Rivas, N. V., Aguilera-Vázquez, L., & Mendoza-Martínez, A. M. (2025). Enhancing Tetradesmus sp. Biomass Recovery: The Influence of Culture Media on Surface Physicochemical Properties. Processes, 13(10), 3099. https://doi.org/10.3390/pr13103099








