1. Introduction
The remarkable development of textile fiber production and its growing commercial activity make their easy recognition and distinction more important and necessary every day, not only for trade, but also for the recycling of fabrics composed of different fibers.
In recent years, sustainability has established new manufacturing habits in the textile industry, as companies are required to comply with laws that protect and ensure the care of the environment. Technological evolution has allowed a variety of products to be manufactured and recycled thanks to the innovation of systems adapted to these materials, in order to give them a new life with appropriate characteristics and functions [
1]. As a point of reference, the global textile and fashion industry is valued at approximately USD 1.7 trillion and employs over 60 million people worldwide, particularly in developing countries such as Bangladesh, India, and Vietnam. Additionally, major exporters like China account for over 40% of global textile exports.
The creation of recycling systems is necessary due to the high consumption and demand by the population. Recycling textile fibers is a crucial aspect for sustainability in different industries, especially the fashion industry. Modern technology allows the reuse of textile fibers to create new fabrics, thus reducing waste and the need to produce new raw materials. This process not only helps to conserve natural resources, but also reduces the environmental impact associated with textile production.
Nowadays, textile waste processing has become one of the major concerns of experts and the general public. Given the wide range of textiles, recycling of cotton (cellulose) fibers, which account for about 85% of the total natural fibers produced, has attracted the attention of many researchers [
2].
According to an analysis carried out in Spain by FER in 2017 (Spanish Federation of Recovery and Recycling), this country generates 890,244 tons of textile waste per year, of which only 11% is reused or recycled and the remaining 87% is landfilled or incinerated before the end of its useful life [
3]. Mixed fabrics, commonly found in clothing and other textile products, require complex processes to separate cellulose from synthetic components. These processes combine advanced physical and chemical techniques to ensure efficient extraction and sustainable reuse of cellulose.
In Europe, textile recycling legislation has been strengthened in recent years with several specific laws and regulations. Different laws in national legislations, such as “Law on Waste and Contaminated Soils for a Circular Economy” [
4], approved in 2022 in Spain, are fundamental in this context. This law establishes that the separate collection of textiles is mandatory before 1 January 2025, and from 2030, it prohibits the disposal in landfills of any textile waste that could be recycled. In addition, the legislation imposes on producers the responsibility of designing more durable and repairable products, promoting the use of recycled materials, and assuming the costs of managing the waste generated by their products. This includes the costs of collection, transportation, treatment and recovery of waste [
4].
For the recycling of textile materials, physical methods are first used, such as mechanical recycling, which consists of shredding and defibering the fabrics that are part of clothing, obtaining as a result short fibers that can be mixed with virgin fibers to obtain yarn again. Currently, mechanical recycling technologies are the most common, but they require fibers composed of a single material. However, it is often not possible to loosen and spin recycled textiles repeatedly. Therefore, they must ultimately be discarded and the problem of repeated reuse of cotton fibers is far from being solved. Spain is the first European producer of recycled yarn, with a production of close to 61,000 tons per year [
2,
3].
In a second process, chemical textile recycling involves breaking down textile fibers into their basic chemical components through processes such as hydrolysis or methanolysis. In these processes, specific solvents are used for dissolving textile polymers, such as polyester or cellulose, breaking down the molecules into their original monomers, generating new high-quality fibers and promoting a circular economy for this waste.
Research such as that by Zhang et al. [
5,
6] has found that cellulose can be readily and rapidly dissolved in any of the precooled aqueous solutions of LiOH/urea, NaOH/urea or NaOH/thiourea, and produce a stable cellulose solution because urea acts as a hydrogen bond acceptor, stabilizing the solution and preventing cellulose regeneration. Later work has used new cellulose solvents in precooled aqueous solutions of LiOH/urea, NaOH/urea and KOH/urea at −5 to −20 °C [
6].
Polyethylene glycol (PEG) is also a typical environmentally friendly molecule, and its aqueous solutions, especially PEG-based aqueous biphasic systems, such as the mixture of PEG with NaOH, Na
2S, Na
2CO
3, etc., have recently been considered as a potential green solvent system [
7], and it has been applied in both lignin separation from wood and organic reactions. The repeating unit of PEG is –(CH
2–CH
2–O)–, and the oxygen atoms in the PEG chain act as a hydrogen bond acceptor, which can be an alternative reagent to urea or thiourea to stabilize cellulose dissolution.
A novel solvent system composed of an aqueous mixture of 1 wt% PEG and 9 wt% NaOH is presented in the study by Yan et al. [
8]. Cellulose is added at room temperature, frozen at −15 °C for 12 h and then thawed with vigorous agitation, resulting in a homogeneous cellulose solution. The dissolved cellulose can be regenerated by adding dilute HCl. After washing and drying, regenerated cellulose powder was obtained.
Several examples have been reported in the literature where hydrothermal processes, at reasonable temperatures, have been presented as potentially efficient, rapid and alternative routes to standard organic synthetic processes [
8,
9,
10]. Research in this field is ongoing, since temperature reduction in hydrothermal processes is important for energy conservation and safety for process maintenance.
The use of subcritical water as an effective solvent for the hydrolysis and carbonization of cotton fibers has been the subject of study for many researchers [
11,
12,
13]. Subcritical water can provide enough energy to destroy cellulose molecules, promoting the formation of carbon spheres and other valuable products under specific temperature and pressure conditions. It offers a sustainable and effective alternative compared to methods using more aggressive chemical reagents.
While previous studies have explored various methods for cellulose recovery from mixed fabrics, this study introduces a novel approach by combining specific chemical and hydrothermal treatments tailored for maximizing cellulose purity and minimizing fiber degradation. This unique combination of methods offers potential improvements in the efficiency and sustainability of textile recycling processes, contributing to the growing field of sustainable material recovery.
2. Materials and Methods
The fabric used (which will be named sample M0) was a pair of men’s olive-green convenience store shorts consisting of 70% cotton, 27% polyester and 3% elastane. Prior to analysis, approximately 100 g of the fabric sample was shredded using a Retsch model SM200 (Haan, Germany) cutting mill with a 2.0 mm sieve. For analysis, different portions of the shredded sample were taken.
Commercially available alkaline hydroxide (NaOH, KOH, LiOH) from Sigma-Aldrich (Saint Louis, MO, USA), and urea obtained from Labkem (Barcelona, Spain) were used without further purification. An aqueous solution mixture was prepared as a cellulose solvent by directly mixing alkaline hydroxide, urea and distilled water. Specifically, solutions of 7% NaOH/12% urea, 9.8% KOH/12% urea and 4.2% LiOH/12% urea, i.e., 0.175 M alkali hydroxide/0.2 M urea, were used. The samples obtained after treatment with these solutions will be coded as M1, M2 and M3, respectively. The experimental conditions are summarized in
Table 1 in order to compile the nomenclature assigned to each of the treatments carried out.
After the treatment of the shredded fibers with the solutions for 3 h, the samples were filtered and washed with distilled water 2 to 3 times and placed in the oven at 60 °C for 1 h for drying and subsequent analysis.
On the other hand, treatment with PEG was performed by using polyethylene glycol 400 (PEG-400) purchased from Corquimia Industrial SL, Barcelona, Spain, and solid NaOH from Sigma-Aldrich (Saint Louis, MO, USA); these were used to extract cellulose from the sample. Ultrapure water with a resistivity of 18 MΩ.cm was used for solution preparation. Following the ratios used previously by other authors [
8], 1 g of PEG-400 and 9.0 g of NaOH were added to 90 mL of ultrapure water to prepare 2 mixtures of aqueous PEG/NaOH solution, which were stirred until homogenized. Each solution was used adding 2 g of the fabric to be treated. The first solution, coded as M4, was allowed to react at room temperature (24 °C) overnight (12 h). Then, the sample was filtered and washed with distilled water 2 to 3 times and placed in the oven at 60 °C for 1 h for drying and subsequent analysis in thermogravimetry (TG). The second solution, coded as M5, was placed on the magnetic stirrer for 3 h at 50 °C. It was allowed to stand at room temperature, filtered and washed with distilled water 2 to 3 times and placed in the oven at 60 °C for 1 h for drying and subsequent analysis.
Huanyu (Wuhan, China) hydrothermal synthesis autoclaves with a Teflon chamber were used for high-pressure treatment. The vessels were heated in a Memmert universal oven model UF30 (Schwabach, Germany). Samples were prepared by mixing 50 mL of distilled water with different amounts of NaOH and HCl. NaOH solutions were prepared at 0.05 M (0.1 g), 0.025 M (0.05 g), 0.013 M (0.025 g) coded as MR1, MR2 and MR3, respectively. Similarly, HCl solutions were prepared in 50 mL of distilled water at 0.164 M (0.3 g), 0.027 M (0.05 g) and 0.013 M (0.025 g) coded as MR4, MR5 and MR6, respectively. To each of these solutions, 1 g of the fabric sample to be analyzed was added. The samples were transferred to an autoclave that was filled to 60% of its capacity and hermetically sealed. Then, the autoclave was quickly heated to 225 °C and allowed to react for 30 min.
Two additional experiments were performed using only distilled water and the fabric sample. Both experiments were carried out under the same concentration and temperature conditions: 50 mL of distilled water and 1 g of the fabric sample, at a constant temperature of 225 °C. However, the reaction times were varied, with one experiment lasting 30 min (MR7) and the other lasting 60 min (MR8).
The choice of PEG/NaOH as a solvent system was based on its demonstrated ability to selectively break down the polyester matrix while preserving the integrity of the cellulose fraction. This system has been previously shown to be effective in separating cotton from polyester in mixed fabrics, as well as being a more sustainable alternative to other chemical treatments (reference). The use of NaOH in combination with PEG enhances the efficiency of cellulose extraction by weakening the polymer structure without causing excessive degradation.
As for the selected hydrothermal conditions (225 °C for 30 min), these were chosen after extensive trials to optimize the balance between effective cellulose extraction and fiber preservation. The temperature and duration were selected based on previous studies, which have shown that these conditions maximize cellulose yield while minimizing the degradation of the fibers (reference). The combination of high temperature and pressure in hydrothermal treatment ensures the effective separation of cellulose from the mixed fabric structure.
A thermobalance was used during the research for analyzing the treated fibers. The equipment used was a PerkinElmer thermobalance, model STA6000 (Shelton, CT, USA). This thermobalance was used in order to compare the thermal behavior of the samples obtained in the different experiments. This was accomplished by heating small samples, of approximately 10 mg, from an initial 30 °C to a temperature of about 800 °C, recording the variation in mass as the temperature increased. The experiments were carried out in an inert atmosphere.
A microscope was used to obtain enlarged photographs of the different fabric samples obtained during the project. The microscope used was the SKYBASIC Wifi Digital Microscope (Dongguan, China). This microscope has a lens with which images can be obtained with a magnification between 50× and 1000×.
For the classification of the different textile fibers, the FTIR technique with ATR module was used. For the recording of spectra, the Jasco FTIR 4700 IRT 5200 model spectrometer (Hachioji, Tokyo, Japan) equipped with the Specac Golden Gate ATR module with a diamond prism was used. The spectra were obtained within the wavenumber range of 7800 and 400 cm−1.
The recorded thermogravimetric runs were analyzed by deconvolution of the peaks observed in the DTG (derivative of weight loss vs. temperature) in each of the thermal decompositions carried out. This deconvolution has been carried out using the Matlab® (v. 2024b) ‘peakfit’ function. This function fits peaks for time series signals and uses a non-linear optimization algorithm to decompose a complex signal with superimposed peaks into its component parts. The type of function into which a given signal is decomposed can be chosen, but for the case at hand, the Gaussian function has been used.
3. Results
3.1. Thermogravimetric Study
The results obtained in the adjustment using the ‘peakfit’ function in Matlab
® are shown in
Table 2. The possible assignment to the materials that comprise the fabrics is also indicated.
It is worth noting that within the TG analysis, the assignment of “degraded cellulose” and “degraded polyester” refers to the states of these materials after being subjected to elevated temperatures that cause their decomposition. “Degraded cellulose” indicates that the cellulose in the cotton has partially been degraded by the corresponding reagent, while “degraded polyester” indicates the degradation of the polymer chains in the polyester.
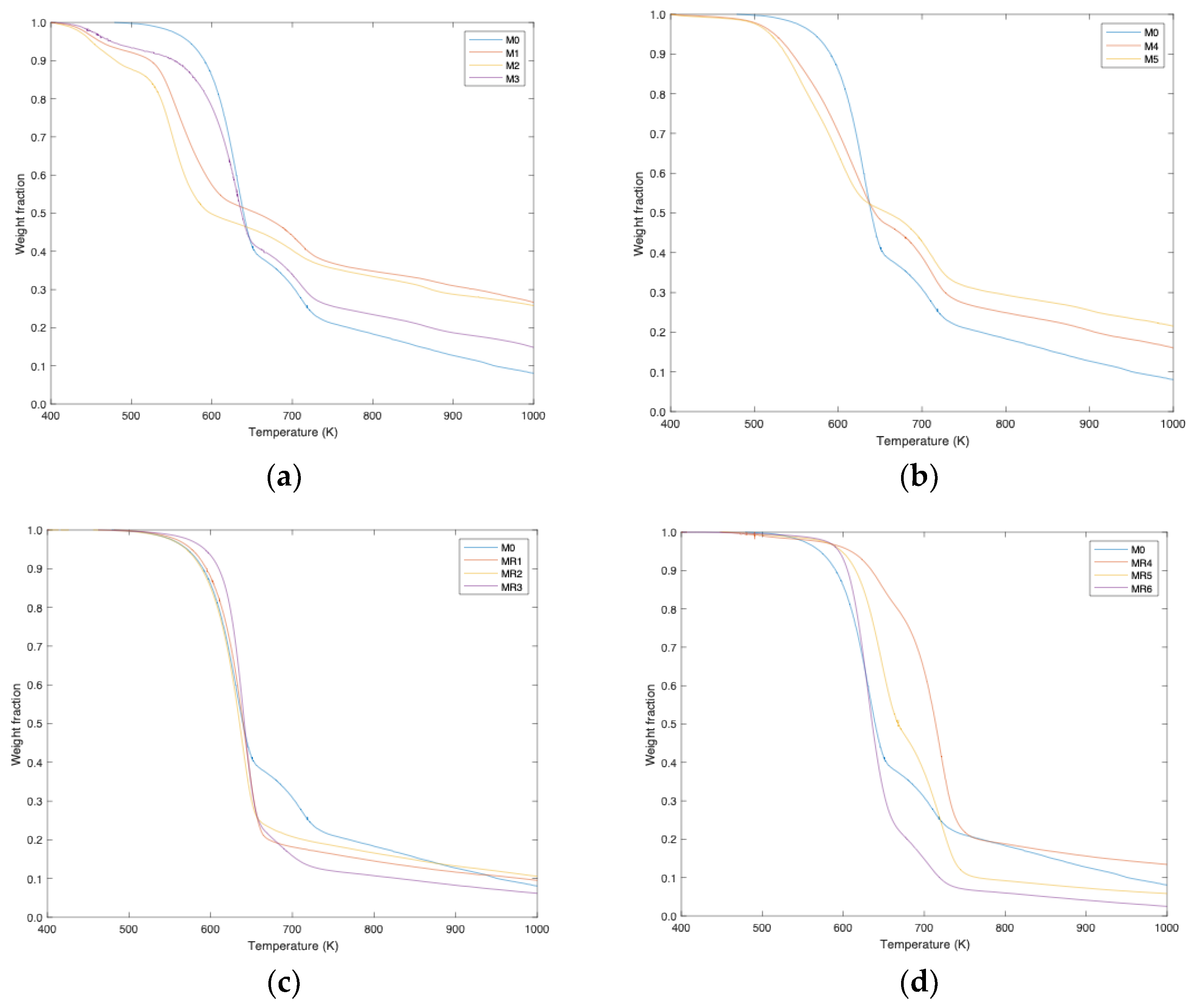
In thermogravimetric (TG) and its derivative (DTG) analysis, the decomposition peaks corresponding to the two main components of the mixed fabric were clearly identified. For sample M0 (original fabric), cotton decomposition peaks were observed at 610.6 K and 633.7 K, contributing 76.3% and 23.7% of the total area, respectively. Polyester showed a peak at 706.3 K, representing 23.7% of the total decomposition, coinciding with the textile composition provided by the manufacturer.
Samples treated with different chemical solutions showed significant variations in the decomposition profiles. For example, in sample M1, treated with NaOH/urea, the decomposition peak of degraded cellulose was observed at 550.0 K, with a contribution of 71.9%, while the peak of polyester appeared at 703.7 K, representing 28.1%. This change indicates that treatment with these reagents affected the structure of cellulose, facilitating its degradation at lower temperatures. Sample M2, treated with KOH/urea, showed a similar behavior, with a decomposition peak of degraded cellulose at 549.7 K and a contribution of 72.3%. Polyester presented a peak at 692.2 K, representing 27.7% of the total area. These results suggest that both NaOH and KOH, in combination with urea (runs M1 and M2), are effective in cellulose degradation, allowing a clearer separation of the components of the mixed fabric. In run M3, in which fabrics were treated with LiOH and urea, a cellulose decomposition peak was presented at 604.5 K, with a contribution of 79.1%, and a polyester peak at 706.5 K, representing 20.9%. This treatment also proved to be effective in the degradation of cellulose, although at a slightly higher temperature compared to the samples treated with NaOH and KOH (
Figure 1a). If we compare this study with that carried out by others [
5,
6], we can conclude that, in the absence of a cooling phase of the reagents, the use of hydroxyls combined with urea, especially LiOH/urea, proved to be effective in the purification of cellulose without external conditions.
PEG- and NaOH-treated samples (samples M4 and M5) showed similar decomposition, indicating degradation of both cellulose and polyester (
Figure 1b). Although a decrease in the cellulose percentage of samples M4 and M5 is seen to 65% and 69%, respectively, it is necessary to evaluate these results in the context of the complete purification process. The goal of using PEG/NaOH treatment is to separate and purify cellulose from polyester, and although there is an apparent loss of cellulose, the process can still be considered successful if it has achieved significant separation of the components. The key is to optimize the experimental conditions to maximize the recovery of pure cellulose and minimize the degradation of cellulose fibers.
To evaluate which of the reagents used is more efficient under high-temperature conditions (225 °C) to obtain a pure cellulose sample, samples MR1 to MR8 were considered; these were treated with different concentrations of NaOH and HCl, as well as hydrothermal treatments with only water.
Samples MR1, MR2 and MR3, treated with NaOH at different concentrations (
Figure 1c), showed significant cellulose purification. In particular, sample MR1, with the highest NaOH concentration (0.1 g in 50 mL), presented 100% cellulose purity according to thermogravimetric analysis.
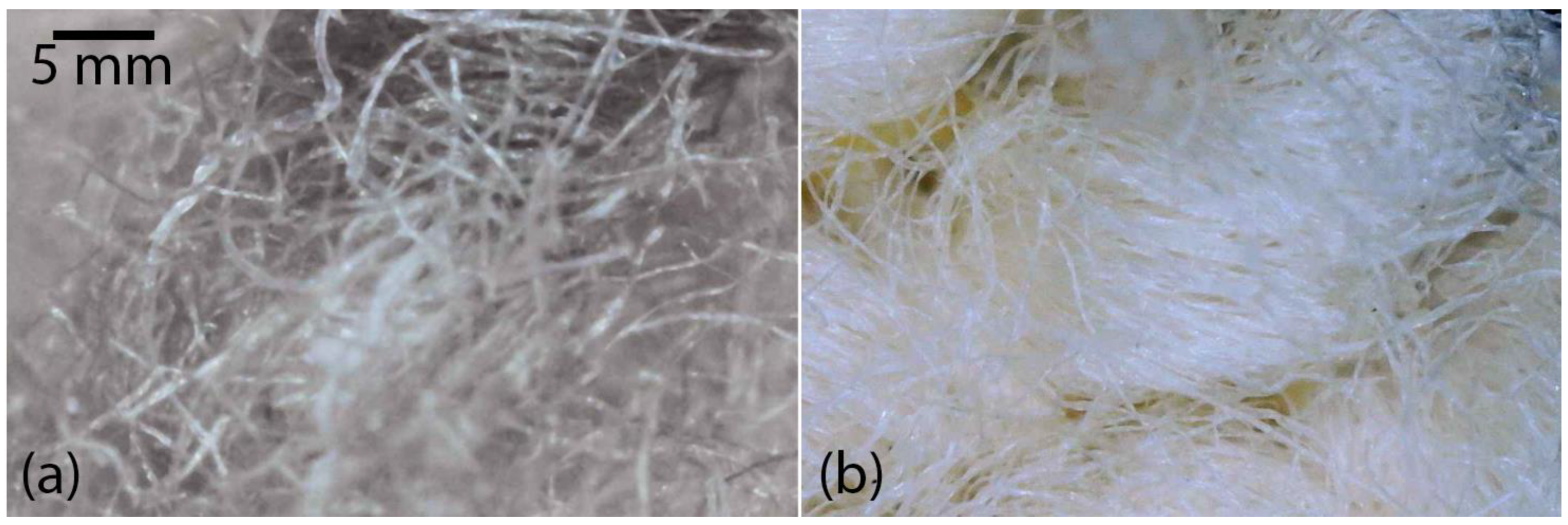
To verify the purity of the 100% cellulose, a microscope was used to obtain a magnified photograph of the most optimal NaOH-treated sample (MR1). A well-defined and clean fibrous structure was observed (
Figure 2b). The cellulose fibers are clearly separated and show high purity, indicating that the MR1 treatment was effective in removing polyester.
In the analysis of the results obtained in this study for samples MR4 and MR5, using HCl to obtain cellulose, it was observed that high concentrations of HCl (>0.05 g) tend to degrade the sample significantly (
Figure 1d). This agrees with what has been reported in various studies, where it is highlighted that the use of strong acids, such as HCl, can be effective for the dissolution of cellulose, but can also lead to a considerable degradation of the cellulose chain [
2], affecting its structural integrity. For sample MR6, where the HCl concentration was 0.025 g, a degradation of polyester and purification of cellulose of 83.6% was shown. However, the purity of cellulose was not as high as that obtained with NaOH.
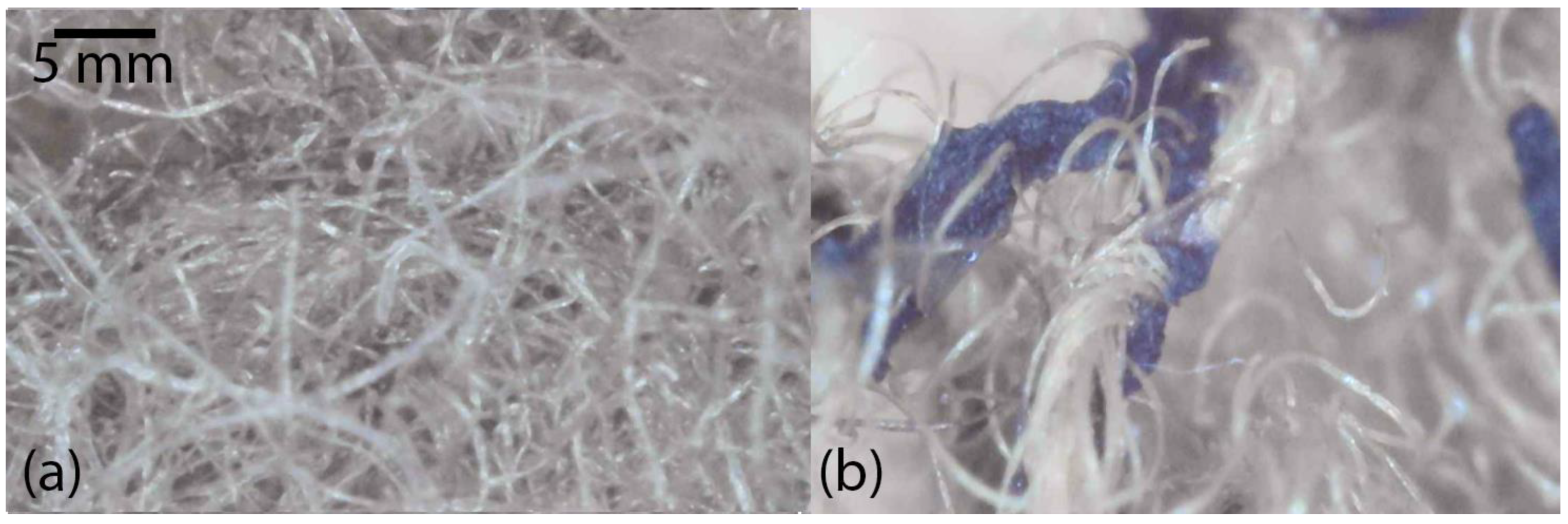
Images taken for sample MR6 (
Figure 3) show a fiber structure in which dark-colored residues can be observed, probably residues of the dyed polyester that were not completely removed. Despite these residues, the cellulose fibers appear to be relatively clean and well defined, suggesting that the HCl treatment at this low concentration was effective in removing the polyester without causing significant degradation of the cellulose.
On the other hand, samples MR7 and MR8, treated with water alone at 225 °C for different lengths of time, showed promising results. Sample MR8, treated with water for 60 min, presented a high purity of cellulose with a cellulose matrix that reached 91.2% purity. Sample MR7, treated for 30 min, showed a cellulose purity of 89.4%. The temperature was kept high in a range of 616 and 618 K. In previous studies, subcritical water has been used for carbonization of cellulose fibers at different pH values, achieving high efficiency in the decomposition of cellulose fibers and the formation of carbon microspheres [
11,
13].
3.2. Analysis with Infrared Spectroscopy (IR)
Infrared (IR) spectroscopy is an essential technique for identifying and characterizing the chemical components of the materials obtained in this study.
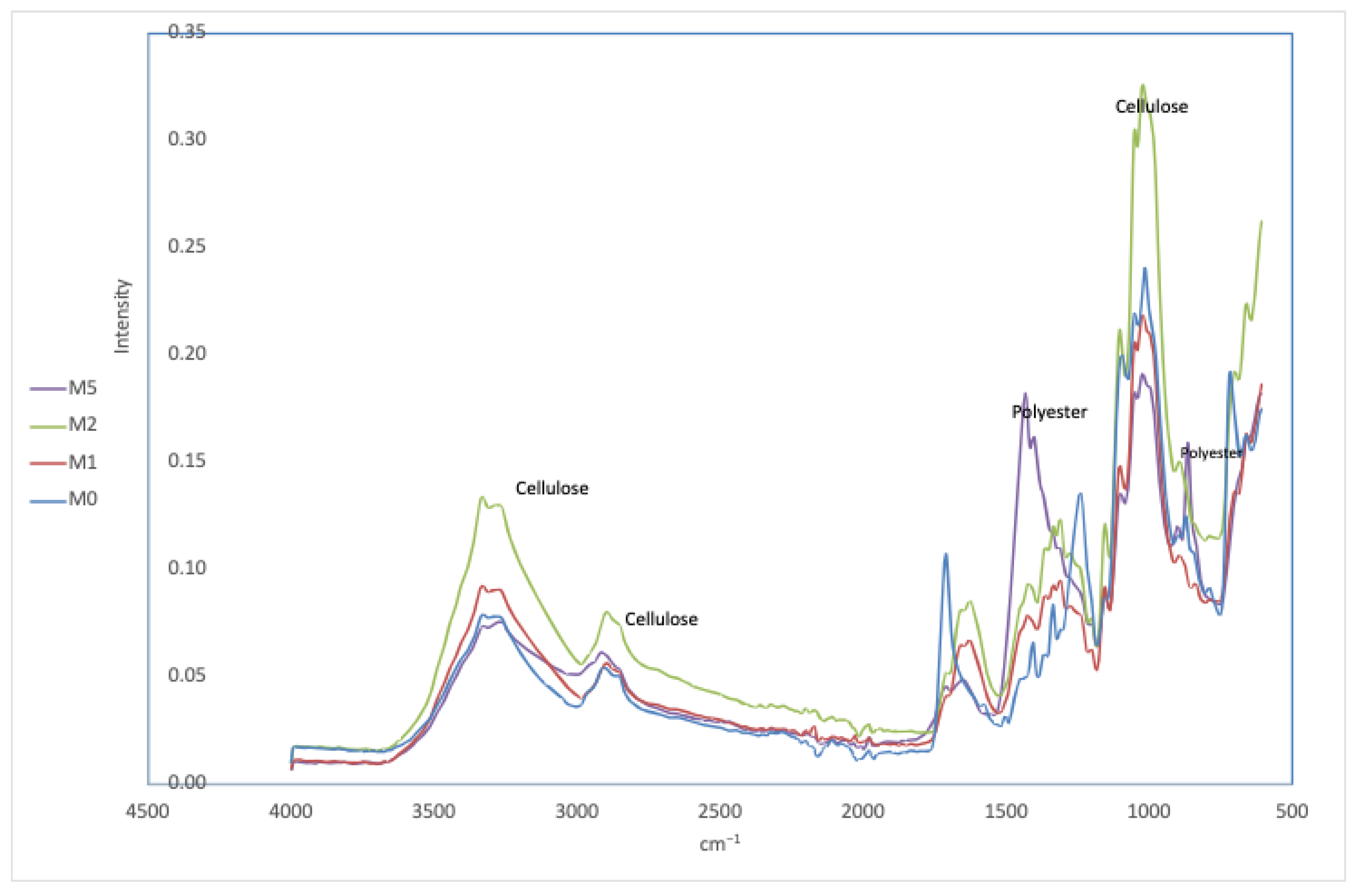
Figure 4 presents the results obtained by IR spectroscopy for the different treated samples.
The IR signal of cellulose is located in the range of 3700–3200 cm
−1 and between 1100 and 1000 cm
−1. While the polyester repeat unit is composed of an aromatic ring and an ester, the first is located approximately in the range of 730–650 cm
−1 and the second is found in the range of 1740–1715 cm
−1 [
14].
In the analysis obtained, the characteristic bands of cellulose are observed in the regions around 3500 cm−1, 2900 cm−1 and between 1050 and 1150 cm−1. These bands correspond to the O-H and C-H stretching vibrations, as well as to the vibrations of the C-O-C bonds in the cellulose structure, while the characteristic bands of polyester are observed in the regions around 1720 cm−1 (C=O) and between 1250 and 1300 cm−1 (C-O) corresponding to the vibrations of the ester groups in the polyester. These coincidences indicate that the spectroscopic analyses performed are consistent with the data reported in the literature, validating the methodology and the results obtained in this study.
As seen in
Figure 4, the spectra of samples M1 and M2 show characteristic cellulose bands in the regions around 3500 cm
−1 and between 1050 and 1150 cm
−1, indicating the presence of cellulose. However, in M1, polyester bands are also observed in the ranges of 1720 cm
−1 and 1250–1300 cm
−1, although with a lower intensity compared to sample M0. This suggests that the NaOH/urea treatment has been partially effective in reducing polyester, but has not completely removed this component. On the other hand, in sample M2, the polyester bands, although present, show a somewhat lower intensity compared to sample M1, suggesting that the KOH/urea treatment has been slightly more effective in removing polyester.
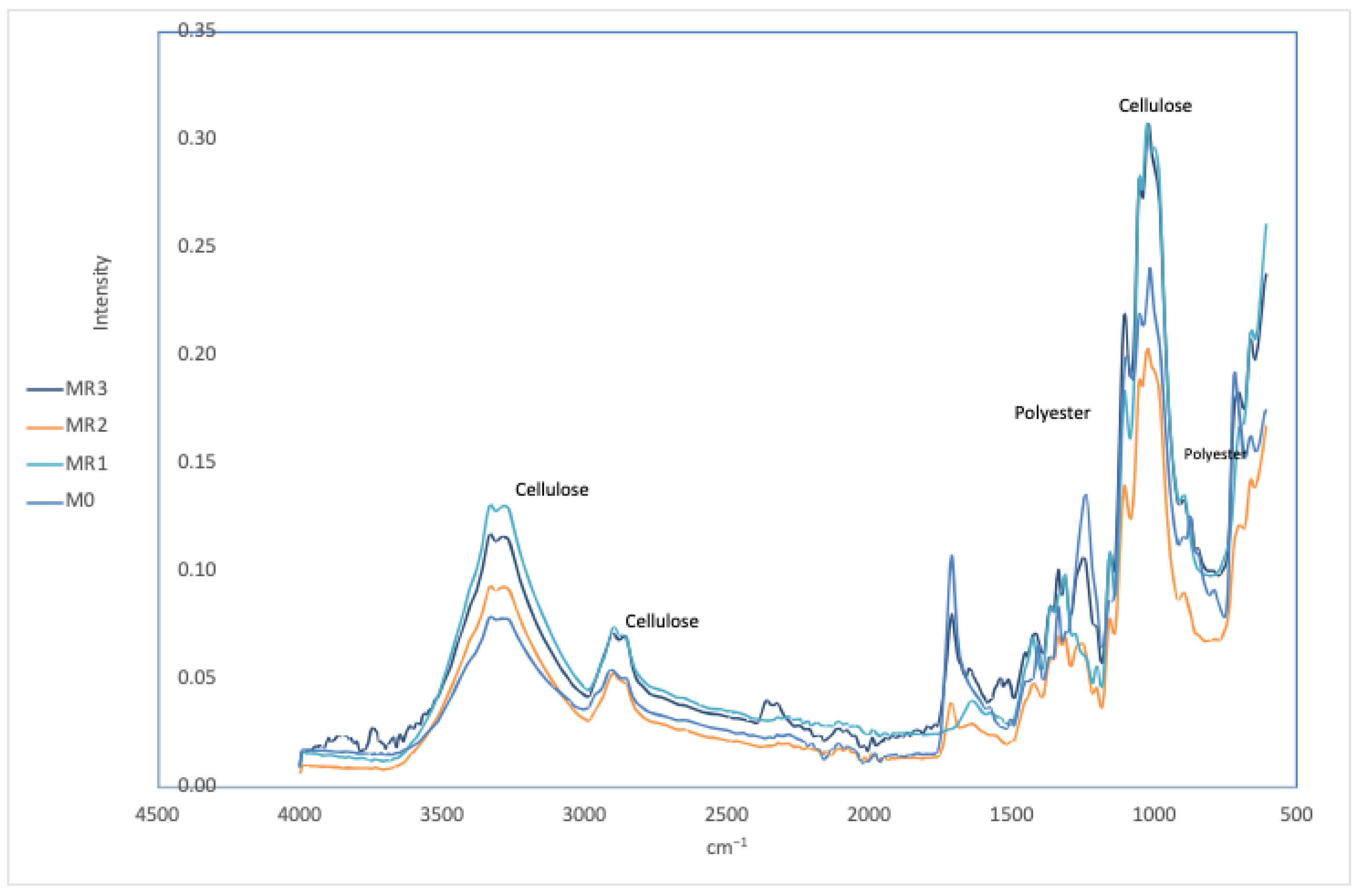
Figure 5 shows the results obtained with NaOH (with MR1 being the most effective). Almost complete removal of polyester is observed, evidenced by the near absence of the C=O band at 1700 cm
−1. The cellulose bands are clear and defined, indicating a high purity of the cellulose obtained. This demonstrates that this treatment is highly effective in removing polyester and preserving cellulose.
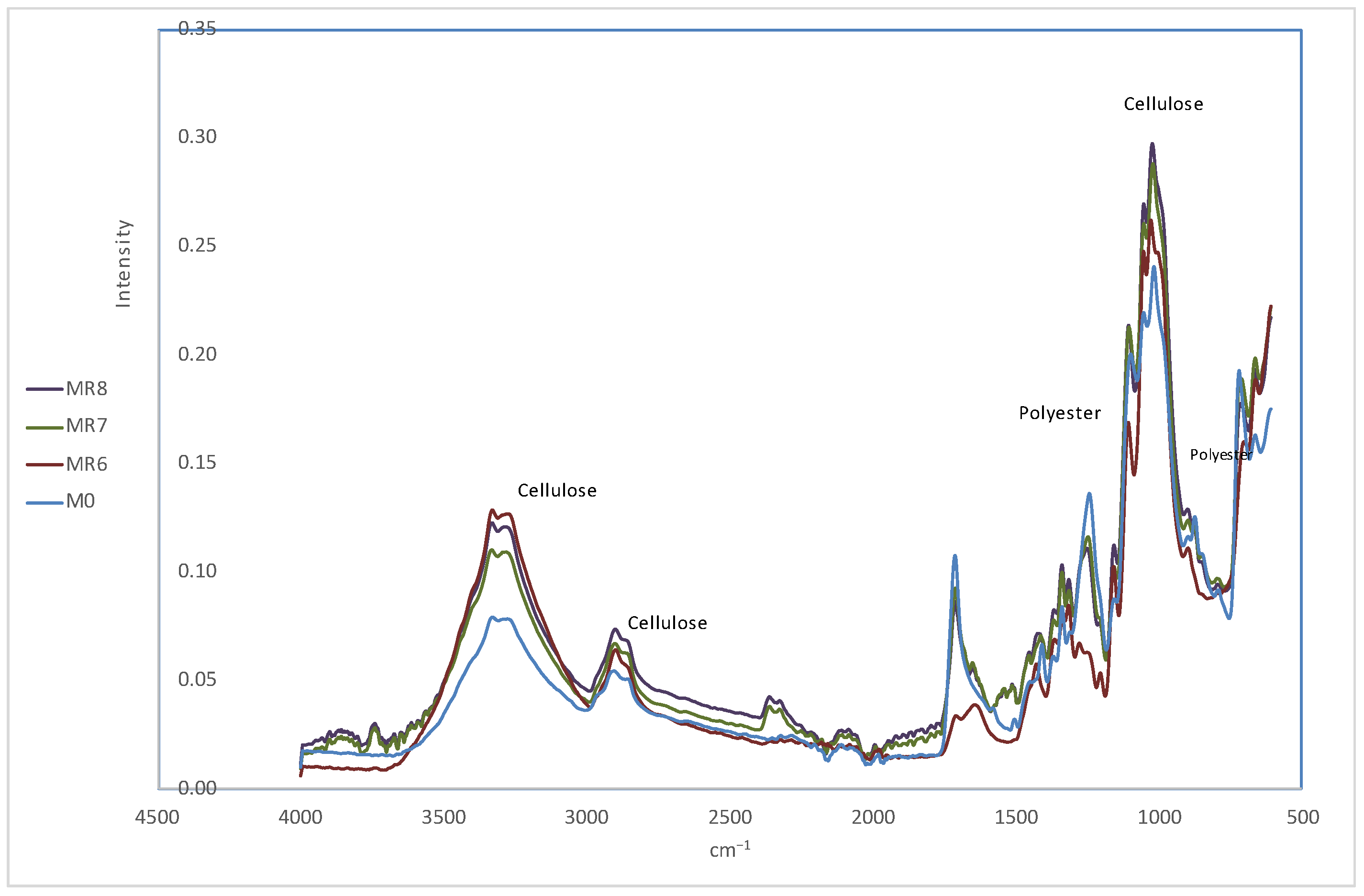
In
Figure 6, we can see the spectra of samples MR6, MR7 and MR8. In sample MR6, the characteristic bands of cellulose are observed in the regions around 3500 cm
−1 and between 1050 and 1150 cm
−1, indicating a good preservation of cellulose. The polyester bands in the ranges of 1720 cm
−1 and 1250–1300 cm
−1 are significantly less intense, suggesting that the HCl treatment has been highly effective in preserving cellulose but less efficient in the complete removal of polyester compared to NaOH.
Samples MR7 and MR8 also retained the characteristic bands of cellulose, indicating that the cellulose remained intact and the polyester bands, although reduced, are still visible. This treatment is effective in removing polyester and reduces the use of additional chemical reagents for obtaining cellulose.
4. Conclusions
The results of the study indicate that NaOH treatments, especially at concentrations of 0.025 g, combined with hydrothermal conditions at 225 °C for 30 min (MR1), are highly effective for obtaining pure cellulose from mixed fabrics. The curves obtained by thermogravimetry (TG) show a clear reduction in the weight fraction corresponding to polyester, confirming the effectiveness of the method. The images obtained by microscopy corroborate a clear fibrillar structure, reinforcing the conclusion derived from the thermogravimetric data.
In the study, it is observed that treatments with subcritical water and strong acids such as HCl are also effective, but can cause significant degradation of the fibers if the experimental conditions are not adequately controlled. In terms of sustainability, the use of NaOH at low concentrations presents advantages due to its lower environmental impact and greater safety in handling.
If maximum polyester removal is the primary goal, NaOH is most suitable. However, for a safer and more environmentally friendly approach, high-temperature water treatment may be sufficient, especially if time and temperature conditions are optimized.
The study not only validates the effectiveness of the applied methods for cellulose extraction, but also underlines the importance of choosing appropriate experimental conditions to maximize purity and minimize fiber degradation. Thermogravimetry, combined with IR spectroscopy, provides a robust approach to evaluate and optimize these processes, which is crucial to advance automation and sustainability of textile recycling.
Future lines of action in this research will explore advanced analytical techniques, optimizing treatment conditions, and investigating the scalability of the processes studied to better address the topic’s practical applications and broader impact.