Formulating an Innovative Emulsion Based on Poloxamer 407 Containing Oregano and Thyme Essential Oils as Alternatives for the Control of Mastitis Caused by Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents and Pharmaceutical Ingredients
2.1.2. Microorganisms
2.2. Characterization of Essential Oils
2.3. Emulsified Systems’ Development
2.4. Selection of the Best Formulation Based on In Vitro Antimicrobial Activity
2.5. Formulation Sterility Research
2.6. Antimicrobial Activity of the Chosen Formulation Against Clinical Strains
2.7. Analysis and Preliminary Evaluation of the Stability of the Chosen Formulation
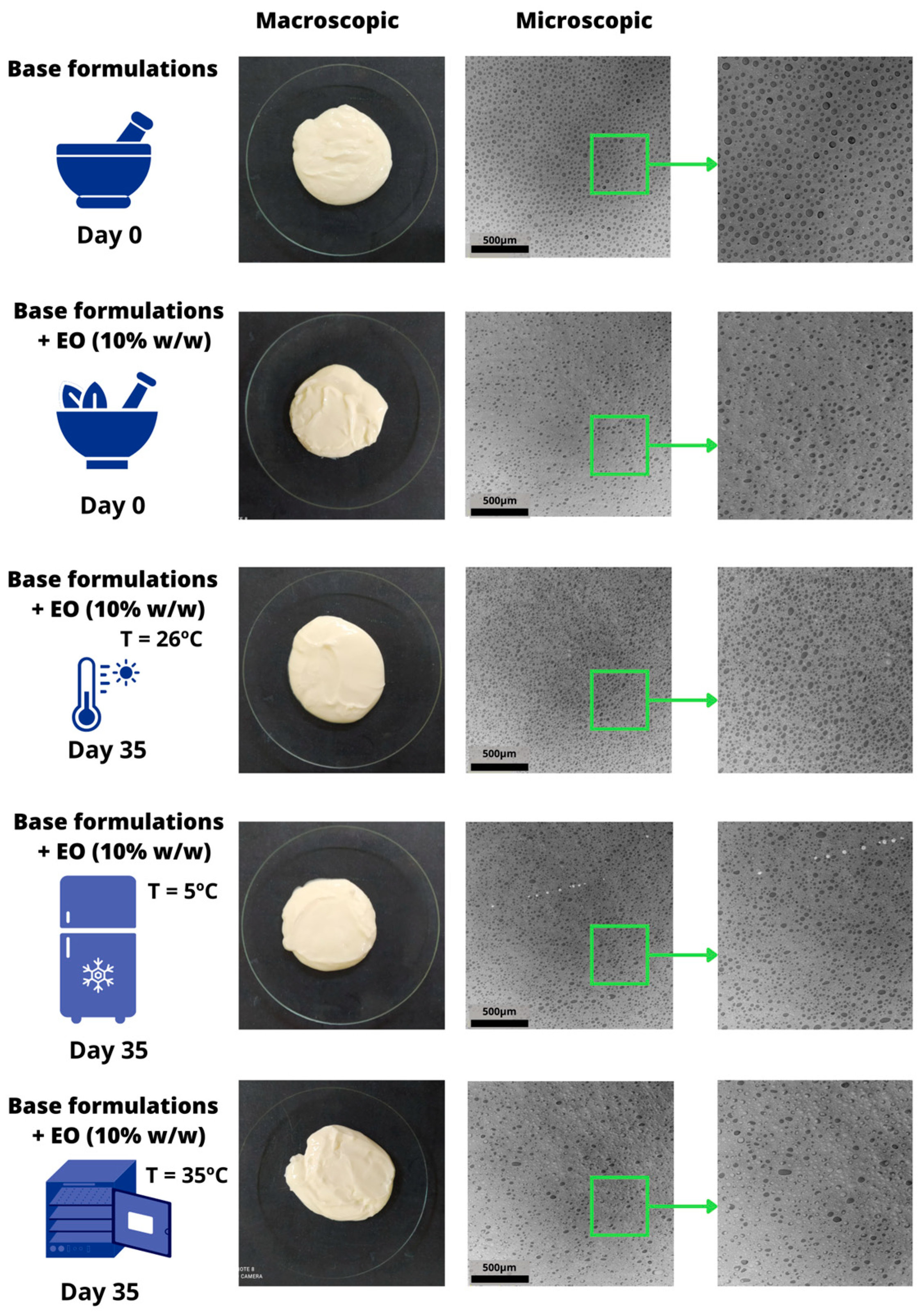
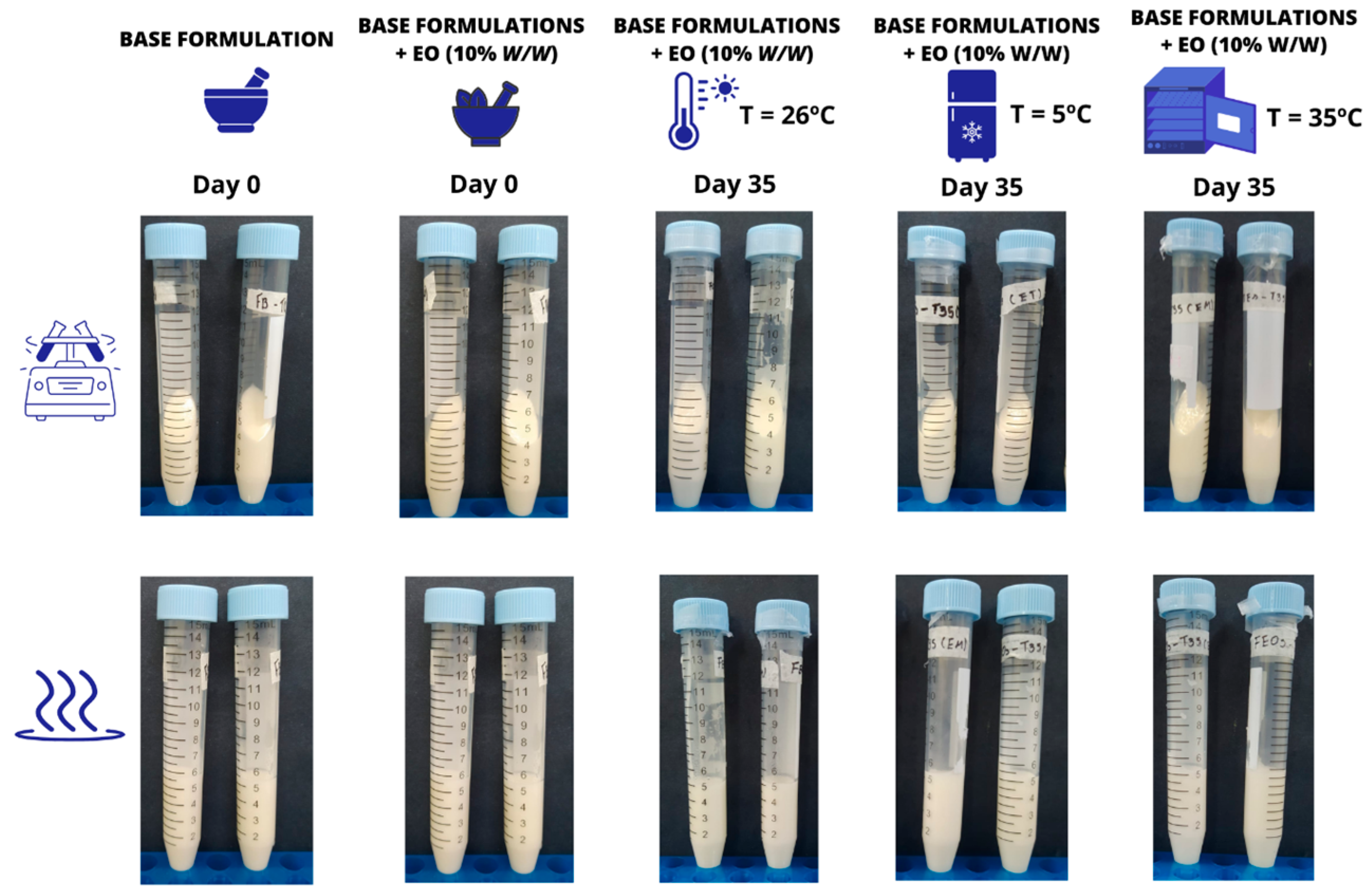
2.7.1. Macro e Microscopic Evaluation, Type of Emulsion and Size of Droplets
2.7.2. Preliminary Stability Assessment
2.7.3. Texture Profile Analysis
2.7.4. Water Loss
2.7.5. Film-Forming Ability of EO Formulations
2.7.6. Statistical Analysis
3. Results
3.1. Chemical Characterization of Essential Oils
3.2. Study of In Vitro Antimicrobial Activity for the Selection of the Best Formulation, Sterility Research, and Investigation of Antimicrobial Activity Against Clinical Strains of Mastitis
3.3. Characterization and Preliminary Stability of the Chosen Formulation
3.4. Texture Profile Analysis, Water Loss and Film-Forming Capacity
4. Discussion
4.1. Composition and Antimicrobial Activity of the Essential Oils of Oregano and Thyme
4.2. Antimicrobial Activity of the Formulation
4.3. Characterization and Preliminary Stability Assessment of the Chosen Formulation
4.4. Texture Profile Analysis, Water Content, and Film-Forming Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kovačević, Z.; Mihajlović, J.; Mugoša, S.; Horvat, O.; Tomanić, D.; Kladar, N.; Samardžija, M. Pharmacoeconomic analysis of the different therapeutic approaches in control of bovine mastitis: Phytotherapy and antimicrobial treatment. Antibiotics 2023, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Suresh, K.P.; Jayamma, K.S.; Shome, B.R.; Patil, S.S.; Amachawadi, R.G. An understanding of the global status of major bacterial pathogens of milk concerning bovine mastitis: A systematic review and meta-analysis (Scientometrics). Pathogens 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- United Nations. General Assembly Resolution A/RES/70/1. Transforming Our World, the 2030 Agenda for Sustainable Development. 2015. Available online: https://sdgs.un.org/2030agenda (accessed on 1 August 2024).
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health, and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors. therapeutic strategies and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Kerro, D.O.; Vidlund, J. Staphylococcal mastitis in dairy cows. Front. Vet. Sci. 2024, 11, 1356259. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef]
- Langer, A.; Sharma, S.; Sharma, N.K.; Nauriyal, D.S. Comparative efficacy of different mastitis markers for diagnosis of sub-clinical mastitis in cows. Int. J. Appl. Sci. Biotechnol. 2014, 2, 121–125. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.; Li, Y.; Han, Q.; Yin, Z.; Zhou, M.; Luo, M.; Chen, J.; Xia, S. Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front. Nutr. 2022, 9, 1024722. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shah, A.M.; Shah, A.; Lone, A.S.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine mastitis: An appraisal of its alternative herbal cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Neculai-Valeanu, A.S.; Ariton, A.M.; Mădescu, B.M.; Rîmbu, C.M.; Creangă, Ş. Nanomaterials and essential oils as candidates for developing novel treatment options for bovine mastitis. Animals 2021, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant essential oils as a tool in the control of bovine mastitis: An update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef] [PubMed]
- Buldain, D.; Gortari, C.L.; Buchamer, A.V.; Bandoni, A.; Marchetti, L.; Mestorino, N. In vitro synergistic interaction between Melaleuca armillaris essential oil and erythromycin against Staphylococcus aureus isolated from dairy cows. Front. Vet. Sci. 2022, 9, 1005616. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Bano, I.; Li, K.; Ul Hassan, I.; Wang, W.; Qadeer, A.; Zhang, J. Evaluation of in vitro antibacterial effect of essential oil and some herbal plant extract used against mastitis pathogens. Vet. Med. Sci. 2022, 8, 2655–2661. [Google Scholar] [CrossRef]
- Rota, C.R.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus yemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Hussein, M.; Roby, H.; Atef, M.; Selim, K.A.; Ibrahim, K. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop Prod. 2013, 43, 827–831. [Google Scholar]
- Khodaei, M.M.; Kazemi, M.; Khaltabadi, F.A.H.; Yahyaei, M.; Rezaei, M.; De Rensis, F.; Taddei, S. Antibacterial effect of medicinal plant essence (Thymus vulgaris) on major bacterial mastitis pathogen in vitro. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 286–294. [Google Scholar]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Mut, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical characterization and biological evaluation of Origanum vulgare L. essential oil formulated as polymeric micelles drug delivery systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef]
- Sánchez-Arribas, N.; Guzmán, E.; Lucia, A.; Toloza, A.C.; Velarde, M.G.; Ortega, F.; Rubio, R.G. Environmentally friendly platforms for encapsulation of an essential oil: Fabrication, characterization and application in pests control. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 473–481. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Hernández-Sánchez, H.; Zambrano-Zaragoza, M.L.; Gutiérrez-López, G.F.; Del-Real, A.; Quintanar-Guerrero, D.; Velasco-Bejarano, B. Influence of stabilizing and encapsulating polymers on antioxidant capacity, stability, and kinetic release of thyme essential oil nanocapsules. Foods 2020, 9, 1884. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Linehan, K.; Flynn, J.; Byrne, N.; Dillon, P.; Conneely, M.; Grimaud, G.; Hill, C.; Stanton, C.; Ross, R.P. Emulsion-based postbiotic formulation is comparable to viable cells in eliciting a localized immune response in dairy cows with chronic mastitis. Front. Microbiol. 2020, 13, 759649. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Hua, S. Physiological and pharmaceutical considerations for rectal drug formulations. Front. Pharmacol. 2019, 10, 1196. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, T.S.; Cha, B.J.; Kim, S.H.; Kim, W.B. Percutaneous absorption, and histopathology of a poloxamer-based formulation of capsaicin analog. Int. J. Pharm. 1997, 159, 105–114. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Zuber, M.; Couarraze, G.; Chaumeil, J.C. Rheological study of a thermoreversible morphine gel. Drug Dev. Ind. Pharm. 1991, 17, 1255–1265. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, S.W.; Choi, H.G.; Lee, M.K.; Gao, Z.G.; Kim, I.S. Trials of in-situ gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int. J. Pharm. 1998, 174, 201–207. [Google Scholar] [CrossRef]
- Beard, M.C.; Cobb, L.H.; Grant, C.S.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of poloxamer 407 hydrogel and its use as a drug delivery vehicle. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 338–347. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Shumkova, M.M.; Sergienko, F.S.; Novozhilova, E.V.; Demina, N.B. Spray film-forming systems as promising topical in situ systems: A review. Saudi Pharm. J. 2023, 31, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Sheskey, P.J.; Hancock, B.C.; Moss, G.P. Handbook of Pharmaceutical Excipients, 9th ed.; Libros Digitales, Pharmaceutical Press: London, UK, 2020; ISBN 978-0-8571-1375-7. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Beloni, M.V. Potencial Antimicrobiano e Antibiofilme da Combinação dos óleos Essenciais de Origanum vulgare e Thymus vulgaris para o Tratamento da Mastite Bovina. Master’s Thesis, Universidade Federal do Espírito Santo, Vitória, Brazil, 2022. [Google Scholar]
- Farmacopeia Brasileira, 6th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brasília, 2019; Volume 1.
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology, 3rd ed.; Informa Healthcare Inc.: New York, NY, USA, 2007; ISBN 978-0-8493-9399-0. [Google Scholar]
- Agência Nacional de Vigilância Sanitária. Guia de Estabilidade dos Produtos Cosméticos, 1st ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2004. [Google Scholar]
- Pianovski, A.R.; Vilela, A.F.G.; da Silva, A.A.S.; Lima, C.G.; da Silva, K.K.; Carvalho, V.F.M.; de Musis, C.R.; Machado, S.R.P.; Ferrari, M. Use of pequi oil (Caryocar brasiliense) in cosmetics emulsions: Development and evaluate of physical stability. Braz. J. Pharm. Sci. 2008, 44, 249–259. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. USP Compounding Standards and Beyond-Use Dates (BUDs). 2019. Available online: https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf (accessed on 18 October 2024).
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiologia, 12th ed.; Artmed: Porto Alegre, Brazil, 2017; ISBN 978-0-3219-2915-0. [Google Scholar]
- Aulton, M.E.; Taylor, K.M.G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 5th ed.; Elsevier: Toronto, ON, Canada, 2018; ISBN 978-0-7020-7005-1. [Google Scholar]
- Allen, L.V., Jr.; Popovich, N.G. Ansel’s: Pharmaceutical Dosage Forms and Drug Delivery Systems, 12th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021; ISBN 978-1-9751-7177-3. [Google Scholar]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules. 2020, 25, 678. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant, and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Rodrigues, M.R.A.; Krause, L.C.; Caramão, E.B.; dos Santos, J.G.; Dariva, C.; de Oliveira, J.V. Chemical composition and extraction yield of the extract of Origanum vulgare obtained from sub-and supercritical CO2. J. Agric. Food Chem. 2004, 52, 3042–3047. [Google Scholar] [CrossRef]
- Pozzatti, P.; Loreto, E.S.; Mario, D.A.N.; Rossato, L.; Santurio, J.M.; Alves, S.H. Activities of essential oils in the inhibition of Candida albicans and Candida dubliniensis germ tube formation. J. Mycol. Med. 2010, 20, 185–189. [Google Scholar] [CrossRef]
- Escobar, P.; Leal, S.M.; Herrera, L.V.; Martinez, R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp. essential oils and their major components. Mem. Inst. Oswaldo Cruz. 2010, 105, 184–190. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Maida, I.; Lo Nostro, A.; Pesavento, G.; Barnabei, M.; Calonico, C.; Perrin, E.; Chiellini, C.; Fondi, M.; Mengoni, A.; Maggini, V.; et al. Exploring the anti-burkholderia cepacia complex activity of essential oils: A preliminary analysis. Evid.-Based Complement. Altern. Med. 2014, 2014, 573518. [Google Scholar] [CrossRef] [PubMed]
- Pensel, P.E.; Maggiore, M.A.; Gende, L.B.; Eguaras, M.J.; Denegri, M.G.; Elissondo, M.C. Efficacy of essential oils of Thymus vulgaris and Origanum vulgare on echinococcus granulosus. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 693289. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.B.; Madrid, I.M.; Silva, A.L.; Dias de Castro, L.L.; Cleff, M.B.; Ferraz, V.; Meireles, M.C.; Zanette, R.; de Mello, J.R. In vitro susceptibility of Sporothrix brasiliensis to essential oils of Lamiaceae family. Mycopathologia 2016, 181, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, S.; Tsvetkova, A.; Georgieva, E.; Vankova, D. Bioactive phyto-compounds with antimicrobial effects and AI: Results of a desk research study. Microorganisms 2024, 12, 1055. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Čolović, R.; Duragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Cleff, M.B.; Meinerz, A.R.M.; Schuch, L.F.D.; Rodrigues, M.R.A.; Meireles, M.C.A.; Mello, J.R.B. In vitro activity of the essential oil of Origanum vulgare against Sporothrix schenckii. Arq. Bras. Med. Vet. Zootec. 2008, 60, 513–516. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A natural phenolic compound with antimicrobial properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Pereira, A.A.; Piccoli, R.H.; Batista, N.N.; Camargos, N.G.; de Oliveira, M.M.M. Thermochemical inactivation of Escherichia coli, Staphylococcus aureus and Salmonella enterica enteritidis by essential oils. Cienc. Rural. 2014, 44, 2022–2028. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils. thymol and carvacrol and their possible synergism. J. Essent. Oil-Bear. Plants. 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Almeida, R.R. Mecanismos de Ação dos Monoterpenos Aromáticos: Timol e Carvacrol. Bachelor’s Thesis, Universidade Federal de São João Del-Rei, São João Del-Rei, Brazil, 2015. [Google Scholar]
- Nobre, M.S.V.; Camargos, L.F.M.; Soares, R.S.L.; Nogueira, T.D.A.M.C.; Freitas, M.L.A.; Oliveira, S.P.D.; Souza, C.N.D.; Almeida, A.C.D. Antibacterial activity of Origanum vulgare essential oil in front of Staphylococcus sp. isolates of minas artesanal cheese. Braz. J. Develop. 2021, 7, 38712–38720. [Google Scholar] [CrossRef]
- Soni, K.A.; Oladunjoye, A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L.; Mikel, B.; Bailey, R.H. Inhibition and inactivation of Salmonella typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Prot. 2013, 76, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Instrução Normativa nº 26, de 9 de julho de 2009. Regulamento Técnico para a Fabricação. o Controle de Qualidade. a Comercialização e o Emprego de Produtos Antimicrobianos de Uso Veterinário; Ministério da Agricultura. Pecuária e Abastecimento (MAPA): Brasília, Brazil, 2009; Seção1; pp. 1–972.
- Dimitrova, E.; Bogdanova, S.; Mitcheva, M.; Tanev, I.; Minkov, E. Development of model aqueous ophthalmic solution of indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 1297–1301. [Google Scholar] [CrossRef]
- Liu, T.; Chu, B. Formation of homogeneous gel-like phases by mixed triblock copolymer micelles in aqueous solution: FCC to BCC phase transition. J. Appl. Cryst. 2000, 33, 727–730. [Google Scholar] [CrossRef]
- Guimarães, L.G.L.; Cardoso, M.G.; Zacaroni, L.M.; de Lima, R.K.; Pimentel, F.A.; Morais, A.R. Influence of light and temperature on the oxidation of lemongrass essential oil (Cymbopogon citratus (DC.) Stapf). Quím. Nova 2008, 31, 1476–1480. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, X.; Wang, X.; Li, H. Detection of antibiotic resistance. virulence gene and drug resistance gene of Staphylococcus aureus isolates from bovine mastitis. Microbiol. Spectr. 2022, 10, e0047122. [Google Scholar] [CrossRef]
- Neelam, J.V.K.; Singh, M.; Joshi, V.G.; Chhabra, R.; Singh, K.; Rana, Y.S. Virulence and antimicrobial resistance gene profiles of Staphylococcus aureus associated with clinical mastitis in cattle. PLoS ONE 2022, 17, e0264762. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil. thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.L.; Duarte-Almeida, J.M.; Perez, D.V.; Franco, B.D.G.M. Oregano essential oil: Influence of the chemical composition on the inhibitory activity against Salmonella enteritidis. Food Sci. Technol. 2010, 30, 136–141. [Google Scholar] [CrossRef]
- Kitching, M.; Mathur, H.; Flynn, J.; Byrne, N.; Dillon, P.; Sayers, R.; Rea, M.C.; Hill, C.; Ross, R.P. A live bio-therapeutic for mastitis, containing Lactococcus lactis DPC3147 with comparable efficacy to antibiotic treatment. Front. Microbiol. 2019, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.P.P.; do Amaral, M.P.; da Silva, D.C.N.; Souza, R.F.S.; Reis, S.A.G.B.; Júnior, F.A.G.S.; de Oliveira, H.P.; Peixoto, R.M.; da Costa, M.M. Efficacy assessment of an intramammary formulation based on soluble polypyrrole in cows with experimentally induced mastites. Cienc. Rural. 2023, 53, e20220047. [Google Scholar] [CrossRef]
- Franzol, A.; Rezende, M.C. Emulsion stability: A case study involving anionic, cationic, and nonionic emulsifiers. Polímeros 2015, 25, 1–9. [Google Scholar] [CrossRef]
- Tadros, T. Viscoelastic properties of sterically stabilised emulsions and their stability. Adv. Colloid. Interface Sci. 2015, 222, 692–708. [Google Scholar] [CrossRef]
- Nugraha, D.H.; Anggadiredja, K.; Rachmawat, H. Mini-review of poloxamer as a biocompatible polymer for advanced drug delivery. Braz. J. Pharm. Sci. 2022, 58, e21125. [Google Scholar] [CrossRef]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive. bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef]
- Ferreira, S.B.S.; Bruschi, M.L. Investigation of the physicochemical stability of emulgels composed of poloxamer 407 and different oil phases using the Quality by Design approach. J. Mol. Liq. 2021, 332, 115856. [Google Scholar] [CrossRef]
- Lucia, A.; Girard, C.; Fanucce, M.; Coviella, C.; Rubio, R.G.; Ortega, F.; Guzmán, E. Development of an environmentally friendly larvicidal formulation based on essential oil compound blend to control Aedes aegypti larvae: Correlations between physicochemical properties and insecticidal activity. ACS Sustain. Chem. Eng. 2020, 8, 10995–11006. [Google Scholar] [CrossRef]
- Ramos, Y.J.; da Silva, N.Q.; Costa-Oliveira, C.; Pereira, R.A.; Morra, M.D.; Cerqueira-Silva, L.C.R. Obtaining and evaluation of micellar systems containing oiti fruit extract [Licania tomentosa (Benth.) Fritsch]. Rev. Fitos 2020, 14, 88–102. [Google Scholar] [CrossRef]
- Gündel, S.S.; de Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; Vaucher, R.A.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development. characterization. stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Prestes, O.S.; Peres, D.D.; de Freitas, A.Z.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Particle size and morphological characterization of cosmetic emulsified systems by Optical Coherence Tomography (OCT). Braz. J. Pharm. Sci. 2016, 52, 273–280. [Google Scholar] [CrossRef]
- Lucia, A.; Toloza, A.C.; Guzmán, E.; Ortega, F.; Rubio, R.G. Novel polymeric micelles for insect pest control: Encapsulation of essential oil monoterpenes inside a triblock copolymer shell for head lice control. Peer J. 2017, 20, e3171. [Google Scholar] [CrossRef]
- Ferreira, S.B.S.; Braga, G.; de Oliveira, E.L.; Rosseto, H.C.; Hioka, N.; Caetano, W.; Bruschi, M.L. Colloidal systems composed of poloxamer 407, different acrylic acid derivatives and curcuminoids: Optimization of preparation method, type of bioadhesive polymer and storage conditions. J. Drug Deliv. Sci. Technol. 2020, 57, 101686. [Google Scholar] [CrossRef]
- Calixto, L.S.; Maia-Campos, P.M.B.G. Physical-mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef]
- Pandit, A.P.; Pol, V.V.; Kulkarni, V.S. Xyloglucan based in situ gel of lidocaine hcl for the treatment of periodontosis. J. Pharm. 2016, 2016, 3054321. [Google Scholar] [CrossRef]
- Erdem, Y.K. Effect of ultrafiltration, fat reduction and salting on textural properties of white brined cheese. J. Food Eng. 2005, 71, 366–372. [Google Scholar] [CrossRef]
- Gilbert, L.; Savary, G.; Grisel, M.; Picard, C. Predicting sensory texture properties of cosmetic emulsions by physical measurements. Chemom. Intell. Lab. Syst. 2013, 124, 21–31. [Google Scholar] [CrossRef]
- Lau, M.H.; Tang, J.; Paulson, A.T. Texture profile and turbidity of gellan/gelatina mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Umar, A.K.; Butarbutar, M.; Sriwidodo, S.; Wathoni, N. Film-forming sprays for topical drug delivery. Drug Des. Devel Ther. 2020, 14, 2909–2925. [Google Scholar] [CrossRef] [PubMed]
- Fakhar-Ud, D.; Khan, G.M. Development and characterisation of levosulpiride-loaded suppositories with improved bioavailability in vivo. Pharm. Dev. Technol. 2019, 24, 63–69. [Google Scholar] [CrossRef] [PubMed]
- The Business Research Company. Veterinary Healthcare Market Reports 2024. 2024. Available online: https://www.thebusinessresearchcompany.com/reports/veterinary-healthcare-market-research (accessed on 20 October 2024).
- Ahmed, I. Pharmaceutical challenges in veterinary product development. Adv. Drug Del. Rev. 2002, 54, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, M.; Brayden, D. Controlled release drug delivery in farmed animals: Commercial challenges and academic opportunities. Curr. Drug Deliv. 2009, 6, 383–390. [Google Scholar] [CrossRef] [PubMed]
- M100 ED30:2020; Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.

| Components | FB | FBSC | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|---|
| Proportions % (w/w) | ||||||
| AP | ||||||
| Poloxamer 407 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Potassium sorbate | 0.15 | - | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin E | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Polysorbate 80 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Purified water SQ | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| OP | ||||||
| OEO | - | - | 2.24 | 3.10 | 4.48 | 8.96 |
| TEO | - | - | 0.26 | 0.40 | 0.52 | 1.04 |
| Sunflower seed oil SQ | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
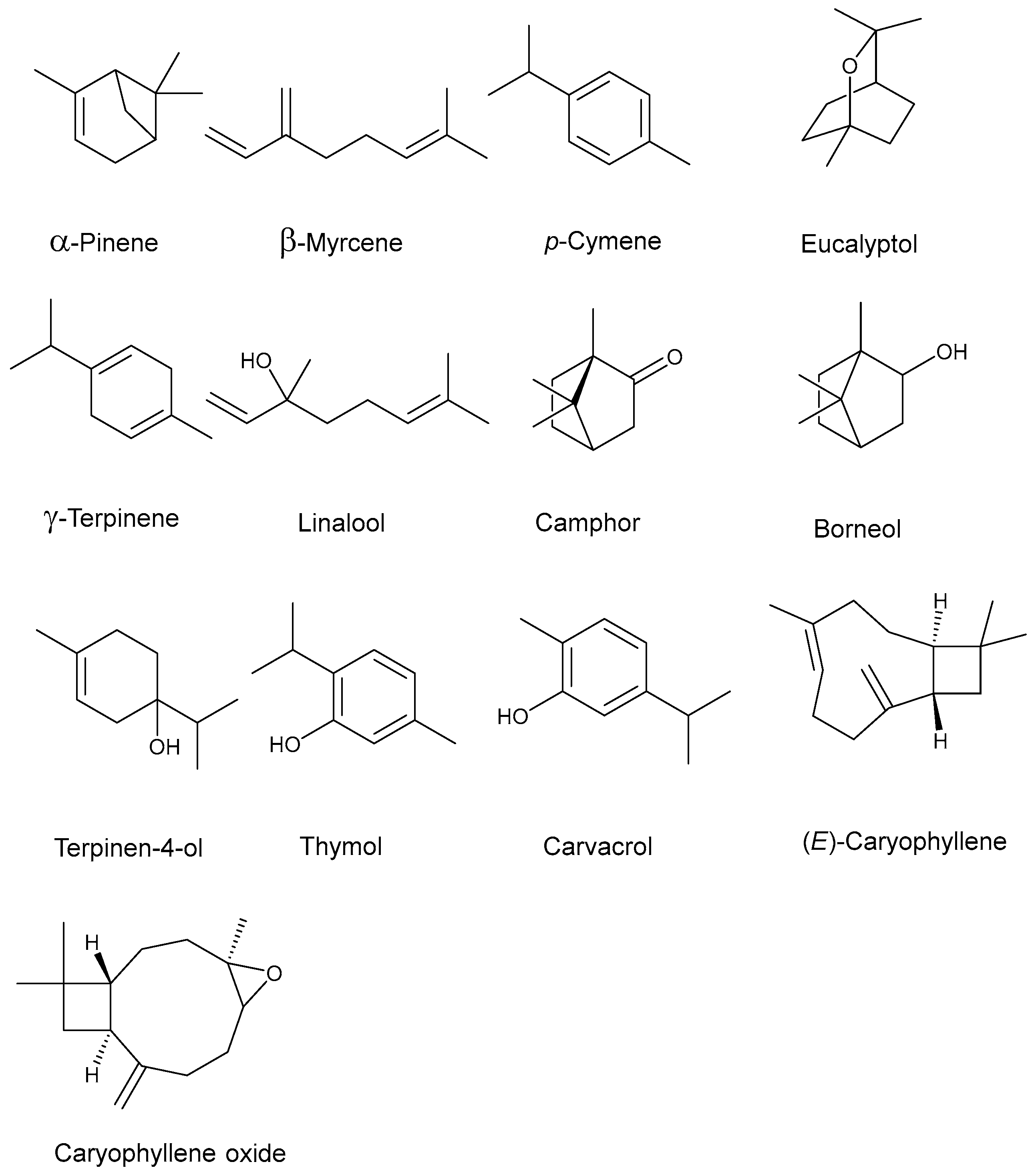
| Components | Kovat’s Index [b] | Kovat’s Index [c] | Area % [d] | |
|---|---|---|---|---|
| TEO | OEO | |||
| α-pinene | 931 | 932 | 2.40 | - |
| β-myrcene | 990 | 988 | 1.08 | - |
| p-cymene | 1021 | 1020 | 20.36 | 7.27 |
| Eucalyptol | 1033 | 1026 | 1.23 | 1.51 |
| γ-terpinene | 1057 | 1054 | 7.54 | 4.84 |
| Linalool | 1099 | 1095 | 8.05 | 4.24 |
| Camphor | 1147 | 1141 | 1.97 | 1.15 |
| Borneol | 1168 | 1165 | 1.25 | 1.30 |
| Terpinen-4-ol | 1190 | 1186 | 1.33 | 1.18 |
| Thymol | 1290 | 1289 | 47.88 | 69.22 |
| Carvacrol | 1298 | 1298 | 4.06 | 3.44 |
| (E)-caryophyllene | 1419 | 1417 | 1.25 | 5.85 |
| Caryophyllene oxide | 1583 | 1582 | 1.60 | - |
| Hydrogenated monoterpenes | 31.38 | 12.11 | ||
| Oxygenated monoterpenes | 65.77 | 82.04 | ||
| Hydrogenated sesquiterpenes | 1.25 | 5.85 | ||
| Oxygenated sesquiterpenes | 1.60 | - | ||
| Products | Population Density (CFU g−1) |
|---|---|
| FB | Countless |
| FBSC | Countless |
| F1 | Countless |
| F2 | Countless |
| F3 | Countless |
| F4 | 0 |
| Growth control | Countless |
| Mastizone® | 0 |
| Mastizone® V.S. | 0 |
| T0 | T1 (35 Days) | |||
|---|---|---|---|---|
| FB | F4 | F4 | ||
| RT | UR | O | ||
| 6.37 | 6.16 | 5.85 | 5.76 | 5.58 |
| (±0.05) NS | (±0.04) NS | (±0.04) S[a] | (±0.07) S[a] | (±0.03) S[a] |
| Parameters | FB (T0) | F4 | |||
|---|---|---|---|---|---|
| T0 | T1 (35 Days) | ||||
| RT | UR | O | |||
| Hardness (g) | 123.33 (± 10.02) S | 191.33 (± 26.10) S | 308.33 (± 27.30) S[a] | 278.00 (± 60.10) S[a] | 194.33 (± 21.57) NS[b] |
| Adhesiveness (mJ) | 6.47 (± 0.76) NS | 6.57 (± 2.02) NS | 16.20 (± 2.39) S[a] | 17.40 (± 4.86) S[a] | 5.67 (± 2.96) NS[b] |
| Elasticity index (mm) | 7.72 (± 0.27) NS | 6.01 (± 1.89) NS | 13.80 (± 1.13) S[a] | 14.37 (± 1.81) S[a] | 11.32 (± 4.69) NS[a] |
| Cohesiveness | 0.77 (± 0.10) NS | 0.65 (± 0.19) NS | 0.62 (± 0.15) NS[a] | 0.49 (± 0.06) NS[b] | 0.64 (± 0.06) NS[a] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, N.M.; Ferreira, N.S.; Menezes, K.V.; Neto, C.S.; Cunha, G.M.; Menini, L.; Resende, J.A.; Villanova, J.C.O. Formulating an Innovative Emulsion Based on Poloxamer 407 Containing Oregano and Thyme Essential Oils as Alternatives for the Control of Mastitis Caused by Staphylococcus aureus. Processes 2024, 12, 2640. https://doi.org/10.3390/pr12122640
Guimarães NM, Ferreira NS, Menezes KV, Neto CS, Cunha GM, Menini L, Resende JA, Villanova JCO. Formulating an Innovative Emulsion Based on Poloxamer 407 Containing Oregano and Thyme Essential Oils as Alternatives for the Control of Mastitis Caused by Staphylococcus aureus. Processes. 2024; 12(12):2640. https://doi.org/10.3390/pr12122640
Chicago/Turabian StyleGuimarães, Nayhara M., Nicolly S. Ferreira, Kássia V. Menezes, Cleveland S. Neto, Gabriel M. Cunha, Luciano Menini, Juliana A. Resende, and Janaina C. O. Villanova. 2024. "Formulating an Innovative Emulsion Based on Poloxamer 407 Containing Oregano and Thyme Essential Oils as Alternatives for the Control of Mastitis Caused by Staphylococcus aureus" Processes 12, no. 12: 2640. https://doi.org/10.3390/pr12122640
APA StyleGuimarães, N. M., Ferreira, N. S., Menezes, K. V., Neto, C. S., Cunha, G. M., Menini, L., Resende, J. A., & Villanova, J. C. O. (2024). Formulating an Innovative Emulsion Based on Poloxamer 407 Containing Oregano and Thyme Essential Oils as Alternatives for the Control of Mastitis Caused by Staphylococcus aureus. Processes, 12(12), 2640. https://doi.org/10.3390/pr12122640









