Phosphite as a Sustainable and Versatile Alternative for Biostimulation, Biocontrol, and Weed Management in Modern Agriculture
Abstract
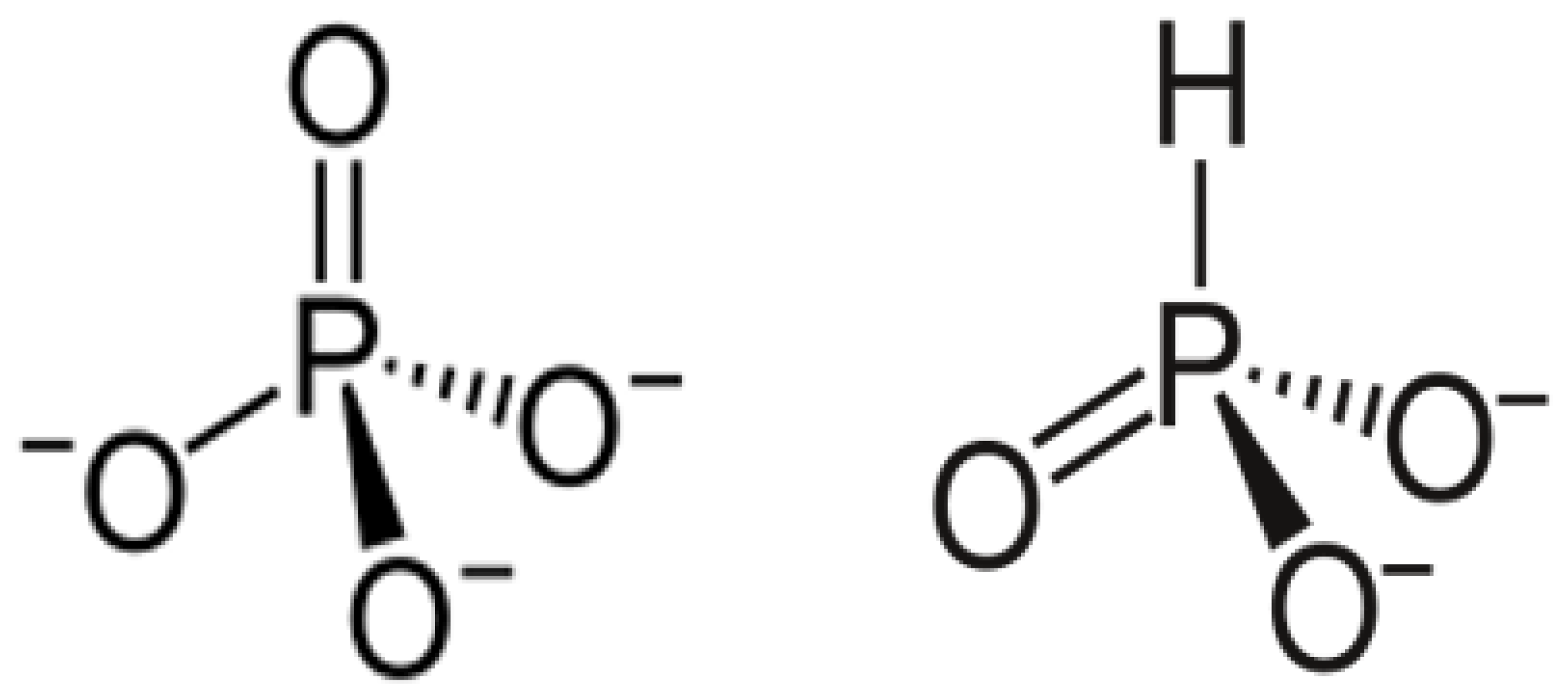
:1. Introduction
2. Use of Phi in Biostimulation and Biocontrol
3. Phytotoxicity of Phosphite and Its Uses as an Herbicide
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcántar-González, G.; Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrición de Cultivos, 2nd ed.; Editorial Colegio de Postgraduados: Texcoco, Mexico, 2016; 443p. [Google Scholar]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. J. Plant Nutr. 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Phosphite as an inductor of adaptive responses to stress and stimulator of better plant performance. In Biotic and Abiotic Stress Tolerance in Plants, 1st ed.; Vats, S., Ed.; Springer: Singapore, 2018; pp. 203–238. [Google Scholar]
- Gómez-Merino, F.C.; Gómez-Trejo, L.F.; Ruvalcaba-Ramírez, R.; Trejo-Téllez, L.I. Application of phosphite as a biostimulant in agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering, 1st ed.; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–153. [Google Scholar]
- Estrada-Ortiz, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Nuñez-Escobar, R.; Sandoval-Villa, M. Biochemical responses in strawberry plants supplying phosphorus in the form of phosphite. Rev. Chapingo Ser. Hortic. 2011, 17, 129–138. [Google Scholar] [CrossRef]
- Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz, T.J.; Medina-López, R. Phosphites as alternative for the management of phytopathological problems. Mex. J. Phytopathol. 2018, 36, 79–94. [Google Scholar]
- Li, Z.; Wang, J.; Wu, Y.; Hu, J.; Cong, H.L.; Yang, C.; Fu, J.; Sun, J. Changes in soil properties and the phoD-harboring bacteria of the alfalfa field in response to phosphite treatment. Front. Microbiol. 2022, 13, e1013896. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotechnol. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, C.J.; Mikkelsen, R.L. Phosphite fertilizers: What are they? Can you use them? What can they do? Better Crop. 2006, 90, 11–13. [Google Scholar]
- Havlin, J.L.; Schlegel, A.J. Review of phosphite as a plant nutrient and fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Morales-Morales, E.J.; Martínez-Campos, A.R.; López-Sandoval, J.A.; Castillo-González, A.M.; Rubí-Arriaga, M. Phosphites and their applications in agriculture. Rev. Mex. Cienc. Agríc. 2022, 13, 345–354. [Google Scholar]
- EPA–Environmental Protection Agency. Mono-and Di-Potassium Salts of Phosphorous Acid (076416) Fact Sheet. 1998. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-076416_1-Oct-98.pdf (accessed on 8 September 2024).
- Cerqueira, A.; Alves, A.; Berenguer, H.; Correia, B.; Gómez-Cadenas, A.; Diez, J.J.; Monteiro, P.; Pinto, G. Phosphite shifts physiological and hormonal profile of Monterey pine and delays Fusarium circinatum progression. Plant Physiol. Biochem. 2017, 114, 88–99. [Google Scholar] [CrossRef]
- Velendia-Monsalve, J.; Viteri-Rosero, S.E.; Rubio-Cárdenas, N.J.; Tovar-Duarte, F.O. Efecto del fosfito de potasio en combinación con el fungicida metalaxyl + mancozeb en el control de mildeo velloso (Peronospora destructor Berk) en cebolla de bulbo (Allium cepa L.). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6317–6325. [Google Scholar]
- Raposo-Junior, L.L.; Gomes, N.J.A.; Silva, S.L.V. Evaluation of different foliar fertilizers on the crop production of sugarcane. J. Plant Nutr. 2013, 36, 459–469. [Google Scholar] [CrossRef]
- Martínez, S. Effects of combined application of potassium phosphite and fungicide on stem and sheath disease control, yield, and quality of rice. Crop Prot. 2016, 89, 259–264. [Google Scholar] [CrossRef]
- Figueira, E.P.P.; Kuhn, O.J.; Martinazzo-Portz, T.; Stangarlin, J.R.; Peliçon, P.M.M.; Lampugnani, C. Histochemical changes induced by Trichoderma spp. and potassium phosphite in common bean (Phaseolus vulgaris) in response to the attack by Colletotrichum lindemuthianum. Semin. Ciênc. Agrár. 2020, 41, 811–828. [Google Scholar] [CrossRef]
- Araujo, L.; Silva, B.W.M.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the phenylpropanoid pathway by acibenzolar-s-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Felipini, R.B.; Boneti, J.I.; Katsurayama, Y.; Rocha, N.A.C.; Veleirinho, B.; Maraschin, M.; di Piero, R.M. Apple scab control and activation of plant defence responses using potassium phosphite and chitosan. Eur. J. Plant Pathol. 2016, 145, 929–939. [Google Scholar] [CrossRef]
- de Oliveira, S.T.A.; Blum, L.E.B.; Duarte, E.A.A.; Luz, E.D.M.N. Reduction of papaya rot (Phytophthora palmivora) with phosphite and acibenzolar-s-methyl in preharvest and postharvest. Biosci. J. 2018, 34, 1522–1531. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Lin, M.; Chen, G.; Ding, X.; Weng, Q.; Chen, Q. Phosphite-induced reactive oxygen species production and ethylene and ABA biosynthesis, mediate the control of Phytophthora capsici in pepper (Capsicum annuum). Funct. Plant Biol. 2016, 43, 563–574. [Google Scholar] [CrossRef]
- Vinas, M.; Mendez, J.C.; Jiménez, V.M. Effect of foliar applications of phosphites on growth, nutritional status and defense responses in tomato plants. Sci. Hortic. 2020, 256, e109200. [Google Scholar] [CrossRef]
- Ramezani, M.; Rahmani, F.; Dehestani, A. Comparison between the effects of potassium phosphite and chitosan on changes in the concentration of cucurbitacin E and on antibacterial property of Cucumis sativus. BMC Complement. Altern. Med. 2017, 17, e295. [Google Scholar] [CrossRef]
- Han, X.; Xi, Y.; Zhang, Z.; Mohammadi, M.A.; Joshi, J.; Borza, T.; Wang-Pruski, G. Effects of phosphite as a plant biostimulant on metabolism and stress response for better plant performance in Solanum tuberosum. Ecotoxicol. Environ. Saf. 2021, 210, e111873. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; Estrada-Ortiz, E.; Gómez-Merino, F.C.; Becker, C.; Krumbein, A.; Schwarz, D. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Front. Plant Sci. 2019, 10, e371. [Google Scholar] [CrossRef]
- Lobato, M.C.; Olivieri, F.P.; Daleo, G.R.; Abreu, A.B. Antimicrobial activity of phosphites against different potato pathogens. J. Plant Dis. Prot. 2010, 1171, 102–109. [Google Scholar] [CrossRef]
- Lobato, M.C.; Machinandiarena, M.F.; Tambascio, C.; Dosio, G.A.A.; Caldiz, D.O.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Effect of foliar applications of phosphite on post-harvest potato tubers. Eur. J. Plant Pathol. 2011, 130, 155–163. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front. Plant Sci. 2015, 6, e16. [Google Scholar]
- Amiri, A.; Bompeix, G. Control of Penicillium expansum with potassium phosphite and heat treatment. Crop Prot. 2011, 30, 222–227. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Drenth, A. Phosphite and metalaxyl rejuvenate macadamia trees in decline caused by Phytophthora cinnamomi. Crop Prot. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Liljeroth, E.; Lankinen, Å.; Wiik, L.; Burra, D.D.; Alexandersson, E.; Andreasson, E. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Prot. 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Pinto, K.M.S.; do Nascimento, L.C.; Gomes, E.C.S.; da Silva, H.F.; Miranda, J.R. Efficiency of resistance elicitors in the management of grapevine downy mildew Plasmopara viticola: Epidemiological, biochemical and economic aspects. Eur. J. Plant Pathol. 2012, 134, 745–754. [Google Scholar] [CrossRef]
- Reuveni, M.; Sheglov, D.; Cohen, Y. Control of moldy-core decay in apple fruits by β-aminobutyric acids and potassium phosphites. Plant Dis. 2003, 87, 933–936. [Google Scholar] [CrossRef]
- Costa, G.B.H.; de Resende, M.L.V.; Ribeiro, J.P.M.; Mathioni, M.S.M.; da Silva, J.P.; da Silva, J.M.B. Suppression of rust and brown eye spot diseases on coffee by phosphites and by-products of coffee and citrus industries. J. Phytopathol. 2014, 162, 635–642. [Google Scholar] [CrossRef]
- Lobato, M.C.; Olivieri, F.P.; González, A.E.A.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O.; Andreu, A.B. Phosphite compounds reduce disease severity in potato seed tubers and foliage. Eur. J. Plant Pathol. 2008, 122, 349–358. [Google Scholar] [CrossRef]
- Quintero-Vargas, C.; Castaño-Zapata, J. Evaluación de inductores de resistencia para el manejo de nematodos fitoparásitos en plántulas de plátano. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2012, 36, 575–586. [Google Scholar] [CrossRef]
- Oka, Y.; Tkachi, N.; Mor, M. Phosphite inhibits development of the nematodes Heterodera avenae and Meloidogyne marylandi in cereals. Phytopathology 2007, 97, 396–404. [Google Scholar] [CrossRef]
- Días-Arieria, C.R.; Marini, P.M.; Fontana, L.F.; Roldi, M.; da Silva, T.R.B. Effect of Azospirillum brasilense, Stimulate® and potassium phosphite to control Pratylenchus brachyurus in soybean and maize. Nematropica 2012, 42, 170–175. [Google Scholar]
- Cohen, Y.; Coffey, M.D. Systemic fungicides and the control of oomycetes. Ann. Rev. Phytopathol. 1986, 24, 311–338. [Google Scholar] [CrossRef]
- Barrett, S.R.; Shearer, B.L.; Hardy, G.E.S. Efficacy of phosphite applied after inoculation on the colonization of Banksia brownii stems by Phytophthora cinnamomi. Australas. Plant Pathol. 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Jackson, T.J.; Burgess, T.; Colquhoun, I.; Hardy, G.E.S.J. Action of the fungicide phosphite on Eucalyptus marginate inoculated with Phytophthora cinnamomic. Plant Pathol. 2000, 49, 147–154. [Google Scholar] [CrossRef]
- Smillie, R.; Grant, B.R.; Guest, D. The mode of action of phosphite: Evidence for both direct and indirect modes of action on three Phytophthora spp. in plants. Phytopathology 1989, 79, 921–926. [Google Scholar] [CrossRef]
- Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. Effect of biocontrol agents and potassium phosphite against Phytophthora crown rot, caused by Phytophthora capsici, on zucchini in a closed soilless system. Sci. Hortic. 2020, 265, 109207. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yu, M.; Xu, J.; Du, H.; Zhang, R.; Wu, D.; Xie, X. Role of phosphite in the environmental phosphorus cycle. Sci. Total Environ. 2023, 881, e163463. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Jost, R.; Pharmawati, M.; Lapis-Gaza, H.R.; Rossig, C.; Berkowitz, O.; Lambers, H.; Finnegan, P.M. Differentiating phosphate-dependent and phosphate-independent systemic phosphate-starvation response networks in Arabidopsis thaliana through the application of phosphite. J. Exp. Bot. 2015, 66, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Herrera-Estrella, L. Engineering phosphorus metabolism in plants to produce a dual fertilization and weed control system. Nat. Biotechnol. 2012, 30, 889–893. [Google Scholar] [CrossRef]
- Manna, M.; Achary, V.M.M.; Islam, T.; Agrawal, P.K.; Reddy, M.K. The development of a phosphite-mediated fertilization and weed control system for rice. Sci. Rep. 2016, 6, 24941. [Google Scholar] [CrossRef]
- Pandeya, D.; López-Arredondo, D.L.; Janga, M.R.; Campbell, L.M.; Estrella-Hernández, P.; Bagavathiannan, M.V.; Herrera-Estrella, L.; Rathore, K.S. Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proc. Natl. Acad. Sci. USA 2018, 115, 6946–6955. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, T.; Lu, W.; Zhao, J.; Zhang, Z.; Xiao, G. The ptxD gene confers rapeseed the ability to utilize phosphite and a competitive advantage against weeds. Agronomy 2024, 14, 727. [Google Scholar] [CrossRef]
- Guo, W.; Lv, L.; Zhang, L.; Li, Q.; Wu, C.; Lu, X.; Lui, W.; Wang, J. Herbicides cross resistance of a multiple resistant short-awn foxtail (Alopecurus aequalis Sobol.) population in wheat field. Chil. J. Agric. Res. 2016, 72, 163–169. [Google Scholar] [CrossRef]
- Furlan-Kashivaqui, E.S.F.; Krzyzaniak, F.; Pires, A.; Moreira, S.A.F.; Albrecht, A.J.P.; Albrecht, L.P. Amaranthus hybridus L. resistant to glyphosate and chlorimuron in Paraguay. Chil. J. Agric. Anim. Sci. 2023, 39, 288–295. [Google Scholar] [CrossRef]
- Lamego, F.P.; Vidal, R.A.; Fleck, N.G.; Bianchi, M.A. Brachiaria plantaginea resistance to ACCase-inhibiting herbicides. Rev. Bras. Herb. 2002, 3, 162–168. [Google Scholar]
- Frenkel, E.; Matzrafi, M.; Rubin, B.; Peleg, Z. Effects of environmental conditions on the fitness penalty in herbicide resistant Brachypodium hybridum. Front. Plant Sci. 2017, 8, e94. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, E.; Martins da Silveira, H.; Alcántara-de la Cruz, R.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; de Prado, R. From tolerance to resistance: Mechanisms governing the differential response to glyphosate in Chloris barbata. Pest Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, J.R.; Hoyos, V.; Plaza, G.; Palma-Bautista, C.; Alcántara-de la Cruz, R.; de Prado, R. Glyphosate resistance in Chloris radiata from Colombian rice fields involves one target-site mechanism. Chemosphere 2021, 281, e130888. [Google Scholar] [CrossRef]
- van Etten, M.; Lee, K.M.; Chang, S.M.; Baucom, R.S. Parallel and nonparallel genomic responses contribute to herbicide resistance in Ipomoea purpurea, a common agricultural weed. PLoS Genet. 2020, 16, e1008593. [Google Scholar] [CrossRef]
- de Freitas, M.N.; Inojosa, F.L.A.; Vital, S.V.F.; Sato, T.C.A.; Padovese, L.M.; de Oliveira, J.R.S. Herbicides applied in pre- and post-emergence to control Chamaesyce hirta. Rev. Ceres Viçosa 2022, 69, 308–313. [Google Scholar] [CrossRef]
- Li, Z.; Boyd, N.; McLean, N.; Rutherford, K. Hexazinone resistance in red sorrel (Rumex acetosella). Weed Sci. 2014, 62, 532–537. [Google Scholar] [CrossRef]
- Bai, S.; Liu, W.; Wang, H.; Zhao, N.; Jia, S.; Zou, N.; Guo, W.; Wan, J. Enhanced herbicide metabolism and metabolic resistance genes identified in tribenuron-methyl resistant Myosoton aquaticum L. J. Agric. Food Chem. 2018, 66, 9850–9857. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; López, M.L. Control of Oxalis latifolia: A review and proposals for its improvement. Cienc. Investig. Agrar. 2008, 35, 121–136. [Google Scholar]
- Masabni, J.G.; Zandstra, B.H. Discovery of a common purslane (Portulaca oleracea) biotype resistant to linuron. Weed Technol. 1999, 13, 599–605. [Google Scholar] [CrossRef]
- Mathukia, R.K.; Sagarka, B.K.; Panara, D.M.; Gohil, B.S. Efficacy of some post-emergence herbicides and their mixtures against complex weed flora in wheat. Int. J. Econ. Plants 2018, 5, 23–26. [Google Scholar] [CrossRef]
- Varanasi, A.; Vara-Prasad, P.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar]
- Vats, S. Herbicides: History, classification and genetic manipulation of plants for herbicide resistance. In Sustainable Agriculture Reviews. Sustainable Agriculture Reviews, 1st ed.; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2015; pp. 153–192. [Google Scholar]
- López-Arredondo, D.L.; Herrera-Estrella, L. A novel dominant selectable system for the selection of transgenic plants under in vitro and greenhouse conditions based on phosphite metabolism. Plant Biotechnol. J. 2023, 11, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Fang, H.; Pan, Q.; Xu, H.; Lv, T.; Fan, X.; Wang, Y.; Guo, Y.; Mou, L.; Xu, J.; et al. Seed microbiome-mediated herbicide resistance evolution in weeds. New Phytol. 2024, 242, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W. Genome evolution in plants and the origins of innovation. New Phytol. 2023, 240, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Manghi, M.C.; Masiol, M.; Calzavara, R.; Graziano, P.L.; Peruzzi, E.; Pavoni, B. The use of phosphonates in agriculture. Chemical, biological properties and legislative issues. Chemosphere 2021, 283, 131187. [Google Scholar] [CrossRef]
- de la Pasture, L. Understanding phosphites. Tech Talk. December 2020, pp. 42–44. Available online: https://www.uniumbioscience.com/wp-content/uploads/2021/04/CPM-PhosphiteTechDec20.pdf (accessed on 10 November 2024).
- Leymonie, J.P. Phosphites and Phosphates: When Distributors and Growers Alike Could Get Confused! New AG International: London, UK, 2007; pp. 36–41. [Google Scholar]
- Mendes, K.F.; Mielke, K.C.; D’Antonino, L.; Alberto da Silva, A. Retention, absorption, translocation, and metabolism of herbicides in plants. In Applied Weed and Herbicide Science, 1st ed.; Mendes, K.F., Alberto da Silva, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 157–186. [Google Scholar]
- Yu, H.; Lu, X.; Miki, T.; Matsubae, K.; Sasaki, Y.; Nagasaka, T. Sustainable phosphorus supply by phosphorus recovery from steelmaking slag: A critical review. Resour. Conserv. Recycl. 2022, 180, 106203. [Google Scholar] [CrossRef]
- Mew, M.C.; Steiner, G.; Geissler, B. Phosphorus supply chain—Scientific, technical, and economic foundations: A transdisciplinary orientation. Sustainability 2018, 10, 1087. [Google Scholar] [CrossRef]
- Shin, N.H.; Cho, L.H. Phosphate depletion: Research status and challenges in agriculture. J. Plant Biotechnol. 2024, 51, 129–142. [Google Scholar] [CrossRef]


| Weed Common Name | Weed Species | Phi Concentration Applied (Source) | Method of Application | Source |
|---|---|---|---|---|
| Shortawn foxtail | Alopecurus aequalis (Solol.: Poaceae) | 100 or 200 mg kg−1 (K2HPO3) | Salt applied to the soil | [51] |
| Green amaranth | Amaranthus hybridus (L.: Amaranthaseae) | 80 or 120 mg kg−1 (KH2PO3) | Salt applied to the soil | [48] |
| Palmer’s amaranth | Amaranthus palmeri (Watson: Amaranthaseae) | 80 or 120 mg kg−1 (K2HPO3) | Salt applied to the soil | [50] |
| Spiny amaranth | Amaranthus sponosus (L.: Amaranthaseae) | 500 mM (Na2HPO3·5H2O) | Solution applied to the soil | [49] |
| Plantain signalgrass | Brachiaria plantaginea [(Link) Hitchcock: Poaceae] | 80 or 120 mg kg−1 (KH2PO3) | Salt applied to the soil | [48] |
| False brome | Brachypodium distachyon [(L.) P.Beauv.: Poaceae] | 80 or 120 mg kg−1 (KH2PO3, K2HPO3) | Salt applied to the soil | [48,50] |
| Swollen fingergrass | Chloris barbata [Swartz: Poaceae] | 500 mM (Na2HPO3·5H2O) | Solution applied to the soil | [49] |
| Snakeweed | Euphorbia hirta (L.: Euphorbiaceae) | 500 mM (Na2HPO3·5H2O) | Foliar application | [49] |
| Common morning glory | Ipomoea purpurea (Roth: Convolvulaceae) | 80 or 120 mg kg−1 (KH2PO3, K2HPO3) | Salt applied to the soil | [48,50] |
| Water chickweed | Malachium aquaticum [(L.) Moench: Caryophyllaceae] | 100 or 200 mg kg−1 (K2HPO3) | Salt applied to the soil | [51] |
| Sourgrass | Oxalis sp. (L.: Oxalidaceae) | 500 mM (Na2HPO3·5H2O) | Foliar application | [49] |
| Purslane | Portulaca oleracea (L.: Portulacaceae) | 500 mM (Na2HPO3·5H2O) | Foliar application | [49] |
| Gale of the wind | Phyllanthus niruri (L.; Phyllanthaceae) | 500 mM (Na2HPO3·5H2O) | Foliar application | [49] |
| Common sorrel | Rumex acetosa (L.: Polygonaceae) | 100 or 200 mg kg−1 (K2HPO3) | Salt applied to the soil | [51] |
| Salt Applied to the Soil | ||||
| Weed Species | Phi Concentration Applied in mg kg−1 (Source) | Dose (kg ha−1) a | Source | |
| Alopecurus aequalis | 100 or 200 (K2HPO3) | 270 or 540 | [51] | |
| Amaranthus hybridus | 80 or 120 (KH2PO3) | 216 or 324 | [48] | |
| Amaranthus palmeri | 80 or 120 (K2HPO3) | 216 or 324 | [50] | |
| Brachiaria plantaginea | 80 or 120 (KH2PO3) | 216 or 324 | [48] | |
| Brachypodium distachyon | 80 or 120 (KH2PO3, K2HPO3) | 216 or 324 | [48,50] | |
| Ipomoea purpurea | 80 or 120 (KH2PO3, K2HPO3) | 216 or 324 | [48,50] | |
| Malachium aquaticum | 100 or 200 (K2HPO3) | 270 or 540 | [51] | |
| Rumex acetosa | 100 or 200 (K2HPO3) | 270 or 540 | [51] | |
| Application in Solution | ||||
| Weed Species | Phi Concentration Applied in mM (Source) | Method of Application | Doses (kg ha−1) b | Source |
| Amaranthus sponosus | 500 (Na2HPO3·5H2O) | Soil application | 21.6 | [49] |
| Chloris barbata | 500 (Na2HPO3·5H2O) | Soil application | 21.6 | [49] |
| Euphorbia hirta | 500 (Na2HPO3·5H2O) | Foliar application | 21.6 | [49] |
| Oxalis sp. | 500 (Na2HPO3·5H2O) | Foliar application | 21.6 | [49] |
| Portulaca oleracea | 500 (Na2HPO3·5H2O) | Foliar application | 21.6 | [49] |
| Phyllanthus niruri | 500 (Na2HPO3·5H2O) | Foliar application | 21.6 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo-Téllez, L.I.; Carbajal-Vázquez, V.H.; Lavín-Castañeda, J.; Gómez-Merino, F.C. Phosphite as a Sustainable and Versatile Alternative for Biostimulation, Biocontrol, and Weed Management in Modern Agriculture. Processes 2024, 12, 2764. https://doi.org/10.3390/pr12122764
Trejo-Téllez LI, Carbajal-Vázquez VH, Lavín-Castañeda J, Gómez-Merino FC. Phosphite as a Sustainable and Versatile Alternative for Biostimulation, Biocontrol, and Weed Management in Modern Agriculture. Processes. 2024; 12(12):2764. https://doi.org/10.3390/pr12122764
Chicago/Turabian StyleTrejo-Téllez, Libia Iris, Víctor Hugo Carbajal-Vázquez, Jazmín Lavín-Castañeda, and Fernando Carlos Gómez-Merino. 2024. "Phosphite as a Sustainable and Versatile Alternative for Biostimulation, Biocontrol, and Weed Management in Modern Agriculture" Processes 12, no. 12: 2764. https://doi.org/10.3390/pr12122764
APA StyleTrejo-Téllez, L. I., Carbajal-Vázquez, V. H., Lavín-Castañeda, J., & Gómez-Merino, F. C. (2024). Phosphite as a Sustainable and Versatile Alternative for Biostimulation, Biocontrol, and Weed Management in Modern Agriculture. Processes, 12(12), 2764. https://doi.org/10.3390/pr12122764







