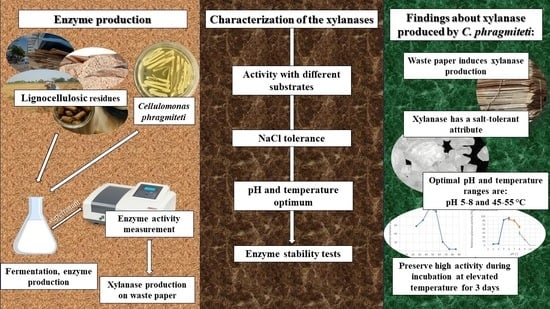
Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Lignocellulosic Materials
2.3. Enzyme Production in Shake Flask
2.4. Enzymatic Activity Measurements
2.5. Investigation of the Time Dependence of Xylanase Production
2.6. Total Protein Content Assay
2.7. Effect of NaCl Concentration on Xylanase Activity and Xylanase Production
2.8. Determination of the pH and Temperature Optima of the Produced Xylanolytic Supernatant
2.9. Enzyme Stability Measurement
2.10. Enzymatic Hydrolysis of Xylan
3. Results and Discussion
3.1. Effect of Different Media and Lignocellulosic Culture Substrates on the Production of a Xylanolytic Enzymatic Supernatant
3.2. Effect of the Amount of Waste Paper on Xylanase Production
3.3. Investigation of the Time Curve of Xylanase Activity Production
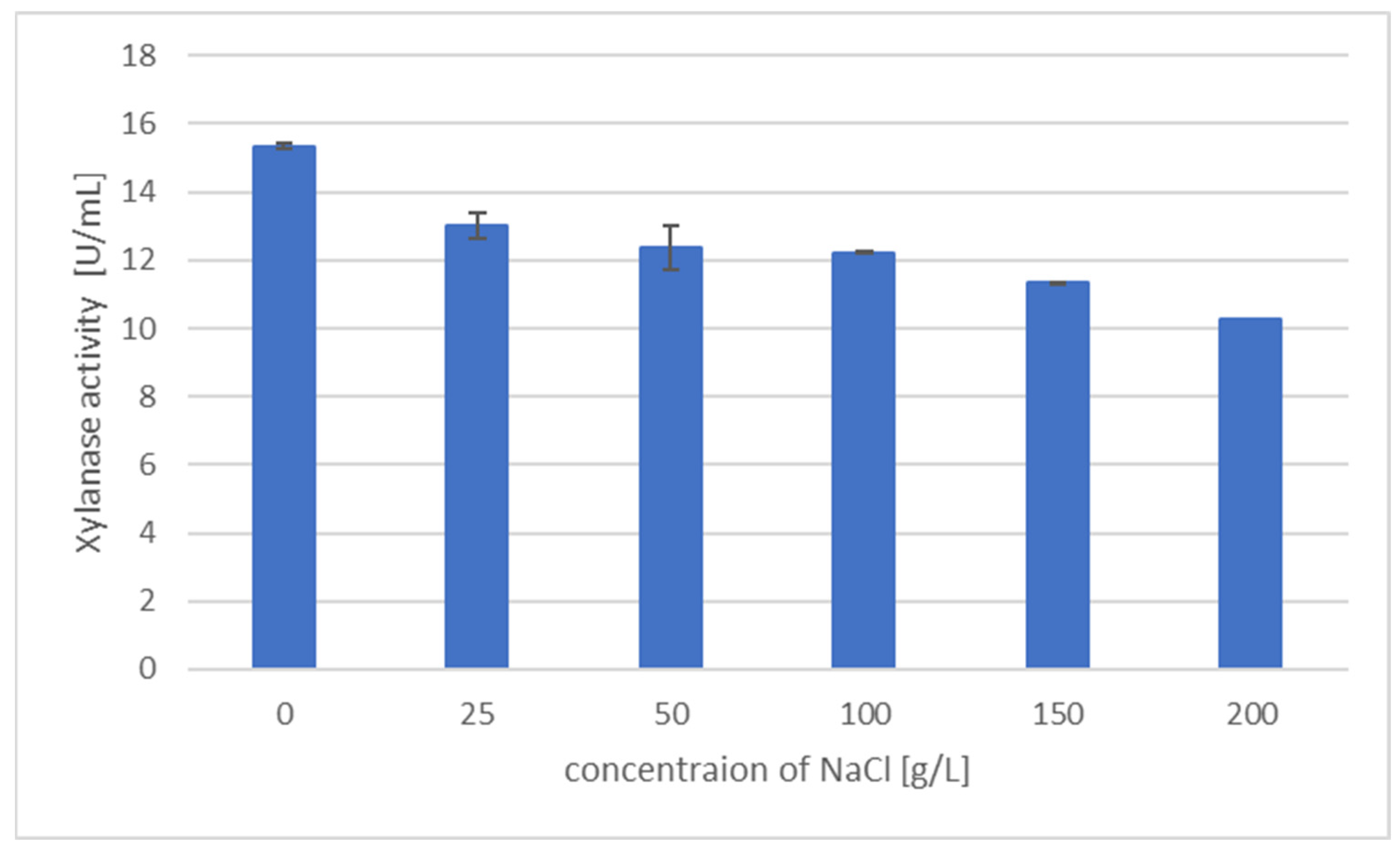
3.4. Effect of NaCl Concentration on Xylanase Activity
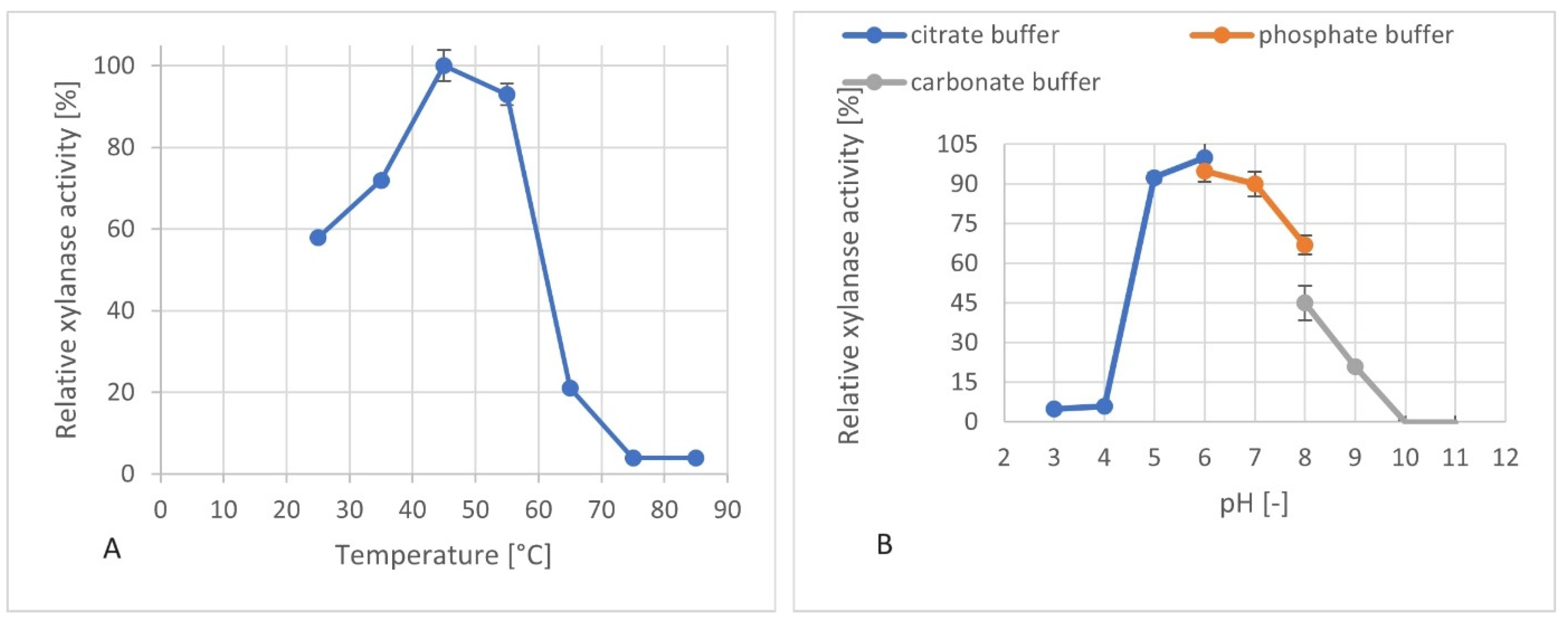
3.5. Investigation of the pH and Temperature Optima of the Produced Xylanases
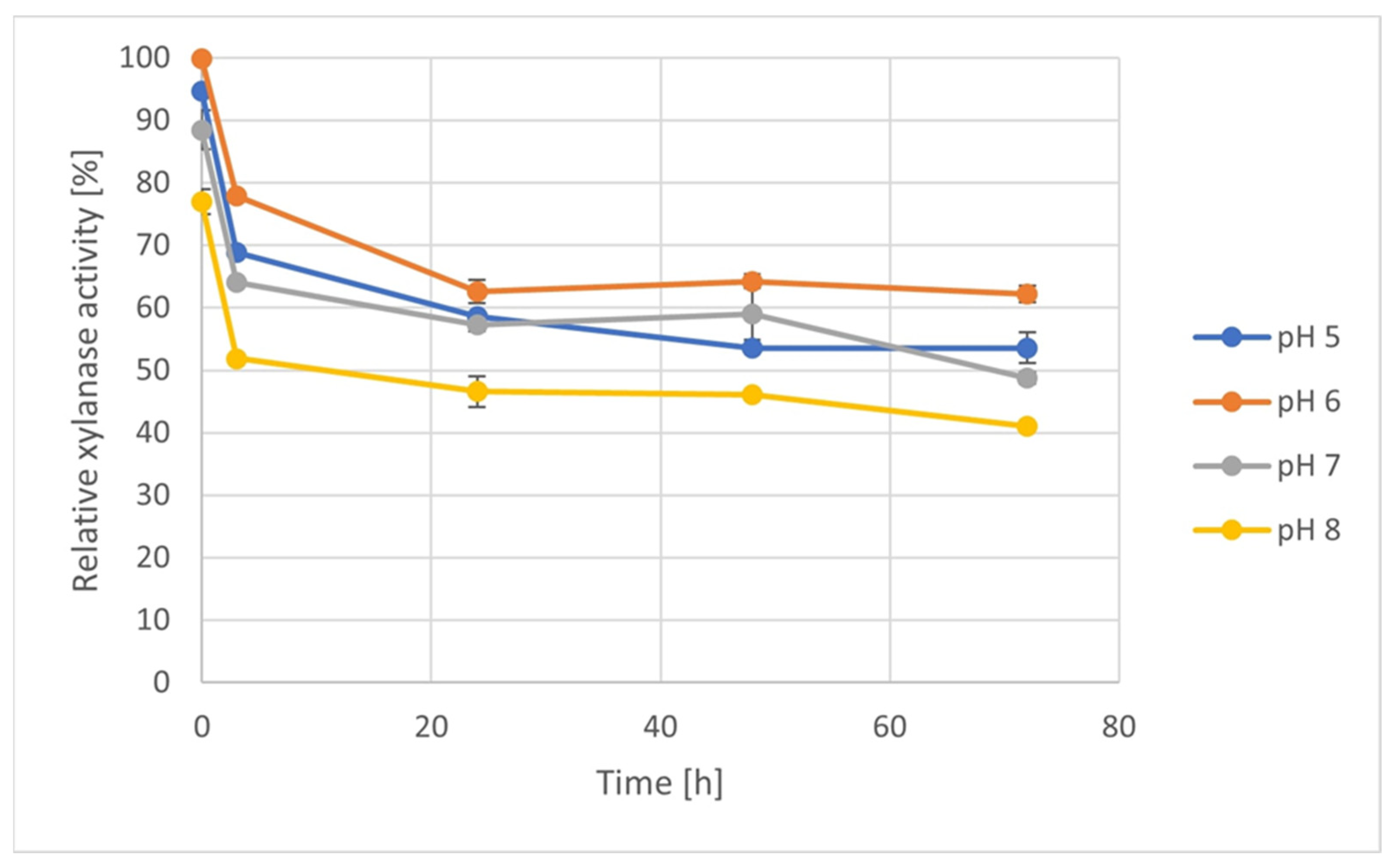
3.6. Enzyme Stability Test
3.7. Enzymatic Hydrolysis of Xylan
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Bomble, Y.J.; Lin, C.Y.; Amore, A.; Wei, H.; Holwerda, E.K.; Ciesielski, P.N.; Donohoe, B.S.; Decker, S.R.; Lynd, L.R.; Himmel, M.E. Lignocellulose Deconstruction in the Biosphere. Curr. Opin. Chem. Biol. 2017, 41, 61–70. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Balan, V. Current Challenges in Commercially Producing Biofuels from Lignocellulosic Biomass. ISRN Biotechnol. 2014, 2014, 463074. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga-Hernández, S.; Velasco-Ayala, C.; Leal-Isla Flores, P.; de Jesús Rostro-Alanis, M.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Biotransformation of Lignocellulosic Biomass into Industrially Relevant Products with the Aid of Fungi-Derived Lignocellulolytic Enzymes. Int. J. Biol. Macromol. 2020, 161, 1099–1116. [Google Scholar] [CrossRef]
- Gonçalves, G.A.L.; Takasugi, Y.; Jia, L.; Mori, Y.; Noda, S.; Tanaka, T.; Ichinose, H.; Kamiya, N. Synergistic Effect and Application of Xylanases as Accessory Enzymes to Enhance the Hydrolysis of Pretreated Bagasse. Enzym. Microb. Technol. 2015, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Woldesenbet, F.; Virk, A.P.; Gupta, N.; Sharma, P. Effect of Microwave Irradiation on Xylanase Production from Wheat Bran and Biobleaching of Eucalyptus Kraft Pulp. Appl. Biochem. Biotechnol. 2012, 167, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed Overview of Xylanases: An Emerging Biomolecule for Current and Future Prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Alokika; Singh, B. Enhanced Production of Bacterial Xylanase and Its Utility in Saccharification of Sugarcane Bagasse. Bioprocess. Biosyst. Eng. 2020, 43, 1081–1091. [Google Scholar] [CrossRef]
- Robinson, R.K.; Batt, C.A.; Patel, P.D. Rajoka1999. In Encyclopedia of Food Microbiology; Elseiver: Amsterdam, The Netherlands, 1999; pp. 365–371. [Google Scholar]
- Lisov, A.V.; Belova, O.V.; Lisova, Z.A.; Vinokurova, N.G.; Nagel, A.S.; Andreeva-Kovalevskaya, Z.I.; Budarina, Z.I.; Nagornykh, M.O.; Zakharova, M.V.; Shadrin, A.M.; et al. Xylanases of Cellulomonas flavigena: Expression, Biochemical Characterization, and Biotechnological Potential. AMB Express 2017, 7, 5. [Google Scholar] [CrossRef]
- Mayorga-Reyes, L.; Morales, Y.; Salgado, L.M.; Ortega, A.; Ponce-Noyola, T. Cellulomonas flavigena: Characterization of an Endo-1,4-Xylanase Tightly Induced by Sugarcane Bagasse. FEMS Microbiol. Lett. 2002, 214, 205–209. [Google Scholar] [CrossRef][Green Version]
- Ontañon, O.M.; Bedő, S.; Ghio, S.; Garrido, M.M.; Topalian, J.; Jahola, D.; Fehér, A.; Valacco, M.P.; Campos, E.; Fehér, C. Optimisation of Xylanases Production by Two Cellulomonas Strains and Their Use for Biomass Deconstruction. Appl. Microbiol. Biotechnol. 2021, 105, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Kalra, K.L.; Sareen, V.K.; Soni, G. Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of trichoderma viride. Braz. J. Microbiol. 2008, 39, 535–541. [Google Scholar] [CrossRef]
- Elisashvili, V.; Metreveli, E.; Khardziani, T.; Sokhadze, K.; Kobakhidze, A.; Kachlishvili, E. Review of Recent Advances in the Physiology of the Regulation of Cellulase and Xylanase Production by Basidiomycetes. Energies 2023, 16, 4382. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from Lignocellulosic Biomass: A Comprehensive Review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef] [PubMed]
- Rusznyák, A.; Tóth, E.M.; Schumann, P.; Spröer, C.; Makk, J.; Szabó, G.; Vladár, P.; Márialigeti, K.; Borsodi, A.K. Cellulomonas phragmiteti sp. Nov., a Cellulolytic Bacterium Isolated from Reed (Phragmites australis) Periphyton in a Shallow Soda Pond. Int. J. Syst. Evol. Microbiol. 2011, 61, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1959, 62, 293–300. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. (LAP) 2008, 1617, 1–16. [Google Scholar]
- Ghio, S.; Sabarís, G.; Lorenzo, D.; Lia, V.; Talia, P.; Cataldi, A.; Grasso, D.; Campos, E. Isolation of Paenibacillus sp. and Variovorax sp. Strains from Decaying Woods and Characterization of Their Potential for Cellulose Deconstruction. Int. J. Biochem. Mol. Biol. 2012, 3, 352. [Google Scholar]
- Miller, G.L. Miller1959. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ontañon, O.M.; Ghio, S.; Marrero Díaz de Villegas, R.; Piccinni, F.E.; Talia, P.M.; Cerutti, M.L.; Campos, E. EcXyl43 β-Xylosidase: Molecular Modeling, Activity on Natural and Artificial Substrates, and Synergism with Endoxylanases for Lignocellulose Deconstruction. Appl. Microbiol. Biotechnol. 2018, 102, 6959–6971. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Ferreira Filho, E.X.; Moreira, L.R.S. An Update on Enzymatic Cocktails for Lignocellulose Breakdown. J. Appl. Microbiol. 2018, 125, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Sárossy, Z.; Tenkanen, M.; Pitkänen, L.; Bjerre, A.B.; Plackett, D. Extraction and Chemical Characterization of Rye Arabinoxylan and the Effect of β-Glucan on the Mechanical and Barrier Properties of Cast Arabinoxylan Films. Food Hydrocoll. 2013, 30, 206–216. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the Structural Features of Arabinoxylans from Brewers’ Spent Grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, M.M.; Kabel, M.A.; Van Eylen, D.; Gruppen, H.; Schols, H.A. Characterization of Oligomeric Xylan Structures from Corn Fiber Resistant to Pretreatment and Simultaneous Saccharification and Fermentation. J. Agric. Food Chem. 2010, 58, 11294–11301. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, F.E.; Ontañon, O.M.; Ghio, S.; Sauka, D.H.; Talia, P.M.; Rivarola, M.L.; Valacco, M.P.; Campos, E. Secretome Profile of Cellulomonas sp. B6 Growing on Lignocellulosic Substrates. J. Appl. Microbiol. 2019, 126, 811–825. [Google Scholar] [CrossRef]
- Wakarchuk, W.W.; Brochu, D.; Foote, S.; Robotham, A.; Saxena, H.; Erak, T.; Kelly, J. Proteomic Analysis of the Secretome of Cellulomonas fimi ATCC 484 and Cellulomonas flavigena ATCC 482. PLoS ONE 2016, 11, e0151186. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Lo, Y.C.; Chang, J.S. Multicomponent Cellulase Production by Cellulomonas biazotea NCIM-2550 and Its Applications for Cellulosic Biohydrogen Production. Biotechnol. Prog. 2010, 26, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, K.C.; Upadhyaya, J.; Joshi, D.R.; Lekhak, B.; Chaudhary, D.K.; Pant, B.R.; Bajgai, T.R.; Dhital, R.; Khanal, S.; Koirala, N.; et al. Production, Characterization, and Industrial Application of Pectinase Enzyme Isolated from Fungal Strains. Fermentation 2020, 6, 59. [Google Scholar] [CrossRef]
- Palaniyappan, M.; Vijayagopal, V.; Viswanathan, R.; Viruthagiri, T. Screening of Natural Substrates and Optimization of Operating Variables on the Production of Pectinase by Submerged Fermentation Using Aspergillus niger MTCC 281. Afr. J. Biotechnol. 2009, 8, 682–686. [Google Scholar]
- Verma, D.; Satyanarayana, T. Xylanolytic Extremozymes Retrieved from Environmental Metagenomes: Characteristics, Genetic Engineering, and Applications. Front. Microbiol. 2020, 11, 551109. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Mladenov, R.; Wulfhorst, H.; Jäger, G.; Spiess, A.C. Point by Point Analysis: How Ionic Liquid Affects the Enzymatic Hydrolysis of Native and Modified Cellulose. Green. Chem. 2010, 12, 1959–1966. [Google Scholar] [CrossRef]
- Akermann, A.; Weiermüller, J.; Chodorski, J.N.; Nestriepke, M.J.; Baclig, M.T.; Ulber, R. Optimization of Bioprocesses with Brewers’ Spent Grain and Cellulomonas Uda. Eng. Life Sci. 2022, 22, 132–151. [Google Scholar] [CrossRef]
- Amaya-Delgado, L.; Vega-Estrada, J.; Flores-Cotera, L.B.; Dendooven, L.; Hidalgo-Lara, M.E.; Montes-Horcasitas, M.C. Induction of Xylanases by Sugar Cane Bagasse at Different Cell Densities of Cellulomonas flavigena. Appl. Microbiol. Biotechnol. 2006, 70, 477–481. [Google Scholar] [CrossRef]
- Ghadikolaei, K.K.; Sangachini, E.D.; Vahdatirad, V.; Noghabi, K.A.; Zahiri, H.S. An Extreme Halophilic Xylanase from Camel Rumen Metagenome with Elevated Catalytic Activity in High Salt Concentrations. AMB Express 2019, 9, 86. [Google Scholar] [CrossRef]
- Garrido, M.M.; Piccinni, F.E.; Landoni, M.; Peña, M.J.; Topalian, J.; Couto, A.; Wirth, S.A.; Urbanowicz, B.R.; Campos, E. Insights into the Xylan Degradation System of Cellulomonas sp. B6: Biochemical Characterization of RCsXyn10A and RCsAbf62A. Appl. Microbiol. Biotechnol. 2022, 106, 5035–5049. [Google Scholar] [CrossRef]




| Percentage of Dry Matter | |
|---|---|
| Glucan | 56 (0.7) |
| Xylan | 15 (0.24) |
| Arabinan | 0 (0.0) |
| Acid-insoluble solid (Klason lignin) | 10.5 (0.07) |
| Enzyme Activity | U/mL |
|---|---|
| β-glucosidase | 0.11 (0.010) |
| β-xylosidase | 0.61 (0.013) |
| Cellobiohydrolase | 0.12 (0.005) |
| Carboxymethyl-cellulase | 0.15 (0.032) |
| α-arabinofuranosidase | 0.007 (0.002) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buda, K.; Fekete, T.; Ontañon, O.M.; Campos, E.; Fehér, C. Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials. Processes 2024, 12, 258. https://doi.org/10.3390/pr12020258
Buda K, Fekete T, Ontañon OM, Campos E, Fehér C. Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials. Processes. 2024; 12(2):258. https://doi.org/10.3390/pr12020258
Chicago/Turabian StyleBuda, Kata, Tünde Fekete, Ornella M. Ontañon, Eleonora Campos, and Csaba Fehér. 2024. "Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials" Processes 12, no. 2: 258. https://doi.org/10.3390/pr12020258
APA StyleBuda, K., Fekete, T., Ontañon, O. M., Campos, E., & Fehér, C. (2024). Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials. Processes, 12(2), 258. https://doi.org/10.3390/pr12020258