Modification of Cotton with Chitosan: Deposition of Copper(II) Sulfate by Complexation Copper Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. COT-CuSO4 and COT-CuSO4-CTS Sample Preparation
2.2.2. Copper Concentration Analysis
2.2.3. Optical and SEM Examination Characterization
2.2.4. Evaluating UV Protection and Transmission of Fabrics
2.2.5. Coagulation Parameters: aPTT and PT Measurement
2.2.6. Antimicrobial Activity
2.2.7. PBM Cells
2.2.8. Cell Viability by Resazurin Assay
2.2.9. DNA Damage by the Comet Assay
2.2.10. Plasmid Relaxation Assay
2.2.11. Statistical Analysis
3. Results
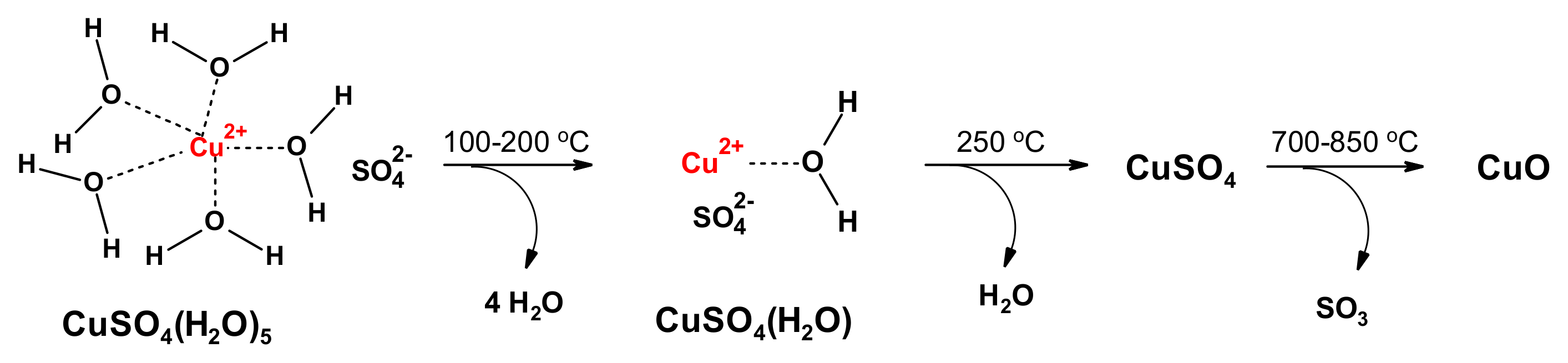
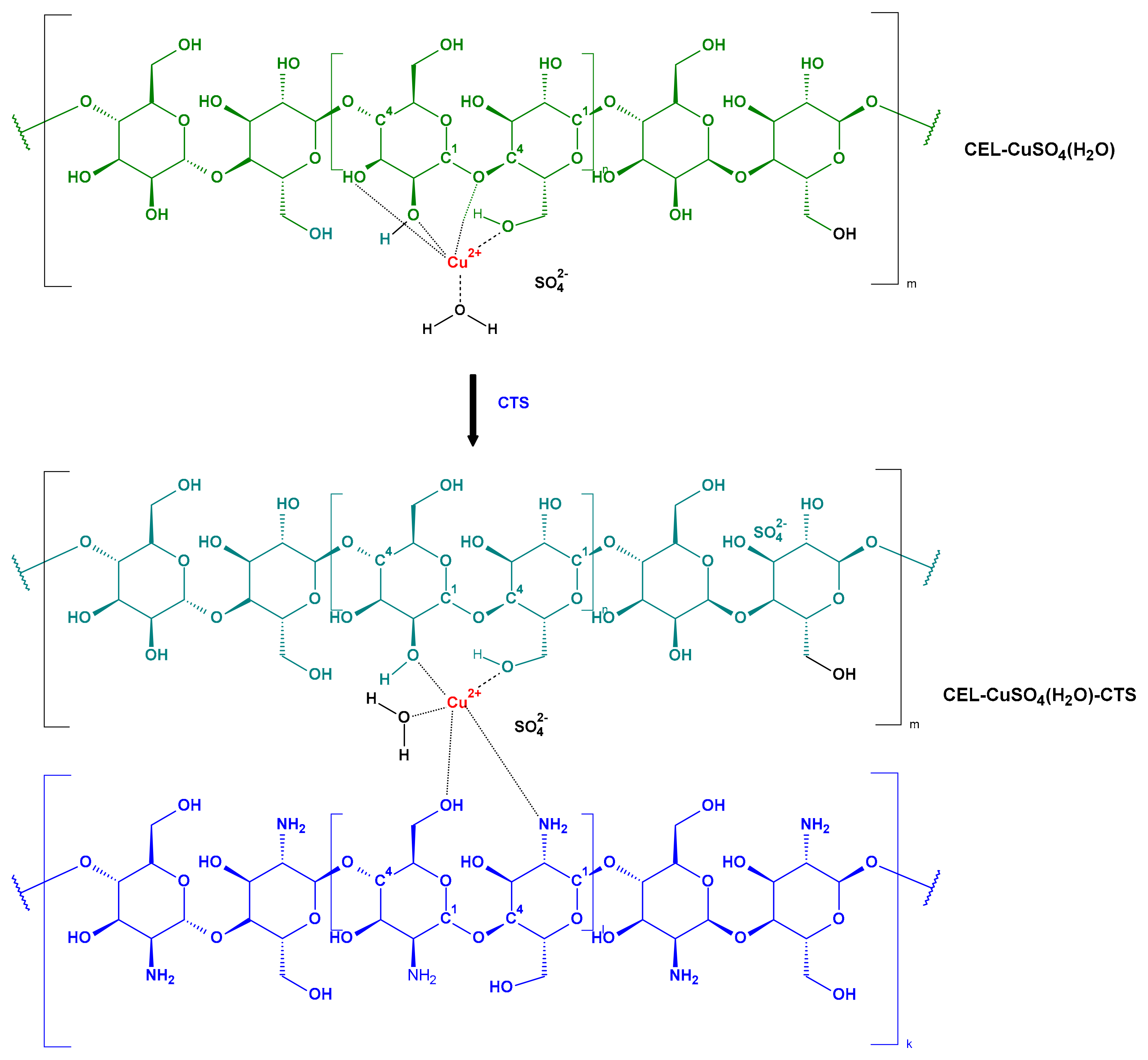
3.1. Preparation of Composite Materials
Preparation of COT-CuSO4
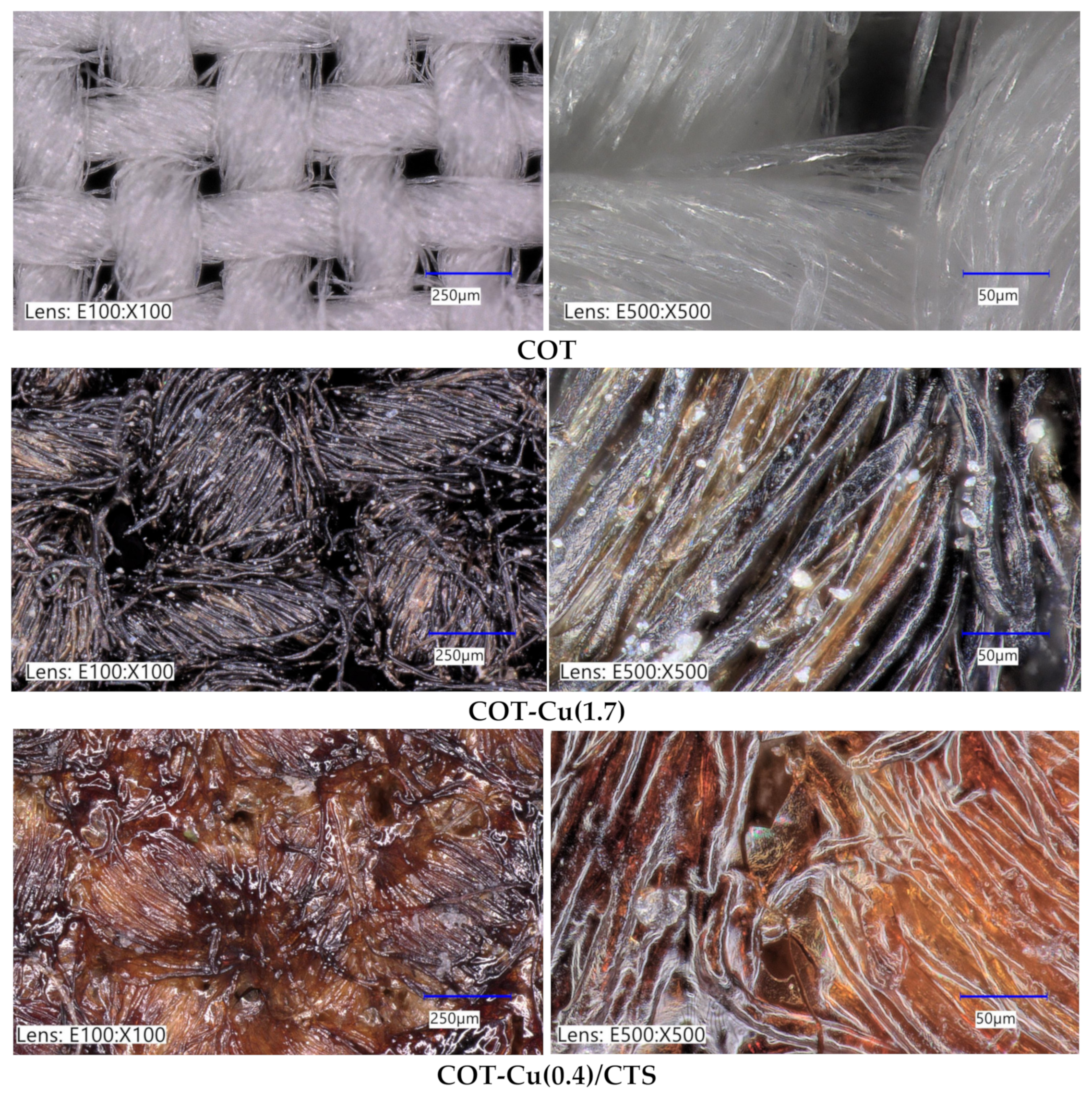
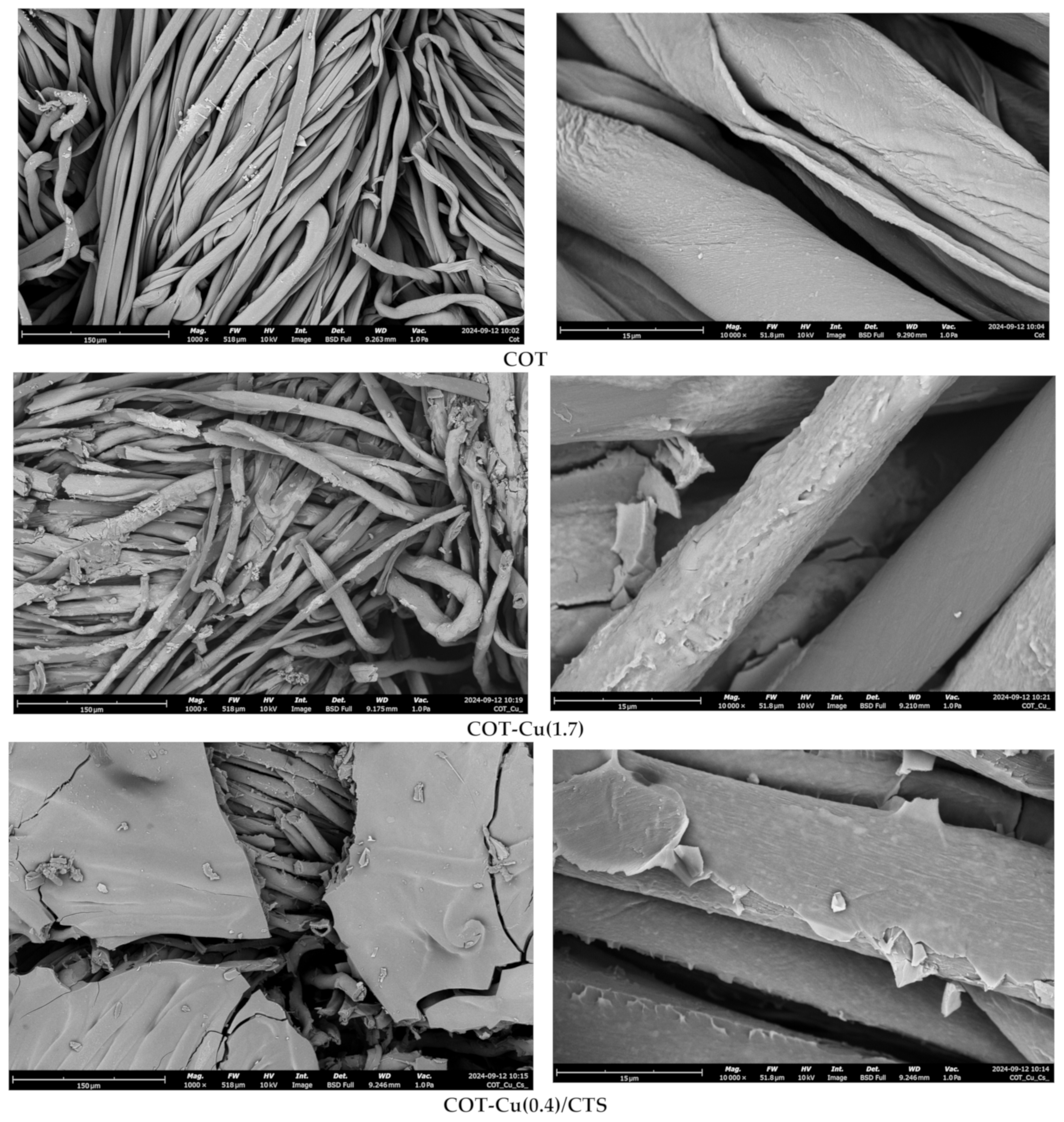
3.2. Optical and SEM Analysis
3.3. EDS Analysis
3.4. UV-VIS Analysis and UV Protection Assessment
3.5. Results of Biological Activity of Materials
3.5.1. Activity Against Microorganisms
3.5.2. Evaluation of Activated aPTT and PT
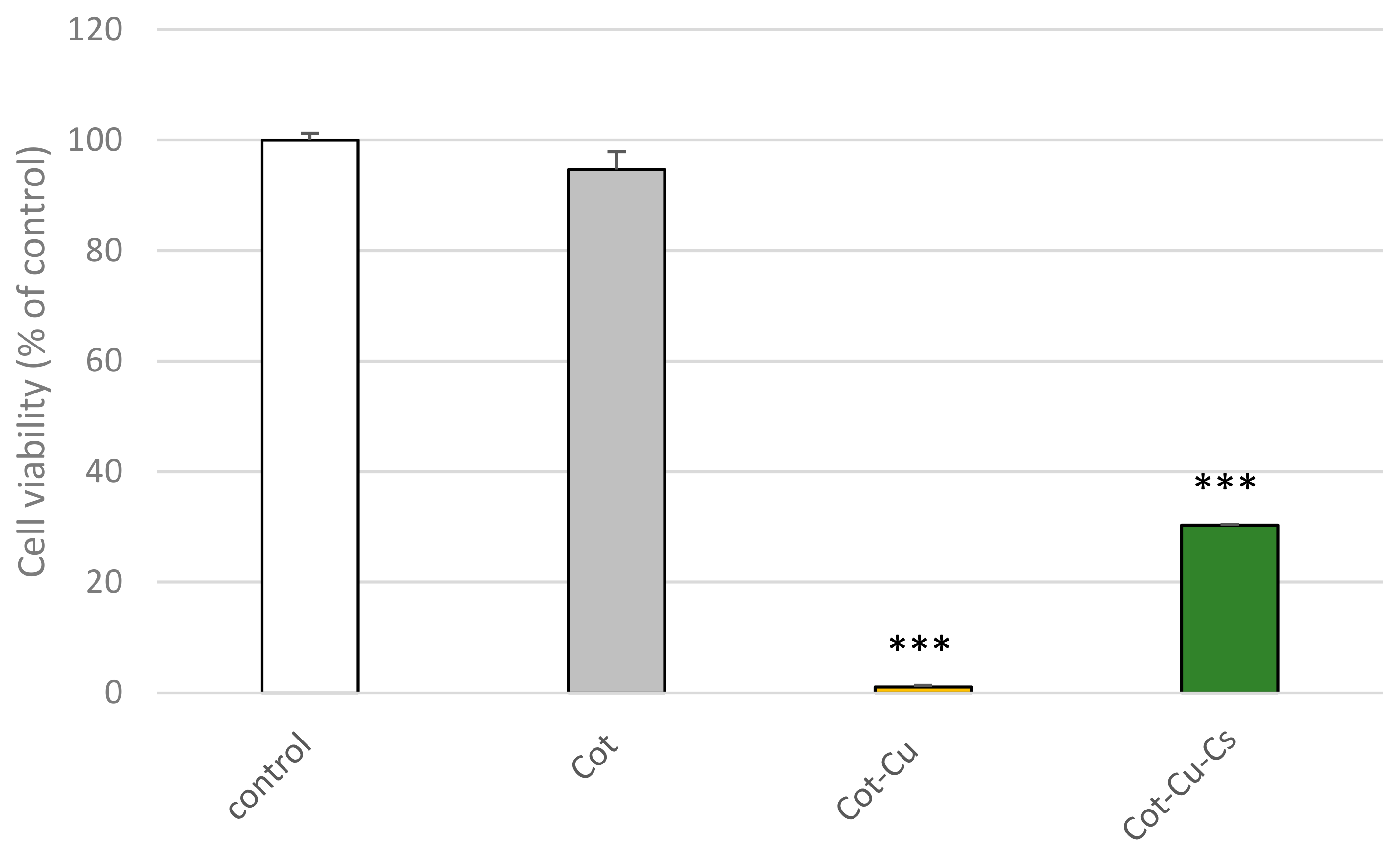
3.5.3. Effect of Cotton Samples on the Viability of PBM Cells
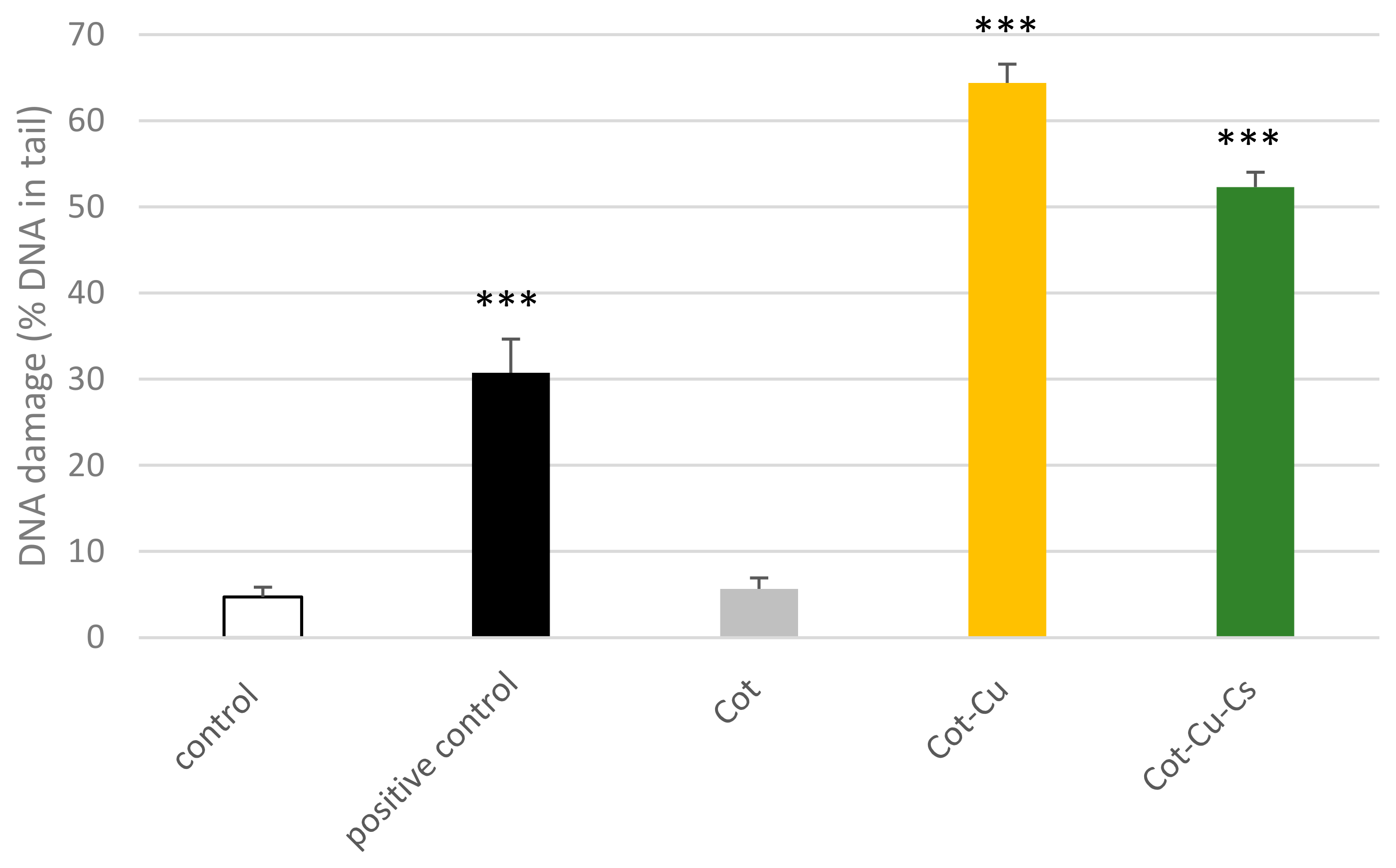
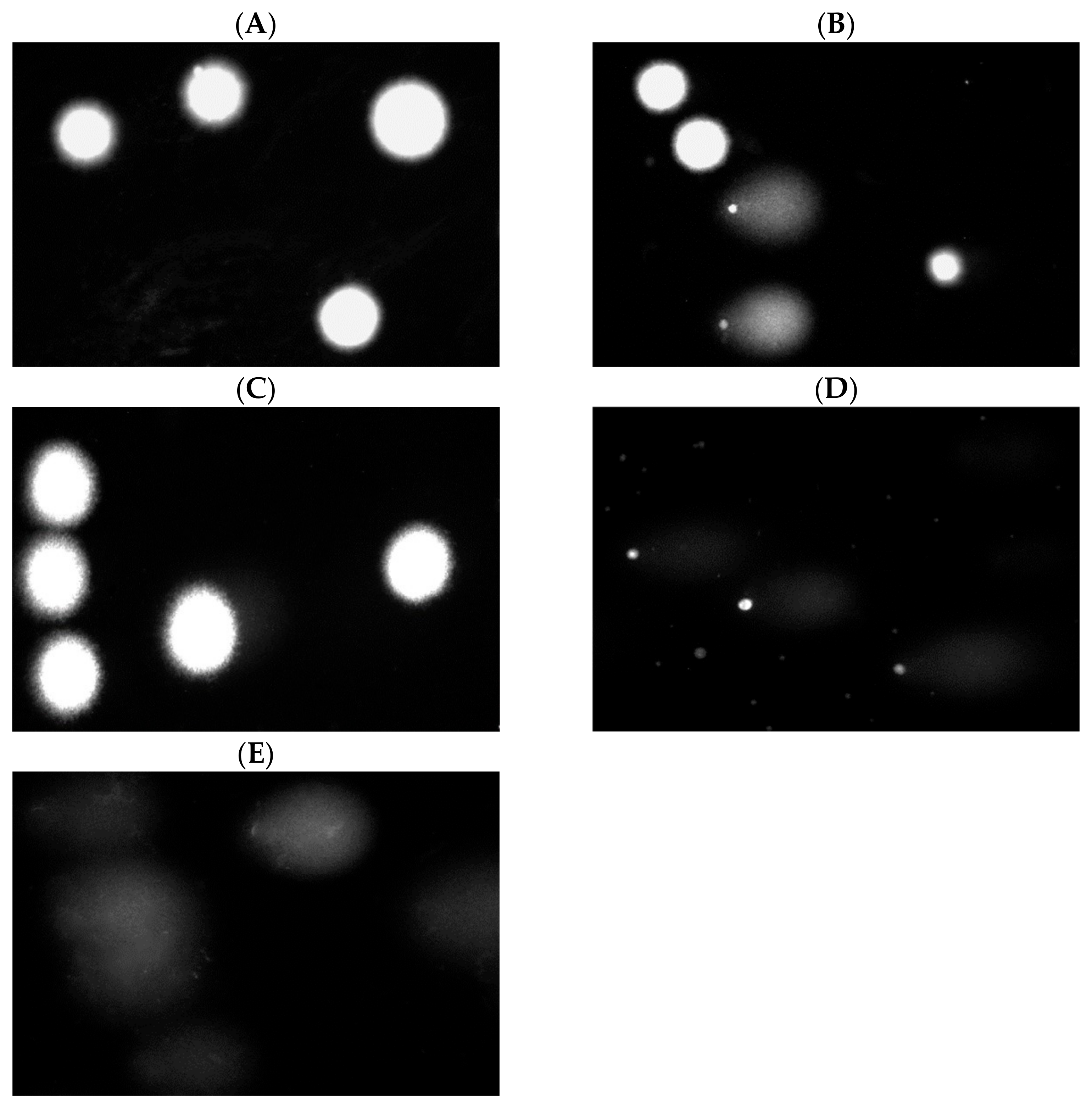
3.5.4. Effect of Cotton Samples on DNA Damage
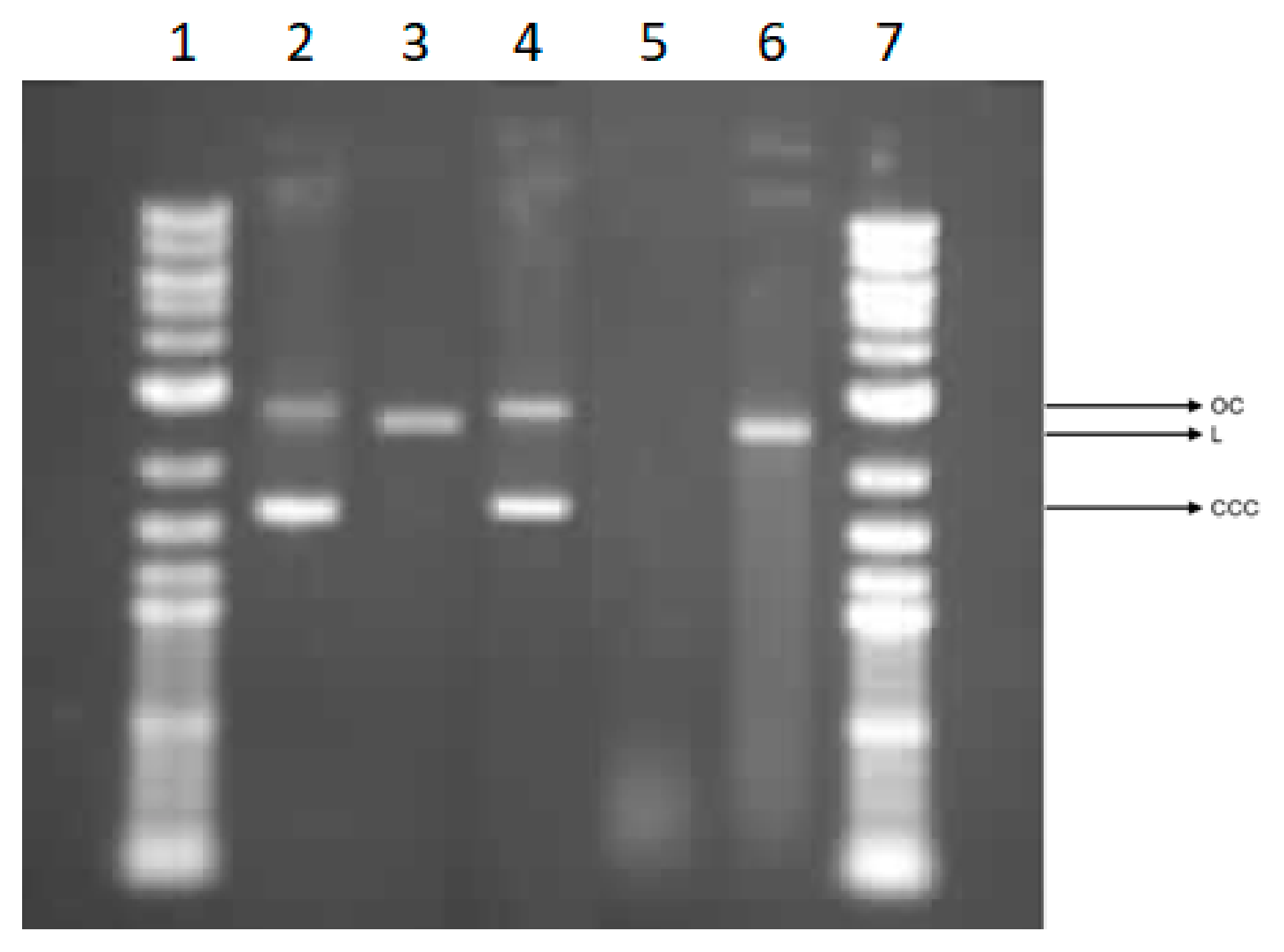
3.5.5. Effect of Cotton Samples on Plasmid DNA
4. Conclusions
- Enhanced Antimicrobial Properties: The modification of medical cotton fibers with copper sulfate and chitosan, followed by thermal processing, enhances the material’s antimicrobial effectiveness, positioning it as a promising candidate for advanced medical textiles. This modified cotton is highly suitable for healthcare and hygiene applications due to its improved functional properties.
- Chemical and Structural Analysis: Detailed analyses of the chemical composition and structural properties of the modified fibers were conducted, employing methods such as flame atomic absorption spectrometry (FAAS); SEM; EDS, etc.
- Impact on Blood Coagulation: Thia study thoroughly examined the biochemical characteristics of the modified material, particularly regarding its effects on hemostatic processes. Blood coagulation effects were evaluated through measurements of activated partial thromboplastin time (aPTT) and prothrombin time (PT), offering insights into the modified cotton’s interactions with blood plasma clotting mechanisms. Thermal reduction of copper(II) sulfate on the cotton surface to copper(II) oxide resulted in notable changes in blood clotting, specifically extending both aPTT and PT times due to the anticoagulant properties of copper. The addition of a chitosan layer on the modified cotton surface reduced the anticoagulant effect, though a prolongation of clotting time persisted when compared to unmodified cotton.
- Cytotoxic and Genotoxic Effects: Pure cotton did not reduce cell viability, cause DNA damage, or interact with plasmid DNA, demonstrating a benign profile for cellular integrity. In contrast, copper-modified cotton COT-Cu(1.7) and copper–chitosan-modified cotton (COT-Cu(0.4)/CTS) showed potential to induce cyto- and genotoxicity in peripheral blood mononuclear (PBM) cells, with COT-Cu(0.4)/CTS exhibiting lower cytotoxic and genotoxic effects than COT-Cu(1.7). COT-Cu(0.4)/CTS fabrics thus demonstrate decreased cyto- and genotoxic potential, suggesting chitosan may mitigate some cytotoxic properties associated with copper modification.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudzin, M.H.; Giełdowska, M.; Król, P.; Sobanska, Z. Preparation of Cotton–Zinc Composites by Magnetron Sputtering Metallization and Evaluation of their Antimicrobial Properties and Cytotoxicity. Materials 2022, 15, 2746. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, M.H.; Giełdowska, M.; Krata, A.A.; Sulak, E.; Urbaniak, P.; Drabowicz, J. Phosphorylation of Chitosan (Chitin) Surface with PCl3. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 625–629. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Rojas, O.J. (Ed.) Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Part of the book series: Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 271, 341p. [Google Scholar] [CrossRef]
- Mahbubul Bashar, M.; Khan, M.A. An Overview on Surface Modification of Cotton Fiber for Apparel Use. J. Polym. Environ. 2013, 21, 181–190. [Google Scholar] [CrossRef]
- Ahmed, F.; Mondal, M.d.I.H. 1—Introduction to Natural Fibres and Textiles. In Fundamentals of Natural Fibres and Textiles; Mondal, M.d.I.H., Ed.; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–32. ISBN 978-0-12-821483-1. [Google Scholar]
- Lam, Y.-L.; Kan, C.-W.; Yuen, C.-W.M. Developments in Functional Finishing of Cotton Fibres—Wrinkle-Resistant, Flame-Retardant and Antimicrobial Treatments. Text. Prog. 2012, 44, 175–249. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Shaid, A.; Khan, M.A. Cationization of Cotton Fiber by Chitosan and Its Dyeing with Reactive Dye without Salt. Chem. Mater. Eng. Cease Publ. 2014, 2, 96–100. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Li, Y.; Lyu, B.; Ren, J.; Ma, J. Long-Acting Antibacterial Activity on the Cotton Fabric. Cellulose 2021, 28, 1221–1240. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal Textiles Can Help Fight Nosocomial Infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Halepoto, H.; Gong, T.; Memon, H. A Bibliometric Analysis of Antibacterial Textiles. Sustainability 2022, 14, 11424. [Google Scholar] [CrossRef]
- Rahman Bhuiyan, M.A.; Hossain, M.A.; Zakaria, M.; Islam, M.N.; Zulhash Uddin, M. Chitosan Coated Cotton Fiber: Physical and Antimicrobial Properties for Apparel Use. J. Polym. Environ. 2017, 25, 334–342. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.E.; Mohamed, M.I.; Ahmed, H.Y.; Elaasser, M.M.; Kandile, N.G. Fabrication and Characterization of Unique Sustain Modified Chitosan Nanoparticles for Biomedical Applications. Sci. Rep. 2024, 14, 13869. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent Applications of Chitosan and Its Derivatives in Antibacterial, Anticancer, Wound Healing, and Tissue Engineering Fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent Advances in Cellulose, Chitosan, and Alginate Based Biopolymeric Composites for Adsorption of Heavy Metals from Wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan Derivatives Obtained by Chemical Modifications for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- da Silva, D.J.; Ferreira, R.R.; da S. Ferreira, G.; Barbosa, R.F.S.; Marciano, J.S.; Camani, P.H.; Souza, A.G.; Rosa, D.S. Multifunctional Cotton Fabrics with Novel Antibacterial Coatings Based on Chitosan Nanocapsules and Polyacrylate. J. Coat. Technol. Res. 2023, 20, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications Chitosan; Springer: Berlin/Heidelberg, Germany, 2020; pp. 457–489. [Google Scholar] [CrossRef]
- Liu, X.D.; Nishi, N.; Tokura, S.; Sakairi, N. Chitosan Coated Cotton Fiber: Preparation and Physical Properties. Carbohydr. Polym. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, G.; Zheng, W.; Fu, J.; Fu, F.; Hu, X.; Jin, Z.; Liu, X. Remarkable Durability of the Antibacterial Function Achieved via a Coordination Effect of Cu(II) Ion and Chitosan Grafted on Cotton Fibers. Cellulose 2022, 29, 1003–1015. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Chelation Capability of Chitosan and Chitosan Derivatives: Recent Developments in Sustainable Corrosion Inhibition and Metal Decontamination Applications. Curr. Res. Green Sustain. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Gamage, A.; Jayasinghe, N.; Thiviya, P.; Wasana, M.L.D.; Merah, O.; Madhujith, T.; Koduru, J.R. Recent Application Prospects of Chitosan Based Composites for the Metal Contaminated Wastewater Treatment. Polymers 2023, 15, 1453. [Google Scholar] [CrossRef]
- Hassabo, A.G.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A.A. Development of Multifunctional Modified Cotton Fabric with Tri-Component Nanoparticles of Silver, Copper and Zinc Oxide. Carbohydr. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Ge, N.; Shen, L.; Zhang, Y.; Fu, F.; Liu, X. Preparation of Copper Nanoparticles Coated Cotton Fabrics with Durable Antibacterial Properties. Fibers Polym. 2018, 19, 1004–1013. [Google Scholar] [CrossRef]
- Anita, S.; Ramachandran, T.; Rajendran, R.; Koushik, C.; Mahalakshmi, M. A Study of the Antimicrobial Property of Encapsulated Copper Oxide Nanoparticles on Cotton Fabric. Text. Res. J. 2011, 81, 1081–1088. [Google Scholar] [CrossRef]
- Qian, J.; Dong, Q.; Chun, K.; Zhu, D.; Zhang, X.; Mao, Y.; Culver, J.N.; Tai, S.; German, J.R.; Dean, D.P.; et al. Highly Stable, Antiviral, Antibacterial Cotton Textiles via Molecular Engineering. Nat. Nanotechnol. 2023, 18, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Devaraji, M.; Thanikachalam, P.V.; Elumalai, K. The potential of Copper Oxide Nanoparticles in Nanomedicine: A Comprehensive Review. Biotechnol. Notes 2024, 5, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Wicaksono, D.H.B.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial Treatment of Different Metal Oxide Nanoparticles: A Critical Review. J. Chin. Chem. Soc. 2016, 63, 385–393. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Agrawal, N.; Low, P.S.; Tan, J.S.J.; Fong, E.W.M.; Lai, Y.; Chen, Z. Durable Easy-Cleaning and Antibacterial Cotton Fabrics Using Fluorine-Free Silane Coupling Agents and CuO Nanoparticles. Nano Mater. Sci. 2020, 2, 281–291. [Google Scholar] [CrossRef]
- Belcastro, S.; Staffolani, N.; Pugliese, M.; D’Alo, F. An in-vitro Study of the Antimicrobial Activity of Copper and Zinc Salts on Pure and Mixed Microbial Cultures. Minerva Stomatol. 1994, 43, 393–396. [Google Scholar]
- Ghoranneviss, M.; Shahidi, S. Effect of Various Metallic Salts on Antibacterial Activity and Physical Properties of Cotton Fabrics. J. Ind. Text. 2013, 42, 193–203. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity Against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- Cheknev, S.B.; Vostrova, E.I.; Apresova, M.A.; Piskovskaya, L.S.; Vostrov, A.V. Deceleration of Bacterial Growth in Staphylococcus Aureus and Pseudomonas Aeruginosa Cultures in the Presence of Copper and Zinc Cations. Zh. Mikrobiol. Epidemiol. Immunobiol. 2015, 2, 9–17, PMID: 26016338. [Google Scholar] [PubMed]
- Cheknev, S.V.; Vostrova, E.L.; Sarycheva, M.A.; Vostrov, A.V. Inhibition of Growth of Bacteria in Staphylococcus Aureus and Pseudomonasaeruginosa Cultures by Copper and Zinc Cations, Applied at Physiological Concentrations. Zh. Mikrobiol. Epidemiol. Immunobiol. 2016, 3, 9–18, PMID: 30695447. [Google Scholar] [PubMed]
- Febré, N.; Silva, V.; Báez, A.; Palza, H.; Delgado, K.; Aburto, I.; Silva, V. Antibacterial Activity of Copper Salts Against Microorganisms Isolated from Chronic Infected Wounds. Rev. Medica Chile 2016, 144, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Fowler, L.; Engqvist, H.; Öhman-Mägi, C. Effect of Copper Ion Concentration on Bacteria and Cells. Materials 2019, 12, 3798. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, L.; Amri, S.; Bensouilah, M.; Ouzrout, R. Antibacterial Effect of Copper Sulfate against Multi-Drug Resistant Nosocomial Pathogens Isolated from Clinical Samples. Pak. J. Med. Sci. 2019, 35, 1322–1328. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Qamar Khan, M.Q.; Ullah, A.; Bie, X.; Taguchi, G.; Kim, I.S. Antibacterial Mechanisms of Various Copper Species Incorporated in Polymeric Nanofibers against Bacteria. Mater. Today Commun. 2020, 25, 101377. [Google Scholar] [CrossRef]
- Popov, S.; Saphier, O.; Popov, M.; Shenker, M.; Entus, S.; Shotland, Y.; Saphier, M. Factors Enhancing the Antibacterial Effect of Monovalent Copper Ions. Curr. Microbiol. 2020, 77, 361–368. [Google Scholar] [CrossRef]
- Pereira, F.S.; de Souza, G.G.; Moraes, P.G.P.; Barroso, R.P.; Lanfredi, S.; Gomes, H.M.; Costa-Filho, A.J.; González, E.R.P. Study of Chitosans Interaction with Cu(II) from the Corresponding Sulfate and Chloride Salts. Cellulose 2015, 22, 2391–2407. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Boguń, M.; Mrozińska, Z.; Kaczmarek, A. Physical Properties, Chemical Analysis, and Evaluation of Antimicrobial Response of New Polylactide/Alginate/Copper Composite Materials. Mar. Drugs 2020, 18, 660. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Kaczmarek, A.; Mrozińska, Z.; Olczyk, J. Deposition of Copper on Polyester Knitwear Fibers by a Magnetron Sputtering System. Physical Properties and Evaluation of Antimicrobial Response of New Multi-Functional Composite Materials. Appl. Sci. 2020, 10, 6990. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Kaczmarek, A.; Lisiak-Kucińska, A. Deposition of Copper on Poly(Lactide) Non-Woven Fabrics by Magnetron Sputtering-Fabrication of New Multi-Functional, Antimicrobial Composite Materials. Materials 2020, 13, 3971. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Giełdowska, M.; Mrozińska, Z.; Boguń, M. Poly(Lactic acid)/zinc/alginate Complex Material: Preparation and Antimicrobial Properties. Antibiotics 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Mrozińska, Z.; Ponczek, M.; Kaczmarek, A.; Boguń, M.; Sulak, E.; Kudzin, M.H. Blood Coagulation Activities of Cotton–Alginate–Copper Composites. Mar. Drugs 2023, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Mrozińska, Z.; Świerczyńska, M.; Juszczak, M.; Woźniak, K.; Kudzin, M.H. Poly(Lactide) Nonwoven Fabric with Iron Coating and its Biological Properties. Coatings 2024, 14, 1050. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kudzin, M.H.; Ponczek, M.B.; Kaczmarek, A.; Król, P.; Lisiak-Kucińska, A.; Żyłła, R.; Walawska, A. Biochemical Approach to Poly(Lactide)–Copper Composite—Impact on Blood Coagulation Processes. Materials 2024, 17, 608. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kaczmarek, A.; Świerczyńska, M.; Juszczak, M.; Kudzin, M.H. Biochemical Behavior, Influence on Cell DNA Condition, and Microbiological Properties of Wool and Wool–Copper Materials. Materials 2024, 17, 2878. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Mrozińska, Z.; Lisiak-Kucińska, A.; Walawska, A.; Kudzin, M.H. Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology. Coatings 2024, 14, 843. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Mrozińska, Z.; Juszczak, J.; Woźniak, K.; Kudzin, M.H. Preparation and Biochemical Activity of Copper-Coated Cellulose Nonwoven Fabric via Magnetron Sputtering and Alginate-Calcium Ion Complexation. Mar. Drugs 2024, 22, 436. [Google Scholar] [CrossRef]
- Sen, P.; Kemppainen, E.; Orešič, M. Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front. Mol. Biosci. 2018, 4, 96. [Google Scholar] [CrossRef]
- Miquelestorena-Standley, E.; da Silva, A.V.V.; Monnier, M.; Chadet, S.; Piollet, M.; Héraud, A.; Lemoine, R.; Bochaton, T.; Derumeaux, G.; Roger, S.; et al. Human Peripheral Blood Mononuclear Cells Display a Temporal Evolving Inflammatory Profile after Myocardial Infarction and Modify Myocardial Fibroblasts Phenotype. Sci. Rep. 2023, 13, 16745. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Vieira-da-Silva, B.; Castanho, M.A.R.B. Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules 2023, 28, 2283. [Google Scholar] [CrossRef]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and Its Consequences. In Molecular, Clinical and Environmental Toxicology: Volume 1: Molecular Toxicology; Luch, A., Ed.; Birkhäuser: Basel, Switzerland, 2009; pp. 87–110. ISBN 978-3-7643-8336-7. [Google Scholar]
- Rojas, E.; Lopez, M.C.; Valverde, M. Single Cell Gel Electrophoresis Assay: Methodology and Applications. J. Chromatogr. B Biomed. Appl. 1999, 722, 225–254. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ganguly, A.; Roy, A.; BoseDasgupta, S.; D’Annessa, I.; Desideri, A.; Majumder, H.K. ATP Independent Type IB Topoisomerase of Leishmania Donovani Is Stimulated by ATP: An Insight into the Functional Mechanism. Nucleic Acids Res. 2011, 39, 3295–3309. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial Applications of Copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 13758-1+A1:2007; Textiles—Protective Properties against UV Radiation—Part 1: Test Method for Flat Textile Products. International Organization for Standardization: Geneva, Switzerland, 2007.
- EN ISO 20645:2006; Textile Fabrics. Determination of Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- EN 14119: 2005 Point 10.5 (B2); Testing of Textiles. Evaluation of the Action of Microfungi. Visual Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-Trans Retinoic Acid Modulates DNA Damage Response and the Expression of the VEGF-A and MKI67 Genes in ARPE-19 Cells Subjected to Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef]
- Gadalla, A.M. Kinetics of Thermal Decomposition of CuSO4·5H2O to CuO. Int. J. Chem. Kinet. 1984, 16, 655–668. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary (=Magyar Állami Földtani Intézet): Budapest, Hungary, 2011; ISBN 978-963-671-288-4. [Google Scholar]
- Pasquarello, A.; Petri, I.; Salmon, P.S.; Parisel, O.; Car, R.; Tóth, É; Powell, D.H.; Fischer, H.E.; Helm, L.; Merbach, A.E. First Solvation Shell of the Cu(II) Aqua Ion: Evidence for Fivefold Coordination. Science 2001, 291, 856–859. [Google Scholar] [CrossRef]
- McNaught, A.D. Nomenclature of carbohydrates (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1919–2008. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Kibria, G.; Repon, M.R.; Hossain, M.F.; Islam, T.; Jalil, M.A.; Aljabri, M.D.; Rahman, M.M. UV-Blocking Cotton Fabric Design for Comfortable Summer Wears: Factors, Durability and Nanomaterials. Cellulose 2022, 29, 7555–7585. [Google Scholar] [CrossRef]
- Tran Thi, V.H.; Lee, B.-K. Development of Multifunctional Self-Cleaning and UV Blocking Cotton Fabric with Modification of Photoactive ZnO Coating via Microwave Method. J. Photochem. Photobiol. A Chem. 2017, 338, 13–22. [Google Scholar] [CrossRef]
- Irfan, M.; Naz, M.Y.; Saleem, M.; Tanawush, M.; Głowacz, A.; Glowacz, W.; Rahman, S.; Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; et al. Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties. Materials 2021, 14, 6998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, K.; Liu, X.; Qiao, X.; Wang, J. Enhancement UV-Resistance and Hydrophobic of Cotton Fabrics through Membrane Formation by Cationic Colored Nanospheres. Surf. Interfaces 2023, 37, 102701. [Google Scholar] [CrossRef]
- Rana, M.; Hao, B.; Mu, L.; Chen, L.; Ma, P.-C. Development of Multi-Functional Cotton Fabrics with Ag/AgBr–TiO2 Nanocomposite Coating. Compos. Sci. Technol. 2016, 122, 104–112. [Google Scholar] [CrossRef]
- Bharadiya, P.; Mahajan, C.; Sharma, A.K.; Mishra, S. Enriched Mechanical, UV Shielding and Flame Retarding Properties of Cotton Fabric Coated with Graphite Nano Platelets Filled Polyaniline–Gum Arabic Nanocomposites. Cellulose 2019, 26, 8135–8151. [Google Scholar] [CrossRef]
- Biliuta, G.; Bostănaru-Iliescu, A.-C.; Mareș, M.; Pavlov-Enescu, C.; Năstasă, V.; Burduniuc, O.; Coseri, S. Antibacterial and Antifungal Silver Nanoparticles with Tunable Size Embedded in Various Cellulose-Based Matrices. Molecules 2022, 27, 6680. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; Amalraj, J. Chitosan Capped Copper Oxide/Copper Nanoparticles Encapsulated Microbial Resistant Nanocomposite Films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Hamed, M.M.; Al-Agamy, M.H.; Gazwi, H.S.S.; Radwan, H.H.; Youssif, A.M. Biosynthesis of Copper Oxide Nanoparticles Using Streptomyces MHM38 and Its Biological Applications. J. Nanomater. 2021, 2021, 6693302. [Google Scholar] [CrossRef]
- Jose, B.; Thomas, F. Photocatalytic Degradation Of Methylene Blue Using Iron Oxide Nanoparticles Synthesized Using Annona Muricata Leaf Extract. Int. J. Pharm. Pharm. Sci. 2020, 12, 46–51. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of Monodispersed Copper Oxide Nanoparticles with Potential Application as Antimicrobial Agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Springer, C.; Humayun, D.; Skouta, R. Cuproptosis: Unraveling the Mechanisms of Copper-Induced Cell Death and its Implication in Cancer Therapy. Cancers 2024, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, J.; Liu, Q.; Zeng, X.; Liu, Y.; Deng, Y.; Lin, Y.; Wu, X.; Deng, H.; Chen, L.; et al. The Characterization and Analysis of the Compound Hemostatic Cotton Based on Ca2+/Poly (Vinyl Alcohol)/Soluble Starch-Fish Skin Collagen. Int. J. Biol. Macromol. 2024, 262, 130084. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Lou, C.-W.; Chen, A.-P.; Lee, M.-C.; Ho, T.-F.; Chen, Y.-S.; Lin, J.-H. Highly Absorbent Antibacterial Hemostatic Dressing for Healing Severe Hemorrhagic Wounds. Materials 2016, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Kobayashi, M.; Rubiy‵atno; Morikawa, M.; Mori, K. Sulfamethoxazole Removal and Fuel-Feedstock Biomass Production from Wastewater in a Phyto-Fenton Process Using Duckweed Culture. Chemosphere 2024, 361, 142592. [Google Scholar] [CrossRef]
- Fernando, P.D.S.M.; Ko, D.O.; Piao, M.J.; Kang, K.A.; Herath, H.M.U.L.; Hyun, J.W. Protective Effect of Luteolin against Oxidative Stress-mediated Cell Injury via Enhancing Antioxidant Systems. Mol. Med. Rep. 2024, 30, 121. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Zhao, Y.; Chen, H.; Li, Q.; Li, X.; Hua, S.; Cao, D.; Chang, Y. Disrupting Redox Homeostasis for Tumor Therapy Based on PDT/Chemo/Ferroptosis Therapeutic Hybrid Liposomes. RSC Adv. 2024, 14, 20152–20162. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Linder, M.C. The Relationship of Copper to DNA Damage and Damage Prevention in Humans. Mutat. Res. Mol. Mech. Mutagen. 2012, 733, 83–91. [Google Scholar] [CrossRef]
- Song, Y.; Xie, X.; Liu, Y.; Zhu, Z.; Sun, L. Nanoscale Study of DNA–Cu2+ Interactions by Liquid-Cell Electron Microscopy. ACS Omega 2023, 8, 26325–26331. [Google Scholar] [CrossRef]
- Jing, M.; Liu, Y.; Song, W.; Yan, Y.; Yan, W.; Liu, R. Oxidative Damage Induced by Copper in Mouse Primary Hepatocytes by Single-Cell Analysis. Environ. Sci. Pollut. Res. 2016, 23, 1335–1343. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of Copper Complexes with Nucleic Acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- de Souza, Í.P.; de P. Machado, B.; de Carvalho, A.B.; Binatti, I.; Krambrock, K.; Molphy, Z.; Kellett, A.; Pereira-Maia, E.C.; Silva-Caldeira, P.P. Exploring the DNA Binding, Oxidative Cleavage, and Cytotoxic Properties of New Ternary Copper(II) Compounds Containing 4-Aminoantipyrine and N,N-Heterocyclic Co-Ligands. J. Mol. Struct. 2019, 1178, 18–28. [Google Scholar] [CrossRef]


| Sample | Cu Concentration | Sample Code | ||
|---|---|---|---|---|
| [mg/kg] ± SD | g/kg | Mol/kg a | ||
| COT | 11 ± 0.0 | 0.011 | 0.0002 b | COT |
| COT-CuSO4 | 107.1 × 103 ± 6.0 × 103 | 107.1 | 1.685 | COT-Cu(1.7) |
| COT-CuSO4/CTS | 24.4 × 103 ± 1.6 × 103 | 24.4 | 0.384 | COT-Cu(0.4)/CTS |
| Samples | Surface Elements Determined | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | Oxygen | Copper | Sulfur | Nitrogen | ||||||
| A.C. | W.C. | A.C. | W.C. | A.C. | W.C. | A.C. | W.C. | A.C. | W.C. | |
| COT | 41.81 | 35.04 | 58.19 | 64.96 | − | − | − | − | − | − |
| COT-Cu(1.7) | 27.69 | 17.42 | 57.81 | 48.45 | 5.92 | 19.72 | 8.58 | 14.41 | − | − |
| COT-Cu(0.4)/CTS | 34.92 | 24.28 | 50.49 | 46.75 | 4.29 | 15.78 | 4.63 | 8.59 | 5.67 | 4.60 |
| Protection Category | Good | Very Good | Excellent |
|---|---|---|---|
| UPF rating [%] | 15–24 | 25–39 | 40–50, 50+ |
| UV radiation blocked | 93.3–95.9 | 96.0–97.4 | 97.5 to 98+ |
| Sample Name | UV Transmittance | UPF [-] | Lit | ||
|---|---|---|---|---|---|
| UV A [%] | UV B [%] | ||||
| COT * | 33.56 | 26.67 | 3.37 | This work | |
| COT-Cu(1.7) * | 6.49 | 6.76 | 14.56 | ||
| COT-Cu(0.4)/CTS * | 0.15 | 0.10 | >50 | ||
| COT-ZnO | 7.43 | 2.36 | 33.56 | [81] | |
| CPNP(R218) | 0.31 g/L | 4.35 | 3.30 | 28.79 | [82] |
| 0.62 g/L | 4.08 | 3.06 | 30.97 | ||
| 1.25 g/L | 3.31 | 2.87 | 33.88 | ||
| 2.50 g/L | 2.95 | 2.59 | 37.85 | ||
| Silane cotton | 1 layer/2 layer * | 33.50 | 36.7 | 2.8/14.1 * | [83] |
| NC-coated cotton | 18.20 | 23.0 | 4.6/41.9 * | ||
| GnP/PANI–GA | 1% wt. % | 2.12 | 2.11 | 50 | [84] |
| 3% wt. % | 1.74 | 1.73 | >50 | ||
| 5% wt. % | 1.95 | 1.97 | >50 | ||
| POLYM- METAL(n)(conc.) or METAL(n)(conc.)NCs | Diameter of Inhibition Zones (±SD) [mm] | Lit. | |||||
|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | ||||||
| Gram-Positive | Gram-Negative | ||||||
| S. aureus | B. subtilis | E. coli | P. aerugin. | A. niger | Ch. glob. | ||
| COTa,b | 0 | 0 | 0 | 0 | This work | ||
| COT-Cu(2+)(1.7) a,b | 3 ± 0.5 | 3 ± 0.5 | 2 ± 0.5 | 2 ± 0.5 | |||
| COT-Cu(+2)(0.4)/CTS a,b | 3 ± 0.5 | 2.5 ± 0.5 | 2 ± 0.5 | 2 ± 0.5 | |||
| CNW-Cu(0)(0.2) a | 1 | 2 | 1 | 2 | [59] | ||
| CNW-Cu(0)(0.4) a | 2 | 3 | 3 | 2 | |||
| MC-AgNPs | 2 | 7 | [85] | ||||
| HPC-AgNPs | 8 | 10 | |||||
| CA-AgNPs | 10 | 8 | |||||
| EC-AgNPs | 9 | 11 | |||||
| COT-ZnO | 3 | 3 | [81] | ||||
| CH-CuO | 16 | [86] | |||||
| CH-Cu | 14 | ||||||
| CuONPs | 18.0 | [87] | |||||
| Fe2O3 NPs (10 µg) | 12 | [88] | |||||
| Fe2O3 NPs (20 µg) | 14 | ||||||
| Ciprofloxacin (30 µg) | 18 | ||||||
| Cu2O NPs (1 mg/mL) | 5.30 | 4.5 | [89] | ||||
| Cu2O NPs (1 mg/mL) | 9.8 | 9.7 | |||||
| POLYM- METAL(n)(conc.) | Metal Concentration | Coagulation Tests | Lit | ||
|---|---|---|---|---|---|
| aPTT | PT | ||||
| g/kg | mol/kg | ||||
| COT-Cu(2+)(1.7) a | 107.1 | 1.685 | 71.30 ± 0.2/ | 16.33 ± 0.4 | This work |
| COT-Cu(2+)(0.4)-CTS a | 24.4 | 0.384 | 40.65 ± 0.25 | 14.27 ± 0.3 | |
| PVA-Ca(2+)(0.47)/SS-FSC b | 18.65 | 0.47 | 40.26 ± 3.66 | 26 | [91] |
| PVA-Ca(2+)(0.47)/SS-FSC c | 18.65 | 0.47 | 35.34 ± 2.99 | 25 | |
| TCP nonwoven d | 50.9 | 14.3 | [92] | ||
| PLA-Fe(3+)(0.04) e | 2.23 | 0.04 | 43 | 14 | [53] |
| PLA-Fe(3+)(0.34) e | 19.0 | 0.34 | 48 | 13.6 | |
| PLA-Fe(3+)(0.08) e | 4.46 | 0.08 | 42 | 13.8 | |
| PLA-Fe(3+)(0.51) e | 28.5 | 0.51 | 46 | 14 | |
| CNW-Cu(0)(0.15) f | 9.59 | 0.15 | 41.2 | 15.6 | [60] |
| CNW-Cu(0)(0.41) f | 26.13 | 0.41 | 50.4 | 16.0 | |
| CNW-Cu(0)(0.23) f | 14.35 | 0.23 | 43.2 | 15.9 | |
| CNW-Cu(0)(0.44) f | 28.11 | 0.44 | 52.1 | 16.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczyńska, M.; Mrozińska, Z.; Juszczak, M.; Woźniak, K.; Kudzin, M.H. Modification of Cotton with Chitosan: Deposition of Copper(II) Sulfate by Complexation Copper Ions. Processes 2024, 12, 2772. https://doi.org/10.3390/pr12122772
Świerczyńska M, Mrozińska Z, Juszczak M, Woźniak K, Kudzin MH. Modification of Cotton with Chitosan: Deposition of Copper(II) Sulfate by Complexation Copper Ions. Processes. 2024; 12(12):2772. https://doi.org/10.3390/pr12122772
Chicago/Turabian StyleŚwierczyńska, Małgorzata, Zdzisława Mrozińska, Michał Juszczak, Katarzyna Woźniak, and Marcin H. Kudzin. 2024. "Modification of Cotton with Chitosan: Deposition of Copper(II) Sulfate by Complexation Copper Ions" Processes 12, no. 12: 2772. https://doi.org/10.3390/pr12122772
APA StyleŚwierczyńska, M., Mrozińska, Z., Juszczak, M., Woźniak, K., & Kudzin, M. H. (2024). Modification of Cotton with Chitosan: Deposition of Copper(II) Sulfate by Complexation Copper Ions. Processes, 12(12), 2772. https://doi.org/10.3390/pr12122772






