Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/274142/global-ethanol-production-since-2000/ (accessed on 10 November 2024).
- Rodopulo, A.K. Fundamentals of Biochemistry of Winemaking, Light and Food Industry; Moscow, Russia, 1983; pp. 112–137. Available online: https://www.livelib.ru/publisher/3663-legkaya-i-pischevaya-promyshlennost (accessed on 10 November 2024).
- Ribereau-Gayon, J.; Peynaud, E.; Ribereau-Gayon, P.; Sudraud, P. Theory and Practice of Winemaking. Characteristics of Wines. Ripening of Grapes. Yeast and Bacteria. Translation from French, Food Industry; Moscow, Russia, 1979; Volume 2, pp. 316–329. Available online: https://fantlab.ru/publisher4692 (accessed on 10 November 2024).
- Hirose, Y.; Ogawa, M.; Kusuda, Y. Constituents of Fusel Oils Obtained Through the Fermentation of Corn, Barley and Sweet Molasses. Agric. Biol. Chem. 1962, 26, 526–531. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, G.; He, Y.; Tang, J.; Zhou, H.; Qiu, S. The formation of higher alcohols in rice wine fermentation using different rice cultivars. Front. Microbiol. 2022, 13, 978323. [Google Scholar] [CrossRef]
- Giudici, P.; Romano, P.; Zambonelli, C. A biometric study of higher alcohol production in Saccharomyces cerevisiae. Can. J. Microbiol. 1990, 36, 61–64. [Google Scholar] [CrossRef]
- Kondo, T.; Tezuka, H.; Ishii, J.; Matsuda, F.; Ogino, C.; Kondo, A. Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae. J. Biotechnol. 2012, 159, 32–37. [Google Scholar] [CrossRef]
- Sanchez, N.; Cobo, M.; Rodriguez-Fontalvo, D.; Uribe-Laverde, M.Á.; Ruiz-Pardo, R.Y. Bioethanol Production from Sugarcane Press-Mud: Assessment of the Fermentation Conditions to Reduce Fusel Alcohol. Fermentation 2021, 7, 194. [Google Scholar] [CrossRef]
- Rosas, J.P.O.; Triana, Y.A.C.; Lozano, M.E.V. Effect of inorganic nutrients supplementation in sugar cane molasses on fusel alcohols production by a Saccharomyces cerevisiae native strain. J. Biotechnol. 2014, 185, 123. [Google Scholar] [CrossRef]
- Ribeiro-Filho, N.; Linforth, R.; Powell, C.D.; Fisk, I.D. Influence of essential inorganic elements on flavour formation during yeast fermentation. Food Chem. 2021, 361, 130025. [Google Scholar] [CrossRef] [PubMed]
- Renger, R.S.; van Hateren, S.H.; Luyben, K.C.A.M. The formation of esters and higher alcohols during the brewery fermentation; the effect of carbon dioxide pressure. J. Inst. Brew. 1992, 98, 509–513. [Google Scholar] [CrossRef]
- González-Jiménez, M.d.C.; Mauricio, J.C.; Moreno-García, J.; Puig-Pujol, A.; Moreno, J.; García-Martínez, T. Endogenous CO2 Overpressure Effect on Higher Alcohols Metabolism during Sparkling Wine Production. Microorganisms 2023, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pedroza, J.d.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G.; Hernández, S.; Orjuela, A. Recovery of alcohol industry wastes: Revaluation of fusel oil through intensified processes. Chem. Eng. Process.—Process Intensif. 2021, 163, 108329. [Google Scholar] [CrossRef]
- GOST 17071-91; Fusel Oil. Specifications. Publishing House of Standards: Moscow, Russia, 1992.
- Massa, T.B.; Raspe, D.T.; Cardozo-Filho, L. Fusel Oil: Chemical Composition and an Overview of Its Potential Application. J. Braz. Chem. Soc. 2023, 34, 153–166. [Google Scholar] [CrossRef]
- Kunakova, R.V.; Akhmetova, V.R.; Zajnullin, R.A.; Kolychev, V.M. Method for Processing Fusel Oils from Distilleries. Federal State Budget Educational Institution of Higher Professional Education “Ufa State Academy of Economy and Service.”. Patent RU2483054C1, 20 March 2012. [Google Scholar]
- Alvesa, D.R.; Finzer, J.R.D.; Teixeira, E.P. Pilot design of a distillation column to obtain isoamyl alcohol from fusel oil. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2022, 86, 89–103. [Google Scholar]
- Montoya, N.; Durán, J.; Córdoba, F.; Gil, I.D.; Trujillo, C.A.; Rodríguez, G. Colombian fusel oil. Ing. E Investig. 2016, 36, 21–27. [Google Scholar] [CrossRef]
- Hilmen, E.K. Separation of Azeotropic Mixtures: Tools for Analysis and Studies on Batch Distillation Operation. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2000. [Google Scholar]
- Cortel, C.; Flordeliza, K.O.; Galvez, S.R.A.; Magalong, M.A.; Mendoza, T.M.G.; Rubi, R.V.C. Recent trends in azeotropic mixture separation: A comprehensive review. Eng. Proc. 2024, 67, 56. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the fusel oil distillation process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351. [Google Scholar] [CrossRef]
- Pedroza, J.d.J.M.; Hernández, J.G.S.; Londoño, Á.O.; Hernández, S. Optimization of a fusel oil separation system using a dividing wall column. Comput. Aided Chem. Eng. 2015, 37, 1031–1036. [Google Scholar] [CrossRef]
- Artyukhov, V.G.; Egorov, A.S.; Osipenko, A.A.; Bereznikova, D.S. Method of High Alcohols Separation from Fusel Oil. Ukrainian Research Institute of Alcohol and Liquor Industry. Patent SU 257487, 20 November 1969. [Google Scholar]
- Báleš, V.; Timár, P.; Hrivnák, M. Separation 2-methylbutan-1-ol and 3-methylbutan-1-ol from fusel oil. J. Int. Sci. Publ. Agric. Food 2021, 9, 20–26. [Google Scholar]
- Liu, Y.; Gao, H.; Sun, J.; Wang, Y.; Wu, P.; Liu, Y.; Ji, Y. Novel packing-enhanced distillation separation of isoamyl alcohol isomer: Experimental and pilot scale study. AIChE J. 2011, 57, 3037–3041. [Google Scholar] [CrossRef]
- Montoya, N.; Córdoba, F.; Trujillo, C.; Gil, I.; Rodríguez, G. Fusel oil process separation. In AIChE Annual Meeting; American Institute of Chemical Engineers: Minneapolis, MN, USA, 2011; pp. 332–337. [Google Scholar]
- Patil, A.G.; Koolwal, S.M.; Butala, H.D. Fusel oil: Composition, removal and potential utilization. Int. Sugar J. 2002, 104, 51–63. [Google Scholar]
- Marinenko, O.V. Development and Mathematical Modeling of a System for Separating Non-Standard Fusel Fractions of Wort Distillation Units. Ph.D. Thesis, Maikop State Technological University, Krasnodar, Russia, 2006. [Google Scholar]
- ASTM D 86-23; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D 5501; Standard Test Method for Determination of Ethanol and Methanol Content in Fuels Containing Greater than 20 % Ethanol by Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D 1218-21; Standard Test Method for Refractive Index and Refractive Dispersion of Hydrocarbon Liquids. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D 445-24; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D 97-22; Standard Test Method for Pour Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D 4006-22; Standard Test Method for Water in Crude Oil by Distillation. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D 130-19; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM A 380; Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D 5865-19; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM: West Conshohocken, PA, USA, 2019.
- Douglas, J.M. Conceptual Design of Chemical Processes; McGraw-Hill Book Company: Singapore, 1988; p. 108. [Google Scholar]
- Stabnikov, V.N. Distillation and Rectification of Ethyl Alcohol; Publishing house “Food Industry”: Moscow, Russia, 1969; pp. 233–242. [Google Scholar]
- Gilyazetdinov, I.M.; Dmitriyev, A.V. Rectification of food-grade ethanol using additional column apparatuses. Bull. Kazan Technol. Univ. 2014, 17, 209–212. [Google Scholar]
- Garasimchuk, S.M.; Azarenkova, A.A.; Boychenko, M.S.; Baranovsky, M.N. Biochemical method for obtaining ethyl alcohol. Sci. Technol. 2014, 18–22. Available online: https://er.nau.edu.ua/bitstream/NAU/38160/1/6038-15169-1-SM.pdf (accessed on 11 November 2024).
- International Finance Corporation (IFC). Available online: https://www.ifc.org/content/dam/ifc/doc/2023/ifc-general-ehs-guidelines.pdf (accessed on 11 November 2024).
- Marinchenko, V.A.; Smirnov, V.A.; Ustinnikov, B.A.; Tsygankov, P.S.; Shvets, V.N.; Yarovenko, V.L. Technology of Alcohol; Smirnov, V.A., Ed.; Light and Food Industry: Moscow, Russia, 1981; pp. 345–346. [Google Scholar]
- Market Research Future. Available online: https://www.marketresearchfuture.com/reports/isoamyl-acetate-market-10823/ (accessed on 7 December 2024).
- Melnikova, G.N.; Anisimova, L.I. Skin Antiseptics for disinfecting the hands of medical personnel in order to optimize the prevention of nosocomial infections in medical institutions. Epidemiol. Infect. Dis. 2012, 51–59. Available online: https://epidemiology-journal.ru/en/archive/article/11415 (accessed on 7 December 2024).
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/isopropyl-alcohol-market-A13841#:~:text=The%20global%20isopropyl%20alcohol%20market,the%20demand%20for%20isopropyl%20alcohol./ (accessed on 7 December 2024).
- Manovyan, A.K. Technology for Processing Natural Energy Resources, Chemistry; KolosS: Moscow, Russia, 2004; pp. 62–63. [Google Scholar]
- Neyland, O.Y. Organic Chemistry: A Textbook for Chemical Specialties at Universities; Higher School: Moscow, Russia, 1990; p. 296. [Google Scholar]
- Transparency Market Research. Available online: https://www.transparencymarketresearch.com/n-butanol-market.html/ (accessed on 7 December 2024).
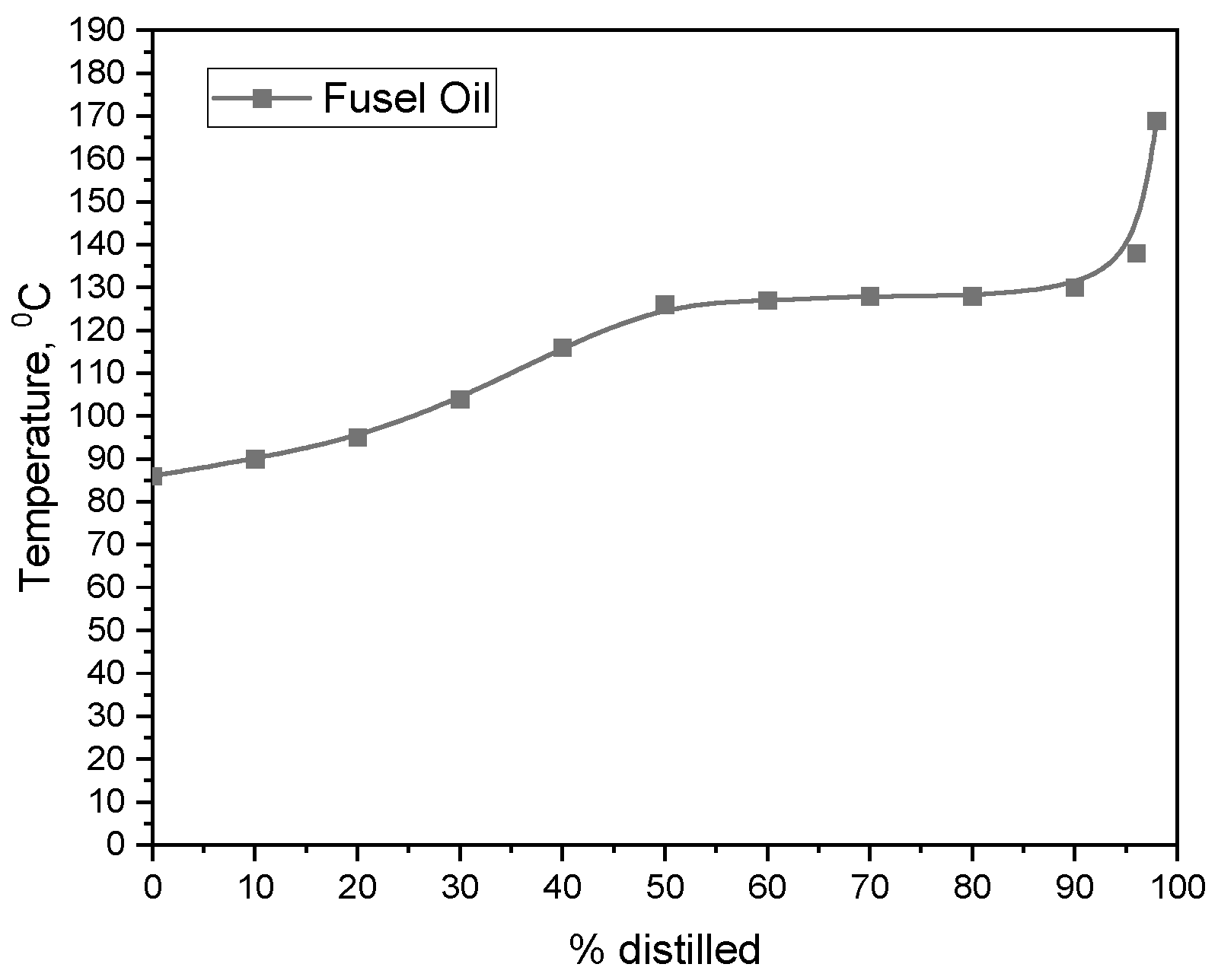

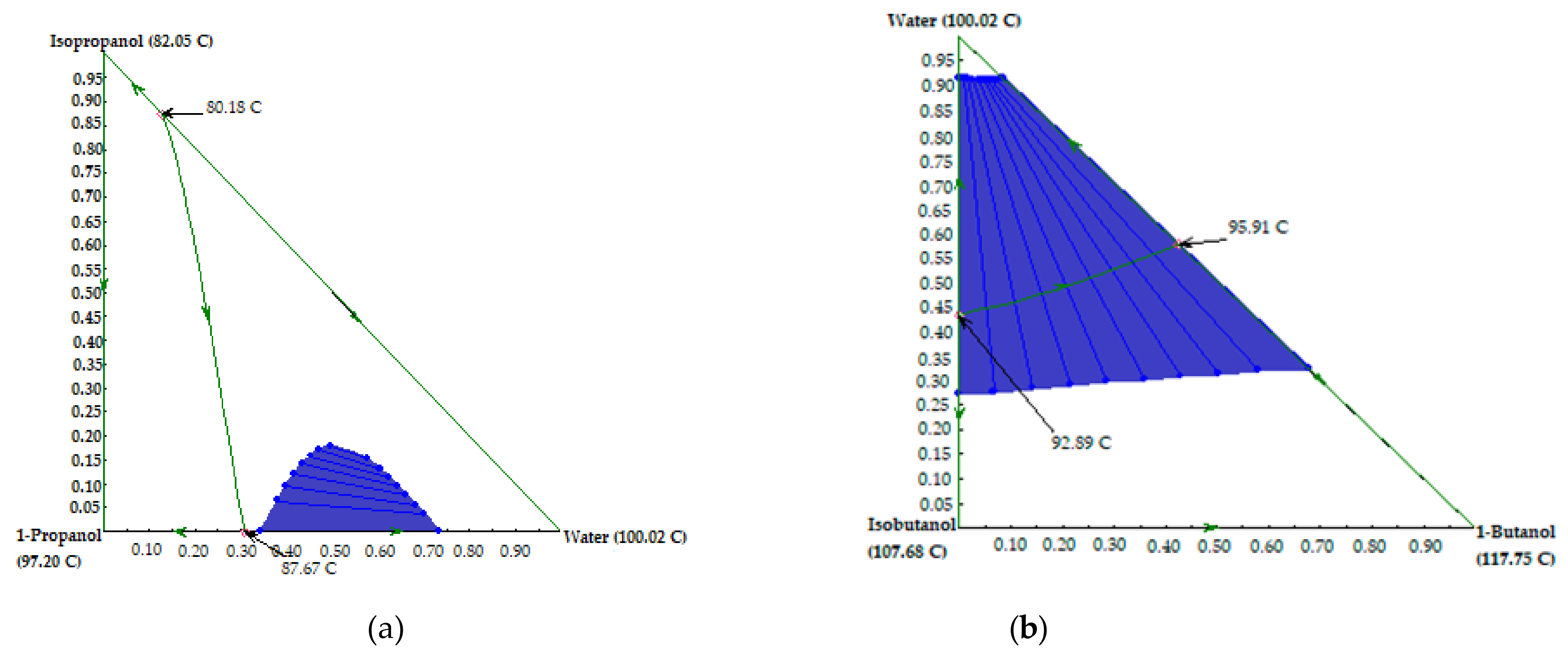
| № | Components | Azeotrope Type | Boiling Point, °C | Composition, Mass Fraction |
|---|---|---|---|---|
| 1 | Ethanol–Water | Homoazeotrope | 78.15 | 0.9562–0.0438 |
| 2 | Isopropanol–Water | Homoazeotrope | 80.18 | 0.8727–0.1273 |
| 3 | 1-Propanol–Water | Homoazeotrope | 87.67 | 0.6923–0.3077 |
| 4 | Isobutanol–Water | Heteroazeotrope | 92.89 | 0.5668–0.4332 |
| 5 | 1-Butanol–Water | Heteroazeotrope | 95.91 | 0.4233–0.5767 |
| 6 | 1-Butanol–3M1B–Water | Heteroazeotrope | 95.63 | 0.1811–0.2741–0.5448 |
| 7 | 2M1B–Water | Heteroazeotrope | 94.92 | 0.5025–0.4975 |
| 8 | 3M1B–Water | Heteroazeotrope | 95.71 | 0.4585–0.5415 |
| 9 | 1-Hexanol–Water | Heteroazeotrope | 98.21 | 0.2793–0.7207 |
| Characteristics | UM | Value | Standard Method |
|---|---|---|---|
| Density at 20 °C | kg/m3 | 829 | ASTM D 5501 [30] |
| Refractive index at 20 °C | - | 1.3990 | ASTM D 1218 [31] |
| Kinematic viscosity at 40 °C | cSt | 2.6 | ASTM D 445 [32] |
| Pour point | °C | −34 | ASTM D 97 [33] |
| Water content | wt.% | 14 | ASTM D 4006 [34] |
| Copper corrosion | - | 2c | ASTM D 130 [35] |
| Stainless steel corrosion | - | No corrosion | ASTM A 380 [36] |
| Calorific value | kJ/kg | 32,858 | ASTM D 5865-12 [37] |
| № | Components | Mass Concentration, wt.% |
|---|---|---|
| 1 | Ethanol | 9.0 |
| 2 | Isopropanol | 1.0 |
| 3 | 1-Propanol | 1.0 |
| 4 | Isobutanol | 1.5 |
| 5 | 1-Butanol | 0.5 |
| 6 | 2-Methyl-1-butanol (optically active amyl alcohol) | 2.0 |
| 7 | 3-Methyl-1-butanol (isoamyl alcohol) | 70.0 |
| 8 | 1-Hexanol | 1.0 |
| 9 | Water | 14.0 |
| Total: | 100 | |
| 1 | Extractor type E-1 | Column |
| 2 | Extraction process type | Continuous |
| 3 | Number of trays in the E-1 extractor | 30 |
| 4 | Top tray pressure, bar | 1.8 |
| 5 | Top tray temperature, °C | 10 |
| 6 | Extractive agent | Water |
| 7 | Extractive agent temperature, °C | 10 |
| 8 | Extractive agent pressure, bar | 2 |
| 9 | Fusel oil pressure, bar | 2 |
| 10 | Fusel oil temperature, °C | 10 |
| 11 | Fusel oil flow rate into the E-1 extractor, kg/h | 1740 |
| 12 | Fusel oil to extractive agent ratio | 1:1 |
| Components | Mass Fraction | |
|---|---|---|
| Extract | Raffinate | |
| Ethanol | 0.0739 | 1.59 × 10−6 |
| Isopropanol | 0.0066 | 0.0025 |
| 1-Propanol | 0.0032 | 0.0078 |
| Isobutanol | 0.0022 | 0.0157 |
| 1-Butanol | 0.0009 | 0.0050 |
| 2M1B | 0.0012 | 0.0237 |
| 3M1B | 0.0331 | 0.8427 |
| 1-Hexanol | 0.0002 | 0.0125 |
| Water | 0.8787 | 0.0901 |
| Total: | 1 | 1 |
| № | Parameter/Characteristic | Water Distillation Column C-1 | Ethanol Column C-2 | First Isoamylic Column C-3 | Second Isoamylic Column C-4 | Water-Stripping C-5 | Propanol Column C-6 |
|---|---|---|---|---|---|---|---|
| 1 | Number of trays | 30 | 50 | 30 | 40 | 10 | 36 |
| 2 | Condenser type | Total | Total | Total | Total | None | Total |
| 3 | Valid phase | Vap-Liq | Vap-Liq | Vap-Liq | Vap-Liq | Vap-Liq | Vap-Liq |
| 4 | Convergence | Standard | Standard | Standard | Standard | Standard | Azeotropic |
| 5 | Distillate rate, kg/h | 380 | 270.2 | 428 | 107.4 | 46 | 50 |
| 6 | Reflux ratio | 4 | 7 | 5 | 5.5 | - | 6 |
| 7 | Feed tray number | 2 | 23 | 2 | 21 | 1 | 21 |
| 8 | Pressure, bar | 1.6 | 0.15 | 0.3 | 0.3 | 1 | 3 |
| 9 | Temperature at the top of the column, °C | 100 | 37 | 64 | 62 | 90 | 116 |
| 10 | Temperature at the bottom of the column, °C | 115 | 56 | 106 | 106 | 102 | 129 |
| № | Indicator Name | Ethanol | Isoamyl Alcohol | Water | Raw Propanol Cut | Raw Butanol Cut |
|---|---|---|---|---|---|---|
| 1 | Average Molecular Weight | 43.39 | 88.31 | 18.02 | 37.44 | 46.95 |
| 2 | Component composition, mass fraction | |||||
| Ethanol | 0.9562 | 1.43 × 10−15 | 8.70 × 10−7 | 0.0931 | 1.11 × 10−7 | |
| Isopropanol | 0.0036 | 6.71 × 10−14 | 7.71 × 10−10 | 0.3268 | 3.21 × 10−10 | |
| 1-Propanol | 2.38 × 10−10 | 9.32 × 10−11 | 1.10 × 10−8 | 0.3327 | 2.73 × 10−5 | |
| Isobutanol | 5.76 × 10−15 | 2.51 × 10−8 | 5.18 × 10−9 | 0.0004 | 0.6122 | |
| 1-Butanol | 3.02 × 10−21 | 7.84 × 10−5 | 2.56 × 10−7 | 4.63 × 10−11 | 0.2019 | |
| 2M1B | 5.26 × 10−24 | 0.0274 | 2.58 × 10−15 | 4.78 × 10−6 | 7.15 × 10−11 | |
| 3M1B | 2.80 × 10−21 | 0.9588 | 6.00 × 10−12 | 9.48 × 10−6 | 1.00 × 10−8 | |
| 1-Hexanol | 2.60 × 10−40 | 0.0137 | 1.36 × 10−15 | 0 | 0 | |
| Water | 0.0402 | 2.42 × 10−12 | 1 | 0.2470 | 0.1859 | |
| Total: | 1 | 1 | 1 | 1 | 1 | |
| Initial conditions: Number of trays 40 Total reflux Condenser pressure 2 bar Subcooled temperature 50 °C Mass boil-up rate 500 kg/h Total initial charge 1255.2 kg | ||||||
| Operating step number | Reflux ratio | Mass boil-up rate, kg/h | Condenser pressure, bar | Liquid distillate receiver number | Ramp time, h | Duration time, h |
| O-1 | Total | 500 | 2 | 1 | ||
| O-2 | 6 | 1200 | 2 | 1 | 1 | 3.5 |
| O-3 | 6 | 1200 | 1.1 | 2 | 0.9 | 3 |
| № | Component | Distillate 1 | Distillate 2 | Bottom 1 |
|---|---|---|---|---|
| Mass Fraction | ||||
| l | Ethanol | 0.1644 | 0.0908 | 0.0007 |
| 2 | Isopropanol | 0.7086 | 0.1981 | 0.0017 |
| 3 | 1-Propanol | 0.0072 | 0.4539 | 0.5932 |
| 4 | Isobutanol | 1.67 × 10−9 | 1.54 × 10−9 | 0.0014 |
| 5 | 1-Butanol | 1.67 × 10−9 | 1.54 × 10−9 | 1.76 × 10−10 |
| 6 | 2M1B | 1.99 × 10−9 | 1.83 × 10−9 | 1.81 × 10−5 |
| 7 | 3M1B | 1.99 × 10−9 | 1.83 × 10−9 | 3.60 × 10−5 |
| 8 | 1-Hexanol | 0 | 0 | 0 |
| 9 | Water | 0.1198 | 0.2572 | 0.4029 |
| Total: | 1 | 1 | 1 | |
| Initial conditions: Number of trays 40 Total reflux Condenser pressure atm Subcooled temperature 50 °C Mass boil-up rate 240 kg/h Total initial charge 480.981 kg | ||||||
| Operating step number | Reflux ration | Mass boil-up rate, kg/h | Condenser pressure, bar | Liquid distillate receiver number | Ramp time, h | Duration time, h |
| O-1 | Total | 240 | 1 | 1 | ||
| O-2 | 6 | 450 | 0.5 | 1 | 1 | 5.7 |
| № | Component | Distillate 3 | Bottom 2 |
|---|---|---|---|
| Mass Fraction | |||
| 1 | Ethanol | 0.1404 | 0.0003 |
| 2 | Isopropanol | 0.3045 | 0.0040 |
| 3 | 1-Propanol | 0.3390 | 0.6637 |
| 4 | Isobutanol | 2.39 × 10−9 | 4.35 × 10−9 |
| 5 | 1-Butanol | 2.39 × 10−9 | 4.35 × 10−9 |
| 6 | 2M1B | 2.84 × 10−9 | 5.18 × 10−9 |
| 7 | 3M1B | 2.84 × 10−9 | 5.18 × 10−9 |
| 8 | 1-Hexanol | 0 | 0 |
| 9 | Water | 0.2161 | 0.3320 |
| Total: | 1 | 1 | |
| Initial conditions: Number of trays 40 Total reflux Condenser pressure 3 bar Subcooled temperature 50 °C Mass boil-up rate 500 kg/h Total initial charge 1022.39 kg | ||||||
| Operating step number | Reflux ration | Mass boil-up rate, kg/h | Condenser pressure, bar | Liquid distillate receiver number | Ramp time, h | Duration time, h |
| O-1 | Total | 500 | 3 | 1 | ||
| O-2 | 4 | 1010 | 3 | 1 | 1 | 8 |
| O-3 | 6 | 1010 | 0.7 | 2 | 1 | 1.7 |
| № | Component | Distillate 1 | Distillate 2 | Bottom 1 |
|---|---|---|---|---|
| Mass Fraction | ||||
| 1 | Ethanol | 1.60 × 10−7 | 2.48 × 10−9 | 0 |
| 2 | Isopropanol | 1.31 × 10−9 | 3.23 × 10−9 | 0 |
| 3 | 1-Propanol | 3.94 × 10−5 | 3.23 × 10−9 | 0 |
| 4 | Isobutanol | 0.7443 | 0.5276 | 0.0068 |
| 5 | 1-Butanol | 4.55 × 10−6 | 0.4239 | 0.9928 |
| 6 | 2M1B | 1.35 × 10−9 | 4.74 × 10−9 | 0 |
| 7 | 3M1B | 1.58 × 10−8 | 4.74 × 10−9 | 0 |
| 8 | 1-Hexanol | 0 | 0 | 0 |
| 9 | Water | 0.2556 | 0.0484 | 0.0004 |
| Total: | 1 | 1 | 1 | |
| Initial conditions: Number of trays 40 Total reflux Condenser pressure 1 bar Subcooled temperature 50 °C Mass boil-up rate 90 kg/h Total initial charge 185.7 kg | ||||||
| Operating step number | Reflux ration | Mass boil-up rate, kg/h | Condenser pressure, bar | Liquid distillate receiver number | Ramp time, h | Duration time, h |
| O-1 | Total | 90 | 1 | 1 | ||
| O-2 | 6 | 170 | 0.7 | 1 | 1 | 4 |
| O-3 | 6 | 170 | 1 | 1 | 1 | 2.5 |
| O-4 | 6 | 170 | 1 | 2 | 3 | |
| № | Component | Distillate 3 | Distillate 4 | Bottom 2 |
|---|---|---|---|---|
| Mass Fraction | ||||
| 1 | Ethanol | 1.11 × 10−8 | 8.29 × 10−9 | 0 |
| 2 | Isopropanol | 1.45 × 10−8 | 1.08 × 10−8 | 0 |
| 3 | 1-Propanol | 1.45 × 10−8 | 1.08 × 10−8 | 6.84 × 10−18 |
| 4 | Isobutanol | 0.8907 | 0.4314 | 0.0021 |
| 5 | 1-Butanol | 0.0009 | 0.5686 | 0.9979 |
| 6 | 2M1B | 1.06 × 10−8 | 1.59 × 10−8 | 1.87 × 10−8 |
| 7 | 3M1B | 1.06 × 10−8 | 1.59 × 10−8 | 1.87 × 10−8 |
| 8 | 1-Hexanol | 0 | 0 | 0 |
| 9 | Water | 0.1084 | 2.20 × 10−6 | 2.85 × 10−8 |
| Total: | 1 | 1 | 1 | |
| № | Components | Propanols | Butanols | ||||
|---|---|---|---|---|---|---|---|
| Isopropanol | Intermediate Cut | 1-Propanol | Isobutanol | Intermediate Cut | 1-Butanol | ||
| Mass Fraction | |||||||
| 1 | Ethanol | 0.1644 | 0.1404 | 0.0006 | 1.49 × 10−7 | 8.29 × 10−9 | 0 |
| 2 | Isopropanol | 0.7086 | 0.3045 | 0.0025 | 2.83 × 10−9 | 1.08 × 10−8 | 0 |
| 3 | 1-Propanol | 0.0072 | 0.3390 | 0.6172 | 3.75 × 10−5 | 1.08 × 10−8 | 1.84 × 10−18 |
| 4 | Isobutanol | 1.67 × 10−9 | 2.39 × 10−9 | 0.0009 | 0.8178 | 0.4314 | 0.0055 |
| 5 | 1-Butanol | 1.67 × 10−9 | 2.39 × 10−9 | 1.60 × 10−9 | 0.0001 | 0.5686 | 0.9942 |
| 6 | 2M1B | 1.99 × 10−9 | 2.84 × 10−9 | 1.20 × 10−5 | 2.51 × 10−9 | 1.59 × 10−8 | 5.01 × 10−9 |
| 7 | 3M1B | 1.99 × 10−9 | 2.84 × 10−9 | 2.37 × 10−5 | 1.64 × 10−8 | 1.59 × 10−8 | 5.01 × 10−9 |
| 8 | 1-Hexanol | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | Water | 0.1198 | 0.2161 | 0.3788 | 0.1820 | 2.20 × 10−6 | 0.0003 |
| Total: | 1 | 1 | 1 | 1 | 1 | 1 | |
| № | Balance Item | Flow Rate, kg/h | Potential Content of Key Component, kg/h | Recovery Rate, Mass Fraction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Isopropanol | 1-Propanol | Isobutanol | 1-Butanol | 2M1B | 3M1B | 1-Hexanol | Water | ||||
| Feed: | ||||||||||||
| 1 | Fusel oil | 1740 | 156.6 | 17.4 | 17.4 | 26.1 | 8.7 | 34.8 | 1218 | 17.4 | 243.6 | - |
| 2 | Water (extractive agent) | 1740 | 1740 | - | ||||||||
| 3 | Ethanol (surplus) | 112 | 106.624 | 0.672 | 4.704 | - | ||||||
| Product: | ||||||||||||
| Ethanol | 270.2 | 258.354 | 0.978823 | 10.8673 | 0.9689 | |||||||
| Isoamyl alcohol | 1270.3 | 0.0996 | 34.7998 | 1218 | 17.4 | Sum of isomers 0.9999 | ||||||
| Raw propanol cut | 52.3 | 4.86929 | 17.0932 | 17.3988 | 0.0197 | 0.0002 | 0.0005 | 12.9183 | Sum of isomers 0.9911 | |||
| Raw butanol cut | 42.5994 | 0.0012 | 26.0801 | 8.5998 | 7.9182 | Sum of isomers 0.9965 | ||||||
| Treated water | 1956.6 | 1956.6 | 0.8892 * | |||||||||
| № | Balance Item | Charge/Flow, kg | Potential Content of Key Component, kg | Recovery Rate, Mass Fraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Isopropanol | 1-Propanol | Isobutanol | 1-Butanol | 2M1B | 3M1B | 1-Hexanol | Water | |||||
| Feed: | |||||||||||||
| 1 | Raw propanol cut | 1255.2 | 116.863 | 410.236 | 417.571 | 0.4728 | 0.0060 | 0.0119 | 310.039 | - | |||
| Product: | |||||||||||||
| 1 | Isopropanol | 443.697 | 72.948 | 314.394 | 3.179 | 53.176 | 0.7664 | ||||||
| 2 | Intermediate cut | 310.738 | 43.6255 | 94.6137 | 105.334 | 67.165 | - | ||||||
| 3 | 1-Propanol | 500.766 | 0.2893 | 1.2289 | 309.059 | 0.4728 | 0.0060 | 0.0119 | 189.698 | 0.7401 | |||
| Feed: | |||||||||||||
| 1 | Raw butanol cut | 1022.39 | 0.0001 | 0.0279 | 625.926 | 206.397 | 190.039 | - | |||||
| Product: | |||||||||||||
| 1 | Isobutanol | 729.841 | 0.0001 | 0.0274 | 596.871 | 0.0800 | 132.863 | 0.9536 | |||||
| 2 | Water (drain) | 61.1768 | 0.0005 | 4.0531 | 0.0007 | 57.1225 | 0.3006 | ||||||
| 3 | Intermediate cut | 55.5672 | 23.9691 | 31.5979 | 0.0001 | - | |||||||
| 4 | 1-Butanol | 175.82 | 0.9732 | 174.799 | 0.0487 | 0.8469 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Missyurin, A.; Cursaru, D.-L.; Neagu, M.; Nicolae, M. Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components. Processes 2024, 12, 2888. https://doi.org/10.3390/pr12122888
Missyurin A, Cursaru D-L, Neagu M, Nicolae M. Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components. Processes. 2024; 12(12):2888. https://doi.org/10.3390/pr12122888
Chicago/Turabian StyleMissyurin, Alexey, Diana-Luciana Cursaru, Mihaela Neagu, and Marilena Nicolae. 2024. "Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components" Processes 12, no. 12: 2888. https://doi.org/10.3390/pr12122888
APA StyleMissyurin, A., Cursaru, D.-L., Neagu, M., & Nicolae, M. (2024). Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components. Processes, 12(12), 2888. https://doi.org/10.3390/pr12122888





