Upcycling Coffee Waste: Key Industrial Activities for Advancing Circular Economy and Overcoming Commercialization Challenges
Abstract
:1. Introduction
2. Coffee Processing and Its Composition
3. Coffee Waste Composition
- -
- Primary waste obtained by coffee processing (to derive green beans)—coffee pulp, husk, mucilage, and parchment.
- -
- Secondary waste obtained by coffee beverage preparation—silverskin and spent coffee grounds.
3.1. Coffee Husk (CH) and Coffee Pulp (CP)
3.2. Coffee Mucilage (CM)
3.3. Coffee Parchment (CPm)
3.4. Coffee Silverskin (CS)
| Compound | Coffee Waste | ||||
|---|---|---|---|---|---|
| Pulp | Husk | Parchment | Silverskin | SCGs | |
| Caffeine (mg/g) | 2.05–31 | 1.33–9.82 | 0.10–58.20 | 0.70–53.30 | 0.03–76.90 |
| Tannins (mg/g) | 1.80–60 | 18–93 | 17 | 2.50–7.66 | 0.99 mg CE/g |
| Trigonelline (mg/g) | - | 1.56–5.43 | 1.20–13.60 | 36.50 | 0.40–2.40 |
| Melanoidins (mg/g) | - | 1.50 | - | 1.70–2.30 | - |
| Phenolic acid and polyphenols | |||||
| Chlorogenic acids (mg/g) | 1.80–3.40 | 1.21–132.50 | 0.05–9.40 | 0.04–21.40 | 1.40–85 |
| 5-Caffeoylquinic acid (mg/g) | 1.74–22.80 | 0.20–1.90 | 6.10 | 0.40–89 | 1.40–37 |
| 4-Caffeoylquinic acid (mg/g) | 0.14–0.43 | 0.01–0.12 | - | 0.18–23.80 | 1.36–16.20 |
| 3-Caffeoylquinic acid (mg/g) | 0.06–0.26 | 0.02–0.05 | - | 0.94–17.90 | 0.02–15 |
| Dicaffeoylquinic acids (mg/g) | - | - | - | - | 3.31–5.79 |
| 3,4-Dicaffeoylquinic acids (mg/g) | 0.29–4.50 | - | - | 10.43 | 2.89 |
| 3,5-Dicaffeoylquinic acids (mg/g) | 0.20–1.13 | - | - | 0.44–9.97 | 1.04 |
| 4,5-Dicaffeoylquinic acids (mg/g) | 0.24–1.22 | - | - | 10.56 | 1.65 |
| Caffeic acid (mg/g) | 0.09–4.29 | 0.06–28.20 | 0.004–0.007 | 0.08–0.54 | 0.004–6.10 |
| Ferulic acid (mg/g) | 0.05–4.30 | 0.01 | 0.001–0.003 | 0.004–0.230 | 0.01–0.03 |
| 5-, and 4-Feruloylquinic acid (mg/g) | 0.01–0.20 | 0.07–0.19 | - | 1.22 | - |
| p-Coumaric acid (µg/g) | 2–160 | 0.10–8.70 | 6.70–34 | 1–18 | 4.42–500 |
| 5-Coumaroylquinic acid (mg/100 g) | - | 3.50–6.20 | - | 5.70 | - |
| 3-Coumaroylquinic acid (mg/100 g) | - | - | - | 2.40 | - |
| Sinapic acid (mg/g) | - | - | - | 0.18 | 0.01 |
| Gallic acid (µg/g) | 4.30 | 1.90–87 | 3–97 | 15.80–31.10 | 32.50 |
| 3-O-Metylgallic acid (µg/g) | - | - | 2.30–5.30 | - | - |
| 4-Hydroxybenzoic acid (µg/g) | - | 13.40 | 1.60–2.40 | 3.40 | 885–1813 |
| Protocatechuic acid (µg/g) | 85–4700 | 37–488 | 9.20–14.10 | 44 | 5.77–530 |
| Gentistic acid (µg/g) | - | 27–77 | - | - | 0.26 |
| Syringic acid (µg/g) | 0.06 | - | 3.50–8.60 | 39–78 | 3.41 |
| Salicylic acid (µg/g) | - | 3.1 | 0.70–1.00 | 2.30 | 7.61 |
| Vanillic acid (µg/g) | 7 | 23 | 15.90–42.80 | 30–345 | 340–1103 |
| Flavonoid | |||||
| Quercetin (µg/g) | - | - | - | 1.53–3.56 | 3.20–3.76 |
| Quercitrin (µg/g) | - | - | - | 0.12–0.59 | 0.13–0.83 |
| Quercetin-3-O-rutinoside (rutin) (µg/g) | 90–700 | 9.85–114.95 | - | 1.25–10.65 | 2.36–9.65 |
| Quercetin-3-O-glucoside (µg/g) | - | 57–69.90 | - | - | - |
| Quercetin-3-O-galactoside (µg/g) | - | 55 | - | - | - |
| Catechin (µg/g) | 60–630 | 0.82–1.70 | - | 10.20 | 14.55 |
| Epicatechin (µg/g) | 630–4360 | 8–25 | - | 151.10 | 10.08 |
| Naringin (µg/g) | - | - | - | 0.32–0.45 | 86.94 |
| Naringenin (µg/g) | - | 1.32–1.94 | - | - | - |
| Kaemferol (µg/g) | - | - | - | 0.76–1.66 | - |
| Kaempferol-3-O-galactoside (µg/g) | - | 123 | - | - | - |
| Anthocyanidins (mg/g) | 40–50 | - | - | - | - |
| Procyanidins (µg/g) | 1200–8500 | 1.3–534 | - | - | - |
| Total flavonoids (mg QE/g) | 21.80–58.80 | 0.071–15.70 | 0.80 mg CE/g | 2.73 | 2.11–8.29 |
| Total phenolic content (mg GAE/g) | 2.55–442 | 1.85–4.55 | 2.28–2.84 | 2.60–36 | 3.33–273.30 |
| Antioxidant capacity (μmol TE/g) | 51–92 | 3136.40 | 14.50 | 21.35 | 20.04 |
| References | [43,60,61,62,63,64,65,66,67,68] | [62,63,65,66,69,70,71,72,73,74,75,76,77] | [38,62,63,65,72,73,75,78,79,80] | [46,62,63,65,66,73,75,76,78,79,81,82,83] | [46,62,65,66,74,78,81,83,84,85,86,87] |
3.5. Spent Coffee Grounds (SCGs)
4. Valorization of Coffee Wastes
4.1. Agricultural Applications
4.1.1. Soil Amendment and Fertilizer
Composting
Vermicomposting
Biochar
4.1.2. Mushroom Cultivation
4.1.3. Pesticides and Herbicides
Biopesticides
Bioherbicides
4.2. Biofuels and Bioenergy
4.2.1. Bioethanol Production
Pretreatment
Hydrolysis
Fermentation
Ethanol Recovery
4.2.2. Biodiesel Production
Combined Processes for Producing Biodiesel and Bioethanol
4.2.3. Bio-Oil Production
4.2.4. Biogas Production
4.2.5. Biorefinery Concept of Coffee Waste Variolization into Biofuels and Bioenergy
4.3. Food Ingredients and Nutraceuticals
4.3.1. Bakery Production
Bread
Cookies, Cake, Biscuits
4.3.2. Yogurt
4.3.3. Innovative Beverages
4.3.4. Food Preservatives
4.4. Biochemicals and Biomaterials
4.4.1. Natural Colorants
4.4.2. Active Food Packaging
5. Navigating the Path to Commercialization: Current Status and Key Challenges
5.1. Present Commercialization Situation
| Main Application | Application Type | Coffee Waste | Company/ Project | City/ Country | Financial Report | Finding | Ref. |
|---|---|---|---|---|---|---|---|
| Agriculture | Substrate for growing mushroom | SCGs | PermaFungi | Brussels, Belgium |
|
| [391,392,393,394] |
| Compost | Coffee silverskin (Chaff) | Imbibe coffee roasters | Dublin, Ireland | N/A |
| [395] | |
| Compost | SCGs | Nestle | Switzerland | FY2023
|
| [397,398] | |
| Compost | SCGs | Starbuck | USA | FY2023
|
| [400,401] | |
| Biofuels and Bioenergy | Biomass pellet, Fire log, Biodiesel | SCGs | Bio-bean | Cambridgeshire, UK | Total equity funding is approximately USD 7.3 M in 7 rounds (2014–2022). |
| [387,388,389,402] |
| Biomass (for steam boiler) | SCGs | Nestle | Australia | As mentioned above in Nestlé company. |
| [403] | |
| Biogas | SCGs | Nestle | Switzerland |
| [397,398] | ||
| Biomass (for steam boiler) | SCGs | Nestle | Spain |
| [404,405] | ||
| Biomass (for steam boiler) | SCG | Veolia | Netherlands | FY2023
|
| [406,407,408,409] | |
| Biochemicals and Biomaterials | Biochemicals, Colorants | SCGs | Caffeinc.nl | Amsterdam, Netherlands | Funding USD 4.4 million (EUR 4 million) by Amsterdam Climate and Energy Fund (ACEF) in 2022. |
| [410,411,412] |
| Polymers | SCGs | Coffeefrom | Italy | Coffeefrom started with bootstrapping and secured seed investment in 2023 through the Terra Next accelerator program. |
| [413,414] | |
| Coffee oil, Antioxidant compounds, Polymers, and Lignin | SCGs | Ecobean | Poland | Total equity funding of USD 9.59 million (2022–2023). |
| [415,416] | |
| Eco-composite polymer to reusable cup | Coffee husk | Huskee | Australia | Raised more than USD 114,000 via Kickstarter campaign in 2018. |
| [417,418,419,420] | |
| Biochemicals, Antioxidant compounds, Bio-oil, and Polymers | SCGs | Kaffe Bueno | Copenhagen, Denmark | Total equity funding of USD 8.13 million (2020–2024) with annual revenue of USD 28.7 K. |
| [421,422,423,424] | |
| Polymer | SCGs | Kaffeeform | Berlin, Germany | Private financing of EUR 40,000 (USD 44,000) in 2016. |
| [425,426] | |
| Polymer | Coffee Pulp + Silverskin | PTT | Thailand |
|
| [427,428,429] | |
| Food ingredients and nutraceuticals | Beverage (called NESCAFÉ NATIV Cascara) | Coffee husk (Cascara) | Nestle | Australia | As mentioned above in Nestlé company. |
| [430,431] |
| Beverages (called Cascara Latte) | Coffee husk (Cascara) | Starbuck | USA | As mentioned above in Starbucks company. |
| [432,433] | |
| Beverage (called Tabifruit) | Coffee husk | Supracafe Ltd. | Spain | The total amount of the operation was EUR 600,000 (USD 658,000) in 2017. |
| [434,435,436] | |
| Functional ingredients for food products | Coffee cherry pulp | The Coffee Cherry Co. | Seattle, USA | N/A |
| [437] |
5.2. Challenges and Constraints
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-Economic Comparison of Process Technologies for Biochemical Ethanol Production from Corn Stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel Production from Waste Cooking Oil: 2. Economic Assessment and Sensitivity Analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Chen, Y.; Zhai, F.; Li, J.; Wang, J.; Wang, X.; Wang, S.; Zhu, W. Biodiesel Production from High Acid Value Waste Frying Oil Catalyzed by Superacid Heteropolyacid. Biotechnol. Bioeng. 2008, 101, 93–100. [Google Scholar] [PubMed]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development: Washington, DC, USA; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0.
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Osorio-Arias, J.; Delgado-Arias, S.; Duarte-Correa, Y.; Largo-Ávila, E.; Montaño, D.; Simpson, R.; Vega-Castro, O. New Powder Material Obtained from Spent Coffee Ground and Whey Protein; Thermal and Morphological Analysis. Mater. Chem. Phys. 2020, 240, 122171. [Google Scholar] [CrossRef]
- Sisti, L.; Celli, A.; Totaro, G.; Cinelli, P.; Signori, F.; Lazzeri, A.; Bikaki, M.; Corvini, P.; Ferri, M.; Tassoni, A.; et al. Monomers, Materials and Energy from Coffee By-Products: A Review. Sustainability 2021, 13, 6921. [Google Scholar] [CrossRef]
- ICO. Coffee Market Report—June 2021; International Coffee Organization (ICO): London, UK, 2021. [Google Scholar]
- ICO. Annual Review Coffee Year 2021/2022; International Coffee Organization (ICO): London, UK, 2022; Volume 95. [Google Scholar]
- ICO. Coffee Report and Outlook; International Coffee Organization (ICO): London, UK, 2023; Volume 1. [Google Scholar]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-Refinery Approach for Spent Coffee Grounds Valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- Navya, P.N.; Pushpa, S.M. Production, Statistical Optimization and Application of Endoglucanase from Rhizopus Stolonifer Utilizing Coffee Husk. Bioprocess Biosyst. Eng. 2013, 36, 1115–1123. [Google Scholar] [CrossRef]
- Arpi, N.; Muzaifa, M.; Sulaiman, M.I.; Andini, R.; Kesuma, S.I. Chemical Characteristics of Cascara, Coffee Cherry Tea, Made of Various Coffee Pulp Treatments. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012030. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of Spent Coffee Grounds as Food Ingredient in Bakery Products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; del Castillo, M.D. Development of Sustainable Novel Foods and Beverages Based on Coffee By-Products for Chronic Diseases. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 307–315. ISBN 978-0-12-812688-2. [Google Scholar]
- Bessada, S.; Alves, R.C.; Oliveira, M.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From Coffee Industry Waste Materials to Skin-friendly Products with Improved Skin Fat Levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In Vitro Health Promoting Properties of Antioxidant Dietary Fiber Extracted from Spent Coffee (Coffee arabica L.) Grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the Health Benefits of the Bioactive Compounds of Coffee Silverskin Extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Ginting, M.; Mubarokah, S.N.; Sebayang, F.; Karo-karo, J.; Nguyen, T.T.; Ginting, J.; Sitepu, E.K. Direct Biodiesel Production from Wet Spent Coffee Grounds. RSC Adv. 2019, 9, 35109–35116. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Ethanol Production from Coffee Mucilage Fermentation by S. cerevisiae Immobilized in Calcium-Alginate Beads. Bioresour. Technol. Rep. 2018, 3, 200–204. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Quyen, V.T.; Pham, T.-H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent Derived from Coffee Husk for Efficient Removal of Toxic Heavy Metals from Wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef]
- Torres Castillo, N.E.; Ochoa Sierra, J.S.; Oyervides-Muñoz, M.A.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldívar, R.; Melchor-Martínez, E.M. Exploring the Potential of Coffee Husk as Caffeine Bio-Adsorbent—A Mini-Review. Case Stud. Chem. Environ. Eng. 2021, 3, 100070. [Google Scholar] [CrossRef]
- Huang, L.; Mu, B.; Yi, X.; Li, S.; Wang, Q. Sustainable Use of Coffee Husks For Reinforcing Polyethylene Composites. J. Polym. Environ. 2018, 26, 48–58. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, Q.T. Hybrid Biocomposites Based on Used Coffee Grounds and Epoxy Resin: Mechanical Properties and Fire Resistance. Int. J. Chem. Eng. 2021, 2021, 1919344. [Google Scholar] [CrossRef]
- Dericiler, K.; Kocanali, A.; Buldu-Akturk, M.; Erdem, E.; Saner Okan, B. Upcycling Process of Transforming Waste Coffee into Spherical Graphene by Flash Pyrolysis for Sustainable Supercapacitor Manufacturing with Virgin Graphene Electrodes and Its Comparative Life Cycle Assessment. Biomass Convers. Biorefinery 2022, 14, 1073–1088. [Google Scholar] [CrossRef]
- Hong, K.H. Effects of Tannin Mordanting on Coloring and Functionalities of Wool Fabrics Dyed with Spent Coffee Grounds. Fash. Text. 2018, 5, 33. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Coffee Processing Solid Wastes: Current Uses and Future Perspectives. In Agricultural Wastes; Nova: New York, NY, USA, 2009; pp. 155–190. ISBN 9781607413059. [Google Scholar]
- Massaya, J.; Prates Pereira, A.; Mills-Lamptey, B.; Benjamin, J.; Chuck, C.J. Conceptualization of a Spent Coffee Grounds Biorefinery: A Review of Existing Valorisation Approaches. Food Bioprod. Process. 2019, 118, 149–166. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical Composition and Value-Adding Applications of Coffee Industry by-Products: A Review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional Properties of Coffee and Coffee By-Products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological Potential of Coffee Pulp and Coffee Husk for Bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical Composition and Health Properties of Coffee and Coffee By-Products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 65–96. ISBN 1043-4526. [Google Scholar]
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; da Rocha, S.J.S.S.; Coimbra, J.S. New Sustainable Perspectives for “Coffee Wastewater” and Other by-Products: A Critical Review. Future Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, M.C.; Nuti, M. Valorisation of the Residues of Coffee Agro-Industry: Perspectives and Limitations. Open Waste Manag. J. 2017, 10, 13–22. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-Zapata, A.; Kim, D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies 2018, 11, 786. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of Coffee Cherry Pulp and Its Utilisation for Production of Cascara Beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Serna-Jiménez, J.A.; Martínez, K. Coffee By-Products: Nowadays and Perspectives. In Coffee—Production and Research; Castanheira, D.T., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Mirón-Mérida, V.A.; Barragán-Huerta, B.E.; Gutiérrez-Macías, P. Coffee Waste: A Source of Valuable Technologies for Sustainable Development. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 173–198. ISBN 978-0-12-824044-1. [Google Scholar]
- Hejna, A. Potential Applications of By-Products from the Coffee Industry in Polymer Technology—Current State and Perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- SCA. The Impact of Mucilage Removers on Coffee Sustainability and Quality. Available online: https://sca.coffee/sca-news/25-magazine/issue-6/english/water-saving-demucilagers (accessed on 14 August 2023).
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative Utilization of Coffee Mucilage Using Bacillus Coagulans and Investigation of Down-Stream Processing of Fermentation Broth for Optically Pure l(+)-Lactic Acid Production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable Electrosprayed Pectin Films: An Alternative to Valorize Coffee Mucilage. Waste Biomass Valorization 2021, 12, 2477–2494. [Google Scholar] [CrossRef]
- Pardo, L.M.F.; Castillo, N.V.; Durán, Y.M.V.; Rosero, J.A.J.; Lozano Moreno, J.A. Comprehensive Analysis of Ethanol Production from Coffee Mucilage under Sustainability Indicators. Chem. Eng. Process. Process Intensif. 2022, 182, 109183. [Google Scholar] [CrossRef]
- Cárdenas, E.L.M.; Zapata-Zapata, A.D.; Kim, D. Hydrogen Production from Coffee Mucilage in Dark Fermentation with Organic Wastes. Energies 2018, 12, 71. [Google Scholar] [CrossRef]
- Hernández, M.A.; Rodríguez Susa, M.; Andres, Y. Use of Coffee Mucilage as a New Substrate for Hydrogen Production in Anaerobic Co-Digestion with Swine Manure. Bioresour. Technol. 2014, 168, 112–118. [Google Scholar] [CrossRef] [PubMed]
- KC, Y.; Subba, R.; Shiwakoti, L.D.; Dhungana, P.K.; Bajagain, R.; Chaudhary, D.K.; Pant, B.R.; Bajgai, T.R.; Lamichhane, J.; Timilsina, S.; et al. Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage. Fermentation 2021, 7, 53. [Google Scholar] [CrossRef]
- Sierra-López, L.D.; Hernandez-Tenorio, F.; Marín-Palacio, L.D.; Giraldo-Estrada, C. Coffee Mucilage Clarification: A Promising Raw Material for the Food Industry. Food Humanit. 2023, 1, 689–695. [Google Scholar] [CrossRef]
- Cantele, C.; Tedesco, M.; Ghirardello, D.; Zeppa, G.; Bertolino, M. Coffee Silverskin as a Functional Ingredient in Vegan Biscuits: Physicochemical and Sensory Properties and In Vitro Bioaccessibility of Bioactive Compounds. Foods 2022, 11, 717. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on Utilization and Composition of Coffee Silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar]
- Behrouzian, F.; Amini, A.M.; Alghooneh, A.; Razavi, S.M.A. Characterization of Dietary Fiber from Coffee Silverskin: An Optimization Study Using Response Surface Methodology. Bioact. Carbohydr. Diet. Fibre 2016, 8, 58–64. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Rodríguez-Durán, L.V.; Favela-Torres, E.; Aguilar, C.N.; Saucedo-Castañeda, G. Coffee Pulp as Potential Source of Phenolic Bioactive Compounds. In Handbook of Research on Food Science and Technology: Volume 1: Food Technology and Chemistry; Chavez-Gonzalez, M., Buenrostro-Figueroa, J.J., Aguilar, C.N., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2018; p. 24. ISBN 9780429487859. [Google Scholar]
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of Coffee Pulp Waste for Rapid Recovery of Pectin and Polyphenols for Sustainable Material Recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Bondam, A.F.; Diolinda da Silveira, D.; Pozzada dos Santos, J.; Hoffmann, J.F. Phenolic Compounds from Coffee By-Products: Extraction and Application in the Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive Potential and Chemical Composition of Coffee By-Products: From Pulp to Silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties With Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590597. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing Nutritional Qualities, Biological Activities and Technological Properties of Coffee Byproducts in Functional Food Formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Strieder, M.M.; Velásquez Piñas, J.A.; Ampese, L.C.; Costa, J.M.; Carneiro, T.F.; Rostagno, M.A. Coffee Biorefinery: The Main Trends Associated with Recovering Valuable Compounds from Solid Coffee Residues. J. Clean. Prod. 2023, 415, 137716. [Google Scholar] [CrossRef]
- Myo, H.; Khat-udomkiri, N. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Coffee Pulp Using Propylene Glycol as a Solvent and Their Antioxidant Activities. Ultrason. Sonochem. 2022, 89, 106127. [Google Scholar] [CrossRef]
- dos Santos, É.M.; de Macedo, L.M.; Ataide, J.A.; Delafiori, J.; de Oliveira Guarnieri, J.P.; Rosa, P.C.P.; Ruiz, A.L.T.G.; Lancellotti, M.; Jozala, A.F.; Catharino, R.R.; et al. Antioxidant, Antimicrobial and Healing Properties of an Extract from Coffee Pulp for the Development of a Phytocosmetic. Sci. Rep. 2024, 14, 4453. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and Hydroxycinnamate Contents of Whole Coffee Fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef]
- das Neves, J.V.G.; Borges, M.V.; Silva, D.d.M.; Leite, C.X.d.S.; Santos, M.R.C.; de Lima, N.G.B.; Lannes, S.C.d.S.; da Silva, M.V. Total Phenolic Content and Primary Antioxidant Capacity of Aqueous Extracts of Coffee Husk: Chemical Evaluation and Beverage Development. Food Sci. Technol. 2019, 39, 348–353. [Google Scholar] [CrossRef]
- Silva, M.d.O.; Honfoga, J.N.B.; de Medeiros, L.L.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a by-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Gil-Ramírez, A.; Benítez, V.; Cañas, S.; Braojos, C.; Martin-Cabrejas, M.A. Biorefinery and Stepwise Strategies for Valorizing Coffee By-Products as Bioactive Food Ingredients and Nutraceuticals. Appl. Sci. 2023, 13, 8326. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Antónia Nunes, M.; Vinha, A.F.; Oliveira, M.B.P.P. State of the Art in Coffee Processing By-Products. In Handbook of Coffee Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–26. ISBN 978-0-12-811290-8. [Google Scholar]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic Composition, Caffeine Content and Antioxidant Capacity of Coffee Silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Castaldo, L.; Graziani, G.; Gaspari, A.; Izzo, L.; Luz, C.; Mañes, J.; Rubino, M.; Meca, G.; Ritieni, A. Study of the Chemical Components, Bioactivity and Antifungal Properties of the Coffee Husk. J. Food Res. 2018, 7, 43. [Google Scholar] [CrossRef]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of Coffee Parchment Waste (Coffea arabica) as a Source of Caffeine and Phenolic Compounds in Antifungal Gellan Gum Films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response Surface Methodology to Optimise the Heat-Assisted Aqueous Extraction of Phenolic Compounds from Coffee Parchment and Their Comprehensive Analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee Silverskin Extracts: Quantification of 30 Bioactive Compounds by a New HPLC-MS/MS Method and Evaluation of Their Antioxidant and Antibacterial Activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee Silver Skin as a Source of Polyphenols: High Resolution Mass Spectrometric Profiling of Components and Antioxidant Activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Hussein, H.; Abouamer, W.; Ali, H.; Elkhadragy, M.; Yehia, H.; Farouk, A. The Valorization of Spent Coffee Ground Extract as a Prospective Insecticidal Agent against Some Main Key Pests of Phaseolus Vulgaris in the Laboratory and Field. Plants 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent Espresso Coffee Grounds as a Source of Anti-Proliferative and Antioxidant Compounds. Innov. Food Sci. Emerg. Technol. 2020, 59, 102254. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, Identification and Quantification of Polyphenols from Spent Coffee Grounds by Chromatographic Methods and Chemometric Analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental Impacts of Biodiesel Production from Waste Spent Coffee Grounds and Its Implementation in a Compression Ignition Engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent Coffee Grounds By-Products and Their Influence on Soil C–N Dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Review: Utilization of Waste From Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Oil Extracted from Spent Coffee Grounds for Bio-Hydrotreated Diesel Production. Energy Convers. Manag. 2016, 126, 1028–1036. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical Fluid Extraction of Spent Coffee Grounds: Measurement of Extraction Curves, Oil Characterization and Economic Analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Bijla, L.; Aissa, R.; Laknifli, A.; Bouyahya, A.; Harhar, H.; Gharby, S. Spent Coffee Grounds: A Sustainable Approach toward Novel Perspectives of Valorization. J. Food Biochem. 2022, 46, e14190. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field Evaluation of Coffee Grounds Application for Crop Growth Enhancement, Weed Control, and Soil Improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Domínguez, J. Vermicompost Derived from Spent Coffee Grounds: Assessing the Potential for Enzymatic Bioremediation. In Handbook of Coffee Processing By-Products; Academic Press: Cambridge, MA, USA, 2017; pp. 369–398. ISBN 9780128112915. [Google Scholar]
- Hardgrove, S.J.; Livesley, S.J. Applying Spent Coffee Grounds Directly to Urban Agriculture Soils Greatly Reduces Plant Growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Yadav, S.K. Retardation in Seedling Growth and Induction of Early Senescence in Plants upon Caffeine Exposure Is Related to Its Negative Effect on Rubisco. Photosynthetica 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Cruz, S.; Marques dos Santos Cordovil, C.S.C. Espresso Coffee Residues as a Nitrogen Amendment for Small-Scale Vegetable Production. J. Sci. Food Agric. 2015, 95, 3059–3066. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Vicente, E.D.; Gomes, A.P.; Nunes, M.I.; Alves, C.; Tarelho, L.A.C. Effect of Industrial and Domestic Ash from Biomass Combustion, and Spent Coffee Grounds, on Soil Fertility and Plant Growth: Experiments at Field Conditions. Environ. Sci. Pollut. Res. 2017, 24, 15270–15277. [Google Scholar] [CrossRef]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of Vegetables Elemental Quality by Espresso Coffee Residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of Spent Coffee Grounds as Organic Amendment on Soil Fertility and Lettuce Growth in Two Mediterranean Agricultural Soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of Different Rates of Spent Coffee Grounds (SCG) on Composting Process, Gaseous Emissions and Quality of End-Product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.Á.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and Chelating Capacity of Spent Coffee Grounds: Two Contrasting Faces in Its Use as Soil Organic Amendment. Sci. Total Environ. 2020, 717, 137247. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting Parameters and Compost Quality: A Literature Review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Cooperband, L.R. Composting: Art and Science of Organic Waste Conversion to a Valuable Soil Resource. Lab. Med. 2000, 31, 283–290. [Google Scholar] [CrossRef]
- Takala, B. Utilization of Coffee Husk and Pulp Waste as Soil Amendment. A Review. J. Nat. Sci. Res. 2021, 12, 10–16. [Google Scholar] [CrossRef]
- Insam, H.; de Bertoldi, M. Chapter 3 Microbiology of the Composting Process. Waste Manag. Ser. 2007, 8, 25–48. [Google Scholar] [CrossRef]
- Hoseini, M.; Cocco, S.; Casucci, C.; Cardelli, V.; Corti, G. Coffee By-Products Derived Resources. A Review. Biomass Bioenergy 2021, 148, 106009. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankagote, P.; Baert, G.; Van Ranst, E. Coffee Waste as an Alternative Fertilizer with Soil Improving Properties for Sandy Soils in Humid Tropical Environments. Soil Use Manag. 2011, 27, 94–102. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Han, J.-C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminum Phytotoxicity in Acidic Environments: A Comprehensive Review of Plant Tolerance and Adaptation Strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. Chapter 31—An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 283–291. ISBN 978-0-12-409517-5. [Google Scholar]
- Nguyen, A.D.; Tran, T.D.; Vo, T.P.K. Evaluation of Coffee Husk Compost for Improving Soil Fertility and Sustainable Coffee Production in Rural Central Highland of Vietnam. Resour. Environ. 2013, 3, 77–82. [Google Scholar]
- Dadi, D.; Daba, G.; Beyene, A.; Luis, P.; Van der Bruggen, B. Composting and Co-Composting of Coffee Husk and Pulp with Source-Separated Municipal Solid Waste: A Breakthrough in Valorization of Coffee Waste. Int. J. Recycl. Org. Waste Agric. 2019, 8, 263–277. [Google Scholar] [CrossRef]
- Ulsido, M.D.; Li, M. Effect of Organic Matter from Coffee Pulp Compost on Yield Response of Chickpeas (Cicer arietinum L.) in Ethiopia. In Proceedings of the 15th Internal Scientific Conference “Engineering for Rural Development”, Jelgava, Latvia, 25–27 May 2016; Malinovska, L., Osadcuks, V., Eds.; Latvia University of Agriculture: Jelgava, Latvia, 2016; pp. 1339–1347. [Google Scholar]
- Jibril, T.; Bekele, G. Effect of Coffee Husk Compost and NPSB Fertilizers on Selected Soil Chemical Properties of Potato Field in Chora District, South West Ethiopia. Appl. Environ. Soil Sci. 2022, 2022, 7397872. [Google Scholar] [CrossRef]
- Nduka, B.A.; Adewale, D.B.; Akanbi, O.S.O.; Adejobi, K.B. Nursery Soil Amendments for Cashew Seedling Production: A Comparative Analysis of Coffee Husk and NPK. J. Agric. Sci. 2015, 7, 111. [Google Scholar] [CrossRef]
- Islam, M.M.; Akhter, S.; Majid, N.M.; Ferdous, J.; Alam, M.S. Integrated Nutrient Management for Potato (Solanum Tuberosum) in Grey Terrace Soil (Aric Albaquipt). Aust. J. Crop Sci. 2013, 7, 1235–1241. [Google Scholar]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of Spent Coffee Residues by a Direct Agronomic Approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Vela-Cano, M.; Cervera-Mata, A.; Purswani, J.; Pozo, C.; Delgado, G.; González-López, J. Bacterial Community Structure of Two Mediterranean Agricultural Soils Amended with Spent Coffee Grounds. Appl. Soil Ecol. 2019, 137, 12–20. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Martín-García, J.M.; Delgado, R.; Párraga, J.; Sánchez-Marañón, M.; Delgado, G. Short-Term Effects of Spent Coffee Grounds on the Physical Properties of Two Mediterranean Agricultural Soils. Int. Agrophysics 2019, 33, 205–216. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Aranda, V.; Ontiveros-Ortega, A.; Comino, F.; Martín-García, J.M.; Vela-Cano, M.; Delgado, G. Hydrophobicity and Surface Free Energy to Assess Spent Coffee Grounds as Soil Amendment. Relationships with Soil Quality. Catena 2021, 196, 104826. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Molinero-García, A.; Martín-García, J.M.; Delgado, G. Sequential Effects of Spent Coffee Grounds on Soil Physical Properties. Soil Use Manag. 2023, 39, 286–297. [Google Scholar] [CrossRef]
- Turek, M.E.; Freitas, K.S.; Armindo, R.A. Spent Coffee Grounds as Organic Amendment Modify Hydraulic Properties in a Sandy Loam Brazilian Soil. Agric. Water Manag. 2019, 222, 313–321. [Google Scholar] [CrossRef]
- Emmanuel, S.A.; Yoo, J.; Kim, E.J.; Chang, J.S.; Park, Y.I.; Koh, S.C. Development of Functional Composts Using Spent Coffee Grounds, Poultry Manure and Biochar through Microbial Bioaugmentation. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 802–811. [Google Scholar] [CrossRef]
- Picca, G.; Plaza, C.; Madejón, E.; Panettieri, M. Compositing of Coffee Silverskin with Carbon Rich Materials Leads to High Quality Soil Amendments. Waste Biomass Valorization 2023, 14, 297–307. [Google Scholar] [CrossRef]
- Prasad, M. Review of the Use of Peat Moss in Horticulture: Final Report of the Chairman of the Working Group; Department of Housing, Local Government and Heritage: Dublin, Ireland, 2021.
- International Peatland Society (IPS). Peat. Available online: https://peatlands.org/peat/ (accessed on 8 July 2024).
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of Municipal Solid Waste Compost (MSWC) as a Growing Medium in the Nursery Production of Tomato Plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Picca, G.; Goñi-Urtiaga, A.; Gomez-Ruano, C.; Plaza, C.; Panettieri, M. Suitability of Co-Composted Biochar with Spent Coffee Grounds Substrate for Tomato (Solanum lycopersicum) Fruiting Stage. Horticulturae 2023, 9, 89. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical Conductivity of Nutrient Solution Influenced Photosynthesis, Quality, and Antioxidant Enzyme Activity of Pakchoi (Brassica campestris L. ssp. Chinensis) in a Hydroponic System. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Fornes, F.; Mendoza-Hernández, D.; García-de-la-Fuente, R.; Abad, M.; Belda, R.M. Composting versus Vermicomposting: A Comparative Study of Organic Matter Evolution through Straight and Combined Processes. Bioresour. Technol. 2012, 118, 296–305. [Google Scholar] [CrossRef]
- Musyoka, S.N.; Liti, D.M.; Ogello, E.O.; Meulenbroek, P.; Waidbacher, H. Using Earthworm, Eisenia Fetida, to Bio-Convert Agro-Industrial Wastes for Aquaculture Nutrition. BioResources 2020, 15, 574–587. [Google Scholar] [CrossRef]
- Martinkosky, L.; Barkley, J.; Sabadell, G.; Gough, H.; Davidson, S. Earthworms (Eisenia fetida) Demonstrate Potential for Use in Soil Bioremediation by Increasing the Degradation Rates of Heavy Crude Oil Hydrocarbons. Sci. Total Environ. 2017, 580, 734–743. [Google Scholar] [CrossRef]
- Vyas, P.; Sharma, S.; Gupta, J. Vermicomposting with Microbial Amendment: Implications for Bioremediation of Industrial and Agricultural Waste. BioTechnologia 2022, 103, 203–215. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling Coffee and Tea Wastes to Increase Plant Available Fe in Alkaline Soils. Plant Soil 2008, 304, 249–255. [Google Scholar] [CrossRef]
- González-Moreno, M.A.; García Gracianteparaluceta, B.; Marcelino Sádaba, S.; Zaratiegui Urdin, J.; Robles Domínguez, E.; Pérez Ezcurdia, M.A.; Seco Meneses, A. Feasibility of Vermicomposting of Spent Coffee Grounds and Silverskin from Coffee Industries: A Laboratory Study. Agronomy 2020, 10, 1125. [Google Scholar] [CrossRef]
- Hanc, A.; Hrebeckova, T.; Grasserova, A.; Cajthaml, T. Conversion of Spent Coffee Grounds into Vermicompost. Bioresour. Technol. 2021, 341, 125925. [Google Scholar] [CrossRef]
- Zergaw, Y.; Kebede, T.; Berhe, D.T. Direct Application of Coffee Pulp Vermicompost Produced from Epigeic Earthworms and Its Residual Effect on Vegetative and Reproductive Growth of Hot Pepper (Capsicum annuum L.). Sci. World J. 2023, 2023, 7366925. [Google Scholar] [CrossRef]
- Raphael, K.; Velmourougane, K. Chemical and Microbiological Changes during Vermicomposting of Coffee Pulp Using Exotic (Eudrilus eugeniae) and Native Earthworm (Perionyx ceylanesis) Species. Biodegradation 2011, 22, 497–507. [Google Scholar] [CrossRef]
- Massaya, J.; Mills-Lamptey, B.; Chuck, C.J. Soil Amendments and Biostimulants from the Hydrothermal Processing of Spent Coffee Grounds. Waste Biomass Valorization 2022, 13, 2889–2904. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Nava-Bravo, I.; Escamilla-Alvarado, C.; Cano-Gómez, J.J.; Valencia-Vázquez, R.; Galván-Arzola, U.; Cuevas-García, R. Bio-Crude and Biochar Production and Properties from Corn Stover at Low Energy-Intensive Hydrothermal Liquefaction. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Rehrah, D.; Reddy, M.R.; Novak, J.M.; Bansode, R.R.; Schimmel, K.A.; Yu, J.; Watts, D.W.; Ahmedna, M. Production and Characterization of Biochars from Agricultural By-Products for Use in Soil Quality Enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Dalias, P.; Polycarpou, P.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Physicochemical and Structural Characterization of Biochar Derived from the Pyrolysis of Biosolids, Cattle Manure and Spent Coffee Grounds. J. Energy Inst. 2020, 93, 2063–2073. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Lustosa Filho, J.F.; Melo, L.C.A.; de Assis, I.R.; de Oliveira, T.S. Influence of Pyrolysis Temperature and Feedstock on the Properties of Biochars Produced from Agricultural and Industrial Wastes. J. Anal. Appl. Pyrolysis 2020, 149, 104839. [Google Scholar] [CrossRef]
- Rodriguez Ortiz, L.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of Pyrolysis Temperature and Bio-Waste Composition on Biochar Characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis Temperature Affects Pore Characteristics of Rice Straw and Canola Stalk Biochars and Biochar-Amended Soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The Effect of Pyrolysis Temperature and Feedstock on Biochar Agronomic Properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, M. Quality Variations of Poultry Litter Biochar Generated at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of Fast Pyrolysis Temperature on Biochar Labile Fraction and Short-Term Carbon Loss in a Loamy Soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan Publishers Ltd.: London, UK, 2009; pp. 1–9. [Google Scholar]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Pouangam Ngalani, G.; Ondo, J.A.; Njimou, J.R.; Nanseu Njiki, C.P.; Prudent, P.; Ngameni, E. Effect of Coffee Husk and Cocoa Pods Biochar on Phosphorus Fixation and Release Processes in Acid Soils from West Cameroon. Soil Use Manag. 2023, 39, 817–832. [Google Scholar] [CrossRef]
- Pouangam Ngalani, G.; Dzemze Kagho, F.; Peguy, N.N.C.; Prudent, P.; Ondo, J.A.; Ngameni, E. Effects of Coffee Husk and Cocoa Pods Biochar on the Chemical Properties of an Acid Soil from West Cameroon. Arch. Agron. Soil Sci. 2023, 69, 744–758. [Google Scholar] [CrossRef]
- Lehmann, J.; Da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vo, T.D.H.; Tran, T.; Nguyen, T.N.; Le, T.N.C.; Bui, X.T.; Bach, L.G. Biochar Derived from the Spent Coffee Ground for Ammonium Adsorption from Aqueous Solution. Case Stud. Chem. Environ. Eng. 2021, 4, 100141. [Google Scholar] [CrossRef]
- Tangmankongworakoon, N. An Approach to Produce Biochar from Coffee Residue for Fuel and Soil Amendment Purpose. Int. J. Recycl. Org. Waste Agric. 2019, 8, 37–44. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of Biochar Amendment on Soil Carbon Balance and Soil Microbial Activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Dawerasha, S.S.; Nebiyu, A.; Ahmed, M.; Haile, B. Effect of Coffee Husk Biochar and Inorganic NP Fertilizer on Soil Properties, Growth and Yield of Potato (Solanum tuberosum L.) on Acidic Soil of Southwest Ethiopia. CABI Agric. Biosci. 2024, 5, 56. [Google Scholar] [CrossRef]
- Gebre, T.; Singh, S.; Zewide, I. Potato Yield Enhancement by Combined Use of NPS Blended Fertilizer and Coffee Husk Biochar and Its Economic Analysis. Trop. Agric. 2020, 97, 240–252. [Google Scholar]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The Effect of Rice Husk Biochar on Soil Nutrient Status, Microbial Biomass and Paddy Productivity of Nutrient Poor Agriculture Soils. CATENA 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil—Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Lima, J.R.d.S.; de Moraes Silva, W.; de Medeiros, E.V.; Duda, G.P.; Corrêa, M.M.; Martins Filho, A.P.; Clermont-Dauphin, C.; Antonino, A.C.D.; Hammecker, C. Effect of Biochar on Physicochemical Properties of a Sandy Soil and Maize Growth in a Greenhouse Experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Filho, A.P.M.; de Medeiros, E.V.; Lima, J.R.S.; da Costa, D.P.; Duda, G.P.; da Silva, J.S.A.; de Oliveira, J.B.; Antonino, A.C.D.; Menezes, R.S.C.; Hammecker, C. Impact of Coffee Biochar on Carbon, Microbial Biomass and Enzyme Activities of a Sandy Soil Cultivated with Bean. An. Acad. Bras. Cienc. 2021, 93, e20200096. [Google Scholar] [CrossRef]
- Asfaw, E.; Nebiyu, A.; Bekele, E.; Ahmed, M.; Astatkie, T. Coffee-Husk Biochar Application Increased AMF Root Colonization, P Accumulation, N2 Fixation, and Yield of Soybean Grown in a Tropical Nitisol, Southwest Ethiopia. J. Plant Nutr. Soil Sci. 2019, 182, 419–428. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Hu, Y.; Abbey, L.; Cesarino, I.; Goonetilleke, A.; He, Q. Exploring the Properties and Potential Uses of Biocarbon from Spent Coffee Grounds: A Comparative Look at Dry and Wet Processing Methods. Processes 2023, 11, 2099. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and Sulfonamide Antibiotics Adsorption Capacity of Spent Coffee Grounds Based Biochar and Hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Mata, A.; Lara, L.; Fernández-Arteaga, A.; Ángel Rufián-Henares, J.; Delgado, G. Washed Hydrochar from Spent Coffee Grounds: A Second Generation of Coffee Residues. Evaluation as Organic Amendment. Waste Manag. 2021, 120, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Hitzl, M.; Mendez, A.; Owsianiak, M.; Renz, M. Making Hydrochar Suitable for Agricultural Soil: A Thermal Treatment to Remove Organic Phytotoxic Compounds. J. Environ. Chem. Eng. 2018, 6, 7029–7034. [Google Scholar] [CrossRef]
- Jeníček, L.; Tunklová, B.; Malaťák, J.; Neškudla, M.; Velebil, J. Use of Spent Coffee Ground as an Alternative Fuel and Possible Soil Amendment. Materials 2022, 15, 6722. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar Soil Amendment on Alleviation of Drought and Salt Stress in Plants: A Critical Review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Kamali, M.; Jahaninafard, D.; Mostafaie, A.; Davarazar, M.; Gomes, A.P.D.; Tarelho, L.A.C.; Dewil, R.; Aminabhavi, T.M. Scientometric Analysis and Scientific Trends on Biochar Application as Soil Amendment. Chem. Eng. J. 2020, 395, 125128. [Google Scholar] [CrossRef]
- Carnier, R.; Coscione, A.R.; Delaqua, D.; Puga, A.P.; de Abreu, C.A. Jack Bean Development in Multimetal Contaminated Soil Amended with Coffee Waste-Derived Biochars. Processes 2022, 10, 2157. [Google Scholar] [CrossRef]
- Al Masud, M.A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A Critical Review of Sustainable Application of Biochar for Green Remediation: Research Uncertainty and Future Directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Mofatteh, S. Biochar for a Sustainable Future: Environmentally Friendly Production and Diverse Applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Chaurra, A.M.; Molina Bastidas, J.C.; Infante Santos, C.; Wilches Rodríguez, J.C. Valorization of Coffee Pulp in the Production of Pleurotus Pulmonarius in Rural Communities of Colombia. ACS Food Sci. Technol. 2023, 3, 1314–1322. [Google Scholar] [CrossRef]
- Leifa, F.; Soccol, C.R.; Pandey, A. Production of Mushrooms on Brazilian Coffee Industry Residues. In Coffee Biotechnology and Quality; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 427–436. ISBN 978-94-017-1068-8. [Google Scholar]
- Martínez-Carrera, D.; Aguilar, A.; Martínez, W.; Bonilla, M.; Morales, P.; Sobal, M. Commercial Production and Marketing of Edible Mushrooms Cultivated on Coffee Pulp in Mexico. In Coffee Biotechnology and Quality; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 471–488. ISBN 978-94-017-1068-8. [Google Scholar]
- Lopez, J.C.C.; Thepanondh, S.; Sachdev, H.; Avelar, A.M.P.; Leon, M.C.D.C. Sustainability and Economic Feasibility through the Production of Oyster Mushroom (Pleurotus ostreatus (Jacq.) p. Kumm.) Derived from the Waste of Coffee-Industry: A Case Study in the Western Area of San Salvador, El Salvador. Pol. J. Environ. Stud. 2021, 30, 5617–5628. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Assemie, A.; Abaya, G. The Effect of Edible Mushroom on Health and Their Biochemistry. Int. J. Microbiol. 2022, 2022, 8744788. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Fourtaka, K.; Melanouri, E.M.; Dedousi, M.; Diamantis, I.; Gardeli, C.; Papanikolaou, S. Examining the Impact of Substrate Composition on the Biochemical Properties and Antioxidant Activity of Pleurotus and Agaricus Mushrooms. Fermentation 2023, 9, 689. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C. In Vitro and in Vivo Antioxidant Activity of Ethanolic Extract of White Button Mushroom (Agaricus Bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sasidharan, S.P.; Yang, X. A Concise Review of Mushrooms Antiviral and Immunomodulatory Properties That May Combat against COVID-19. Food Chem. Adv. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Nozaki, H.; Itonori, S.; Sugita, M.; Nakamura, K.; Ohba, K.; Suzuki, A.; Kushi, Y. Mushroom Acidic Glycosphingolipid Induction of Cytokine Secretion from Murine T Cells and Proliferation of NK1.1 α/β TCR-Double Positive Cells in Vitro. Biochem. Biophys. Res. Commun. 2008, 373, 435–439. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Xie, X.; Holman, C.D.J. Dietary Intakes of Mushrooms and Green Tea Combine to Reduce the Risk of Breast Cancer in Chinese Women. Int. J. Cancer 2009, 124, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Kim, J.-S.; Kim, H.-J.; Choi, M.S.; Park, B.R.; Kim, S.-G.; Ahn, H.; Chun, H.S.; Shin, Y.K.; Kim, J.-J.; et al. Purification and Characterization of a Novel Fibrinolytic α Chymotrypsin like Serine Metalloprotease from the Edible Mushroom, Lyophyllum Shimeji. J. Biosci. Bioeng. 2014, 117, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.; Simon, J.; Sliva, D. Anti-Inflammatory Activity of Edible Oyster Mushroom Is Mediated through the Inhibition of NF-ΚB and AP-1 Signaling. Nutr. J. 2011, 10, 52. [Google Scholar] [CrossRef]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of Edible Mushroom as a Potent Therapeutics for the Diabetes and Obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Morales, D.; Tabernero, M.; Largo, C.; Polo, G.; Piris, A.J.; Soler-Rivas, C. Effect of Traditional and Modern Culinary Processing, Bioaccessibility, Biosafety and Bioavailability of Eritadenine, a Hypocholesterolemic Compound from Edible Mushrooms. Food Funct. 2018, 9, 6360–6368. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, Z.; Meydani, S.N.; Wu, D. White Button Mushroom Enhances Maturation of Bone Marrow-Derived Dendritic Cells and Their Antigen Presenting Function in Mice. J. Nutr. 2008, 138, 544–550. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Elkanah, F.A.; Oke, M.A.; Adebayo, E.A. Substrate Composition Effect on the Nutritional Quality of Pleurotus Ostreatus (MK751847) Fruiting Body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.-L.; Wang, C.-H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Atila, F. Comparative Study on the Mycelial Growth and Yield of Ganoderma Lucidum (Curt.: Fr.) Karst. on Different Lignocellulosic Wastes. Acta Ecol. Sin. 2020, 40, 153–157. [Google Scholar] [CrossRef]
- Muswati, C.; Simango, K.; Tapfumaneyi, L.; Mutetwa, M.; Ngezimana, W. The Effects of Different Substrate Combinations on Growth and Yield of Oyster Mushroom (Pleurotus ostreatus). Int. J. Agron. 2021, 2021, 9962285. [Google Scholar] [CrossRef]
- Dissasa, G. Cultivation of Different Oyster Mushroom (Pleurotus Species) on Coffee Waste and Determination of Their Relative Biological Efficiency and Pectinase Enzyme Production, Ethiopia. Int. J. Microbiol. 2022, 2022, 5219939. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Antunes, M.B.; Rodrigues, D.; Sousa, S.; Amorim, M.; Barroso, M.F.; Carvalho, A.; Ferrador, S.M.; Gomes, A.M. Use of Coffee By-products for the Cultivation of Pleurotus Citrinopileatus and Pleurotus Salmoneo-stramineus and Its Impact on Biological Properties of Extracts Thereof. Int. J. Food Sci. Technol. 2018, 53, 1914–1924. [Google Scholar] [CrossRef]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C.R. Use of Various Coffee Industry Residues for the Cultivation of Pleurotus Ostreatus in Solid State Fermentation. Acta Biotechnol. 2000, 20, 41–52. [Google Scholar] [CrossRef]
- Calzada, J.F.; de Leon, R.; de Arriola, M.C.; Rolz, C. Growth of Mushrooms on Wheat Straw and Coffee Pulp: Strain Selection. Biol. Wastes 1987, 20, 217–226. [Google Scholar] [CrossRef]
- Velázquez-Cedeño, M.A.; Mata, G.; Savoie, J.M. Waste-Reducing Cultivation of Pleurotus Ostreatus and Pleurotus Pulmonarius on Coffee Pulp: Changes in the Production of Some Lignocellulolytic Enzymes. World J. Microbiol. Biotechnol. 2002, 18, 201–207. [Google Scholar] [CrossRef]
- Yoshimura, H.; Washio, H.; Yoshida, S.; Seino, T.; Otaka, M.; Matsubara, K.; Matsubara, M. Promoting Effect of Wood Vinegar Compounds on Fruit-Body Formation of Pleurotus Ostreatus. Mycoscience 1995, 36, 173–177. [Google Scholar] [CrossRef]
- Fan, L.; Soccol, A.T.; Pandey, A.; Vandenberghe, L.P.D.S.; Soccol, C.R. Effect of Caffeine and Tannins on Cultivation and Fructification of Pleurotus on Coffee Husks. Braz. J. Microbiol. 2006, 37, 420–424. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Jasińska, A.; Budzyńska, S.; Rzymski, P.; Kalač, P.; Niedzielski, P.; Pankiewicz, J.; Mleczek, M. Effect of Thymus Vulgaris Post-Extraction Waste and Spent Coffee Grounds on the Quality of Cultivated Pleurotus Eryngii. J. Food Process. Preserv. 2020, 44, e14648. [Google Scholar] [CrossRef]
- Mata, G.; Salmones, D.; Pérez-Merlo, R. Hydrolytic Enzyme Activities in Shiitake Mushroom (Lentinula edodes) Strains Cultivated on Coffee Pulp. Rev. Argent. Microbiol. 2016, 48, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine Metabolism during Cultivation of Oyster Mushroom (Pleurotus ostreatus) with Spent Coffee Grounds. Appl. Microbiol. Biotechnol. 2019, 103, 5831–5841. [Google Scholar] [CrossRef] [PubMed]
- Alsanad, M.A.; Sassine, Y.N.; El Sebaaly, Z.; Abou Fayssal, S. Spent Coffee Grounds Influence on Pleurotus Ostreatus Production, Composition, Fatty Acid Profile, and Lignocellulose Biodegradation Capacity. CyTA J. Food 2021, 19, 11–20. [Google Scholar] [CrossRef]
- Grogan, R.A. Agricultural Pesticides: Usage Trends and Analysis of Data Sources; Nova: New York, NY, USA, 2011; ISBN 9781611225310. [Google Scholar]
- Chitara, M.K.; Singh, R.P.; Gupta, P.K.; Mishra, D.; Jatav, S.S.; Sharma, S.; Jatav, H.S. The Risk Associated with Crop Ecosystem Management and Pesticides Pollution. In Ecosystem Services: Types, Management and Benefits; Nova: New York, NY, USA, 2022; pp. 151–164. ISBN 9781685077471. [Google Scholar]
- Rao, M.S. Innovations, Commercialization and Registration of Biopesticides. In Biopesticides in Horticultural Crops; CRC Press: London, UK, 2021; pp. 1–11. ISBN 9781000486803. [Google Scholar]
- Swapan, C.; Mainak, B.; Deewa, B.; Tanmoy, M. Natural Pesticides for Pest Control in Agricultural Crops: An Alternative and Eco-Friendly Method. Plant Sci. Today 2023, 11, 433–450. [Google Scholar] [CrossRef]
- Usha Rani, P.; Pratyusha, S. Defensive Role of Gossypium Hirsutum L. Anti-Oxidative Enzymes and Phenolic Acids in Response to Spodoptera Litura F. Feeding. J. Asia. Pac. Entomol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Bedmutha, R.; Booker, C.J.; Ferrante, L.; Briens, C.; Berruti, F.; Yeung, K.K.C.; Scott, I.; Conn, K. Insecticidal and Bactericidal Characteristics of the Bio-Oil from the Fast Pyrolysis of Coffee Grounds. J. Anal. Appl. Pyrolysis 2011, 90, 224–231. [Google Scholar] [CrossRef]
- Breedlove, B. Deadly, Dangerous, and Decorative Creatures. Emerg. Infect. Dis. 2022, 28, 495–496. [Google Scholar] [CrossRef]
- Poopathi, S.; Mani, C. Use of Coffee Husk Waste for Production of Biopesticides for Mosquito Control. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: San Diego, CA, USA, 2015; pp. 293–300. ISBN 9780124167162. [Google Scholar]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Martínez, S.S.; Sánchez, J.V. Herbicides: Applications, Degradation, and Environmental Impact. In Herbicides: Properties, Crop Protection and Environmental Hazards; Nova: New York, NY, USA, 2011; pp. 67–120. [Google Scholar]
- Gaur, N.; Diwan, B.; Choudhary, R. Bioremediation of Organic Pesticides Using Nanomaterials. In Nano-Bioremediation: Fundamentals and Applications; Iqbal, H.M.N., Bilal, M., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 517–540. [Google Scholar]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva, L.F.S.; Corrêa, A.P.F.; Bach, E.; Brandelli, A. Total Polyphenols, Antioxidant, Antimicrobial and Allelopathic Activities of Spend Coffee Ground Aqueous Extract. Waste Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Huang, J.; Li, B.; Xian, X.; Hu, Y.; Lin, X. Efficient Bioethanol Production from Spent Coffee Grounds Using Liquid Hot Water Pretreatment without Detoxification. Fermentation 2024, 10, 436. [Google Scholar] [CrossRef]
- Choi, I.S.; Wi, S.G.; Kim, S.-B.; Bae, H.-J. Conversion of Coffee Residue Waste into Bioethanol with Using Popping Pretreatment. Bioresour. Technol. 2012, 125, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Biological Pretreatment of Lignocellulosic Biomass—Current Trends and Future Perspectives. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. [Google Scholar]
- Nguyen, Q.A.; Cho, E.; Trinh, L.T.P.; Jeong, J.; Bae, H.-J. Development of an Integrated Process to Produce D-Mannose and Bioethanol from Coffee Residue Waste. Bioresour. Technol. 2017, 244, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-F.; Yang, S.; Sun, R.-C. Recent Advances in Alcohol and Organic Acid Fractionation of Lignocellulosic Biomass. Bioresour. Technol. 2016, 200, 971–980. [Google Scholar] [CrossRef]
- Chiyanzy, I.; Brienzo, M.; García-Aparicio, M.; Agudelo, R.; Görgens, J. Spent Coffee Ground Mass Solubilisation by Steam Explosion and Enzymatic Hydrolysis. J. Chem. Technol. Biotechnol. 2015, 90, 449–458. [Google Scholar] [CrossRef]
- Dadi, D.; Beyene, A.; Simoens, K.; Soares, J.; Demeke, M.M.; Thevelein, J.M.; Bernaerts, K.; Luis, P.; Van der Bruggen, B. Valorization of Coffee Byproducts for Bioethanol Production Using Lignocellulosic Yeast Fermentation and Pervaporation. Int. J. Environ. Sci. Technol. 2018, 15, 821–832. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Christakopoulos, P. Hydrolysis and Fermentation for Cellulosic Ethanol Production. In Advances in Bioenergy; Lund, P.D., Byrne, J., Berndes, G., Vasalos, I.A., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 11–31. ISBN 9781118957844. [Google Scholar]
- Meng, Q.-S.; Liu, C.-G.; Zhao, X.-Q.; Bai, F.-W. Engineering Trichoderma Reesei Rut-C30 with the Overexpression of Egl1 at the Ace1 Locus to Relieve Repression on Cellulase Production and to Adjust the Ratio of Cellulolytic Enzymes for More Efficient Hydrolysis of Lignocellulosic Biomass. J. Biotechnol. 2018, 285, 56–63. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P.W. Applications of Fungal Cellulases in Biofuel Production: Advances and Limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Selvam, K.; Govarthanan, M.; Kamala-Kannan, S.; Govindharaju, M.; Senthilkumar, B.; Selvankumar, T.; Sengottaiyan, A. Process Optimization of Cellulase Production from Alkali-Treated Coffee Pulp and Pineapple Waste Using Acinetobacter Sp. TSK-MASC. RSC Adv. 2014, 4, 13045–13051. [Google Scholar] [CrossRef]
- Catalán, E.; Komilis, D.; Sánchez, A. Environmental Impact of Cellulase Production from Coffee Husks by Solid-State Fermentation: A Life-Cycle Assessment. J. Clean. Prod. 2019, 233, 954–962. [Google Scholar] [CrossRef]
- Marín, M.; Artola, A.; Sánchez, A. Optimization of Down-Stream for Cellulases Produced Under Solid-State Fermentation of Coffee Husk. Waste Biomass Valorization 2019, 10, 2761–2772. [Google Scholar] [CrossRef]
- Morales-Martínez, J.L.; Aguilar-Uscanga, M.G.; Bolaños-Reynoso, E.; López-Zamora, L. Optimization of Chemical Pretreatments Using Response Surface Methodology for Second-Generation Ethanol Production from Coffee Husk Waste. BioEnergy Res. 2021, 14, 815–827. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for Detoxification of Diluted-Acid Lignocellulosic Hydrolyzates for Use in Fermentative Processes: A Review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of Ethanol Production from Coffee Husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Madson, P.W.; Lococo, D.B. Recovery of Volatile Products from Dilute High-Fouling Process Streams. Appl. Biochem. Biotechnol. 2000, 84–86, 1049–1062. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; de Matos, L.J.B.L.; de Lima, L.P.; Figueiredo, P.M.d.S.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-Assisted Production of Biodiesel and Ethanol from Spent Coffee Grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Rasul, M.G.; Hassan, N.M.S.; Mofijur, M. Waste Coffee Oil: A Promising Source for Biodiesel Production. Energy Procedia 2019, 160, 677–682. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of Coffee Oil Extraction from Spent Coffee Grounds Using Four Solvents and Prototype-Scale Extraction Using Circulation Process. Agric. Nat. Resour. 2017, 51, 181–189. [Google Scholar] [CrossRef]
- Todaka, M.; Kowhakul, W.; Masamoto, H.; Shigematsu, M. Improvement of Oxidation Stability of Biodiesel by an Antioxidant Component Contained in Spent Coffee Grounds. Biofuels 2021, 12, 227–235. [Google Scholar] [CrossRef]
- Bui, H.N.; Do, H.Q.; Duong, H.T.G.; Perng, Y.-S.; Dam, V.N.; Nguyen, V.-T.; Bui, H.M. Taguchi Optimization and Life Cycle Assessment of Biodiesel Production from Spent Ground Coffee. Environ. Dev. Sustain. 2022, 24, 12900–12916. [Google Scholar] [CrossRef]
- Veitía-de-Armas, L.; Reynel-Ávila, H.E.; Bonilla-Petriciolet, A.; Jáuregui-Rincón, J. Green Solvent-Based Lipid Extraction from Guava Seeds and Spent Coffee Grounds to Produce Biodiesel: Biomass Valorization and Esterification/Transesterification Route. Ind. Crops Prod. 2024, 214, 118535. [Google Scholar] [CrossRef]
- Leow, Y.; Yew, P.Y.M.; Chee, P.L.; Loh, X.J.; Kai, D. Recycling of Spent Coffee Grounds for Useful Extracts and Green Composites. RSC Adv. 2021, 11, 2682–2692. [Google Scholar] [CrossRef]
- Im, G.; Yeom, S.H. Repeated Biodiesel Production from Waste Coffee Grounds via a One-Step Direct Process with a Cartridge Containing Solid Catalysts Manufactured from Waste Eggshells. Biotechnol. Bioprocess Eng. 2020, 25, 623–632. [Google Scholar] [CrossRef]
- Son, J.; Kim, B.; Park, J.; Yang, J.; Lee, J.W. Wet in Situ Transesterification of Spent Coffee Grounds with Supercritical Methanol for the Production of Biodiesel. Bioresour. Technol. 2018, 259, 465–468. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-Situ Transesterification Process for Biodiesel Production Using Spent Coffee Grounds from the Instant Coffee Industry. Ind. Crops Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Najdanovic-Visak, V.; Lee, F.Y.-L.; Tavares, M.T.; Armstrong, A. Kinetics of Extraction and in Situ Transesterification of Oils from Spent Coffee Grounds. J. Environ. Chem. Eng. 2017, 5, 2611–2616. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-Situ Transesterification of Wet Spent Coffee Grounds for Sustainable Biodiesel Production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Son, J.; Lee, J.W. Solvo-Thermal in Situ Transesterification of Wet Spent Coffee Grounds for the Production of Biodiesel. Bioresour. Technol. 2018, 249, 494–500. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Nickel Sulfide, Nickel Phosphide and Nickel Carbide Catalysts for Bio-Hydrotreated Fuel Production. Energy Convers. Manag. 2017, 151, 324–333. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Pongsiriyakul, K.; Lim, J.W.; Kiatkittipong, K.; Wongsurakul, P.; Yodpetch, V.; Boonyasuwat, S.; Assabumrungrat, S. Bioresources and Biofuels—From Classical to Perspectives and Trends. In A-Z of Biorefinery; Thongchul, N., Kokossis, A., Assabumrungrat, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–220. ISBN 978-0-12-819248-1. [Google Scholar]
- Passadis, K.; Fragoulis, V.; Stoumpou, V.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Study of Valorisation Routes of Spent Coffee Grounds. Waste Biomass Valorization 2020, 11, 5295–5306. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential Co-Production of Biodiesel and Bioethanol with Spent Coffee Grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete Utilization of Spent Coffee Grounds To Produce Biodiesel, Bio-Oil, and Biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Czekała, W.; Łukomska, A.; Pulka, J.; Bojarski, W.; Pochwatka, P.; Kowalczyk-Juśko, A.; Oniszczuk, A.; Dach, J. Waste-to-Energy: Biogas Potential of Waste from Coffee Production and Consumption. Energy 2023, 276, 127604. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic Co-Digestion of Spent Coffee Grounds with Different Waste Feedstocks for Biogas Production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Kim, D.; Cha, J.; Lee, C. Enhanced Methane Production with Co-Feeding Spent Coffee Grounds Using Spare Capacity of Existing Anaerobic Food Waste Digesters. Sci. Rep. 2024, 14, 4472. [Google Scholar] [CrossRef]
- Albarracin, L.T.; Mas, I.R.; Fuess, L.T.; Rodriguez, R.P.; Volpi, M.P.C.; de Souza Moraes, B. The Bioenergetic Potential from Coffee Processing Residues: Towards an Industrial Symbiosis. Resources 2024, 13, 21. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Dinesh Kumar, M.; Preethi; Atabani, A.E.; Kumar, G. Biorefinery of Spent Coffee Grounds Waste: Viable Pathway towards Circular Bioeconomy. Bioresour. Technol. 2020, 302, 122821. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food Waste Valorization Advocating Circular Bioeconomy—A Critical Review of Potentialities and Perspectives of Spent Coffee Grounds Biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- del Castillo Bilbao, M.D.; Ibáñez Ezequiel, M.E.; Amigo Benavent, M.; Herrero Calleja, M.; Plaza del Moral, M.; Ullate Artiz, M. Application of Products of Coffee Silverskin in Anti-Ageing Cosmetics and Functional Food. Patent No. WO/2013/004873, 2013. [Google Scholar]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; Del Castillo, M.D. Nutritional Quality, Potential Health Promoting Properties and Sensory Perception of an Improved Gluten-Free Bread Formulation Containing Inulin, Rice Protein and Bioactive Compounds Extracted from Coffee Byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Rios, M.B.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Herrera, T.; Velasco, D.; Gómez-Alonso, S.; Callejo, M.J.; Del Castillo, M.D. Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation. Molecules 2020, 25, 1358. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castaño-Tostado, E. Effect of Extrusion Process on the Obtention of a Flour from Coffee Pulp Coffea arabica Variety Red Caturra and Its Use in Bakery Products. J. Food Sci. Technol. 2023, 60, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, İ.A. Use of Coffee Silverskin to Improve the Functional Properties of Cookies. J. Food Sci. Technol. 2019, 56, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Ateş, G.; Elmacı, Y. Physical, Chemical and Sensory Characteristics of Fiber-Enriched Cakes Prepared with Coffee Silverskin as Wheat Flour Substitution. J. Food Meas. Charact. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and Dialysable Polyphenols in Selected Apple Varieties Following in Vitro Digestion vs. Their Native Patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Coffee Silverskin as Fat Replacer in Cake Formulations and Its Effect on Physical, Chemical and Sensory Attributes of Cakes. LWT 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Koay, H.Y.; Azman, A.T.; Mohd Zin, Z.; Portman, K.L.; Hasmadi, M.; Rusli, N.D.; Aidat, O.; Zainol, M.K. Assessing the Impact of Spent Coffee Ground (SCG) Concentrations on Shortbread: A Study of Physicochemical Attributes and Sensory Acceptance. Future Foods 2023, 8, 100245. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Hochkogler, C.; Somoza, V.; Del Castillo, M. Biscuits with No Added Sugar Containing Stevia, Coffee Fibre and Fructooligosaccharides Modifies α-Glucosidase Activity and the Release of GLP-1 from HuTu-80 Cells and Serotonin from Caco-2 Cells after In Vitro Digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef]
- Fåk, F.; Jakobsdottir, G.; Kulcinskaja, E.; Marungruang, N.; Matziouridou, C.; Nilsson, U.; Stålbrand, H.; Nyman, M. The Physico-Chemical Properties of Dietary Fibre Determine Metabolic Responses, Short-Chain Fatty Acid Profiles and Gut Microbiota Composition in Rats Fed Low- and High-Fat Diets. PLoS ONE 2015, 10, e0127252. [Google Scholar] [CrossRef]
- Abioye, R.O.; Nwamba, O.C.; Okagu, O.D.; Udenigwe, C.C. Synergistic Effect of Acarbose–Chlorogenic Acid on α-Glucosidase Inhibition: Kinetics and Interaction Studies Reveal Mixed-Type Inhibition and Denaturant Effect of Chlorogenic Acid. ACS Food Sci. Technol. 2023, 3, 1255–1268. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as α-Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-P.; Fink, H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee Silverskin as Nutraceutical Ingredient in Yogurt: Its Effect on Functional Properties and Its Bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Pagadigorria, C.L.; Bazotte, R.B.; da Silva, R.S.d.S.F.; de Souza, H.M. Chlorogenic Acid Reduces the Plasma Glucose Peak in the Oral Glucose Tolerance Test: Effects on Hepatic Glucose Release and Glycaemia. Cell Biochem. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S. Investigation of the Anticoagulant and Antithrombotic Effects of Chlorogenic Acid. J. Biochem. Mol. Toxicol. 2017, 31, e21865. [Google Scholar] [CrossRef]
- Glade, M.J. Caffeine—Not Just a Stimulant. Nutrition 2010, 26, 932–938. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Iriondo-DeHond, A.; Herrera, T.; Fernández-Fernández, A.M.; Sorzano, C.O.S.; Miguel, E.; del Castillo, M.D. Sensory Acceptance, Appetite Control and Gastrointestinal Tolerance of Yogurts Containing Coffee-Cascara Extract and Inulin. Nutrients 2020, 12, 627. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A Novel Antioxidant Beverage for Body Weight Control Based on Coffee Silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Manjunatha, M.R.; Sulochannama, G.; Naidu, M.M. Extraction, Characterization and Bioactivity of Coffee Anthocyanins. Eur. J. Biol. Sci. 2012, 4, 13–19. [Google Scholar]
- Patil, S.; Pimpley, V.; Warudkar, K.; Murthy, P.S. Valorisation of Coffee Pulp for Development of Innovative Probiotic Beverage Using Kefir: Physicochemical, Antioxidant, Sensory Analysis and Shelf Life Studies. Waste Biomass Valorization 2022, 13, 905–916. [Google Scholar] [CrossRef]
- Brand, D.; Pandey, A.; Roussos, S.; Brand, I.; Soccol, C.R. Microbial Degradation of Caffeine and Tannins from Coffee Husk. In Coffee Biotechnology and Quality; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 393–400. ISBN 978-94-017-1068-8. [Google Scholar]
- Lopes, A.C.A.; Andrade, R.P.; de Oliveira, L.C.C.; Lima, L.M.Z.; Santiago, W.D.; de Resende, M.L.V.; das Graças Cardoso, M.; Duarte, W.F. Production and Characterization of a New Distillate Obtained from Fermentation of Wet Processing Coffee By-Products. J. Food Sci. Technol. 2020, 57, 4481–4491. [Google Scholar] [CrossRef]
- Sampaio, A.; Dragone, G.; Vilanova, M.; Oliveira, J.M.; Teixeira, J.A.; Mussatto, S.I. Production, Chemical Characterization, and Sensory Profile of a Novel Spirit Elaborated from Spent Coffee Ground. LWT 2013, 54, 557–563. [Google Scholar] [CrossRef]
- Machado, E.; Mussatto, S.I.; Teixeira, J.; Vilanova, M.; Oliveira, J. Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages. Beverages 2018, 4, 105. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Liu, S.Q. The Potential of Spent Coffee Grounds Hydrolysates Fermented with Torulaspora Delbrueckii and Pichia Kluyveri for Developing an Alcoholic Beverage: The Yeasts Growth and Chemical Compounds Modulation by Yeast Extracts. Curr. Res. Food Sci. 2021, 4, 489–498. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, W.; Lu, Y.; Liu, S.Q. Biotransformation of Spent Coffee Grounds by Fermentation with Monocultures of Saccharomyces Cerevisiae and Lachancea Thermotolerans Aided by Yeast Extracts. LWT 2021, 138, 110751. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor Impacts of Glycerol in the Processing of Yeast Fermented Beverages: A Review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Rosas-Sánchez, G.A.; Hernández-Estrada, Z.J.; Suárez-Quiroz, M.L.; González-Ríos, O.; Rayas-Duarte, P. Coffee Cherry Pulp By-Product as a Potential Fiber Source for Bread Production: A Fundamental and Empirical Rheological Approach. Foods 2021, 10, 742. [Google Scholar] [CrossRef]
- Littardi, P.; Rinaldi, M.; Grimaldi, M.; Cavazza, A.; Chiavaro, E. Effect of Addition of Green Coffee Parchment on Structural, Qualitative and Chemical Properties of Gluten-Free Bread. Foods 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee Silverskin as a Source of Dietary Fiber in Bread-Making: Optimization of Chemical Treatment Using Response Surface Methodology. LWT Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; del Castillo, M.D. Use of Coffee Silverskin and Stevia to Improve the Formulation of Biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins 2022, 14, 109. [Google Scholar] [CrossRef]
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Padilla-Camberos, E.; Corona-González, R.I.; Mondragón-Cortez, P.M.; Arriola-Guevara, E. Evaluation of the Use of a Coffee Industry By-Product in a Cereal-Based Extruded Food Product. Foods 2020, 9, 1008. [Google Scholar] [CrossRef]
- Damat, D.; Anggriani, R.; Setyobudi, R.H.; Soni, P. Dietary Fiber and Antioxidant Activity of Gluten-Free Cookies with Coffee Cherry Flour Addition. Coffee Sci. 2019, 14, 493–500. [Google Scholar] [CrossRef]
- Cubero-Castillo, E.; Bonilla-Leiva, A.R.; García-Velasques, E. Coffee Berry Processing By-Product Valorization: Coffee Parchment as a Potential Fiber Source to Enrich Bakery Goods. J. Food Nutr. Popul. Health 2017, 1, 1–7. [Google Scholar]
- Han, I.; Lee, C.S. Quality Properties and Bioactivities of American Cookies with Coffee Extract Residues. LWT 2021, 151, 112173. [Google Scholar] [CrossRef]
- Trà, T.T.T.; Phúc, L.N.; Yến, V.T.N.; Sang, L.T.; Thu, N.T.A.; Nguyệt, T.N.M.; Mẫn, L.V.V. Use of Wheat Flour and Spent Coffee Grounds in the Production of Cookies with High Fiber and Antioxidant Content: Effects of Spent Coffee Grounds Ratio on the Product Quality. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012044. [Google Scholar] [CrossRef]
- Aguilar-Raymundo, V.G.; Sánchez-Páez, R.; Gutiérrez-Salomón, A.L.; Barajas-Ramírez, J.A. Spent Coffee Grounds Cookies: Sensory and Texture Characteristics, Proximate Composition, Antioxidant Activity, and Total Phenolic Content. J. Food Process. Preserv. 2019, 43, e14223. [Google Scholar] [CrossRef]
- Meerasri, J.; Sothornvit, R. Novel Development of Coffee Oil Extracted from Spent Coffee Grounds as a Butter Substitute in Bakery Products. J. Food Process. Preserv. 2022, 46, e16687. [Google Scholar] [CrossRef]
- Parra-Campos, A.; Ordóñez-Santos, L.E. Natural Pigment Extraction Optimization from Coffee Exocarp and Its Use as a Natural Dye in French Meringue. Food Chem. 2019, 285, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Benincá, D.B.; do Carmo, L.B.; Grancieri, M.; Aguiar, L.L.; Lima Filho, T.; Costa, A.G.V.; Oliveira, D.d.S.; Saraiva, S.H.; Silva, P.I. Incorporation of Spent Coffee Grounds in Muffins: A Promising Industrial Application. Food Chem. Adv. 2023, 3, 100329. [Google Scholar] [CrossRef]
- Severini, C.; Caporizzi, R.; Fiore, A.G.; Ricci, I.; Onur, O.M.; Derossi, A. Reuse of Spent Espresso Coffee as Sustainable Source of Fibre and Antioxidants. A Map on Functional, Microstructure and Sensory Effects of Novel Enriched Muffins. LWT 2020, 119, 108877. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef]
- Nguyen Le, B.X.; Van, T.P.; Phan, Q.K.; Pham, G.B.; Quang, H.P.; Do, A.D. Coffee Husk By-Product as Novel Ingredients for Cascara Kombucha Production. J. Microbiol. Biotechnol. 2024, 34, 673–680. [Google Scholar] [CrossRef]
- Masino, F.; Montevecchi, G.; Calvini, R.; Foca, G.; Antonelli, A. Sensory Evaluation and Mixture Design Assessment of Coffee-Flavored Liquor Obtained from Spent Coffee Grounds. Food Qual. Prefer. 2022, 96, 104427. [Google Scholar] [CrossRef]
- Phan, D.T.A.; Ha, H.T.; Ho, T.T. An Extract and Fractions from Coffea arabica Sediment on Antioxidant and Anti-Tyrosinase Activities, and on the Quality of Whiteleg Shrimp (Litopenaus vannamei) during Refrigerated Storage. Prev. Nutr. Food Sci. 2021, 26, 346–356. [Google Scholar] [CrossRef]
- Lin, C.; Toto, C.; Were, L. Antioxidant Effectiveness of Ground Roasted Coffee in Raw Ground Top Round Beef with Added Sodium Chloride. LWT 2015, 60, 29–35. [Google Scholar] [CrossRef]
- Hashimoto, T.A.; Caporaso, F.; Toto, C.; Were, L. Antioxidant Capacity and Sensory Impact of Coffee Added to Ground Pork. Eur. Food Res. Technol. 2019, 245, 977–986. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Shehta, H.A.; Rashad, S.; Rawash, E.-S.A.; Eid, H.R. Evaluation of the Antioxidant and Antimicrobial Activities of the Spent Coffee Extracts and Their Applications as Natural Food Preservatives of Chicken Fillets. Nov. Res. Microbiol. J. 2024, 8, 2303–2319. [Google Scholar] [CrossRef]
- Estévez, M.; Cava, R. Lipid and Protein Oxidation, Release of Iron from Heme Molecule and Colour Deterioration during Refrigerated Storage of Liver Pâté. Meat Sci. 2004, 68, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein Oxidation in Muscle Foods: A Review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, D.U.; Eun, J.B.; Moon, S.H. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncales, P. Antioxidant Action of Borage, Rosemary, Oregano, and Ascorbic Acid in Beef Patties Packaged in Modified Atmosphere. J. Food Sci. 2003, 68, 339–344. [Google Scholar] [CrossRef]
- Estévez, M.; Cava, R. Effectiveness of Rosemary Essential Oil as an Inhibitor of Lipid and Protein Oxidation: Contradictory Effects in Different Types of Frankfurters. Meat Sci. 2006, 72, 348–355. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M. Stabilisation of Edible Oils with Natural Antioxidants. Eur. J. Lipid Sci. Technol. 2001, 103, 752–767. [Google Scholar] [CrossRef]
- Qi, J.; Xu, Y.; Zhang, W.; Xie, X.; Xiong, G.; Xu, X. Short-Term Frozen Storage of Raw Chicken Meat Improves Its Flavor Traits upon Stewing. LWT 2021, 142, 111029. [Google Scholar] [CrossRef]
- Jully, K.M.M.; Toto, C.S.; Were, L. Antioxidant Effect of Spent, Ground, and Lyophilized Brew from Roasted Coffee in Frozen Cooked Pork Patties. LWT Food Sci. Technol. 2016, 66, 244–251. [Google Scholar] [CrossRef]
- de Farias Marques, A.D.J.; de Lima Tavares, J.; de Carvalho, L.M.; Leite Abreu, T.; Alves Pereira, D.; Moreira Fernandes Santos, M.; Suely Madruga, M.; de Medeiros, L.L.; Kênia Alencar Bezerra, T. Oxidative Stability of Chicken Burgers Using Organic Coffee Husk Extract. Food Chem. 2022, 393, 133451. [Google Scholar] [CrossRef]
- Sasse, A.; Colindres, P.; Brewer, M.S. Effect of Natural and Synthetic Antioxidants on the Oxidative Stability of Cooked, Frozen Pork Patties. J. Food Sci. 2009, 74, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Lourith, N.; Xivivadh, K.; Boonkong, P.; Kanlayavattanakul, M. Spent Coffee Waste: A Sustainable Source of Cleansing Agent for a High-Performance Makeup Remover. Sustain. Chem. Pharm. 2022, 29, 100826. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Valorization of Spent Coffee Grounds as the Specialty Material for Dullness and Aging of Skin Treatments. Chem. Biol. Technol. Agric. 2021, 8, 55. [Google Scholar] [CrossRef]
- Shankaranand, V.S.; Lonsane, B.K. Coffee Husk: An Inexpensive Substrate for Production of Citric Acid by Aspergillus Niger in a Solid-State Fermentation System. World J. Microbiol. Biotechnol. 1994, 10, 165–168. [Google Scholar] [CrossRef]
- Machado, C.M.M.; Soccol, C.R.; de Oliveira, B.H.; Pandey, A. Gibberellic Acid Production by Solid-State Fermentation in Coffee Husk. Appl. Biochem. Biotechnol. 2002, 102, 179–191. [Google Scholar] [CrossRef]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Lactic Acid. Lett. Appl. Microbiol. 2018, 66, 306–312. [Google Scholar] [CrossRef]
- Dessie, W.; Zhu, J.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Bio-Succinic Acid Production from Coffee Husk Treated with Thermochemical and Fungal Hydrolysis. Bioprocess Biosyst. Eng. 2018, 41, 1461–1470. [Google Scholar] [CrossRef]
- Niglio, S.; Procentese, A.; Russo, M.E.; Sannia, G.; Marzocchella, A. Investigation of Enzymatic Hydrolysis of Coffee Silverskin Aimed at the Production of Butanol and Succinic Acid by Fermentative Processes. BioEnergy Res. 2019, 12, 312–324. [Google Scholar] [CrossRef]
- Kim, B.; Yang, J.; Kim, M.; Lee, J.W. One-Pot Selective Production of Levulinic Acid and Formic Acid from Spent Coffee Grounds in a Catalyst-Free Biphasic System. Bioresour. Technol. 2020, 303, 122898. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.-M. Corrigendum: Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 652–655. [Google Scholar] [CrossRef]
- Schneider, R.; Mehlmann, K.; Venus, J.; Alexandri, M. Recent Advances in D-Lactic Acid Production from Renewable Resources. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef]
- Pettinato, M.; Drago, E.; Campardelli, R.; Perego, P. Spent Coffee Grounds Extract for Active Packaging Production. Chem. Eng. Trans. 2021, 87, 583–588. [Google Scholar] [CrossRef]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural Bio-Colorant and Pigments: Sources and Applications in Food Processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Prata, E.R.B.A.; Oliveira, L.S. Fresh Coffee Husks as Potential Sources of Anthocyanins. LWT Food Sci. Technol. 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; dos Reis, K.C.; Schwan, R.F.; Silva, C.F. Solid Coffee Waste as Alternative to Produce Carotenoids with Antioxidant and Antimicrobial Activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Troise, A.D.; Fogliano, V. Melanoidins from Coffee, Cocoa, and Bread Are Able to Scavenge α-Dicarbonyl Compounds under Simulated Physiological Conditions. J. Agric. Food Chem. 2019, 67, 10921–10929. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Pastoriza, S. Chapter 20—Melanoidins in Coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 183–188. ISBN 978-0-12-409517-5. [Google Scholar]
- Sutakwa, A.; Nadia, L.S.; Suharman, S. Addition of Blue Pea Flower (Clitoria ternatea L.) Extract Increase Antioxidant Activity in Yogurt from Various Types of Milk. J. Agercolere 2021, 3, 31–37. [Google Scholar] [CrossRef]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; de Mejía, E.G.; Martín-Cabrejas, M.A. Black Bean Coats: New Source of Anthocyanins Stabilized by β-Cyclodextrin Copigmentation in a Sport Beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin Food Colorant and Its Application in PH-Responsive Color Change Indicator Films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of Temperature and PH on Stability of Anthocyanin Obtained from Blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Huang, C.; Hu, Y.; Ji, H.; Liu, S.; Gao, J. Improvement of Solubility, Stability and Antioxidant Activity of Carotenoids Using Deep Eutectic Solvent-Based Microemulsions. Colloids Surf. B Biointerfaces 2022, 217, 112591. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed Electric Field Assisted Extraction of Natural Food Pigments and Colorings from Plant Matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Ramírez, J.D.; Guerrero-Moras, M.C.; González-Peña, M.A.; Silva-Pereira, T.S.; Anaya de Parrodi, C.; Ortega-Regules, A.E. Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity. Molecules 2023, 28, 1353. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of Natural Dyes with Biopolymers for Application in Food: A Review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Tores de la Cruz, S.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; del Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Deng, Y. Latest Advances in Active Materials for Food Packaging and Their Application. Foods 2023, 12, 4055. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Ghazvini, A.K.A.; Ormondroyd, G.; Curling, S.; Saccani, A.; Sisti, L. An Investigation on the Possible Use of Coffee Silverskin in PLA/PBS Composites. J. Appl. Polym. Sci. 2022, 139, 52264. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dufresne, A. Sustainable Biodegradable Coffee Grounds Filler and Its Effect on the Hydrophobicity, Mechanical and Thermal Properties of Biodegradable PBAT Composites. J. Appl. Polym. Sci. 2017, 134, 44498. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of Different Compatibilizers on Sustainable Composites Based on a PHBV/PBAT Matrix Filled with Coffee Silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of Torrefied Coffee Grounds as Reinforcing Agent to Produce High-Quality Biodegradable PBAT Composites for Food Packaging Applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Schutz, G.F.; Alves, R.M.V.; Vieira, R.P. Development of Starch-Based Films Reinforced with Coffee Husks for Packaging Applications. J. Polym. Environ. 2023, 31, 1955–1966. [Google Scholar] [CrossRef]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Foruzanmehr, R.; Medina, D.I. Assessing the Reinforcement Effect by Response Surface Methodology of Holocellulose from Spent Coffee Grounds on Biopolymeric Films as Food Packaging Materials. Biopolymers 2024, 115, e23585. [Google Scholar] [CrossRef] [PubMed]
- Turan, D.; Wang, Y.; Grundmann, D.; Paillart, M.; Dieleman, R.; Rahn, A. Coffee By-Product Cascara as an Edible Active Coating for Enhancing Hazelnut Preservation and Packaging. Food Packag. Shelf Life 2024, 45, 101350. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl Cellulose-Based Multifunctional Film Integrated with Polyphenol-Rich Extract and Carbon Dots from Coffee Husk Waste for Active Food Packaging Applications. Food Chem. 2024, 448, 139143. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Lappa, I.K.; Andriotis, H.; Eriotou, E.; Mandala, I.; Kopsahelis, N. Tuning the Physical and Functional Properties of Whey Protein Edible Films: Effect of PH and Inclusion of Antioxidants from Spent Coffee Grounds. Sustain. Chem. Pharm. 2022, 27, 100700. [Google Scholar] [CrossRef]
- Dordevic, D.; Gablo, N.; Zelenkova, L.; Dordevic, S.; Tremlova, B. Utilization of Spent Coffee Grounds as a Food By-Product to Produce Edible Films Based on κ-Carrageenan with Biodegradable and Active Properties. Foods 2024, 13, 1833. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Using Lignocellulosic Fractions of Coffee Husk to Improve Properties of Compatibilised Starch-PLA Blend Films. Food Packag. Shelf Life 2019, 22, 100423. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of Polylactic Acid Nanocomposite Films Reinforced with Cellulose Nanocrystals Derived from Coffee Silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef]
- Cavanagh, Q.; Brooks, M.S.-L.; Rupasinghe, H.P.V. Innovative Technologies Used to Convert Spent Coffee Grounds into New Food Ingredients: Opportunities, Challenges, and Prospects. Future Foods 2023, 8, 100255. [Google Scholar] [CrossRef]
- Hoinkes, C.; Blumer, F. Why the Nescafé Plan Fails to Benefit Farmers. Available online: https://www.publiceye.ch/en/topics/soft-commodity-trading/why-the-nescafe-plan-fails-to-benefit-farmers (accessed on 3 October 2024).
- Foreign Agricultural Service (USDA). Production—Coffee. Available online: https://fas.usda.gov/data/production/commodity/0711100 (accessed on 3 October 2024).
- Rennie, D. Leading the World of Coffee; Nestlé: Vevey, Switzerland, 2022. [Google Scholar]
- Nestlé. Creating Shared Value and Sustainability Report 2023; Nestlé: Vevey, Switzerland, 2023. [Google Scholar]
- Lekto Woodfuels Ltd. The Rise and Fall of Coffee Logs: From Richard Branson’s Backing to Bankruptcy (Updated November 2023). Available online: https://www.lektowoodfuels.co.uk/blogs/news/bio-bean-coffee-logs?srsltid=AfmBOorcAbevaXwlEtwrVMf0YfPj9qWxCuOVC0ML55BmbDMQ1WJj65Lo (accessed on 16 August 2024).
- Mattinson, A. Coffee Logs Supplier Bio-Bean Collapses Following on-Site Fire. Available online: https://www.thegrocer.co.uk/news/coffee-logs-supplier-bio-bean-collapses-following-on-site-fire/678767.article (accessed on 16 August 2024).
- Envar. Envar Composting Ltd. Acquires Bio-Bean Ltd. Available online: https://www.envar.co.uk/envar-composting-ltd-acquires-bio-bean-ltd/ (accessed on 19 August 2024).
- Langenbahn, H.J. Upcycled Coffee By-Products and Food Safety. Challenges for Coffee Producers. Available online: https://www.thezerowastecoffeeproject.com/post/upcycled-coffee-by-products-and-food-safety (accessed on 1 October 2024).
- NEFF. Permafungi: Breeding Mushrooms with Old Coffee Grounds. Available online: https://www.neff-home.com/theingredient/story/mushrooms (accessed on 18 August 2024).
- The European Circular Economy Stakeholder Platform. PermaFungi—Recycling Coffee Grounds into Upcycled Products. Available online: https://circulareconomy.europa.eu/platform/en/good-practices/permafungi-recycling-coffee-grounds-upcycled-products (accessed on 18 August 2024).
- Permafungi. Press Kit 2019; PermaFungi: Brussels, Belgium, 2019; Available online: https://www.permafungi.be/wp-content/uploads/2019/02/Press-Kit-2019.pdf (accessed on 18 August 2024).
- Marnix, A. Financial Data of Permafungi. Available online: https://www.companyweb.be/en/0546704074/permafungi (accessed on 5 October 2024).
- Imbibe Coffee Roasters. Zero Waste Coffee. Available online: https://imbibe.ie/wholesale/zero-waste/ (accessed on 18 August 2024).
- Nestlé. Financial Statements 2023; Nestlé: Vevey, Switzerland, 2024. [Google Scholar]
- Arthur, R. Coffee Power: Nestlé Waters Announces Swiss Biogas Plant—With a Little Help from Nespresso. Available online: https://www.beveragedaily.com/Article/2015/04/02/Coffee-power-Nestle-Waters-announces-Swiss-biogas-plant-with-a-little-help-from-Nespresso (accessed on 16 August 2024).
- Nestle. Nestlé Waters Starts Building Swiss Biogas Plant. Available online: https://www.nestle.com/media/news/nestle-waters-swiss-biogas-plant (accessed on 16 August 2024).
- Starbucks Corporation. Starbucks Fiscal 2023 Annual Report; Starbucks Corporation: Seattle, WA, USA, 2024. [Google Scholar]
- Starbucks. Starbucks’ Eco-Friendly Mission Reinforced by #GroundsForYourGarden Encouraging Customers to Enrich Soil with Coffee Grounds. Available online: https://www.starbucks.co.th/ground-for-your-garden/ (accessed on 20 August 2024).
- Starbucks. Starbucks Grounds For Your Garden: Rooted in a Mission to Help Local Communities Grow. Available online: https://stories.starbucks.ca/en-ca/stories/2021/starbucks-grounds-for-your-garden-rooted-in-a-mission-to-help-local-communities-grow/ (accessed on 20 August 2024).
- Tracxn. Bio-Bean. Available online: https://platform.tracxn.com/a/d/company/Y0xg7jiQSNapicDNFM0soH0BXhp8t_dmfyPZoQmuvlk/bio-bean.com#a:key-metrics (accessed on 3 October 2024).
- Nestle Spent Coffee Grounds Help Power Queensland Factory. Available online: https://www.nestle.com.au/en/stories/recycled-coffee (accessed on 16 August 2024).
- Agència Catalana de Notícies (ACN). Nestlé to Invest €17.2m in Biomass Boiler in Girona Plant. Available online: https://www.catalannews.com/business/item/nestle-to-invest-17-2m-in-biomass-boiler-in-girona-plant (accessed on 16 August 2024).
- Invest in Spain (ICEX). Nestlé Boosts Environmental Sustainability in Spanish Factories. Available online: https://www.investinspain.org/content/icex-invest/en/noticias-main/2023/nestle.html (accessed on 16 August 2023).
- Veolia Coffee Grounds. Available online: https://www.anz.veolia.com/services/recycling-waste-services/recycling/organics/coffee-grounds (accessed on 22 August 2024).
- Veolia Turning Waste into Renewable Energy. Available online: https://www.veolia.hu/en/our-services/industrial/case-studies/food-beverage/netherlands-douwe-egberts-master-blenders-demb (accessed on 22 August 2024).
- Veolia Coffee Grounds Turned into Renewable Energy. Available online: https://www.veolia.in/about-us/cop21/our-solutions/coffee-grounds-turned-renewable-energy (accessed on 22 August 2024).
- Veolia Environnement. Veolia—Operating & Financial Review; Veolia Environnement: Aubervilliers, France, 2023; Volume 2023. [Google Scholar]
- Caffee Inc. Start Creating Eco-Friendly Coffee Based Products Your Customers Will Love. Available online: https://caffeinc.nl/ (accessed on 19 August 2024).
- Caffe Inc. Caffee Inc.—Linkedin. Available online: https://www.linkedin.com/posts/caffeinc_last-thursday-caffe-inc-hosted-an-open-day-activity-7211691073075621888-xwJW (accessed on 3 October 2024).
- Caffee Inc. Caffe Inc. Signs Contract for €4 Million to Build Recycling Plant for Coffee Waste in Amsterdam. Available online: https://caffeinc.nl/updates/caffe-inc-signs-contract-for-e4-million-to-build-recycling-plant-for-coffee-waste-in-amsterdam/ (accessed on 3 October 2024).
- Coffeefrom Materials. Available online: https://coffeefrom.it/en/materials/#bio (accessed on 20 August 2024).
- Gallo, L. Turning Coffee Waste into Thermoplastics with CoffeeFrom. Available online: https://www.forestvalley.org/article/turning-coffee-waste-into-thermoplastics-with-coffee-from (accessed on 3 October 2024).
- Ecobean Products with Potential. Available online: https://ecobean.pl (accessed on 20 August 2024).
- Tracxn. Ecobean. Available online: https://platform.tracxn.com/a/d/company/-9qWzxUeU6zV3nwbCOvZnbmm5LHXzIbi0-RXF4_9fSU/ecobean.pl#a:key-metrics (accessed on 3 October 2024).
- Huskee. HuskeeLoop. Available online: https://huskee.co/huskeeloop/ (accessed on 20 August 2024).
- Melo, C. Sustainable Solutions: Repurposing Coffee Waste for a Greener Future. Available online: https://www.eraofwe.com/coffee-lab/en/articles/sustainable-solutions-repurposing-coffee-waste-for-a-greener-future (accessed on 20 August 2024).
- Huskee. HuskeeCup: Waste Made Beautiful. Available online: https://www.kickstarter.com/projects/1366930566/huskeecup-waste-made-beautiful?ref=discovery&term=Huskee&total_hits=1&category_id=28 (accessed on 3 October 2024).
- Australian Circular Economy Hub Huskee. Creating Reusable Coffee Cups from Coffee Husk Waste. Available online: https://acehub.org.au/knowledge-hub/case-studies/huskee (accessed on 3 October 2024).
- Kaffe Bueno. We Make It Natural. Available online: https://www.kaffebueno.com/ (accessed on 17 August 2024).
- Monagas, D.C. World’s First Coffee Biorefinery Opens in Denmark. Available online: https://worldbiomarketinsights.com/worlds-first-coffee-biorefinery-opens-in-denmark/ (accessed on 3 October 2024).
- Monagas, D.C. Kaffe Bueno Secures €6.2 Million in Series A Round Led by Global Biorefinery Leader Borregaard. Available online: https://worldbiomarketinsights.com/kaffe-bueno-secures-e6-2-million-in-series-a-round-led-by-global-biorefinery-leader-borregaard/ (accessed on 3 October 2024).
- Tracxn Kaffe Bueno. Available online: https://platform.tracxn.com/a/d/company/MlFsjODwQdnKEyy1IltTK-2W5XVdAOJJhwLddqj827Y/kaffebueno.com#a:key-metrics (accessed on 3 October 2024).
- Kaffeeform. Available online: https://www.kaffeeform.com/en/material/ (accessed on 20 August 2024).
- Morrison, L. The People Getting Rich off the Discards of Our Addiction. Available online: https://www.bbc.com/worklife/article/20160818-the-people-getting-rich-off-the-discards-of-our-addiction (accessed on 3 October 2024).
- Krasaephol, S. PTT InI’s Product—Coffee Chaff. Available online: https://www.pttplc.com/en/Products/Ourbusinessbypttplc/Technologyandengineeringunit/Technologyandengineering/Pttinis-Product/Content-26913.aspx (accessed on 17 September 2024).
- PTT Public Company Limited. Financial Report 2023; PTT Public Company Limited: Bangkok, Thailand, 2023. [Google Scholar]
- The Stock Exchange of Thailand (SET). PTT—Factsheet. Available online: https://www.set.or.th/en/market/product/stock/quote/ptt/factsheet (accessed on 5 October 2024).
- Nestle. NESCAFÉ NATIV Cascara. Available online: https://www.nestle.com.au/en/brands/cascara (accessed on 16 August 2024).
- Nestle. Nestlé Tackles Upstream Food Waste Through Product and Technology Innovation. Available online: https://www.nestle.com/aboutus/research-development/news/nestle-science-technology-new-value-agricultural-side-streams (accessed on 16 August 2024).
- Hedlund, D. What Is a Cascara Latte? Available online: https://www.eraofwe.com/coffee-lab/en/articles/what-is-a-cascara-latte (accessed on 20 August 2024).
- Starbucks Introducing Starbucks First New Beverage of 2017, the Cascara Latte. Available online: https://stories.starbucks.com/stories/2017/starbucks-cascara-latte/ (accessed on 20 August 2024).
- Supracafe ¿Conoces La Infusión de Cáscara de Café? Supracafé Maximiza Su Sostenibilidad Con Tabifruit. Available online: https://www.supracafe.com/blogs/news/conoces-la-infusion-de-cascara-de-cafe-supracafe-maximiza-su-sostenibilidad-con-tabifruit (accessed on 16 September 2024).
- Invest in Spain (ICEX). Supracafé. Available online: https://www.investinspain.org/content/icex-invest/en/casos-exito-main/NEW2015493859_EN_US.html (accessed on 5 October 2024).
- COFIDES. Supracafe Expands Its Colombian Subsidiary with COFIDES Financial Support. Available online: https://www.cofides.es/en/noticias/notas-de-prensa/supracafe-expands-its-colombian-subsidiary-cofides-financial-support (accessed on 5 October 2024).
- The Coffee Cherry, Co. Available online: https://coffeecherryco.com (accessed on 17 August 2024).
- Kirchherr, J.; Piscicelli, L.; Bour, R.; Kostense-Smit, E.; Muller, J.; Huibrechtse-Truijens, A.; Hekkert, M. Barriers to the Circular Economy: Evidence From the European Union (EU). Ecol. Econ. 2018, 150, 264–272. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Sumadi; Ardhiarisca, O.; Putra, R. Production and Calculation of Economic Value of the Coffee Skin Waste Products. In Proceedings of the First International Conference on Social Science, Humanity, and Public Health (ICOSHIP 2020), Jember, Indonesia, 7–8 November 2020; Atlantis Press: Paris, France, 2021; pp. 41–44. [Google Scholar]
- Kamil, M.; Ramadan, K.M.; Olabi, A.G.; Al-Ali, E.I.; Ma, X.; Awad, O.I. Economic, Technical, and Environmental Viability of Biodiesel Blends Derived from Coffee Waste. Renew. Energy 2020, 147, 1880–1894. [Google Scholar] [CrossRef]
- Duarte, A.; Uribe, J.C.; Sarache, W.; Calderón, A. Economic, Environmental, and Social Assessment of Bioethanol Production Using Multiple Coffee Crop Residues. Energy 2021, 216, 119170. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Cho, E.-J.; Maskey, S.; Nguyen, D.-T.; Bae, H.-J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. [Google Scholar] [CrossRef]
- Proença, J.F.; Torres, A.C.; Marta, B.; Silva, D.S.; Fuly, G.; Pinto, H.L. Sustainability in the Coffee Supply Chain and Purchasing Policies: A Case Study Research. Sustainability 2022, 14, 459. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent Coffee Grounds: A Review on Current Utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Nanda, P.V.; Usman, I. Green Business Opportunity of Coffee Ground Waste through Reverse Logistics. J. Glob. Bus. Adv. 2017, 10, 721. [Google Scholar] [CrossRef]
- Nguyen, G.N.T.; Sarker, T. Sustainable Coffee Supply Chain Management: A Case Study in Buon Me Thuot City, Daklak, Vietnam. Int. J. Corp. Soc. Responsib. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- van Keulen, M.; Kirchherr, J. The Implementation of the Circular Economy: Barriers and Enablers in the Coffee Value Chain. J. Clean. Prod. 2021, 281, 125033. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; López-Eccher, C.; Muñoz, E.; Salvador, R.; Cano-Londoño, N.A.; Barros, M.V.; Bernal, D.C.; Mendoza, J.M.F.; Nadal, A.; Guerrero, A.B. Circular Economy in Latin America and the Caribbean: Drivers, Opportunities, Barriers and Strategies. Sustain. Prod. Consum. 2024, 51, 118–136. [Google Scholar] [CrossRef]
- de Jesus, A.; Mendonça, S. Lost in Transition? Drivers and Barriers in the Eco-Innovation Road to the Circular Economy. Ecol. Econ. 2018, 145, 75–89. [Google Scholar] [CrossRef]
- Bonnín Roca, J.; Vaishnav, P.; Morgan, M.G.; Mendonça, J.; Fuchs, E. When Risks Cannot Be Seen: Regulating Uncertainty in Emerging Technologies. Res. Policy 2017, 46, 1215–1233. [Google Scholar] [CrossRef]
- Vidal-Ayuso, F.; Akhmedova, A.; Jaca, C. The Circular Economy and Consumer Behaviour: Literature Review and Research Directions. J. Clean. Prod. 2023, 418, 137824. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Mattsson, M.; Ulvenblad, P. Perception and Awareness of Circular Economy within Water-Intensive and Bio-Based Sectors: Understanding, Benefits and Barriers. J. Clean. Prod. 2024, 464, 142725. [Google Scholar] [CrossRef]
- Peluso, M. Coffee By-Products: Economic Opportunities for Sustainability and Innovation in the Coffee Industry. Proceedings 2023, 89, 6. [Google Scholar] [CrossRef]
- Ungerman, O.; Dědková, J. Consumer Behavior in the Model of the Circular Economy in the Field of Handling Discarded Items. PLoS ONE 2024, 19, e0300707. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Ares, G.; Thøgersen, J.; Monteleone, E. A Sense of Sustainability?—How Sensory Consumer Science Can Contribute to Sustainable Development of the Food Sector. Trends Food Sci. Technol. 2019, 90, 180–186. [Google Scholar] [CrossRef]

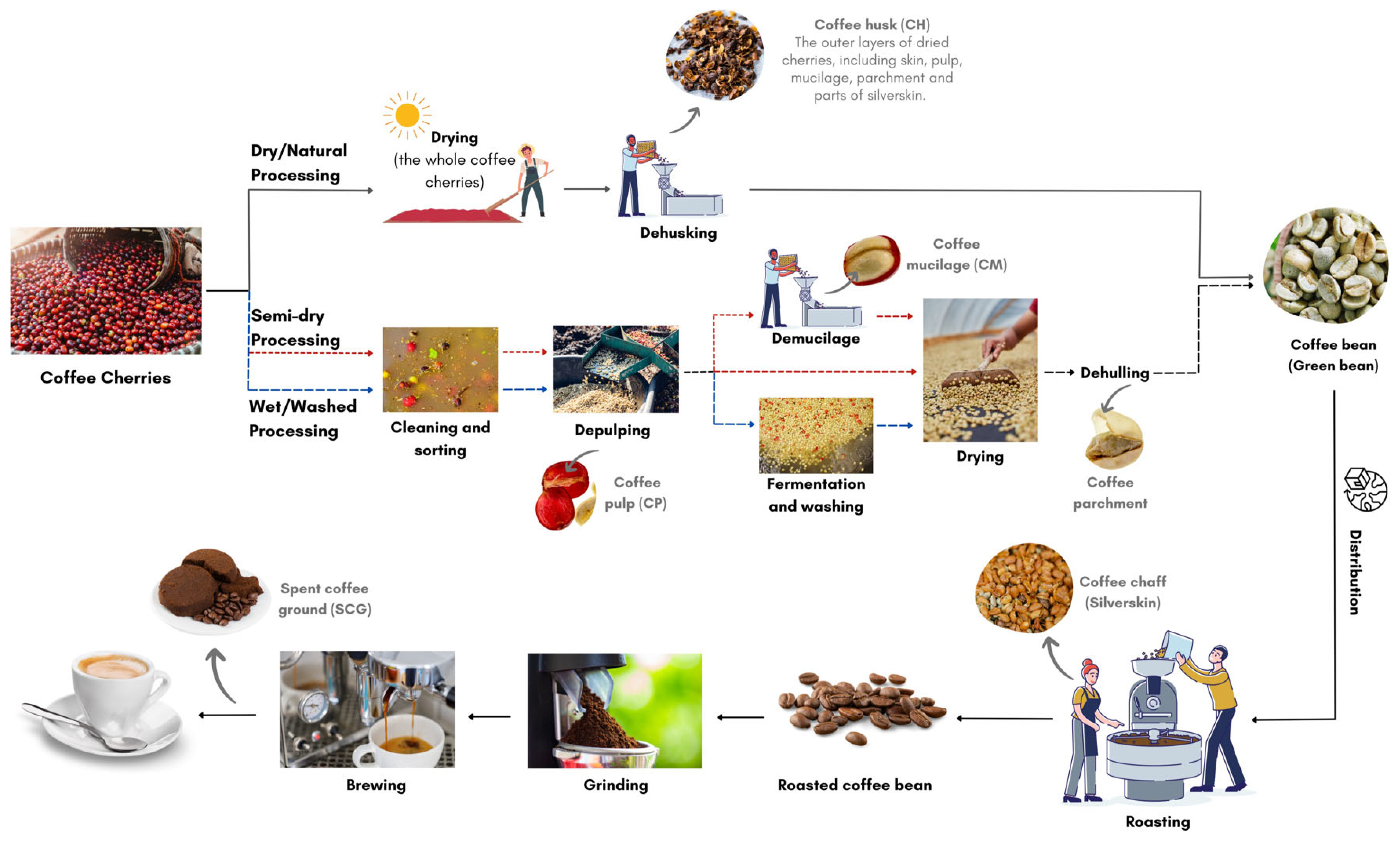
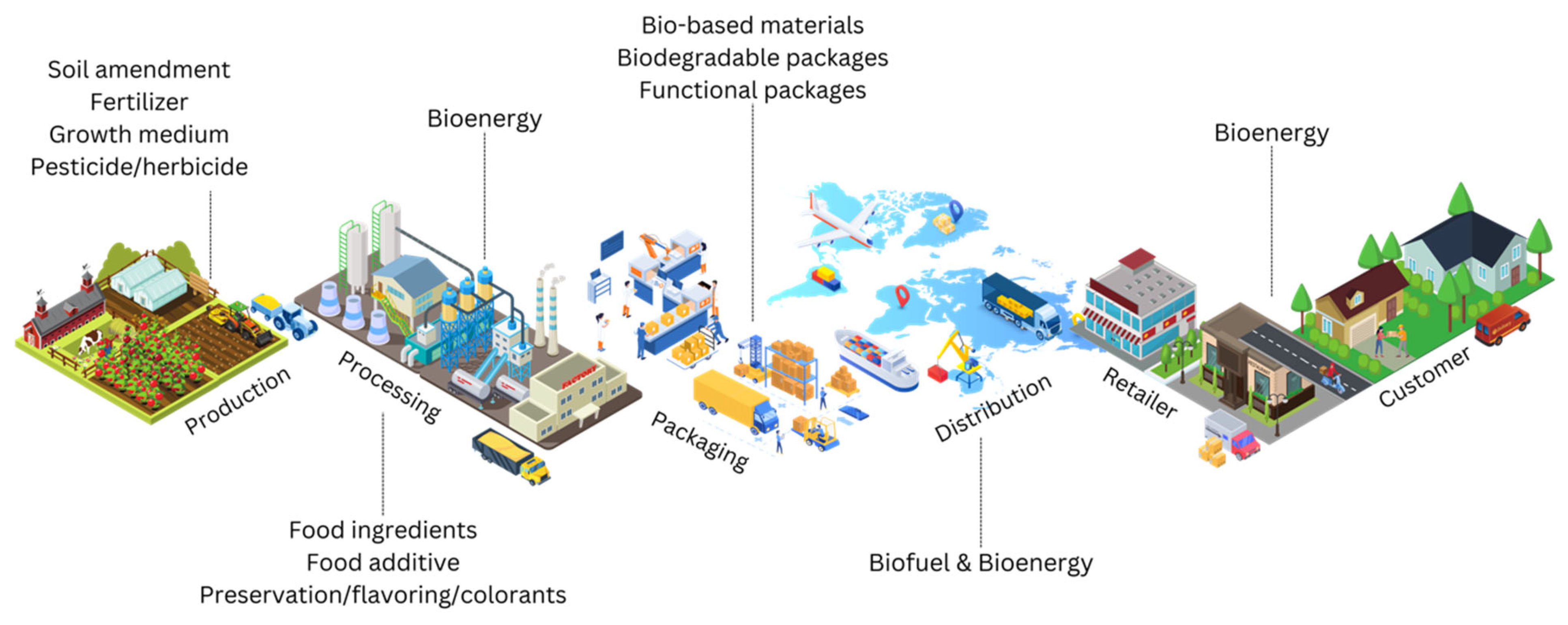
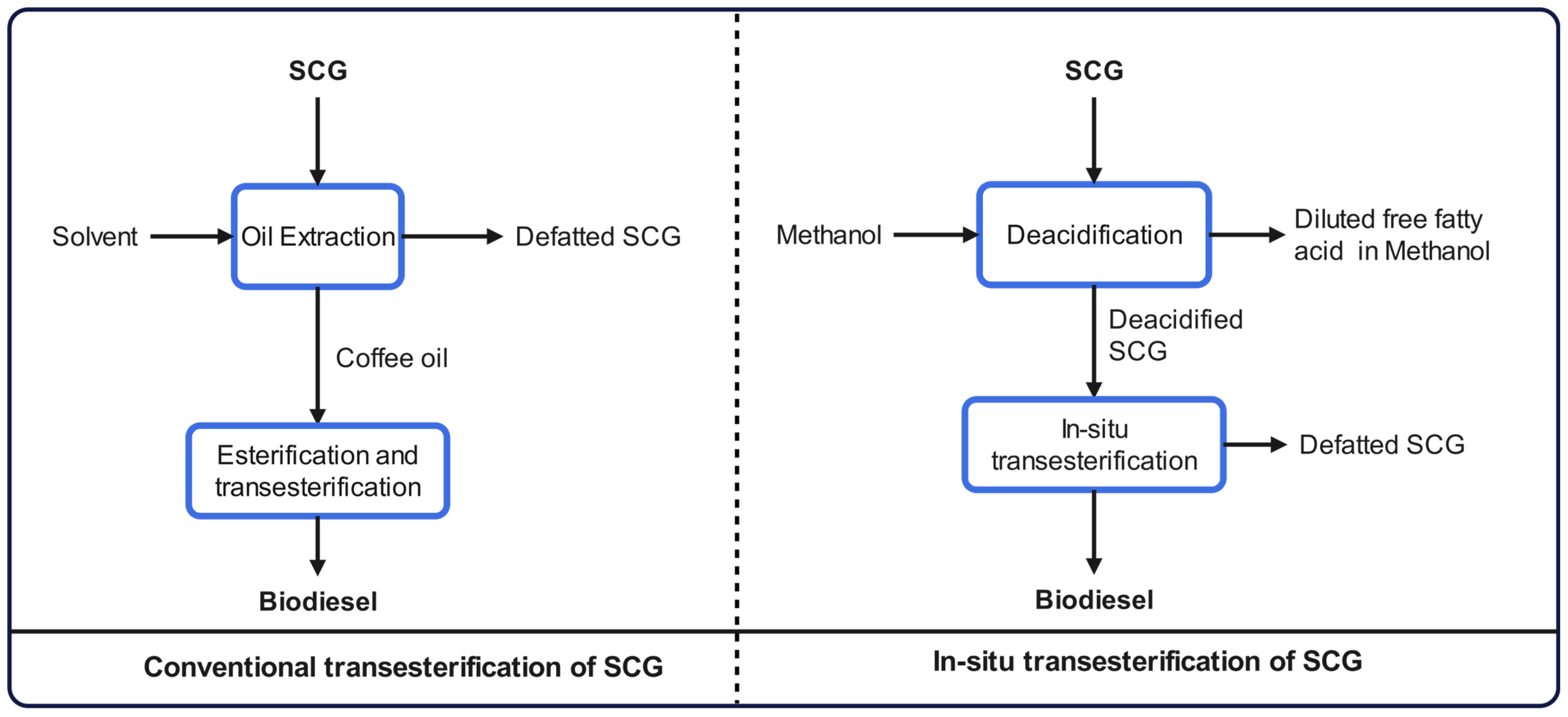

| Component | Husk | Pulp | Mucilage | Parchment | Silverskin | Spent Coffee Grounds |
|---|---|---|---|---|---|---|
| Processing | Dry | Wet | Wet | Semi-Dry/Wet | Roasting | Brewing |
| Yield | kg/100 kg of coffee cherry | kg/100 kg of coffee bean | ||||
| 12–18 | 37–45 | 11.8 | 5.8–6.1 | 4.2 | 65 | |
| Moisture | 7–18 | 75–90 | 80–94 | 4–9 | 5–7 | 50–85 |
| Carbohydrates | 58–85 | 35–89 | 5–46 | 0.4 | 44 | 54–82 |
| Celluloses | 16–43 | 18–63 | 8 | 35–54 | 10–24 | 7–23 |
| Hemicelluloses | 4–30 | 2–29 | 18 | 20–38 | 4–22 | 15–42 |
| Lignin | 6–24 | 14–26 | - | 27–35 | 18–31 | 0–26 |
| Proteins | 3–13 | 4–14 | 0.5–12 | 0.4–3 | 15–23 | 10–18 |
| Lipids | 2–7 | 1–5 | 0.12 | 0.3–0.9 | 2–6 | 2–24 |
| mineral | 3–7 | 6–10 | ||||
| Pectins | 2–12 | 5.5–12.4 | 0.6–2.6 | - | 1 | 0.01 |
| Total fiber | 18–30 | 18–64 | - | 89–91 | 56–72 | 21–59 |
| Ash | 6 | 0.3–8.9 | 0.4 | 0.5–1 | 4–8 | 1–3 |
| References | [12,31,46] | [12,37,46] | [12,35] | [12,35] | [12,31,35] | [12,31,35,47] |
| Feedstock | Hydrolysis Method | Sugar Yield | Ethanol Concentration | Yield and Productivity | Ref. |
|---|---|---|---|---|---|
| Spent coffee grounds | Dilute acid pretreatment | Glucose: 192 mg/g SCG | 19.0 g/L | Ethanol yield: 0.50 g/g Productivity:1.90 g/L·h | [245] |
| Coffee husk | Dilute acid hydrolysis followed by peroxide alkaline pretreatment, then Enzymatic Hydrolysis | Glucose: 115.6 g/L | 48.19 g/L | Ethanol yield: 0.41 g/g Productivity: 2.4 g/L·h | [240] |
| Coffee husk | Acid hydrolysis and enzymatic hydrolysis | Glucose: 11.5 g/L Xylose: 7.4 g/L | After fermentation: 36.6 ± 0. 2 g/L After pervaporation: 132.2 ± 40 g/L | Productivity: 3.05 g/L·h | [233] |
| Spent coffee grounds | Acid hydrolysis and enzymatic hydrolysis | Glucose: 16.0 g/L Xylose: 15.6 g/L | After fermentation: 47.9 ± 0.06 g/L After pervaporation: 51.7 ± 7.4 g/L | Productivity: 4.00 g/L·h | [233] |
| Spent coffee grounds | Ethanol pretreatment at high temperature, then enzymatic hydrolysis | Glucose: 17.5 g/L Galactose: 8.0 g/L Mannose: 31.7 g/L | After fermentation: 16.4 g/L After pervaporation: 11.3 g DW of ethanol from 150 g DW of ethanol-pretreated CRW | Productivity: 0.23 g/L·h | [230] |
| Spent coffee grounds | Popping pretreatment followed by enzymatic pretreatment | Fermentable sugar: 40.2 g/L | 15.3 g/L (45.9 g of ethanol per 300 g of CRW) | Productivity: 0.15 g/L·h | [228] |
| Spent coffee grounds | Acid hydrolysis followed by peroxide alkaline pretreatment | Sugar concentration: ~50 g/L | 11.7 g/L | Ethanol yield = 0.26 g/g Productivity= 0.49 g/L·h | [243] |
| Food Products | Coffee Waste | Process | Functions | Ref. |
|---|---|---|---|---|
| Bread | CP powder | Baking |
| [301] |
| CP flour | Extrusion/Baking |
| [272] | |
| Coffee cascara isolated fiber | Water extraction/Baking |
| [271] | |
| CPm ground | Baking |
| [302] | |
| CS powder | Alkaline hydrogen peroxide pretreatment/Baking |
| [303] | |
| CH and CS extracts | Water extraction/Baking |
| [270] | |
| Biscuits | SCG powder | Baking |
| [277] |
| CS fiber/SCG powder/dietary fiber extracts | Extraction/Baking |
| [16,20,278,304] | |
| Brownies | SCG extracts | Isopropanol extraction/Baking |
| [305] |
| Cake | CS powder | Water treatment/Baking |
| [274,276] |
| Cereal | CS grounds | Extrusion and Baking |
| [306] |
| Cookies | coffee flour | Baking |
| [307] |
| CPm powder | Ultrasound treatment/Baking |
| [308] | |
| CS powder | Baking |
| [273] | |
| SCG powder | Baking |
| [309] | |
| SCG powder | Baking |
| [310] | |
| SCG powder | Baking |
| [311] | |
| SCG oil | Ethanol extraction/Baking |
| [312] | |
| French Meringue | Coffee fresh skin extract | Ethanol extraction |
| [313] |
| Muffins | SCG powder | Baking |
| [314,315] |
| Yogurt | Cascara extracts | Water extraction/Inoculated fermentation |
| [290] |
| CS powder | Inoculated fermentation |
| [284] | |
| Antioxidant Beverage | CS extracts | Extraction and/Infusion |
| [291] |
| Instant Cascara (IC) | CH extract powder | Extraction-Freeze dried/Infusion |
| [316] |
| Cascara Beverage | CP powder | Milled/Infusion |
| [43] |
| Cascara Kombucha | Dried whole CH | Steeping/Fermentation |
| [317] |
| Probiotic Beverage | Dried whole CP | Viscozyme or steam pretreatment/Infusion/Inoculated Fermentation |
| [293] |
| Distilled Beverage | Wet or dried CP in coffee wastewater | Fermentation/Distillation |
| [295] |
| Alcoholic Beverage | Wet CP and CM | Fermentation |
| [54] |
| SCG Spirit | SCG extract | Hydrothermal extraction/Fermentation/Distillation |
| [296] |
| Alcoholic Beverage | SCG hydrolysate | Acidic-enzymatic hydrolysis/Fermentation |
| [298] |
| Coffee-Flavored Liquor | SCG hydroalcoholic solution | Ethanol extraction |
| [318] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongsiriyakul, K.; Wongsurakul, P.; Kiatkittipong, W.; Premashthira, A.; Kuldilok, K.; Najdanovic-Visak, V.; Adhikari, S.; Cognet, P.; Kida, T.; Assabumrungrat, S. Upcycling Coffee Waste: Key Industrial Activities for Advancing Circular Economy and Overcoming Commercialization Challenges. Processes 2024, 12, 2851. https://doi.org/10.3390/pr12122851
Pongsiriyakul K, Wongsurakul P, Kiatkittipong W, Premashthira A, Kuldilok K, Najdanovic-Visak V, Adhikari S, Cognet P, Kida T, Assabumrungrat S. Upcycling Coffee Waste: Key Industrial Activities for Advancing Circular Economy and Overcoming Commercialization Challenges. Processes. 2024; 12(12):2851. https://doi.org/10.3390/pr12122851
Chicago/Turabian StylePongsiriyakul, Kanokthip, Peerawat Wongsurakul, Worapon Kiatkittipong, Aerwadee Premashthira, Kulapa Kuldilok, Vesna Najdanovic-Visak, Sushil Adhikari, Patrick Cognet, Tetsuya Kida, and Suttichai Assabumrungrat. 2024. "Upcycling Coffee Waste: Key Industrial Activities for Advancing Circular Economy and Overcoming Commercialization Challenges" Processes 12, no. 12: 2851. https://doi.org/10.3390/pr12122851
APA StylePongsiriyakul, K., Wongsurakul, P., Kiatkittipong, W., Premashthira, A., Kuldilok, K., Najdanovic-Visak, V., Adhikari, S., Cognet, P., Kida, T., & Assabumrungrat, S. (2024). Upcycling Coffee Waste: Key Industrial Activities for Advancing Circular Economy and Overcoming Commercialization Challenges. Processes, 12(12), 2851. https://doi.org/10.3390/pr12122851












