Carbon-Free H2 Production from Ammonia Decomposition over 3D-Printed Ni-Alloy Structures Activated with a Ru/Al2O3 Catalyst
Abstract
1. Introduction
- (1)
- Ammonia adsorption:
- (2)
- 1st N-H cleavage:
- (3)
- 2nd N-H cleavage:
- (4)
- 3rd N-H cleavage:
- (5)
- H2 recombination:
- (6)
- N2 recombination:
2. Materials and Methods
2.1. Materials
POCS and TPMS Structures and Used Catalyst
2.2. Activation of the Structured Supports
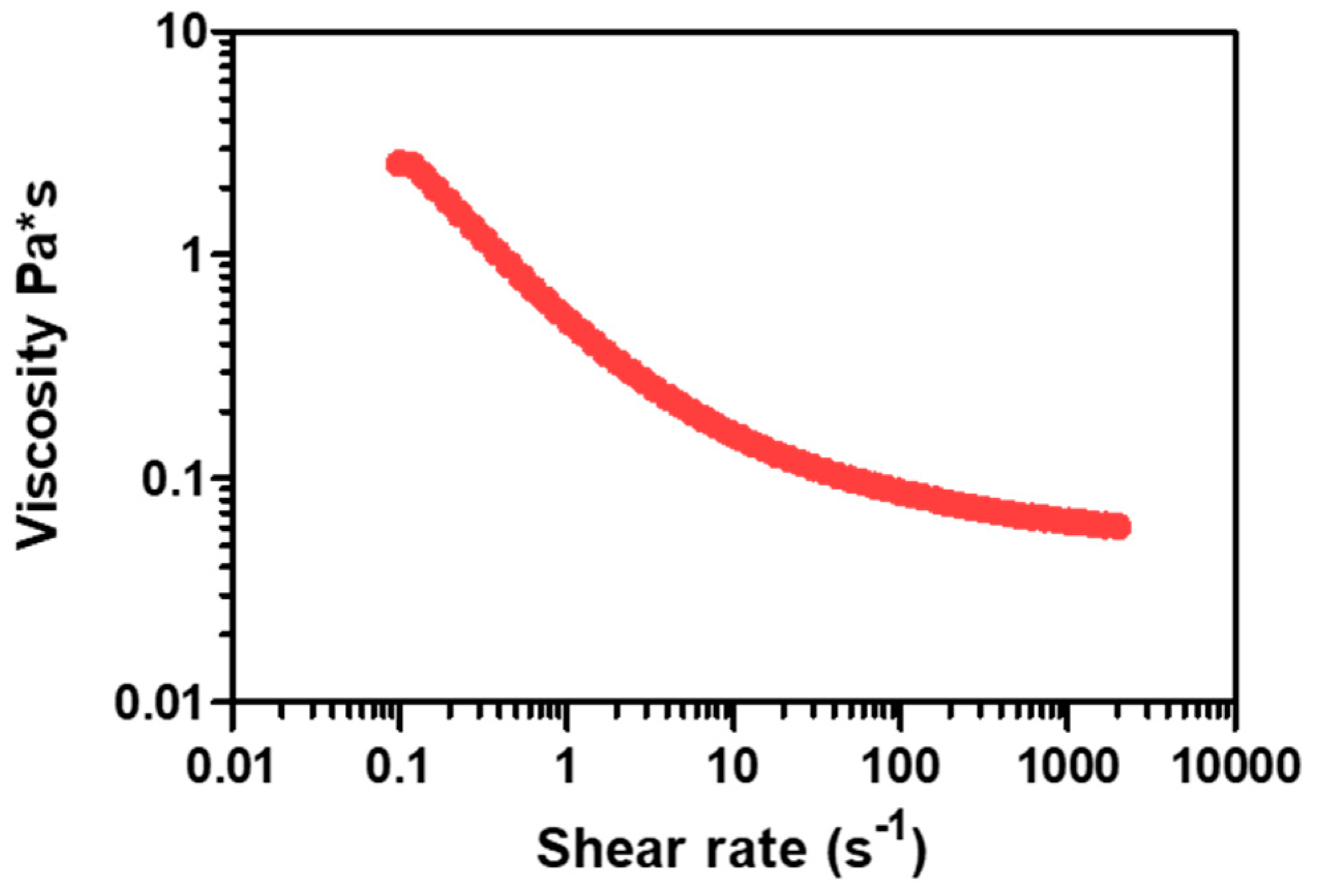
2.2.1. Slurry Preparation and Composition
2.2.2. Support Pretreatment and Slurry Deposition
2.3. Test Setup
2.4. Characterizations
3. Results and Discussion
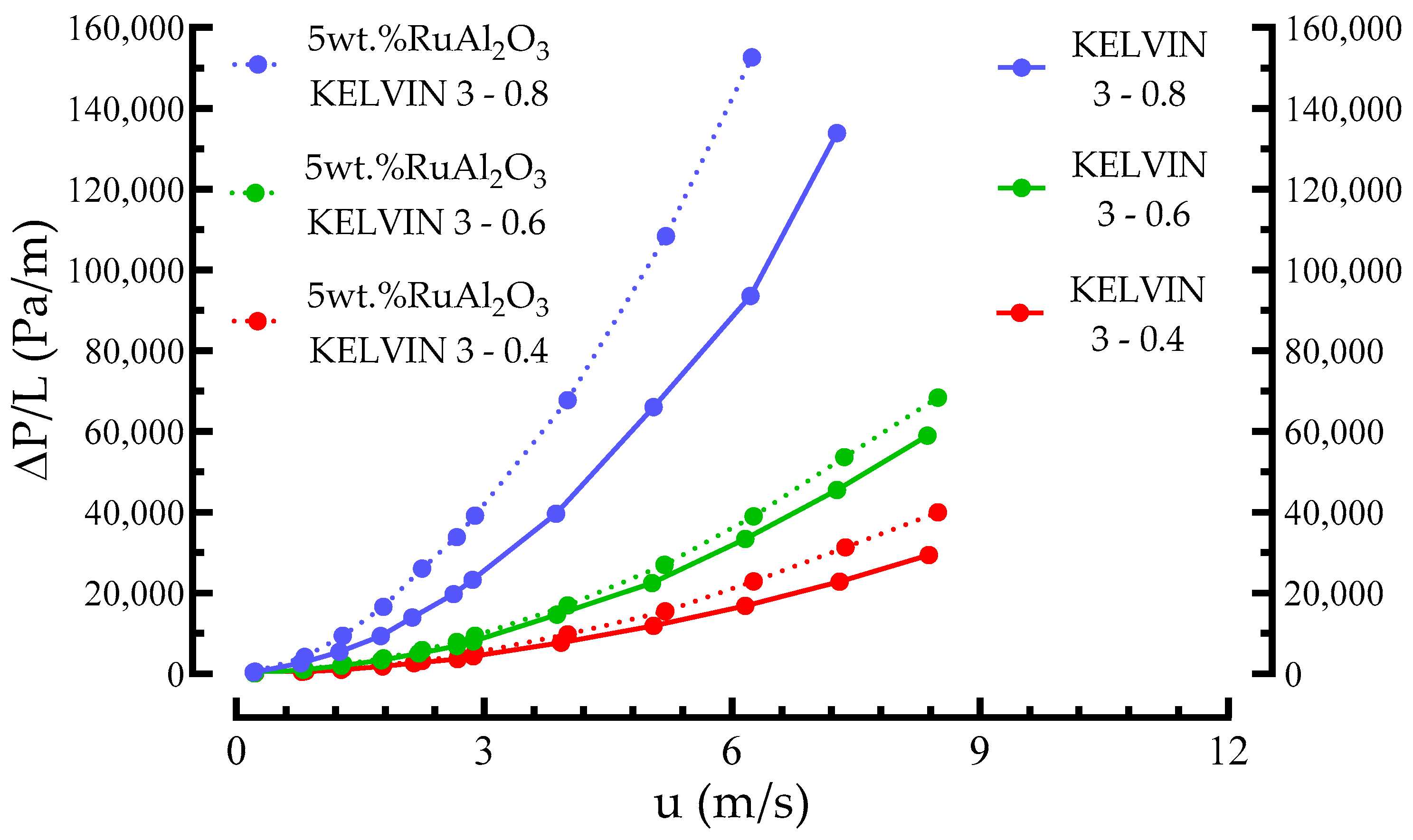
3.1. Morphology, Pressure Drop, and Mechanical Stability
3.2. Catalytic Activity
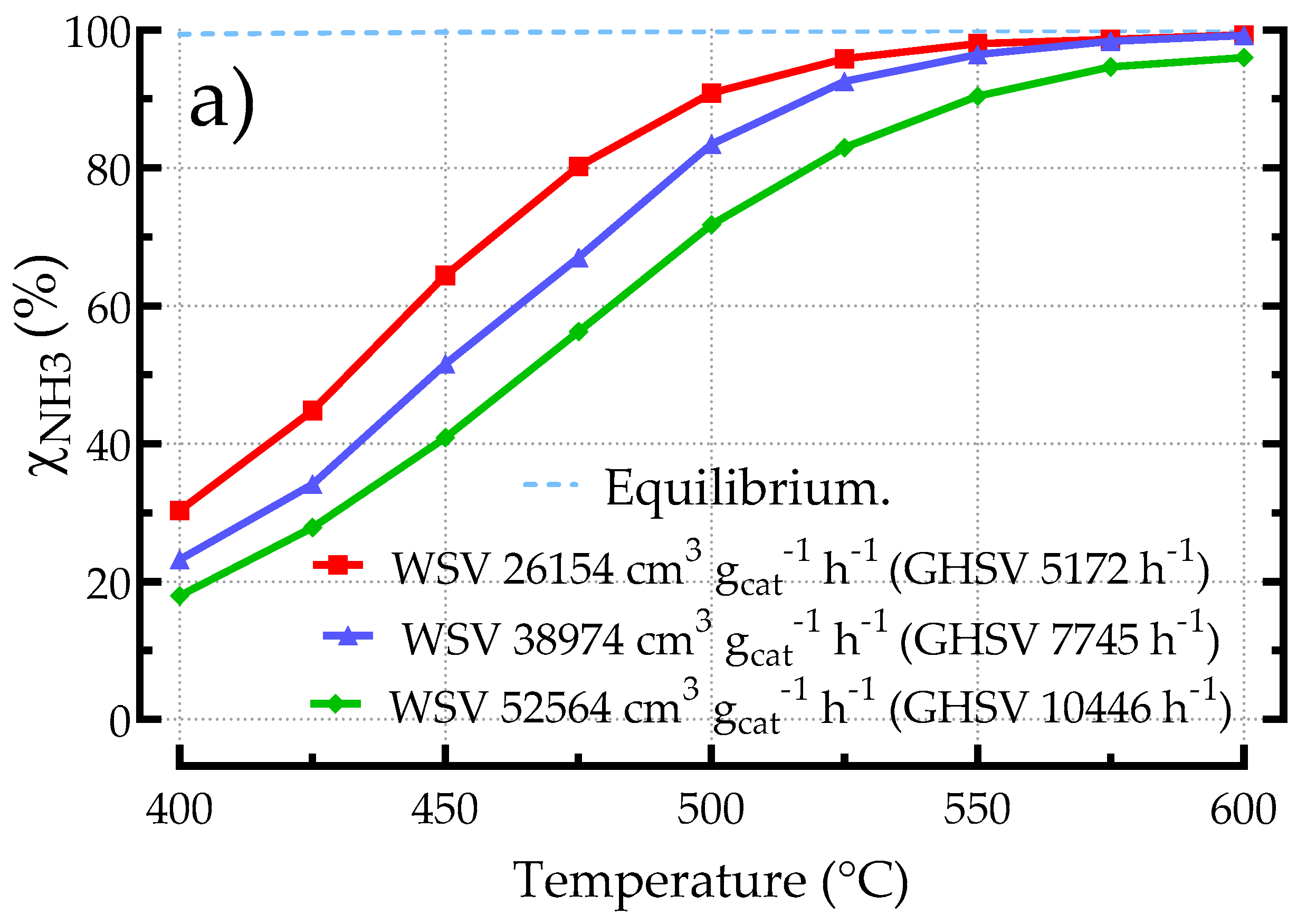
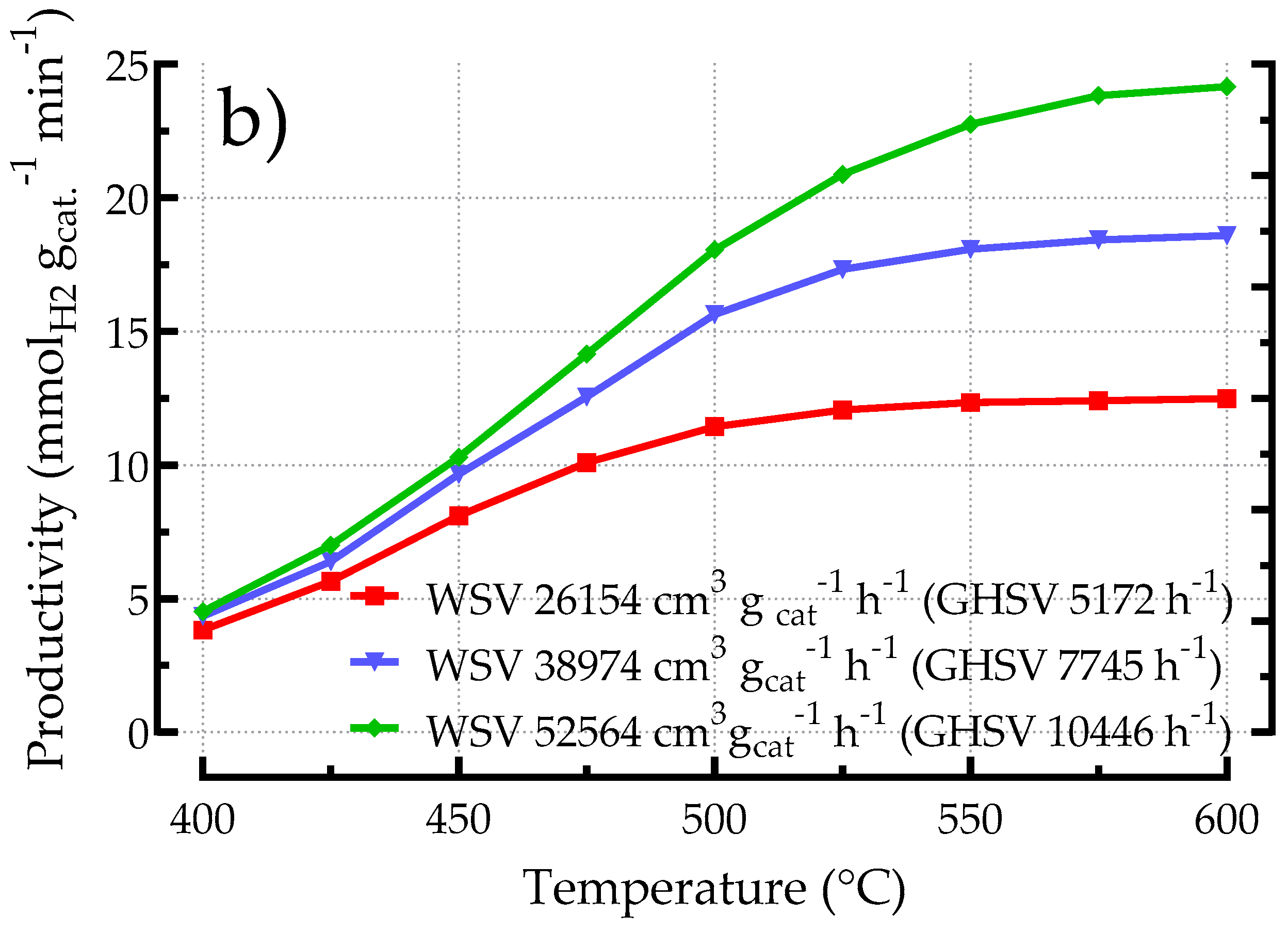
3.2.1. Influence of Space Velocity on the Performances of Kelvin-Based POCS
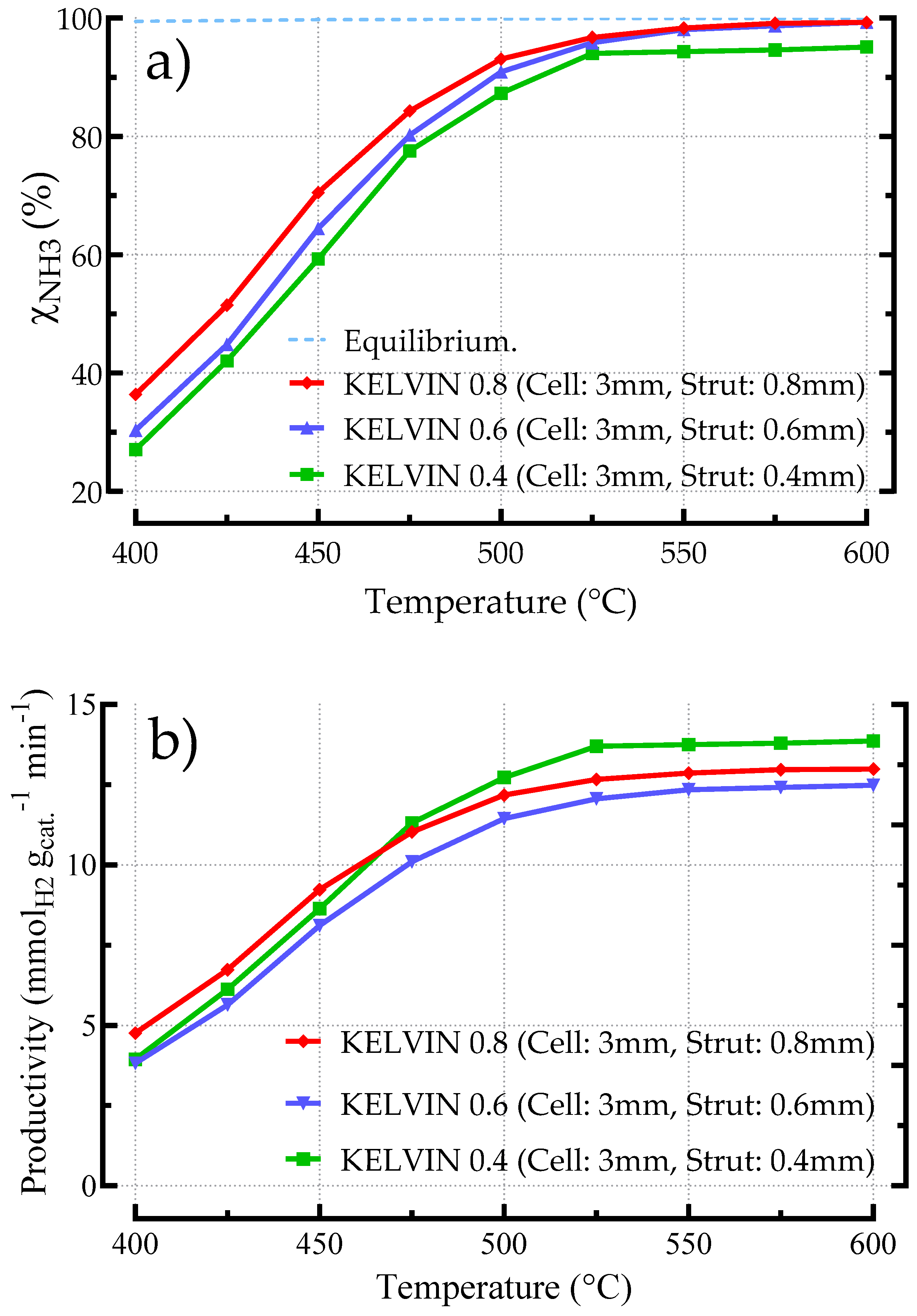
3.2.2. Influence of Geometric Features on the Performances of Kelvin-Based Catalyst
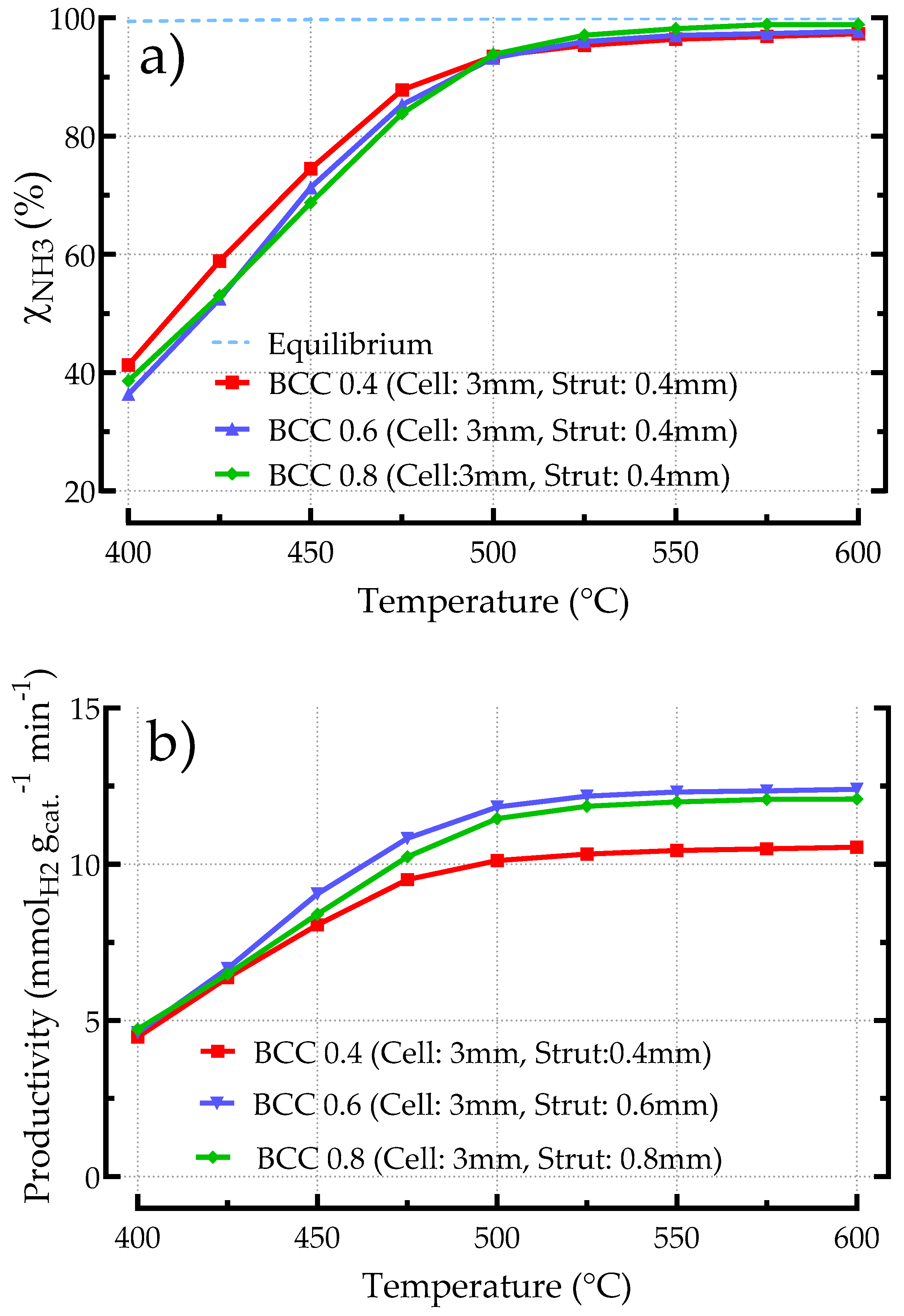
3.2.3. Influence of Geometric Features on the Performances of BCC-Based Catalysts
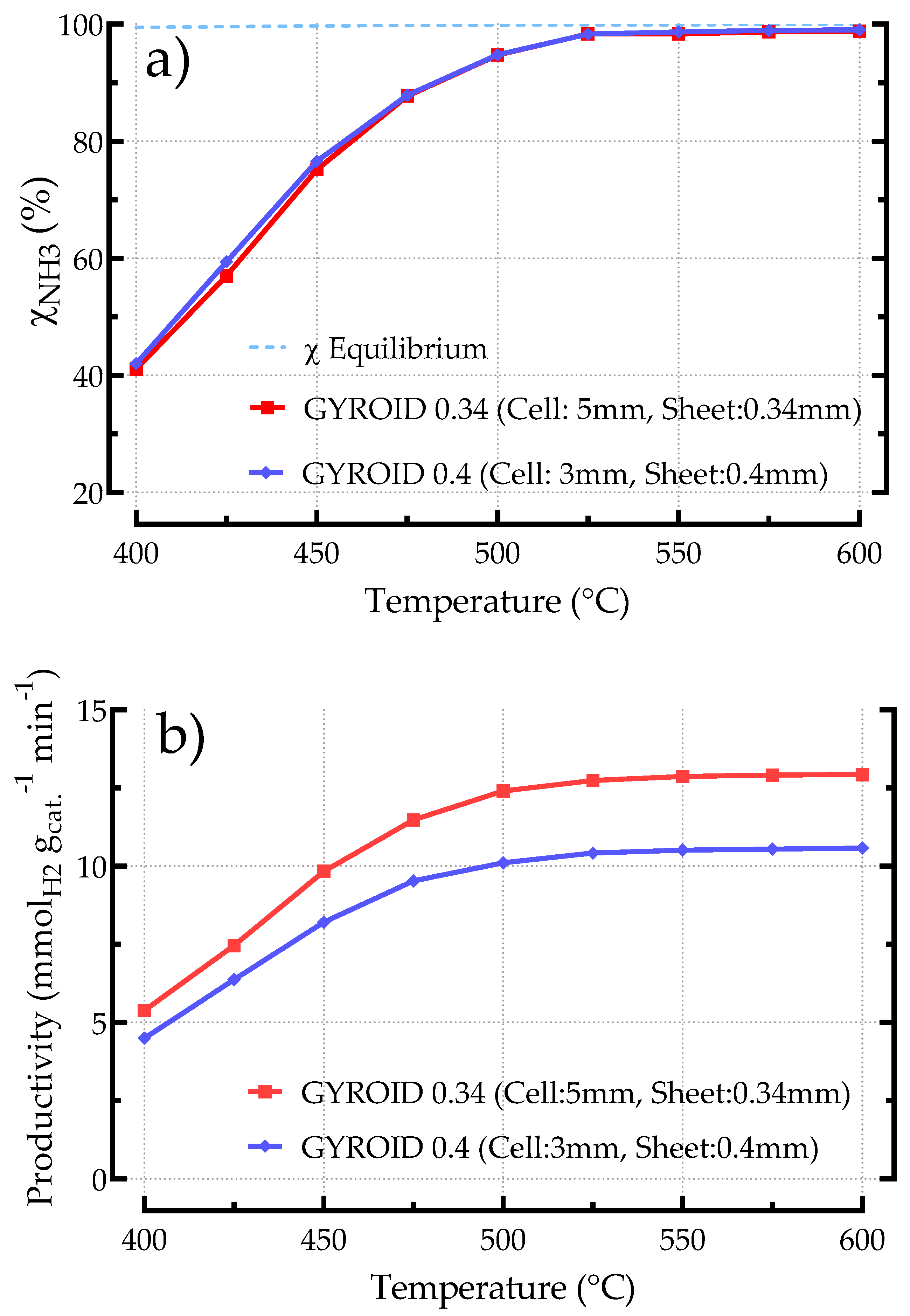
3.2.4. Influence of Geometric Features on the Performances of Gyroid Based Catalysts
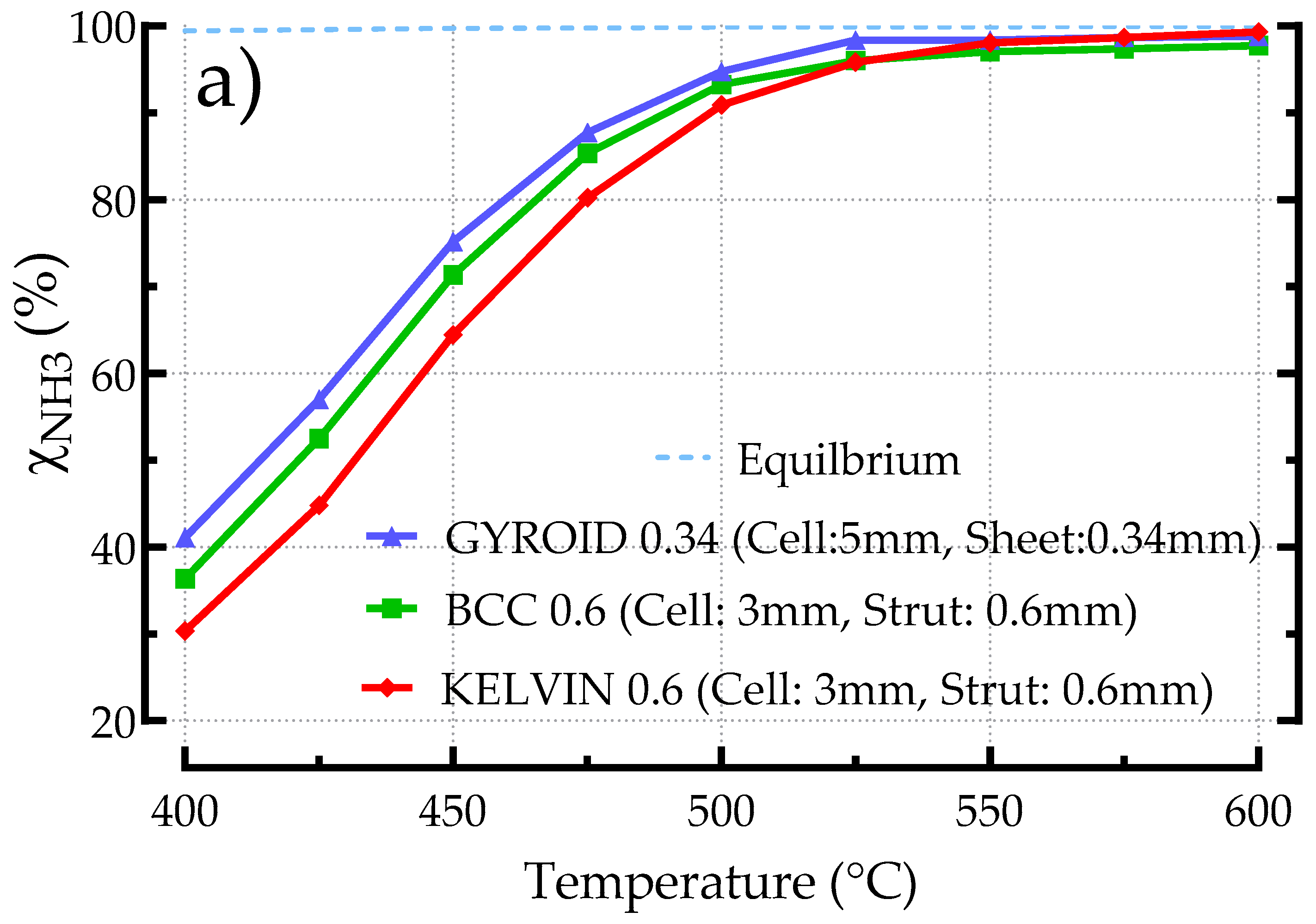
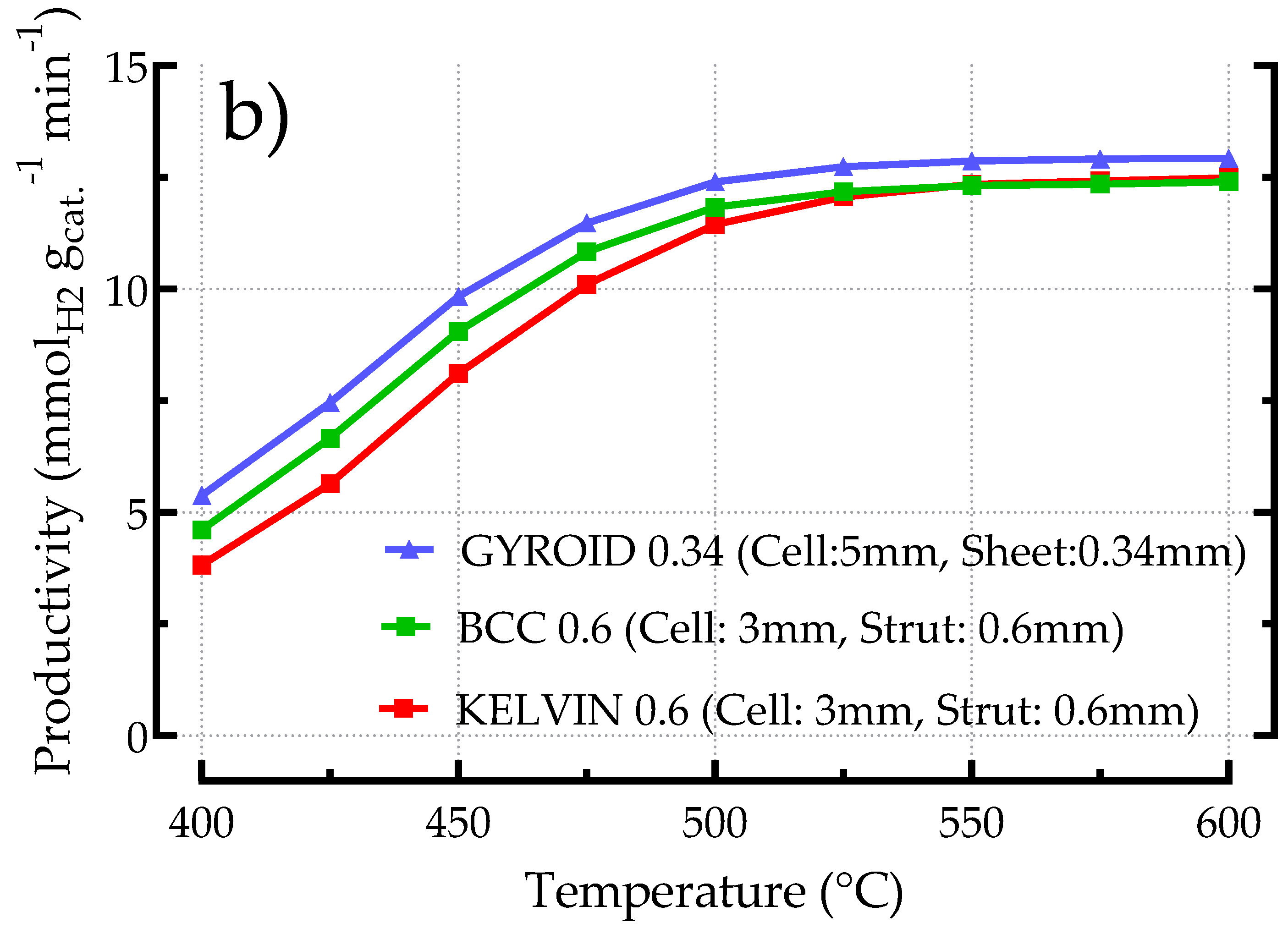
3.2.5. Comparing Different Structured Catalysts (BCC, Kelvin, Gyroid)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamran, M.; Turzyński, M. Exploring hydrogen energy systems: A comprehensive review of technologies, applications, prevailing trends, and associated challenges. J. Energy Storage 2024, 96, 112601. [Google Scholar] [CrossRef]
- Papadias, D.D.; Peng, J.K.; Ahluwalia, R.K. Hydrogen carriers: Production, transmission, decomposition, and storage. Int. J. Hydrogen Energy 2021, 46, 24169–24189. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Lipman, T.; Shah, N. Ammonia as an Alternative Energy Storage Medium for Hydrogen Fuel Cells: Scientific and Technical Review for Near-Term Stationary Power Demonstration Projects, Final Report|Transportation Sustainability Research Center. 2017. Available online: https://tsrc.berkeley.edu/publications/ammonia-alternative-energy-storage-medium-hydrogen-fuel-cells-scientific-and-technical (accessed on 7 July 2024).
- Aziz, M.; TriWijayanta, A.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Li, N.; Zhang, C.; Li, D.; Jiang, W.; Zhou, F. Review of reactor systems for hydrogen production via ammonia decomposition. Chem. Eng. J. 2024, 495, 153125. [Google Scholar] [CrossRef]
- Ashcroft, J.; Goddin, H. Centralised and Localised Hydrogen Generation by Ammonia Decomposition: A technical review of the ammonia cracking process. Johns. Matthey Technol. Rev. 2022, 66, 375–385. [Google Scholar] [CrossRef]
- Trangwachirachai, K.; Rouwenhorst, K.; Lefferts, L.; Faria Albanese, J.A. Recent progress on ammonia cracking technologies for scalable hydrogen production. Curr. Opin. Green Sustain. Chem. 2024, 49, 100945. [Google Scholar] [CrossRef]
- Galley, M.R.; Atomique, E.; Limitea, C.; Operations, C.; Candu, O. Future Trends in Heavy Water Production Tendances Futures la Production D’eau Lourde. In Proceedings of the Fourth Pacific Basin Conference, Vancouver, BC, Canada, 11–15 September 1983; Atomic Energi of Canada Limited: Chalk River, ON, Canada, 1983. CANDU Operations Canada KOJ 1J0 (ISSN 0067-0367). [Google Scholar]
- Bell, T.E.; Torrente-Murciano, L. H2 Production via Ammonia Decomposition Using Non-Noble Metal Catalysts: A Review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef]
- WMakepeace, J.; David, W.I. Structural Insights into the Lithium Amide-Imide Solid Solution. J. Phys. Chem. C. 2017, 121, 12010–12017. [Google Scholar] [CrossRef]
- Zou, X.; Su, H.-Y.; Sun, X.; Pang, W.; Hao, X.; Xu, Y.; Sun, K. Interplay between surface structure, reaction condition and mechanism for ammonia decomposition on Ru catalyst. Appl. Surf. Sci. 2024, 649, 159175. [Google Scholar] [CrossRef]
- Schüth, F.; Palkovits, R.; Schlögl, R.; Su, D.S. Ammonia as a possible element in an energy infrastructure: Catalysts for ammonia decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Chen, C.; Wu, K.; Ren, H.; Zhou, C.; Luo, Y.; Lin, L.; Au, C.; Jiang, L. Ru-Based Catalysts for Ammonia Decomposition: A Mini-Review. Energy Fuels 2021, 35, 11693–11706. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Mathhey, J. PGM Management. 2024. Available online: https://matthey.com/products-and-markets/pgms-and-circularity/pgm-management (accessed on 7 July 2024).
- Cechetto, V.; Di Felice, L.; Medrano, J.A.; Makhloufi, C.; Zuniga, J.; Gallucci, F. H2 production via ammonia decomposition in a catalytic membrane reactor. Fuel Process. Technol. 2021, 216, 106772. [Google Scholar] [CrossRef]
- Cechetto, V.; Anello, G.; Rahimalimamaghani, A.; Gallucci, F. Carbon Molecular Sieve Membrane Reactors for Ammonia Cracking. Processes 2024, 12, 1168. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Maestri, M.; Groppi, G.; Tronconi, E. A fundamental investigation of gas/solid mass transfer in open-cell foams using a combined experimental and CFD approach. Chem. Eng. J. 2018, 352, 558–571. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Vita, A.; Pino, L.; Cipit, F.; Lagan, M.; Recupero, V. Structured reactors as alternative to pellets catalyst for propane oxidative steam reforming. Int. J. Hydrogen Energy 2010, 35, 9810–9817. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Sindera, K.; Iwaniszyn, M.; Piątek, M.; Macek, W.; Jodłowski, P.J.; Wroński, S.; Sitarz, M.; Łojewska, J.; Kołodziej, A. Metal foams as novel catalyst support in environmental processes. Catalysts 2019, 9, 587. [Google Scholar] [CrossRef]
- Laguna, O.H.; Lietor, P.F.; Godino, F.J.I.; Corpas-Iglesias, F.A. A review on additive manufacturing and materials for catalytic applications: Milestones, key concepts, advances and perspectives. Mater. Des. 2021, 208, 109927. [Google Scholar] [CrossRef]
- Schwieger, W. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed]
- Busse, C.; Freund, H.; Schwieger, W. Intensification of heat transfer in catalytic reactors by additively manufactured periodic open cellular structures (POCS). Chem. Eng. Process. Process Intensif. 2018, 124, 199–214. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Mi, U.H. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Balzarotti, R.; Cristiani, C.; Francis, L.F. Combined dip-coating/spin-coating depositions on ceramic honeycomb monoliths for structured catalysts preparation. Catal. Today 2019, 334, 90–95. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ambrosetti, M.; Arnesano, M.; Anglani, A.; Groppi, G.; Tronconi, E. Periodic open cellular structures (POCS) as enhanced catalyst supports: Optimization of the coating procedure and analysis of mass transport. Appl. Catal. B Environ. 2021, 283, 119651. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ferrante, G.D.; Italiano, C.; Laganà, M.; Francis, L.F.; Vita, A.; Cristiani, C.; Pino, L. RhNi/CeO2 catalytic activation of alumina open cell foams by dip-spin coating for the CO2 methanation of biogas. Surf. Coatings Technol. 2022, 441, 128563. [Google Scholar] [CrossRef]
- Chyrkin, A.; Gunduz, K.O.; Fedorova, I.; Sattari, M.; Visibile, A.; Halvarsson, M.; Froitzheim, J.; Stiller, K. High-temperature oxidation behavior of additively manufactured IN625: Effect of microstructure and grain size. Corros. Sci. 2022, 205, 110382. [Google Scholar] [CrossRef]
- Boettge, D.; Standke, G.; Fuessel, A.; Adler, J. Functionalization of open-celled foams by homogeneous slurry based coatings. J. Mater. Res. 2013, 28, 2220–2233. [Google Scholar] [CrossRef]
- Ibáñez, M.; Ibáñez, M.; Sanz, O.; Sanz, O.; Egaña, A.; Egaña, A.; Reyero, I.; Reyero, I.; Bimbela, F.; Bimbela, F.; et al. Performance comparison between washcoated and packed-bed monolithic reactors for the low-temperature Fischer-Tropsch synthesis. Chem. Eng. J. 2021, 425, 130424. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Ali, M.; Khalil, M.; Rowshan, R.; Khan, K.A.; Abu Al-Rub, R.K. Forced Convection Computational Fluid Dynamics Analysis of Architected and Three-Dimensional Printable Heat Sinks Based on Triply Periodic Minimal Surfaces. J. Therm. Sci. Eng. Appl. 2021, 13, 21010. [Google Scholar] [CrossRef]
- Ansari, D.; Duwig, C. A gyroid TPMS heat sink for electronic cooling. Energy Convers. Manag. 2024, 319, 118918. [Google Scholar] [CrossRef]
- Richard, S.; Tasso, D.; Rajana, M.; Saker, A.; Santos, A.R.; Makhloufi, C.; Meynet, N.; Hary, B.; Nardone, S.; Marino, G.; et al. Comparison of thermo-hydraulic performance among different 3D printed periodic open cellular structures. Chem. Eng. J. 2024, 492, 152005. [Google Scholar] [CrossRef]
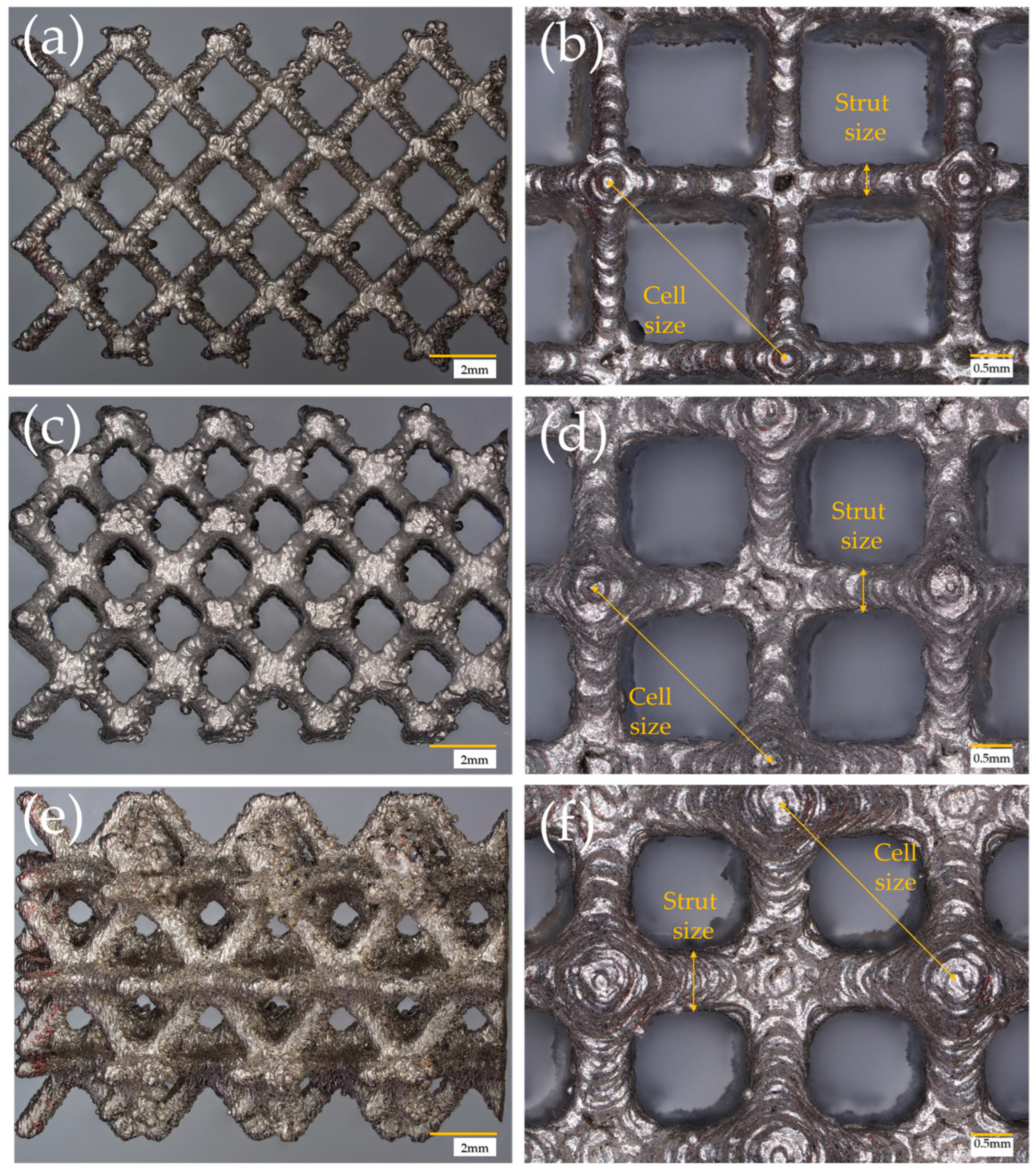
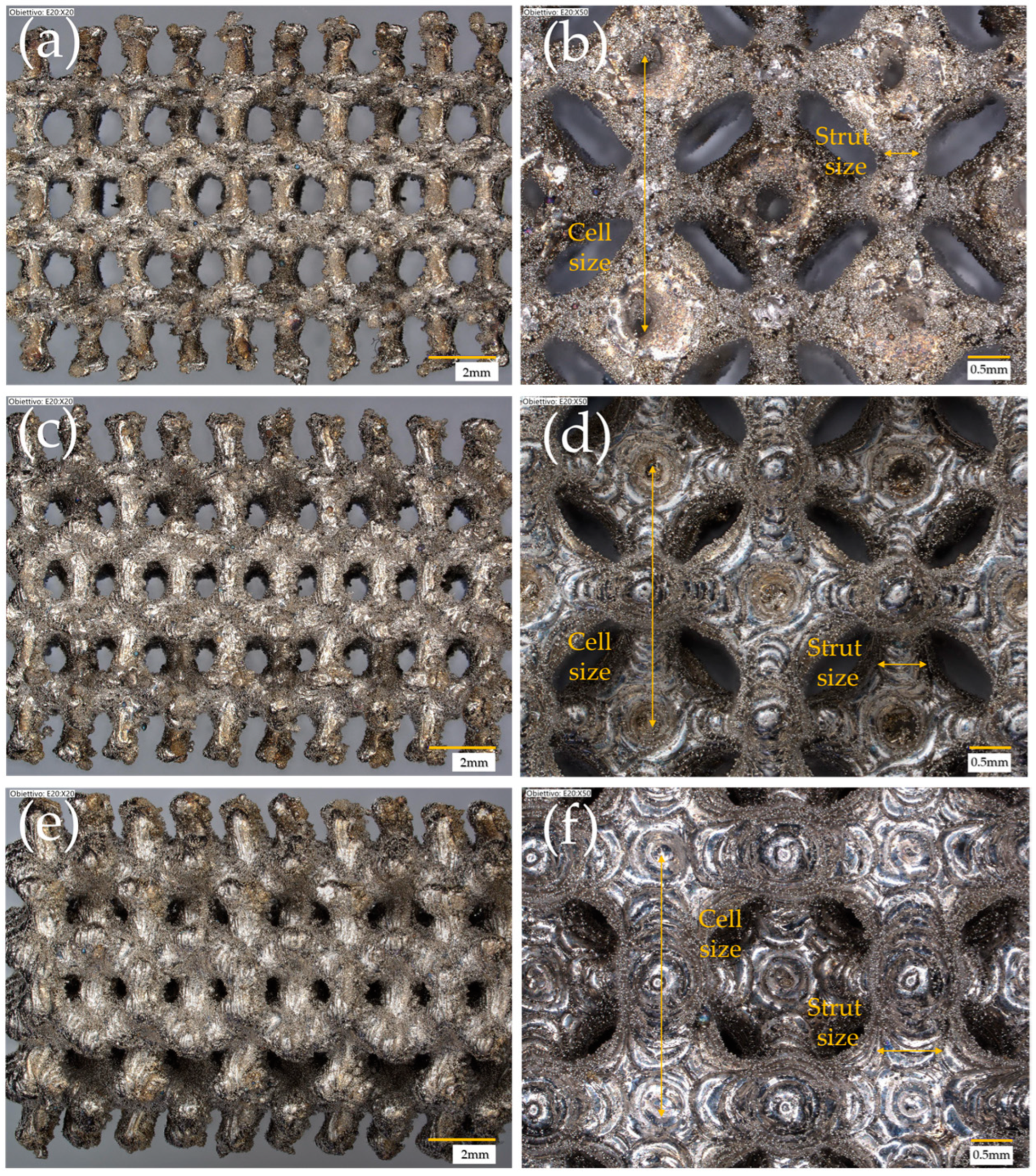
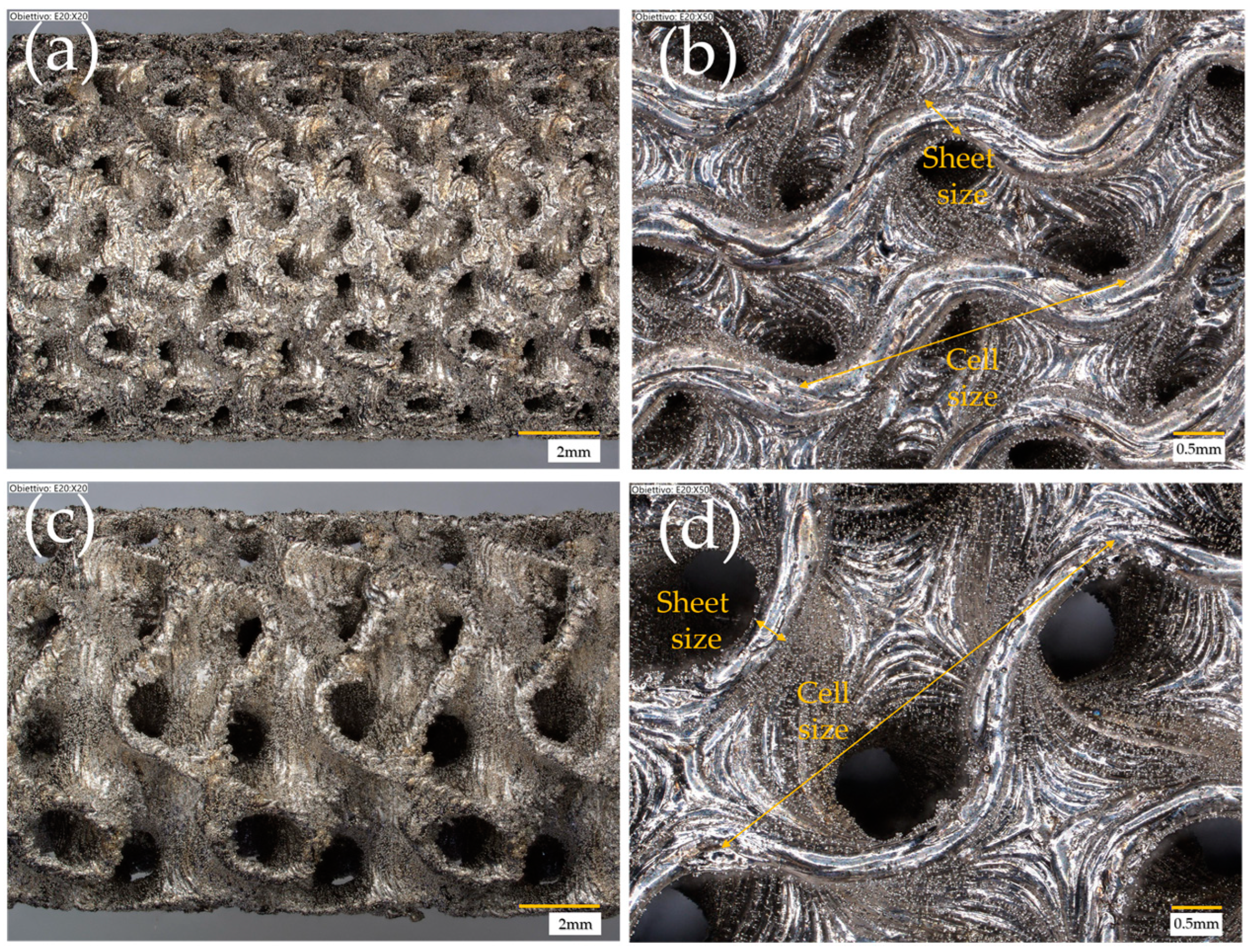
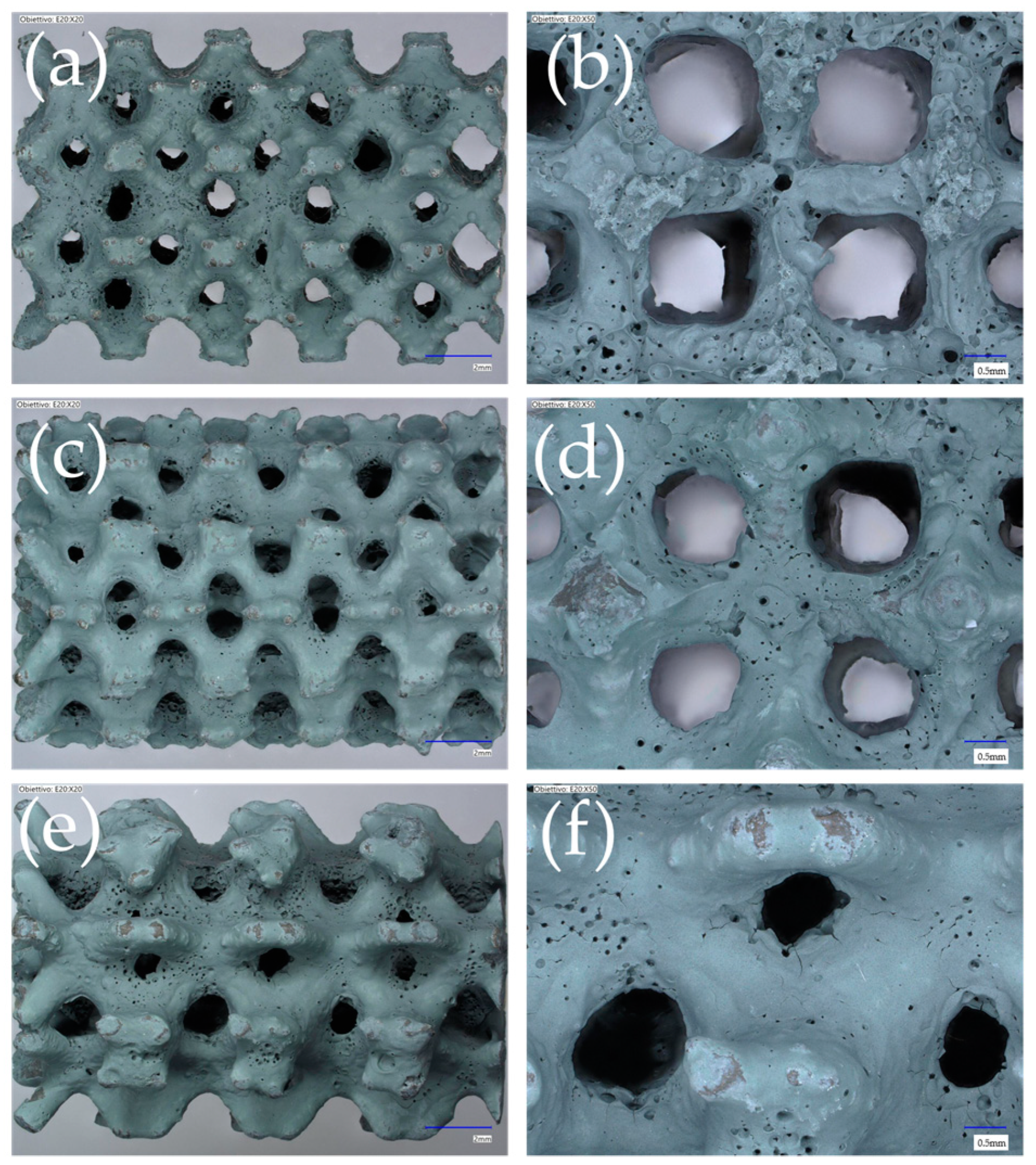
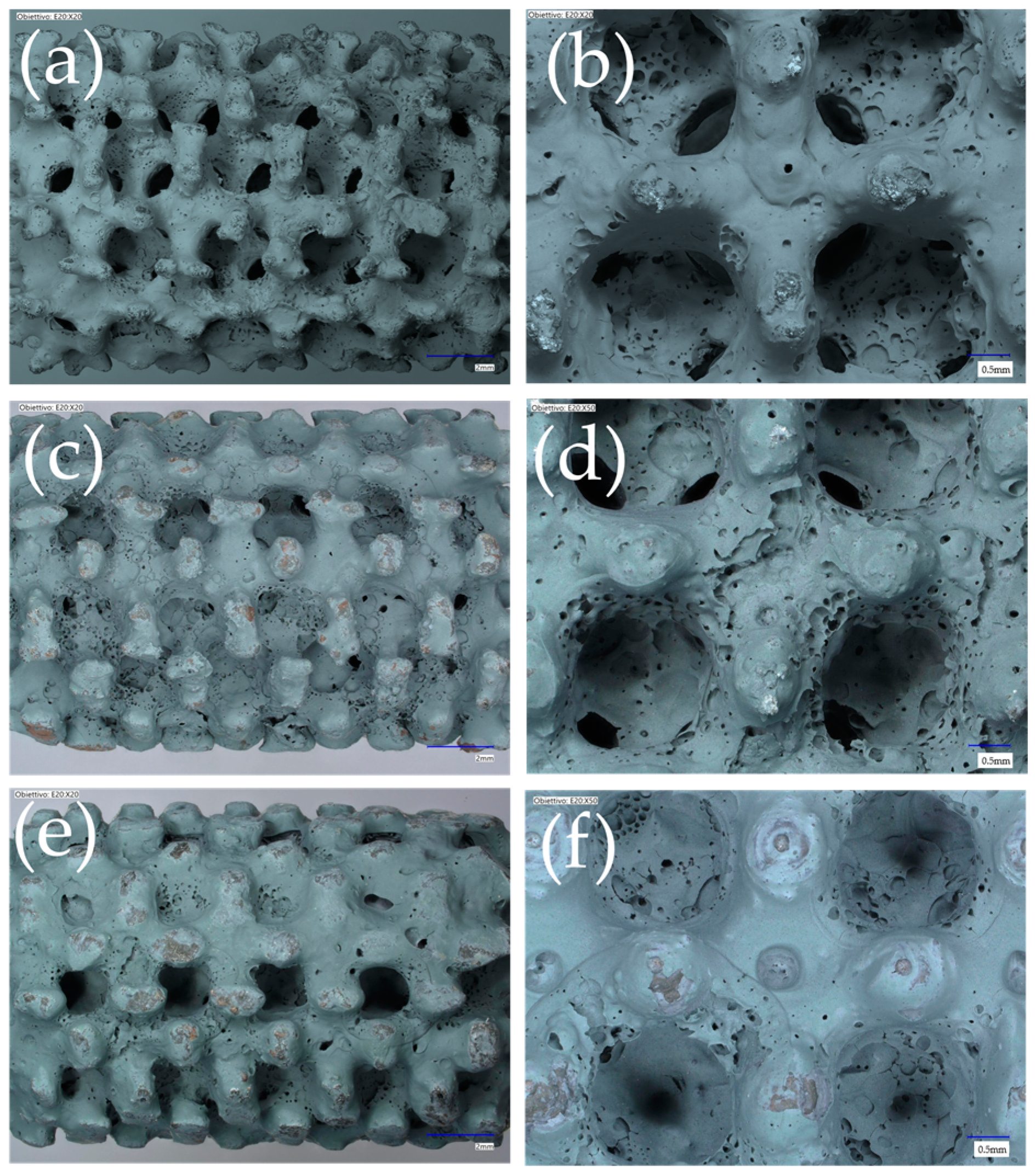
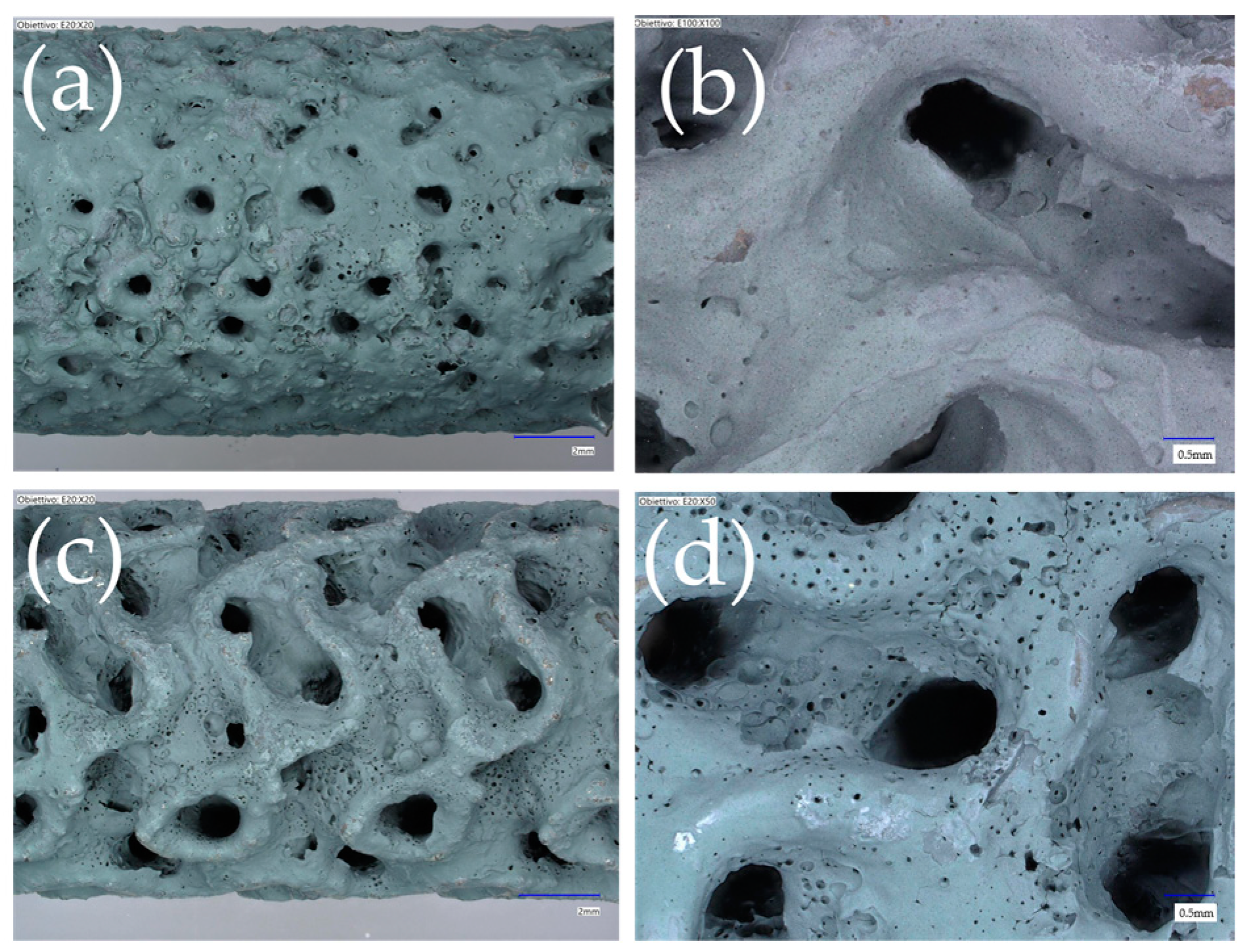


| Cell Type | Cell Size (mm) | Strut/Sheet Size (mm) | Solid Volume (cm3) | Solid Density (g/cm3) | Internal Surface Area (cm2) | Porosity (%) | Surface/Solid Vol. (SSA-cm2/cm3) | Cat. Layer Thickness (µm) |
|---|---|---|---|---|---|---|---|---|
| BCC 0.4 | 3 (3 *) | 0.4 (0.4 *) | 0.099 (0.163 **) | 11.31 (8.3 **) | 9.47 | 92.1 (86.17 ***) | 95.66 | (17.24 ****) |
| BCC 0.6 | 3 (3 *) | 0.6 (0.59 *) | 0.220 (0.284 **) | 8.86 (8.40 **) | 12.94 | 83.3 (75.89 ***) | 58.82 | (23.92 ****) |
| BCC 0.8 | 3 (3 *) | 0.8 (0.75 *) | 0.395 (0.392 **) | 4.41 (3.41 **) | 15.41 | 71.5 (66.71 ***) | 39.01 | (37.46 ****) |
| Kelvin 0.4 | 3 (3.04 *) | 0.4 (0.44 *) | 0.126 (0.213 **) | 14.21 (8.40 **) | 11.21 | 90.4 (81.94 ***) | 88.97 | (13.77 ****) |
| Kelvin 0.6 | 3 (3 *) | 0.6 (0.61 *) | 0.290 (0.344 **) | 10.14 (8.50 **) | 15.23 | 78.7 (70.75 ***) | 52.52 | (27.02 ****) |
| Kelvin 0.8 | 3 (3 *) | 0.8 (0.81 *) | 0.518 (0.569 **) | 9.42 (8.53 **) | 16.93 | 64.4 (51.64 ***) | 32.68 | (41.74 ****) |
| Gyroid 0.4 | 3 (3.10 *) | 0.4 (0.41 *) | 0.476 (0.624 **) | 11.12 (8.49 **) | 23.01 | 59.6 (47.01 ***) | 48.34 | (28.22 ****) |
| Gyroid 0.34 | 5 (5.18 *) | 0.34 (0.34 *) | 0.245 (0.325 **) | 11.26 (8.49 **) | 14.51 | 79.2 (72.40 ***) | 59.22 | (26.11 ****) |
| Slurry | Catalyst | Powder Catalyst (%) | Glycerol (%) | PVA (%) | Water (%) | Slurry Density (g/cm3) |
|---|---|---|---|---|---|---|
| 5 g | 5 wt% Ru/Al2O3 Engelhard | 22.4 | 42.50 | 1.50 | 33.60 | 1.4 |
| Structure Type | Cell Size (mm) | Strut/Sheet Size (mm) | Solid Volume (cm³) | Mass of Support (g) | Surface/Volume Ratio (cm2/cm3) | HPUV (T 450 °C) (mmol H2 min−1/ Vol. Support, cm3) | HPUW (T 450 °C) (mmol H2 min−1/ Weight Support, g) |
|---|---|---|---|---|---|---|---|
| Kelvin 0.6 | 3 | 0.6 | 0.290 | 2.93 | 52.52 | 1.61 | 0.65 |
| BCC 0.6 | 3 | 0.6 | 0.220 | 2.38 | 58.82 | 1.78 | 0.88 |
| Gyroid 0.34 | 5 | 0.34 | 0.325 | 2.76 | 59.22 | 2.13 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italiano, C.; Marino, G.; Thomas, M.; Hary, B.; Nardone, S.; Richard, S.; Saker, A.; Tasso, D.; Meynet, N.; Olivier, P.; et al. Carbon-Free H2 Production from Ammonia Decomposition over 3D-Printed Ni-Alloy Structures Activated with a Ru/Al2O3 Catalyst. Processes 2024, 12, 2663. https://doi.org/10.3390/pr12122663
Italiano C, Marino G, Thomas M, Hary B, Nardone S, Richard S, Saker A, Tasso D, Meynet N, Olivier P, et al. Carbon-Free H2 Production from Ammonia Decomposition over 3D-Printed Ni-Alloy Structures Activated with a Ru/Al2O3 Catalyst. Processes. 2024; 12(12):2663. https://doi.org/10.3390/pr12122663
Chicago/Turabian StyleItaliano, Cristina, Gabriel Marino, Minju Thomas, Benjamin Hary, Steve Nardone, Simon Richard, Assia Saker, Damien Tasso, Nicolas Meynet, Pierre Olivier, and et al. 2024. "Carbon-Free H2 Production from Ammonia Decomposition over 3D-Printed Ni-Alloy Structures Activated with a Ru/Al2O3 Catalyst" Processes 12, no. 12: 2663. https://doi.org/10.3390/pr12122663
APA StyleItaliano, C., Marino, G., Thomas, M., Hary, B., Nardone, S., Richard, S., Saker, A., Tasso, D., Meynet, N., Olivier, P., Gallucci, F., & Vita, A. (2024). Carbon-Free H2 Production from Ammonia Decomposition over 3D-Printed Ni-Alloy Structures Activated with a Ru/Al2O3 Catalyst. Processes, 12(12), 2663. https://doi.org/10.3390/pr12122663









