Formulation and In Vitro Assessment of Tragacanth Gum-Based Hydrogel Loaded with Artemisia vestita Leaf Extract for Wound Healing
Abstract
1. Introduction
2. Materials and Methods
- Collection of Plant and Extract Preparation
- Gel Preparation
- Drug–Excipient Compatibility Study
- Preformulation Studies
Physiochemical Characterization
- Physical appearance: The physical appearance of a formulated gel is an essential aspect with regard to its overall quality and user acceptance. Evaluation of color, appearance, and clarity helps detect any abnormalities or changes in the formulation that may affect its effectiveness or esthetics. To evaluate the color, appearance, and clarity of the hydrogel formed by incorporating A. vestita leaf extract (ALE) into tragacanth gum, the following procedures were systematically and reliably performed. Samples were prepared in identical containers to ensure uniformity. Color assessment was performed by trained observers in triplicate. Standardized color charts were used for precise measurement. Assessments were performed under standardized lighting conditions. The same trained observers evaluated the hydrogel thrice to observe visible particulates, uniformity, and texture. Clarity was measured using a transparent grid behind the gel, and a numerical scale (ranging from 1 to 5) with detailed criteria was used for scoring. Turbidity or transmittance was spectrophotometrically measured at 600 nm, with lower turbidity or higher transmittance indicating higher clarity.
- pH: The pH of the A. vestita leaf extract hydrogel (ALEH) was measured thrice using a calibrated Seven Excellence S400 pH meter (Mettler Toledo, India) to ensure accuracy. To determine the pH of the formulations using the calibrated pH meter, an optimal pH range was required (e.g., for skin compatibility). Hence, 1 g of the gel formulation was dispersed in 100 mL of distilled water and allowed to stand for 2 h. The pH of the resultant solution was then measured. Data were presented as the mean ± SEM.
- Spreadability: In total, 1 mL of the prepared ALEH was transferred onto a glass plate using a sterile syringe. A calibrated plate was placed on top of the hydrogel. Weights of increasing mass (25, 50, 100, 200, 300, 400, and 500 g) were then sequentially placed on the plate at 20 s time intervals to enable ALEH to stabilize and spread under the applied weight. After placing each weight, the radii of the ALEH formulation were measured. To ensure the reliability and variability of the results, spreadability was assessed in triplicate at ambient temperature. The area covered by the prepared ALEH was calculated using the following formula:where P is the surface area covered by ALEH (cm2) and r is the radius of ALEH (cm).P = πr2,
- Rheological properties: The viscosity of the formulation was assessed using a Brookfield viscometer. Viscosity affects spreadability, product handling, and release kinetics. The digital viscometer was loaded with the hydrogel and placed into the flow jacket of the viscometer. The flow jacket is a component of the apparatus used to regulate temperature or create specific conditions during the measurement process. An adapter was used to measure the viscosity of the formulated gel at a rotation speed of 20 rotations per minute (rpm). The temperature was kept constant at 24.8 °C by circulating water through a thermostated water jacket. Before taking readings, the sample was allowed to settle for 5–6 min. The viscosity of the hydrogels was determined by increasing the shear rate.
- Extrudability: The formulated gel (10 g) was filled into standard caps containing eight collapsible aluminum tubes. They were sealed at the end by crimping. To assess extrudability, the weights of the tubes were recorded. Subsequently, the tubes were securely positioned between two glass slides and firmly clamped in place. A 500 g weight was placed on top of the slides, following which the cap was removed to allow the extrusion of the gel. The gel extruded from the tubes was gathered and weighed. Based on the amount of gel extruded, the percentage of extruded gel was calculated.
- Excellent: more than 90% extrudability;
- Good: more than 80% extrudability;
- Fair: more than 70% extrudability.
- Drug Release Study
- Fickian diffusion or case I transport: n = 0.45;
- Non-Fickian or anomalous transport: 0.45 < n < 0.89;
- Case II transport or relaxation-controlled release: n = 0.89;
- Super case II transport: n > 0.89.
- Antimicrobial Activity Assay
- Agar Well Diffusion Technique
- Minimum Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC) Assays
- Cell Culture
- Wound Healing Assay
- Statistical Analysis
3. Results and Discussion
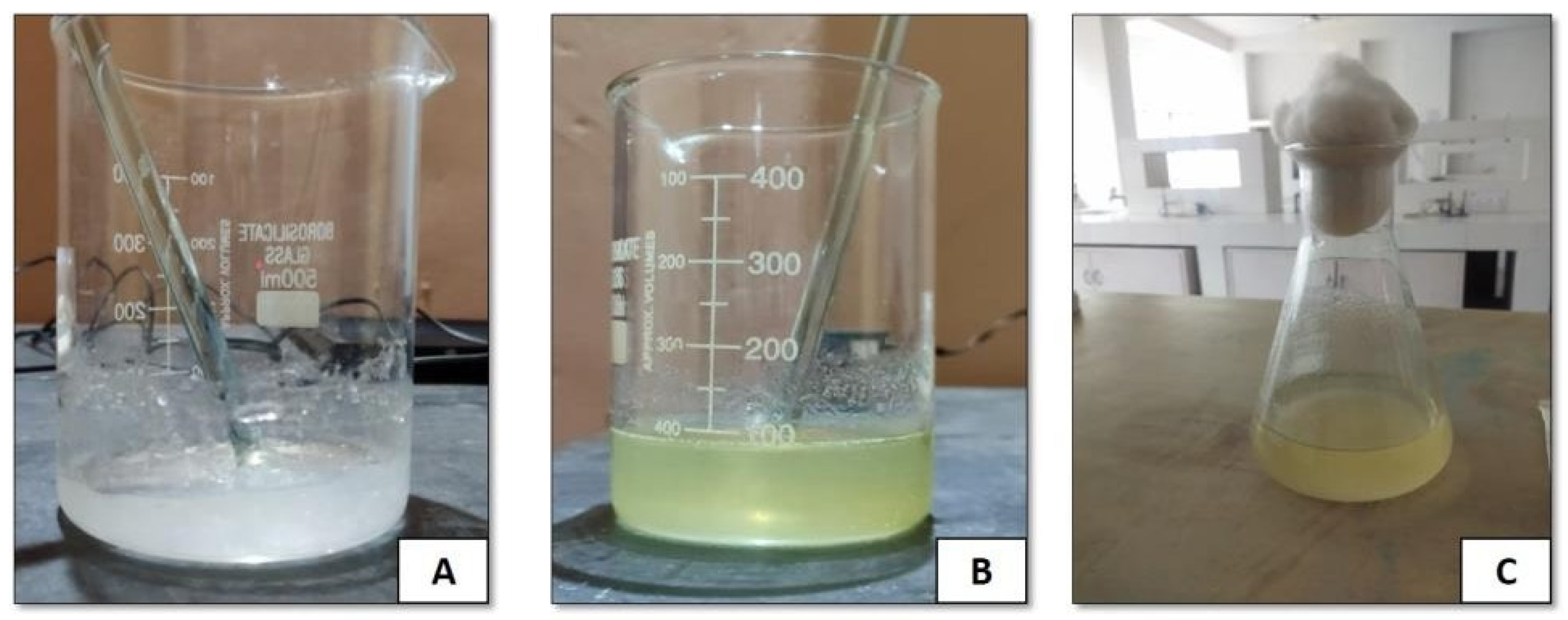
3.1. ALEH Preparation
Physical Assessment of Compatibility
3.2. Physiochemical Characterization
3.2.1. Physical Appearance
3.2.2. pH
3.2.3. Spreadability
3.2.4. Rheological Properties
3.2.5. Extrudability
3.2.6. Optimization
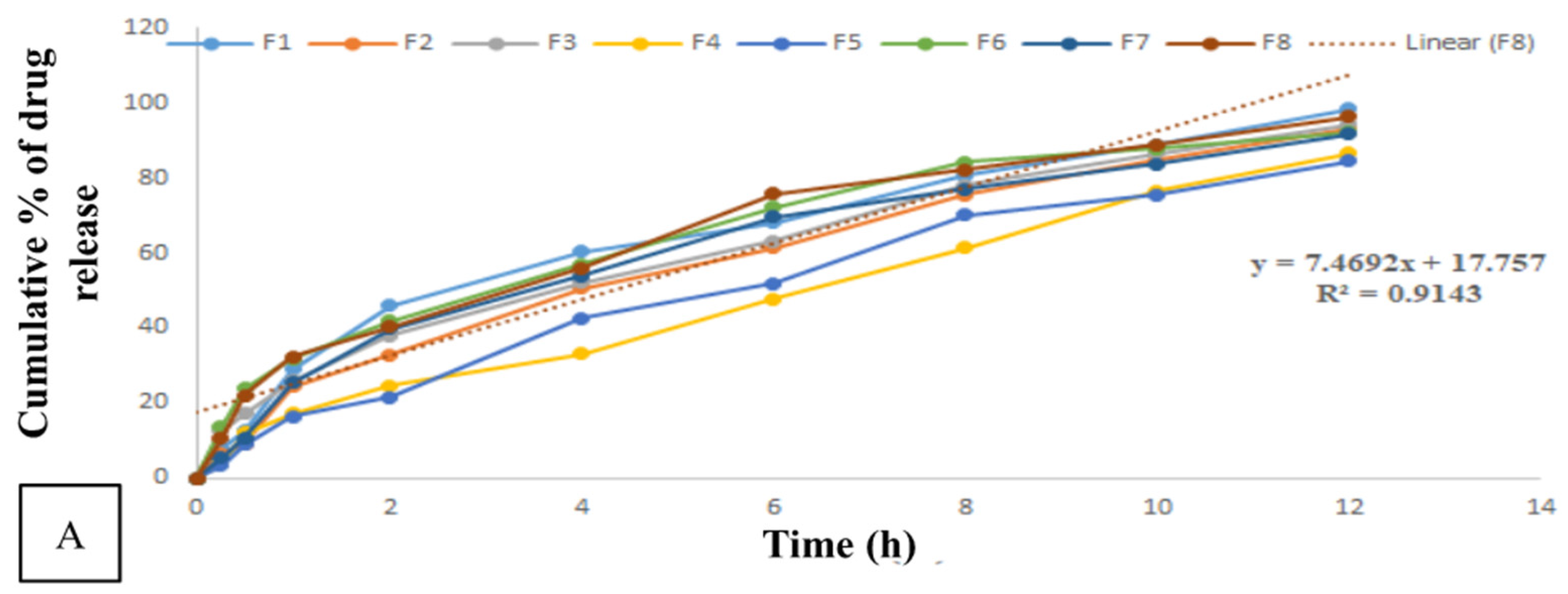
3.3. Drug Release Study
- Antimicrobial Activity
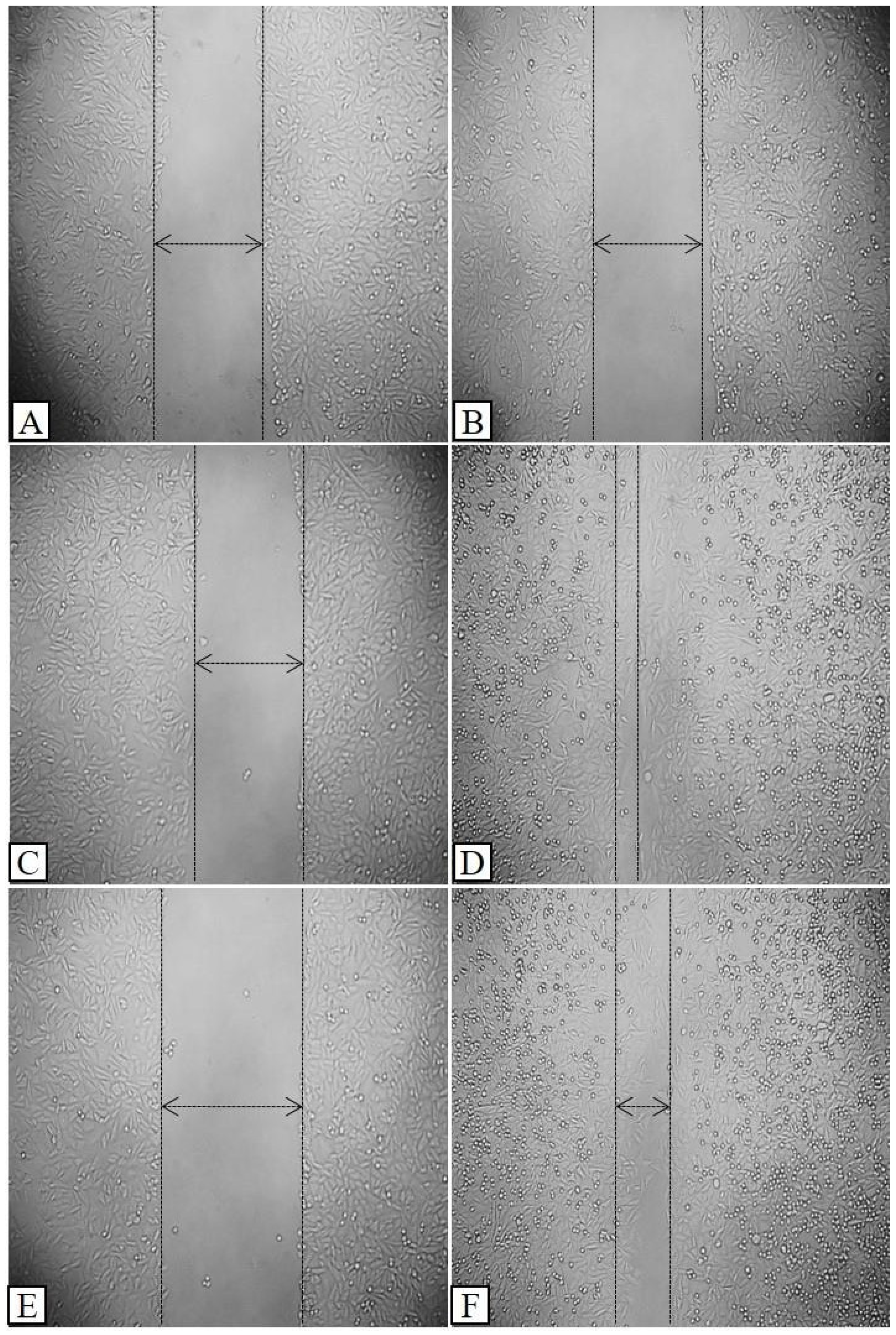
3.4. Wound Healing Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, F.; Passariello, R.; Imparato, G.; Casale, C.; Netti, P.A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020, 6, 21. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The skin microbiome: Current landscape and future opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M. The relevance of quantitative ethnobotanical indices for ethnopharmacology and ethnobotany. J. Ethnopharmacol. 2022, 288, 115008. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From traditional ethnopharmacology to modern natural drug discovery: A methodology discussion and specific examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Warude, D.; Pushpangadan, P.; Bhatt, N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid. Based Complement. Altern. Med. 2005, 2, 465–473. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Choudhary, A.; Bains, A.; Sridhar, K.; Dhull, S.B.; Goksen, G.; Sharma, M.; Chawla, P. Recent advances in modifications of exudate gums: Functional properties and applications. Int. J. Biol. Macromol. 2024, 271, 132688. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Hydrocolloid Applications: Gum Technology in the Food and Other Industries; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Glicksman, M. Gum Technology in the Food Industry; Academic Press: New York, NY, USA; London, UK, 1969. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Dogra, S.; Singh, J.; Koul, B.; Yadav, D. Artemisia vestita: A Folk Medicine with Hidden Herbal Fortune. Molecules 2023, 28, 2788. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Khatri, T. The Artemisia Genus: Panacea to Several Maladies. In Bioactive Natural Products in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–95. [Google Scholar]
- Dograa, S.; Singh, J.; Vashist, H.R. Anthology of pharmacological activities from folklore medicine Artemisia. NVEO-Nat. Volatiles Essent. Oils J.|NVEO 2021, 8, 3678–3693. [Google Scholar]
- Dograa, S.; Singha, J.; Vashistb, H. Extraction, isolation and pharmacognostical characterization of components from Artemisia vestita Wall ex Besser. NVEO-Nat. Volatiles Essent. Oils J. NVEO 2021, 8, 12955–12976. [Google Scholar]
- Dogra, S.; Koul, B.; Singh, J.; Mishra, M.; Yadav, D. Phytochemical Analysis, Antimicrobial Screening and In Vitro Pharmacological Activity of Artemisia vestita Leaf Extract. Molecules 2024, 29, 1829. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Allen, L.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013. [Google Scholar]
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Giron, D. Thermal Analysis of Drugs and Drug Products. In Encyclopedia of Pharmaceutical Technology; CRC Press: Boca Raton, FL, USA, 2002; Volume 15, pp. 12–17. [Google Scholar]
- Giron, D. Physicochemical Characterization of the Solid State in Drug Development. In Pharmacokinetic Profiling in Drug Research: Biological, Physicochemical, and Computational Strategies; Wileys: Hoboken, NJ, USA, 2006; pp. 307–329. [Google Scholar]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use; Pharmaceutical Press: London, UK, 2015. [Google Scholar]
- Varshosaz, J.; Dehghan, Z. Development and characterization of buccoadhesive nifedipine tablets. Eur. J. Pharm. Biopharm. 2002, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Aslani, A.; Shahmoradi, Z.; Abtahi Fahliani, F. Preparation and clinical evaluation of skin lightening cream contain arbutin, kojic dipalmitate, licorice extract and ascorbyl palmitate. Isfahan Univ. Med. Sci. 2010, 17, 72–79. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Kumar, B.; Vijayakumar, M.; Govindarajan, R.; Pushpangadan, P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J. Ethnopharmacol. 2007, 114, 103–113. [Google Scholar] [CrossRef] [PubMed]
- James, O.; Friday, E.T. Phytochemical composition, bioactivity and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. Int. J. Pharm. Biomed. Res. 2010, 1, 54–63. [Google Scholar]
- Schürer, N. pH and Acne. In pH of the Skin: Issues and Challenges; Karger Publishers: Basel, Switzerland, 2018; Volume 54, pp. 115–122. [Google Scholar]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and Microbial Infections. In pH of the Skin: Issues and Challenges; Karger Publishers: Basel, Switzerland, 2018; Volume 54, pp. 87–94. [Google Scholar]
- Lynde, C.; Tan, J.; Beecker, J.; Claveau, J.; Li, M.; Rao, J.; Salsberg, J.; Sauder, M.; Zip, C. Skin surface pH. J. Drugs Dermatol. JDD 2019, 18, 214. [Google Scholar] [PubMed]
- Giri, M.; Abhale, A.; Ahire, M.; Bhalke, R.D. Formulation, Characterization, and Evaluation of Topical Anti-inflammatory Herbal Gel. Int. J. Pharm. Biol. Arch. 2019, 10, 190–195. [Google Scholar]
- Das, S.; Samanta, A.; Bose, A. Design, development and evaluation of fluconazole topical gel. Asian J. Pharma. Clin. Res. 2015, 8, 132–135. [Google Scholar]
- Mohamed, H.A.; Radwan, R.R.; Raafat, A.I.; Ali, A.E. Antifungal activity of oral (Tragacanth/acrylic acid) Amphotericin B carrier for systemic candidiasis: In vitro and in vivo study. Drug Deliv. Transl. Res. 2018, 8, 191–203. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, D.J. Soil-related bacterial and fungal infections. J. Am. Board Fam. Med. 2012, 25, 734–744. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Nazarnezhad, S.; Mousavi, S.H.; Sadegh Amiri, M.; Darroudi, M.; Baino, F.; Kargozar, S. Gum tragacanth (GT): A versatile biocompatible material beyond borders. Molecules 2021, 26, 1510. [Google Scholar] [CrossRef]
- Fayazzadeh, E.; Rahimpour, S.; Ahmadi, S.M.; Farzampour, S.; Anvari, M.S.; Boroumand, M.A.; Ahmadi, S.H. Acceleration of skin wound healing with tragacanth (Astragalus) preparation: An experimental pilot study in rats. Acta Med. Iran. 2014, 52, 3–8. [Google Scholar]
- Koyyada, A.; Orsu, P. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431. [Google Scholar] [CrossRef]
- Nazemi, Z.; Sahraro, M.; Janmohammadi, M.; Nourbakhsh, M.S.; Savoji, H. A review on tragacanth gum: A promising natural polysaccharide in drug delivery and cell therapy. Int. J. Biol. Macromol. 2023, 241, 124343. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, M.; Gupta, R.K.; Rani, A. Natural gums and their derivatives based hydrogels: In biomedical, environment, agriculture, and food industry. Crit. Rev. Biotechnol. 2024, 44, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Abdi, G.; Jain, M.; Patil, N.; Tariq, M.; Choudhary, S.; Kumar, P.; Raj, N.S.; Mohsen Ali, S.S.; Uthappa, U. Tragacanth gum-based hydrogels for drug delivery and tissue engineering applications. Front. Mater. 2024, 11, 1296399. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Kumar, R.; Kaushal, D.; Chauhan, V.; Thakur, S.; Shandilya, P.; Sharma, P.P. Gum acacia based hydrogels and their composite for waste water treatment: A review. Int. J. Biol. Macromol. 2024, 262, 129914. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lunardi, V.B.; Irawaty, W.; Yuliana, M.; Santoso, S.P.; Puspitasari, N.; Wenten, I.G.; Ismadji, S. Polysaccharides gums in drug delivery systems: A review. Int. J. Biol. Macromol. 2023, 253, 127020. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum Tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef]
- Rohilla, S.; Chawla, G.; Bhagwat, D.; Rohilla, A. Natural gum: An option to prepare nanocarriers. Med. Theor. Hypothesis 2023, 6, 15. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, J.; Othman, N.; Khan, J.; Alolayan, S.O.; Al thagfan, S.S.; Kaleemullah, M. A review of moisturizers; history, preparation, characterization and applications. Cosmetics 2022, 9, 61. [Google Scholar] [CrossRef]
- Afzal, S.; Zahid, M.; Nimra, S.; Fatima, Z.; Shakir, H.; Rehan, Z. Ultrasound hydrogel: A review on materials and method. J. Mod. Polym. Chem. Mater. 2022, 1, 2. [Google Scholar]
- Boamah, P.O.; Afoakwah, N.A.; Onumah, J.; Osei, E.D.; Mahunu, G.K. Physicochemical properties, biological properties and applications of gum tragacanth—A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100288. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. Potential of tragacanth gum in the industries: A short journey from past to the future. Polym. Bull. 2023, 80, 4643–4662. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.; Gaytán-Martínez, M.; Reyes-Vega, M.; Mendoza, S. Protein-gum-based gels: Effect of gum addition on microstructure, rheological properties, and water retention capacity. Trends Food Sci. Technol. 2021, 116, 303–317. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic force microscopy (AFM) on biopolymers and hydrogels for biotechnological applications—Possibilities and limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Li, Q.; Yang, L.; Liu, H.; Yan, R.; Xiao, L.; Liu, H.; Wang, J.; Yang, B. Transparent conductive supramolecular hydrogels with stimuli-responsive properties for on-demand dissolvable diabetic foot wound dressings. Macromol. Rapid Commun. 2020, 41, 2000441. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J. Application of tragacanth gum and alginate in hydrogel wound dressing’s formation using gamma radiation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100058. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ramezani, S.; Rashidi, M.-R. Fabrication of honey-loaded ethylcellulose/gum tragacanth nanofibers as an effective antibacterial wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126615. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, K.; Dutt, S. Dietary fiber tragacanth gum based hydrogels for use in drug delivery applications. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100208. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ma, Z.; Wu, X.; Wei, H.; Zhang, H.; Li, G.; Qian, Y.; Shahriari-Khalaji, M.; Hou, K.; Cao, R. Advances in stimuli- responsive chitosan hydrogels for drug delivery systems. Macromol. Biosci. 2023, 24, 2300399. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Chuku, A.; Nwankiti, O.O. Association of bacteria with fungal infection of skin and soft tissue lesions in Plateau State, Nigeria. Br. Microbiol. Res. J. 2013, 3, 470–477. [Google Scholar] [CrossRef]
- Lam, P.L.; Lee, K.K.H.; Wong, R.S.M.; Cheng, G.Y.M.; Bian, Z.X.; Chui, C.H.; Gambari, R. Recent advances on topical antimicrobials for skin and soft tissue infections and their safety concerns. Crit. Rev. Microbiol. 2018, 44, 40–78. [Google Scholar] [CrossRef]






| Composition | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| Aqueous extract (g) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Tragacanth gum (g) | 0.25 | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 | 1.75 | 2.0 |
| Milli-Q water (mL) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Methylparaben (mL) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Propylparaben (mL) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Propylene glycol (mL) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Triethanolamine (mL) | Adjust pH to 6–6.5 | |||||||
| Batch | Discoloration | Caking | Liquefaction | |||
|---|---|---|---|---|---|---|
| Initial | After 30 Days | Initial | After 30 Days | Initial | After 30 Days | |
| Aqueous extract | No | Consistent | No | Consistent | No | Consistent |
| Tragacanth gum | No | Consistent | No | Consistent | No | Consistent |
| Aqueous extract + Tragacanth gum | No | Consistent | No | Consistent | No | Consistent |
| Formulation Code | pH | Spreadability (g/cm2) | Viscosity (cP) |
|---|---|---|---|
| F1 | 6.86 ± 0.1 | 15.08 ± 0.085 | 2318 ± 3.78 |
| F2 | 6.93 ± 0.15 | 14.75 ± 0.16 | 2540 ± 2.51 |
| F3 | 6.83 ± 0.05 | 13.03 ± 0.09 | 2463 ± 5.13 |
| F4 | 7.03 ± 0.11 | 15.75 ± 0.19 | 2450 ± 2.08 |
| F5 | 7.16 ± 0.05 | 12.84 ± 0.08 | 2637 ± 2.08 |
| F6 | 6.90 ± 1.08 | 11.63 ± 0.07 | 2429 ± 3.21 |
| F7 | 6.83 ± 0.05 | 12.83 ± 0.11 | 2318 ± 2.08 |
| F8 | 6.9 ± 0.10 | 14.31 ± 0.11 | 2411 ± 1.15 |
| Time (h) | Cumulative Percentage of Drug Release (25 °C) | |||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
| 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.25 | 7.982 ± 0 | 6.390 ± 0 | 12.58 ± 0 | 3.878 ± 0 | 3.291 ± 0 | 13.593 ± 0 | 5.553 ± 0 | 10.578 ± 0 |
| 0.5 | 12.664 ± 0 | 9.282 ± 0 | 17.362 ± 0 | 11.995 ± 0 | 8.969 ± 0 | 23.831 ± 0 | 10.940 ± 0 | 22.096 ± 0 |
| 1 | 29.078 ± 0 | 24.540 ± 0 | 25.912 ± 0 | 17.259 ± 0 | 16.349 ± 0 | 31.840 ± 0 | 25.395 ± 0 | 32.333 ± 0 |
| 2 | 46.067 ± 0 | 32.814 ± 0 | 38.144 ± 0 | 24.633 ± 0 | 21.613 ± 0 | 42.010 ± 0 | 39.883 ± 0 | 40.337 ± 0 |
| 4 | 60.369 ± 0 | 50.540 ± 0 | 52.285 ± 0 | 33.069 ± 0 | 42.721 ± 0 | 57.061 ± 0 | 53.896 ± 0 | 56.109 ± 0 |
| 6 | 68.155 ± 0 | 61.488 ± 0 | 63.093 ± 0 | 47.778 ± 0 | 52.014 ± 0 | 72.312 ± 0 | 69.515 ± 0 | 75.699 ± 0 |
| 8 | 80.596 ± 0 | 75.400 ± 0 | 78.201 ± 0 | 61.512 ± 0 | 70.020 ± 0 | 84.246 ± 0 | 77.050 ± 0 | 82.176 ± 0 |
| 10 | 88.988 ± 0 | 84.709 ± 0 | 86.468 ± 0 | 76.508 ± 0 | 75.468 ± 0 | 87.848 ± 0 | 83.787 ± 0 | 88.752 ± 0 |
| 12 | 98.440 ± 0 | 93.254 ± 0 | 94.200 ± 0 | 86.587 ± 0 | 84.431 ± 0 | 92.244 ± 0 | 91.636 ± 0 | 96.267 ± 0 |
| Time (h) | Cumulative Percentage of Drug Release (F4) | |||
|---|---|---|---|---|
| pH 4.5 Temperature 37 °C | pH 4.5 Temperature 40 °C | pH 6.8 Temperature 37 °C | pH 6.8 Temperature 40 °C | |
| 0 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.25 | 4.811 | 1.604 | 1.815 | 11.028 |
| 0.5 | 11.248 | 9.510 | 5.961 | 15.542 |
| 1 | 18.702 | 14.781 | 15.818 | 22.267 |
| 2 | 23.784 | 23.031 | 22.083 | 33.138 |
| 4 | 32.819 | 29.448 | 29.361 | 40.785 |
| 6 | 42.532 | 34.260 | 43.272 | 50.274 |
| 8 | 49.760 | 40.792 | 47.879 | 65.198 |
| 10 | 64.329 | 51.677 | 68.147 | 74.411 |
| 12 | 74.380 | 54.656 | 81.044 | 79.110 |
| Microbes | Zone of Inhibition (mm) | MIC and MBC/MFC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| 25 µg/mL | 50 µg/mL | 100 µg/mL | Standard Drug (Azithromycin/Fluconazole) | MIC | MBC/MFC | |
| S. aureus | - | 12.14 ±0.52 | 15.0 ± 0.26 | 20.4 ± 0.22 | 110 | 220 |
| E. coli | 6.4 ± 0.84 | 14.6 ± 0.28 | 19.6 ± 0.51 | 22.2 ± 0.31 | 200 | 240 |
| B. subtilis | - | 13.46 ± 0.40 | 15.1 ± 0.17 | 17.4 ± 0.23 | 160 | 200 |
| S. pyogenes | 12.12 ± 0.9 | 18.1 ±0.70 | 20.3 ± 0.60 | 23.1± 0.20 | 200 | 250 |
| A. flavus | 12.3 ± 0.22 | 15.2 ± 0.31 | 17.5 ± 0.21 | 20.3 ± 0.23 | 100 | 260 |
| A. niger | - | 10.7 ± 0.42 | 14.8 ± 0.50 | 16.8 ± 0.14 | 100 | 220 |
| C. albicans | 10.2 ± 0.36 | 12.1 ± 0.32 | 19.4 ± 0.01 | 22.1 ± 0.24 | 200 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogra, S.; Koul, B.; Singh, J.; Mishra, M.; Rabbee, M.F. Formulation and In Vitro Assessment of Tragacanth Gum-Based Hydrogel Loaded with Artemisia vestita Leaf Extract for Wound Healing. Processes 2024, 12, 2750. https://doi.org/10.3390/pr12122750
Dogra S, Koul B, Singh J, Mishra M, Rabbee MF. Formulation and In Vitro Assessment of Tragacanth Gum-Based Hydrogel Loaded with Artemisia vestita Leaf Extract for Wound Healing. Processes. 2024; 12(12):2750. https://doi.org/10.3390/pr12122750
Chicago/Turabian StyleDogra, Shivani, Bhupendra Koul, Joginder Singh, Meerambika Mishra, and Muhammad Fazle Rabbee. 2024. "Formulation and In Vitro Assessment of Tragacanth Gum-Based Hydrogel Loaded with Artemisia vestita Leaf Extract for Wound Healing" Processes 12, no. 12: 2750. https://doi.org/10.3390/pr12122750
APA StyleDogra, S., Koul, B., Singh, J., Mishra, M., & Rabbee, M. F. (2024). Formulation and In Vitro Assessment of Tragacanth Gum-Based Hydrogel Loaded with Artemisia vestita Leaf Extract for Wound Healing. Processes, 12(12), 2750. https://doi.org/10.3390/pr12122750









