Study of Mixed Combustion Behavior of Pulverized Municipal Solid Waste and Anthracite Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Methods
2.2.1. Heat Treatment and Crushing and Screening
2.2.2. Thermogravimetric Analysis
2.2.3. Kinetic Model
3. Results and Discussion
3.1. Comparative Analysis of the Combustion Curves of a Single Fuel
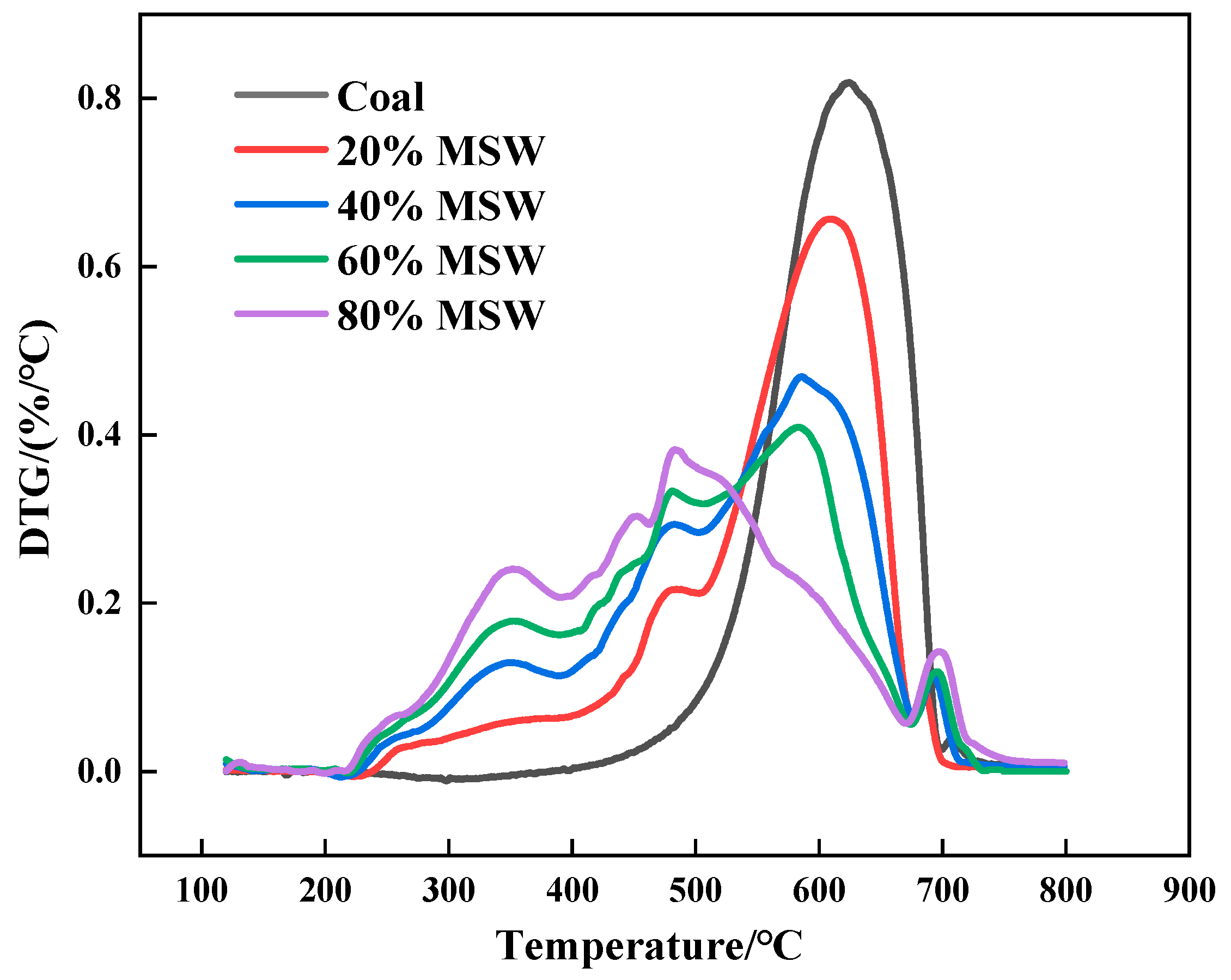
3.2. Characteristic Analysis of the Combustion Curves of MSW–Pulverized Anthracite Mixtures
3.3. Quantitative Analysis of the Effect of MSW on the Combustibility of Pulverized Anthracite
3.4. Kinetic Analysis of the Combustion Process of MSW–Pulverized Anthracite Mixtures
4. Conclusions
- (1)
- Compared to anthracite, the weight loss curve for the combustion process of MSW, though smooth, is divided into multiple stages, whereas anthracite experiences weight loss in only one stage. This indicates that the combustion process of MSW is more complex. The initial ignition temperature of MSW is 334 °C, while that of anthracite is 551 °C, suggesting that MSW has better combustibility than anthracite.
- (2)
- As the proportion of MSW increases, the number of weight loss stages in the combustion curve of the mixture increases, and the ignition temperature gradually decreases. When the MSW proportion is 20%, the combustion curve closely resembles that of anthracite. The injection of MSW promotes the combustion of anthracite powder.
- (3)
- By calculating the Rm, the co-combustion process of MSW and anthracite has been quantitatively characterized. As the mixing ratio of MSW increases, the index Rm exhibits an upward trend, rising from 0.131 to 0.235. By increasing the proportion of MSW in the mixture, the combustion performance of the mixed fuel is significantly enhanced.
- (4)
- The combustion process of MSW and anthracite mixture was fitted using the RPM, URCM, and VM. The results indicate that the VM is the most suitable for describing the co-combustion process of MSW and anthracite. As the proportion of MSW increases, the activation energy of the combustion process gradually decreases from 152.05 kJ/mol to 32.17 kJ/mol.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Bureau of China. Available online: https://www.stats.gov.cn/sj/ndsj/2024/indexch.htm (accessed on 18 November 2024).
- Zhang, G.; Liu, K.; Lv, L.; Gao, W.; Li, W.; Ren, Z.; Yan, W.; Wang, P.; Liu, X.; Sun, L. Enhanced landfill process based on leachate recirculation and micro-aeration: A comprehensive technical, environmental, and economic assessment. Sci. Total Environ. 2023, 857, 159535. [Google Scholar] [CrossRef] [PubMed]
- He, J.N.; Liu, Y.L.; Li, L.L.; Qiu, Z.W. Energy consumption analysis of municipal solid waste classification and transportation. Environ. Eng. 2021, 39, 136–142. [Google Scholar]
- Deng, H.; Zhang, D.; Yu, H.; Man, Y.; Wang, Y. Assessing life-cycle GHG emissions of recycled paper products under imported solid waste ban in China: A case study. Sci. Total Environ. 2023, 891, 164407. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Structure characteristics and development sustainability of municipal solid waste treatment in China. Ecol. Indic. 2023, 152, 110391. [Google Scholar] [CrossRef]
- Moya, D.; Aldás, C.; López, G.; Kaparaju, P. Municipal solid waste as a valuable renewable energy resource: A worldwide opportunity of energy recovery by using Waste-To-Energy Technologies. Energy Procedia 2017, 134, 286–295. [Google Scholar] [CrossRef]
- Agarwal, M.; Tardio, J.; Venkata Mohan, S. Critical analysis of pyrolysis process with cellulosic based municipal waste as renewable source in energy and technical perspective. Bioresour. Technol. 2013, 147, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xu, S.; Zhang, L.; Zhang, H.; Liu, C.; Zhu, H.; Liu, S. Characteristics of fast pyrolysis of biomass in a free fall reactor. Fuel Process. Technol. 2006, 87, 863–871. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Lu, L.; Namioka, T.; Yoshikawa, K. Effects of hydrothermal treatment on characteristics and combustion behaviors of municipal solid wastes. Appl. Energy 2011, 88, 3659–3664. [Google Scholar] [CrossRef]
- García, R.; González-Vázquez, M.; Rubiera, F.; Pevida, C.; Gil, M. Co-pelletization of pine sawdust and refused derived fuel (RDF) to high-quality waste-derived pellets. J. Clean. Prod. 2021, 328, 129635. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.S.; Xue, Q.G. Efficient utilization of waste plastics as raw material for metallic iron and syngas production by combining heat treatment pulverization and direct reduction. Process Saf. Environ. Prot. 2020, 137, 49–57. [Google Scholar]
- Zhang, H.; Wang, G.; Wang, J.S.; Xue, Q.G. Low-temperature treatment of polyethylene plastics and semi-coke mixture and CO2 gasification of finely ground products. Fuel 2021, 285, 119215. [Google Scholar] [CrossRef]
- Nie, Z.; Wang, G.; Zhang, H.; Wang, J.; Xue, Q. New technology of efficient pulverization of simulated municipal solid waste based on low temperature heat treatment and properties of pulverized product for blast furnace injection. Fuel 2023, 337, 127245. [Google Scholar]
- Li, Y.; Wu, X.; Sun, C.S.; Geng, Z.; Zhang, Q. Optimization of Beijing municipal solid waste treatment model based on low-carbon development. Resour. Sci. 2021, 43, 1574–1588. [Google Scholar]
- Li, X.-G.; Ma, B.-G.; Xu, L.; Hu, Z.-W.; Wang, X.-G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar] [CrossRef]
- Prabowo, B.; Aziz, M.; Umeki, K.; Susanto, H.; Yan, M.; Yoshikawa, K. CO2-recycling biomass gasification system for highly efficient and carbon-negative power generation. Appl. Energy 2015, 158, 97–106. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.P.; Wang, Y.G.; Argyle, M.D.; Fan, M. CO2 gasification of powder River Basin coal catalyzed by a cost-effective and environ-mentally friendly iron catalyst. Appl. Energy 2015, 145, 295–305. [Google Scholar]
- Wang, G.W.; Zhang, J.L.; Hou, X.M.; Shao, J.; Geng, W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour. Technol. 2015, 177, 66–73. [Google Scholar]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Li, K.; Zuo, H. Investigation of non-isothermal and isothermal gasification process of coal char using different kinetic model. Int. J. Min. Sci. Technol. 2015, 25, 15–21. [Google Scholar]
- Liao, X.J.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel 2021, 287, 119355. [Google Scholar]
- Wang, G.; Zhang, H.Q.; Wang, J.S.; Xue, Q.G. Gasification kinetics of char formed from waste polyvinyl chloride for efficient utilization in ironmaking process. J. Iron Steel Res. Int. 2022, 29, 1535–1544. [Google Scholar] [CrossRef]
- Muthuraman, M.; Namioka, T.; Yoshikawa, K. A comparative study on co-combustion performance of municipal solid waste and Indonesian coal with high ash Indian coal: A thermogravimetric analysis. Fuel Process. Technol. 2010, 91, 550–558. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Rubiera, F.; Pis, J. Effect of biomass blending on coal ignition and burnout during oxy-fuel combustion. Fuel 2008, 87, 2753–2759. [Google Scholar] [CrossRef]
- Chen, Y.; Mori, S.; Pan, W.-P. Estimating the Combustibility of Various Coals by TG-DTA. Energy Fuels 1995, 9, 71–74. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.W.; Zhang, J.L.; Lee, J.Y.; Wang, H.; Wang, C. Combustion behaviors and kinetics analysis of coal, biomass and plastic. Thermochim. Acta 2018, 669, 140–148. [Google Scholar]
- Ding, L.; Wang, P.Y.; Kong, L.X.; Bai, J.; Yu, G.; Li, W.; Wang, F. Progress on reaction models for coal gasification processes. Chem. Ind. Eng. Prog. 2024, 43, 3593–3612. [Google Scholar]




| Kitchen Waste | Paper | Plastics | Textiles | Wood | Dust | Glasses | Metal | Others |
|---|---|---|---|---|---|---|---|---|
| 50.65 | 20.98 | 21.62 | 0.47 | 3.53 | 0.23 | 1.67 | 0.35 | 0.53 |
| Sample | Proximate Analysis/% | Ultimate Analysis/% | LHV/(kJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vd | Ad | FCd | C | H | O | N | S | Cl | ||
| Pulverized MSW | 75.81 | 7.85 | 16.34 | 65.70 | 9.70 | 8.26 | 0.38 | 0.38 | 2.63 | 28,829 |
| Anthracite | 10.93 | 9.56 | 79.52 | 77.71 | 1.21 | 8.19 | 0.55 | 1.12 | 0.12 | 25,558 |
| Sample | Tmax1 | rmax1 | Tmax2 | rmax2 | Tmax3 | rmax3 | Tmax4 | rmax4 | Rm |
|---|---|---|---|---|---|---|---|---|---|
| Anthracite | - | - | - | - | 624 | 0.82 | - | - | 0.131 |
| 20% MSW-80% anthracite | - | - | 480 | 0.21 | 610 | 0.66 | 685 | 0.10 | 0.167 |
| 40% MSW-60% anthracite | 346 | 0.12 | 479 | 0.29 | 588 | 0.47 | 693 | 0.12 | 0.192 |
| 60% MSW-40% anthracite | 348 | 0.18 | 480 | 0.33 | 584 | 0.41 | 696 | 0.12 | 0.208 |
| 80% MSW-20% anthracite | 353 | 0.24 | 452 | 0.31 | 485 | 0.38 | 699 | 0.14 | 0.235 |
| Sample | Anthracite | 20% MSW-80% Anthracite | 40% MSW-60% Anthracite | 60% MSW-40% Anthracite | 80% MSW-20% Anthracite | MSW | |
|---|---|---|---|---|---|---|---|
| RPM | E (kJ/mol) | 152.05 | 77.73 | 50.46 | 40.67 | 33.43 | 32.32 |
| A | 1.13 × 107 | 961.05 | 28.59 | 8.62 | 3.65 | 3.81 | |
| φ | −1.03 × 1026 | −7.11 × 1025 | −3.58 × 1025 | −1.05 × 1028 | −3.07 × 1028 | 9.44 × 1024 | |
| R2 | 0.99969 | 0.99687 | 0.99717 | 0.99818 | 0.99949 | 0.99768 | |
| URCM | E (kJ/mol) | 50.36 | 37.31 | 30.65 | 26.44 | 22.51 | 19.93 |
| A | 11.08 | 2.79 | 1.36 | 0.87 | 0.57 | 0.44 | |
| R2 | 0.95678 | 0.97911 | 0.99167 | 0.99486 | 0.99585 | 0.99008 | |
| VM | E (kJ/mol) | 152.05 | 80.51 | 51.05 | 40.87 | 33.41 | 32.17 |
| A | 1.13 × 107 | 1.41 × 103 | 31.10 | 8.89 | 3.65 | 3.71 | |
| R2 | 0.99969 | 0.99692 | 0.99718 | 0.99819 | 0.99949 | 0.99768 | |
| Sample | DEV(x)/% | ||
|---|---|---|---|
| RPM | URCM | VM | |
| Anthracite | 0.79 | 9.30 | 0.79 |
| 20% MSW-80% anthracite | 2.42 | 6.26 | 2.40 |
| 40% MSW-60% anthracite | 2.22 | 3.82 | 2.22 |
| 60% MSW-40% anthracite | 1.75 | 2.95 | 1.75 |
| 80%MSW-20% anthracite | 0.91 | 2.61 | 0.91 |
| MSW | 1.96 | 4.05 | 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Wang, G.; Wang, J.; Xue, Q. Study of Mixed Combustion Behavior of Pulverized Municipal Solid Waste and Anthracite Coal. Processes 2024, 12, 2853. https://doi.org/10.3390/pr12122853
Zuo X, Wang G, Wang J, Xue Q. Study of Mixed Combustion Behavior of Pulverized Municipal Solid Waste and Anthracite Coal. Processes. 2024; 12(12):2853. https://doi.org/10.3390/pr12122853
Chicago/Turabian StyleZuo, Xiaojian, Guang Wang, Jingsong Wang, and Qingguo Xue. 2024. "Study of Mixed Combustion Behavior of Pulverized Municipal Solid Waste and Anthracite Coal" Processes 12, no. 12: 2853. https://doi.org/10.3390/pr12122853
APA StyleZuo, X., Wang, G., Wang, J., & Xue, Q. (2024). Study of Mixed Combustion Behavior of Pulverized Municipal Solid Waste and Anthracite Coal. Processes, 12(12), 2853. https://doi.org/10.3390/pr12122853




