Comprehensive Study of Sustainable Pressurized Liquid Extractions to Obtain Bioavailable Antioxidant Phenolic Compounds from Grape Seed By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Experimental Design
2.4. Pressurized Liquid Extraction (PLE)
2.5. HPLC-PAD Analysis of Phenolic Composition
2.6. Analysis of Total Flavan-3-ol Mono-Oligomers and Total Polymers by NP HPLC
2.7. Determination of Mean Degree of Procyanidin Polymerization (mDP)
2.8. TPC
2.9. Antioxidant Activity
2.10. In Vitro Digestion Process
2.11. Caco-2 Cell Transepithelial Transport
2.12. Statistical Analyses
3. Results and Discussion
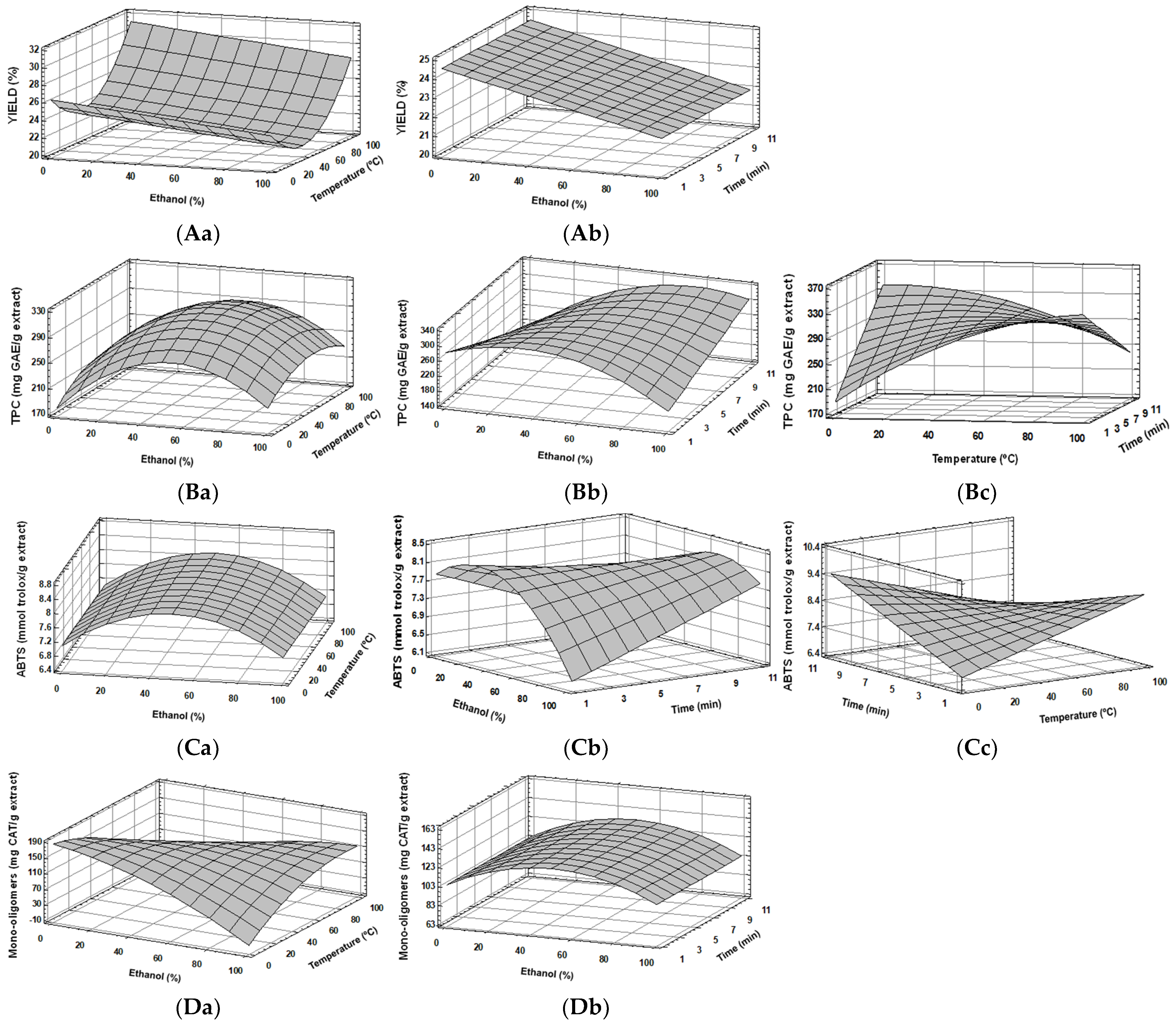
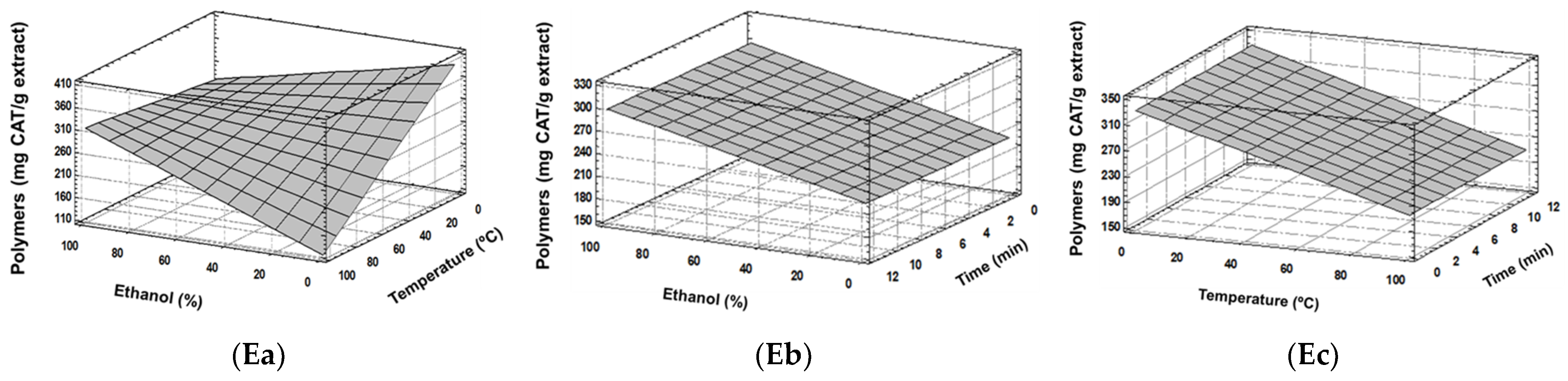
3.1. Experimental Model Fitting
3.2. Optimal Conditions and Validation of the Model
3.3. Correlation Between Response Variables
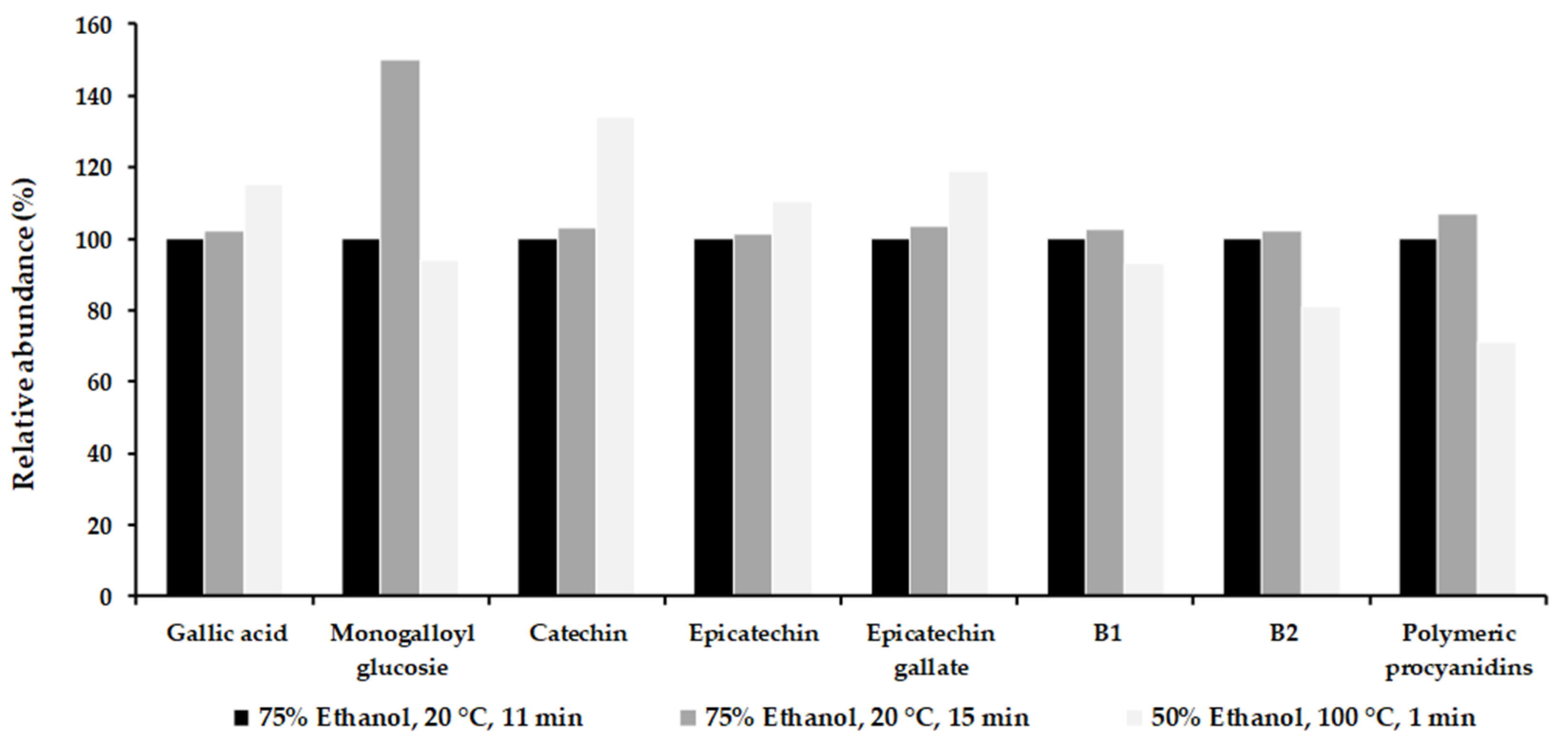
3.4. Phenolic Composition of the Optimum Extract
3.5. Changes in Phenolic Composition of the Optimal Extract During In Vitro Gastrointestinal Digestion
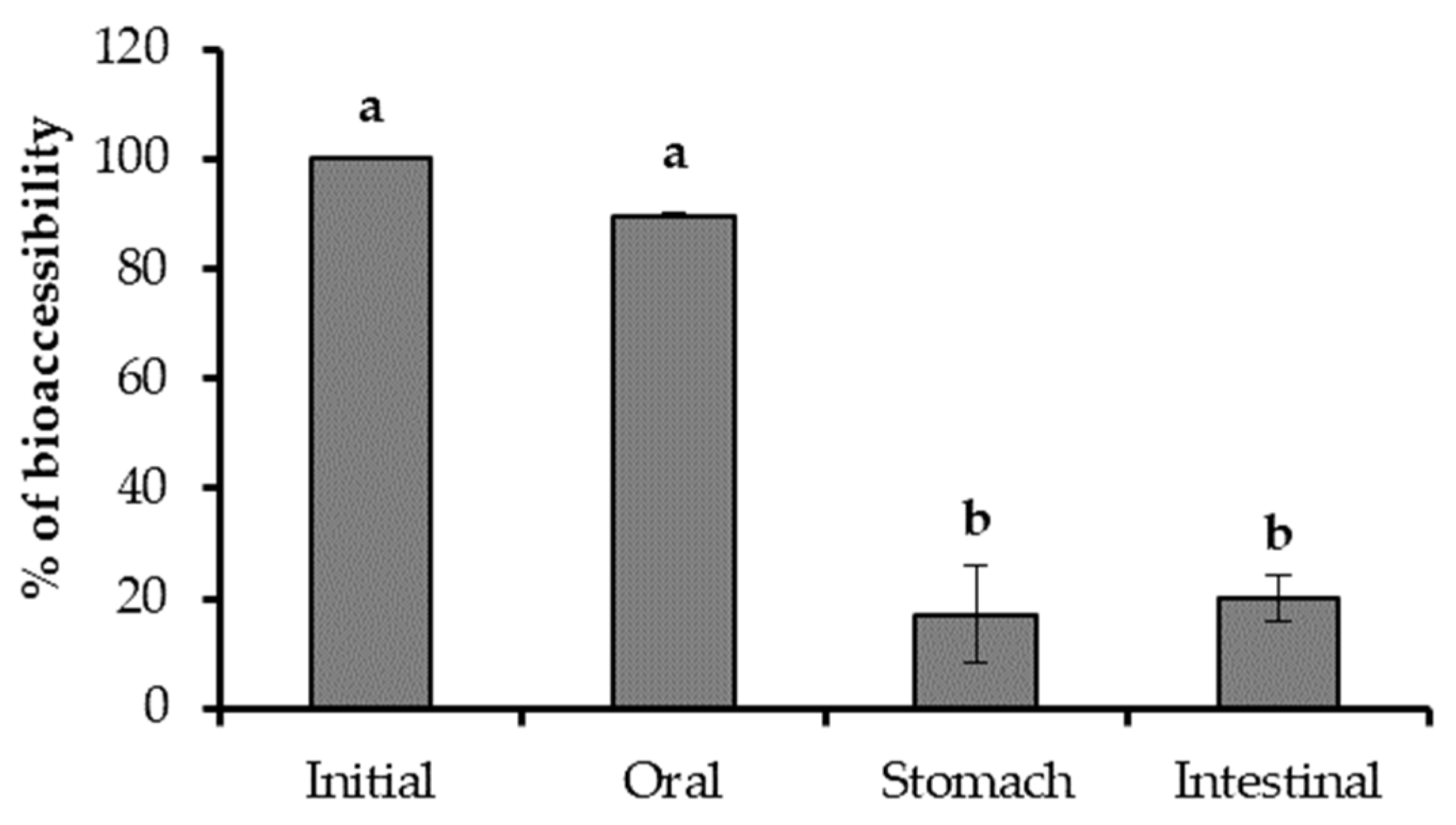
3.6. Changes in TPC and Antioxidant Activity of the Optimal Extract during In Vitro Gastrointestinal Digestion
3.7. Caco-2 Cell Transport Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ran, X.L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D. Characterization of polyphenolic and oligosaccharidic fractions extracted from grape seeds followed by the evaluation of prebiotic activity related to oligosaccharides. Int. J. Food Sci. Technol. 2019, 54, 1283–1291. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. Fortification using grape extract polyphenols—A review on functional food regulations. Int. J. Food Sci. Technol. 2021, 56, 3742–3751. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace-Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of identification and quantification of polyphenolic compounds in skins and seeds of four grape varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo Da Silva, J.M. Monomeric, oligomeric, and polymeric Flavan-3-Ol composition of wines and grapes from Vitis vinifera L. cv. Graciano, Tempranillo, and cabernet sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Yan, B.; Lu, G.; Wang, R.; Kang, S.; Huang, C.; Wu, H.; Yong, Q. Protective effects of lignin fractions obtained from grape seeds against bisphenol AF neurotoxicity via antioxidative effects mediated by the Nrf2 pathway. Front. Chem. Sci. Eng. 2023, 17, 976–989. [Google Scholar] [CrossRef]
- Talebi, M.; Esmaeeli, H.; İlgün, S.; Talebi, M.; Farkhondeh, T.; Mishra, G.; Samarghandian, S. The Protective Role of Grape Seed in Obesity and Lipid Profile: An Updated Narrative Overview of Preclinical and Clinical Studies. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 46–62. [Google Scholar]
- Ghanbari, P.; Raiesi, D.; Alboebadi, R.; Zarejavid, A.; Dianati, M.; Razmi, H.; Bazyar, H. The effects of grape seed extract supplementation on cardiovascular risk factors, liver enzymes and hepatic steatosis in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. BMC Complement. Med. Ther 2024, 24, 192. [Google Scholar] [CrossRef]
- Nieto, J.A.; Fernández-Jalao, I.; Siles-Sánchez, M.D.L.N.; Santoyo, S.; Jaime, L. Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules 2023, 28, 2461. [Google Scholar] [CrossRef]
- Gomes, T.M.; Toaldo, I.M.; da Silva Haas, I.C.; Burin, V.M.; Caliari, V.; Luna, A.S.; Santos de Gois, J.; Bordignon-Luiz, M.T. Differential contribution of grape peel, pulp, and seed to bioaccessibility of micronutrients and major polyphenolic compounds of red and white grapes through simulated human digestion. J. Funct. Foods 2019, 52, 699–708. [Google Scholar] [CrossRef]
- Elejalde, E.; Villarán, M.C.; Esquivel, A.; Alonso, R.M. Bioaccessibility and Antioxidant Capacity of Grape Seed and Grape Skin Phenolic Compounds After Simulated In Vitro Gastrointestinal Digestion. Plant Foods Hum. Nutr. 2024, 79, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Skouroumounis, J.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Ma, Y.; Zhu, P.; He, J.; Lei, J. Optimization of subcritical water extraction of resveratrol from grape seeds by response surface methodology. Appl. Sci. 2017, 7, 321. [Google Scholar] [CrossRef]
- Amador-Luna, V.M.; Herrero, M.; Domínguez-Rodríguez, G.; Ibáñez, E.; Montero, L. Enhancing the bioactivity of Dunaliella salina extracts through ultra-high pressure supercritical fluid extraction (UHP-SFE). Innov. Food Sci. Emerg. Technol. 2024, 95, 103697. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Prodanov, M.; Villamiel, M. Effects of high intensity ultrasound on disaggregation of a macromolecular procyanidin-rich fraction from Vitis vinifera L. seed extract and evaluation of its antioxidant activity. Ultrason. Sonochem. 2019, 50, 74–81. [Google Scholar] [CrossRef]
- Sun, B.S.; Leandro, M.C.; Ricardo-da-Silva, J.M.; Spranger, M.I. Separation of grape and wine proanthocyanidins according to their degree of polymerisation. J. Agric. Food Chem. 1998, 46, 1390–1396. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuveleir, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulosa, N.; Karathanosa, V.T. Deployment of response surface methodology to optimize recovery of grape (Vitis vinifera) stem and seed polyphenols. 11th International Congress on Engineering and Food (ICEF11). Procedia Food Sci. 2011, 1, 1686–1693. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica-Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crop. Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Teixeira, A.; Rosa, E.; Barros, A. Assessment of (poly)phenols in grape (Vitis vinifera L.) stems by using food/pharma industry compatible solvents and response surface methodology. Food Chem. 2014, 164, 339–346. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot pressurized liquid extraction of polyphenols from the skin and seeds of Vitis vinifera L. Cv. negra criolla pomace a peruvian native pisco industry waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant white grape seed phenolics: Pressurized liquid extracts from different varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Turner, C. Pressurized solvent extraction and monolithic column HPLC/DAD analysis of anthocyanins in red cabbage. Talanta 2008, 74, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, S.; Stoylov, B.L.; Saykova, I.; Tchaoushev, S.T. Mass transfer intensification in bioactive compounds recovery by alternative extraction methods: Effects of solvent. Glob. Nest J. 2019, 21, 30–36. [Google Scholar]
- Kadri, S.; El Ayed, M.; Mabrouk, M.; Limam, F.; Elkahoui, S.; Aouani, E.; Mokni, M. Characterization, anti-oxidative effect of grape seed powder and in silico affinity profiling of polyphenolic and extra-phenolic compounds for calpain inhibition. J. Pharm. Biomed. Anal. 2019, 164, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Chamorro, S.; Goñi, I.; Viveros, A.; Hervert-Hernández, D.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial potential, antioxidant activity, and phenolic content of grape seed extracts from four grape varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Lorrain, B.; Chira, K.; Teissedre, P.L. Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: Comparison to 2006, 2007 and 2008 vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of organic pollutans from environmental solids with subcritical and supercritical water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Hanc, L.; Sun, B. Extraction yields and anti-oxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Souquet, J.M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.Y.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.P.; Valls, J.; Blade, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Ferreyra, S.; Torres-Palazzolo, C.; Bottini, R.; Camargo, A.; Fontana, A. Assessment of in-vitro bioaccessibility and antioxidant capacity of phenolic compounds extracts recovered from grapevine bunch stem and cane by-products. Food Chem. 2021, 348, 129063. [Google Scholar] [CrossRef]
- Garbetta, A.; Nicassio, L.; D’Antuono, I.; Cardinali, A.; Linsalata, V.; Attolico, G.; Minervini, F. Influence of in vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food Res. Int. 2018, 106, 878–884. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Rodríguez, M.; Bianchi, F.; Simonato, B.; Rizzi, C.; Fontana, A.; Tironi, V.A. Exploration of grape pomace peels and amaranth flours as functional ingredients in the elaboration of breads: Phenolic composition, bioaccessibility, and antioxidant activity. Food Func. 2024, 15, 608–624. [Google Scholar] [CrossRef]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer and polymer across monolayers of human intestinal epithelial caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Toro-Uribe, S.; López-Giraldo, L.J.; Alvarez-Rivera, G.; Ibáñez, E.; Herrero, M. Insight of Stability of Procyanidins in Free and Liposomal Form under an In Vitro Digestion Model: Study of Bioaccessibility, Kinetic Release Profile, Degradation, and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Theumer, M.G.; Kruzynski, P.; Wunderlin, D.A.; Baroni, M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019, 122, 496–505. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of simulated digestion on the phenolic components of red grapes and their corresponding wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Abia, R.; Fry, S.C. Degradation and metabolism of 14C-labelled proanthocyanidins from carob (Ceratonia siliqua) pods in the gastrointestinal tract of the rat. J. Sci. Food Agric. 2001, 81, 1156–1165. [Google Scholar] [CrossRef]
- Laurent, C.; Besancon, P.; Caporiccio, B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/caco-2 cell culture model. Food Chem. 2007, 100, 1704–1712. [Google Scholar] [CrossRef]
- Henning, S.M.; Zhang, Y.; Rontoyanni, V.G.; Huang, J.; Lee, R.P.; Trang, A.; Nuernberger, G.; Heber, D. Variability in the Antioxidant Activity of Dietary Supplements from Pomegranate, Milk Thistle, Green Tea, Grape Seed, Goji, and Acai: Effects of In Vitro Digestion. J. Agric. Food Chem. 2014, 62, 4313–4321. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Silva, M.; Molinero, N.; Miralles, B.; Bartolomé, B.; Moreno-Arribas, M.V. Gastrointestinal co-digestion of wine polyphenols with glucose/whey proteins affects their bioaccessibility and impact on colonic microbiota. Food Res. Int. 2022, 155, 111010. [Google Scholar] [CrossRef]
- Said, I.H.; Truex, J.D.; Heidorn, C.; Retta, M.B.; Petrov, D.D.; Haka, S.; Kuhnert, N. LC-MS/MS based molecular networking approach for the identification of cocoa phenolic metabolites in human urine. Food Res. Int. 2020, 132, 109119. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vézin, H.; Demigné, C.; Rémésy, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Juaristi, M.; Sarria, B.; Goya, L.; Bravo-Clemente, L.; Mateos, R. Experimental confounding factors affecting stability, transport and metabolism of flavanols and hydroxycinnamic acids in Caco-2 cells. Food Res. Int. 2020, 129, 108797. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Lu, Q.Y.; Massie, L.; Qualls, C.; Mao, J.T. Grape seed procyanidin extract against lung cancer: The role of microrna-106b, bioavailability, and bioactivity. Oncotarget 2018, 9, 15579. [Google Scholar] [CrossRef]
| Factor | Coded Factor | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| Ethanol content (%) | X1 | 0 | 20 | 50 | 80 | 100 |
| Temperature (°C) | X2 | 20 | 36 | 60 | 84 | 100 |
| Time (min) | X3 | 1 | 3 | 6 | 9 | 11 |
| Factor | Response Variable | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | Yield | TPC | ABTS | Mono-Oligomers | Total Polymers |
| (%) | (°C) | (min) | (g Extract/100 Seed) | (g GAE/g Extract) | (mmol Trolox/g Extract) | (mg Catechin/g Extract) | (mg Catechin/g Extract) | |
| 1 | 20 | 36 | 3 | 21.7 | 269.14 | 7.56 | 132.12 | 267.70 |
| 2 | 80 | 36 | 3 | 21.5 | 238.44 | 7.30 | 135.61 | 272.77 |
| 3 | 20 | 84 | 3 | 26.5 | 297.89 | 8.05 | 63.47 | 154.32 |
| 4 | 80 | 84 | 3 | 24.7 | 279.19 | 7.87 | 139.11 | 311.34 |
| 5 | 20 | 36 | 9 | 24.5 | 263.00 | 7.75 | 144.67 | 273.22 |
| 6 | 80 | 36 | 9 | 21.8 | 297.89 | 8.05 | 127.54 | 326.63 |
| 7 | 20 | 84 | 9 | 27.5 | 205.23 | 6.47 | 66.52 | 162.55 |
| 8 | 80 | 84 | 9 | 24.8 | 286.17 | 7.96 | 143.86 | 240.64 |
| 9 | 0 | 60 | 6 | 23.1 | 230.91 | 7.05 | 128.67 | 232.05 |
| 10 | 100 | 60 | 6 | 21.1 | 255.75 | 7.41 | 65.91 | 242.36 |
| 11 | 50 | 20 | 6 | 22.4 | 310.17 | 8.29 | 131.14 | 305.33 |
| 12 | 50 | 100 | 6 | 30.7 | 281.70 | 7.87 | 74.00 | 235.78 |
| 13 | 50 | 60 | 1 | 23.5 | 304.31 | 8.17 | 134.96 | 283.79 |
| 14 | 50 | 60 | 11 | 23.3 | 323.01 | 8.49 | 140.01 | 285.07 |
| 15 | 50 | 60 | 6 | 25.6 | 308.22 | 8.20 | 95.88 | 274.16 |
| 16 | 50 | 60 | 6 | 23.2 | 314.64 | 8.50 | 134.93 | 276.96 |
| 17 | 50 | 60 | 6 | 24.3 | 297.05 | 8.01 | 137.80 | 280.99 |
| 18 | 50 | 60 | 6 | 22.3 | 308.78 | 8.22 | 131.68 | 276.45 |
| 19 | 50 | 60 | 6 | 23.4 | 295.94 | 7.93 | 134.62 | 225.56 |
| Model Optimal Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Polynomial Equation of Fitted Model | R | Lack of Fit (p-Value) | Et (%) | T (°C) | t (min) | Predicted | Real * |
| Yield (g extract/g seed) | Y = 26.2984 − 0.0263927(Et) − 0.14602(T) + 0.00194226(T)2 | 0.91 | 0.38 | 0 | 100 | 11 | 22.17 | 21.20 |
| TPC (mg GAE/g extract) | Y = 159.929 + 1.71911(Et) + 2.88061(T) + 3.08083(t) − 0.0286387(Et)2 + 0.235371(Et × t) − 0.0116306(T)2 − 0.24749(T × t) | 0.93 | 0.12 | 75 | 20 | 11 | 354.02 | 350.80 |
| ABTS (mmol Trolox/g extract) | Y = 6.44972 + 0.0235014(Et) + 0.0259616(T) + 0.100783(t) –0.000425612(Et)2 + 0.00317664(Et × t) − 0.00432693(T × t) | 0.85 | 0.25 | 30 | 100 | 1 | 9.12 | 9.31 |
| Total Polymers (mg catechin/g extract) | Y = 387.285 − 1.11844(Et) − 2.77271(T) + 0.0314506(Et × T) | 0.62 | 0.14 | 0.2 | 20 | 11 | 331.11 | 327.20 |
| mg/g of Extract | |
|---|---|
| Hydroxybenzoic acids | |
| Gallic acid | 1.355 ± 0.004 |
| Protocatechuic acid | 0.008 ± 0.000 |
| Monogalloyl glucoside | 0.524 ± 0.001 |
| Ellagic acid | 0.016 ± 0.002 |
| Protocatechuic aldehyde | 0.022 ± 0.001 |
| Hydroxycinnamic acids | |
| Caftaric acid | 0.029 ± 0.000 |
| p-Coumaric acid | 0.040 ± 0.000 |
| 3-Coumaric acid | 0.025 ± 0.000 |
| Flavan-3-ols | |
| Catechin | 32.405 ± 0.755 |
| Epicatechin | 44.217 ± 0.089 |
| Catechin gallate | 0.034 ± 0.004 |
| Epicatechin gallate | 3.927 ± 0.019 |
| Dimer B1 | 7.761 ± 0.017 |
| Dimer B2 | 11.856 ± 0.015 |
| Dimer A2 | 0.440 ± 0.018 |
| Flavonols | |
| Quercetin-3-O-glucuronide | 0.013 ± 0.000 |
| Quercetin-3-O-glucoside | 0.101 ± 0.000 |
| Quercetin | Traces |
| Σ Phenolic compounds | 102.77 ± 0.054 |
| Initial | Oral | Stomach | Intestine | Apical | Basolateral | |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | ||||||
| Gallic acid | 1.355 ± 0.004 a | 1.267 ± 0.008 b | 1.278 ± 0.011 b | 1.214 ± 0.009 c | 0.845 ± 0.004 d | 0.052 ± 0.003 e |
| Protocatechuic acid | 0.008 ± 0.000 c | 0.007 ± 0.000 c | 0.008 ± 0.001 c | 0.010 ± 0.000 b | 0.018 ± 0.000 a | 0.002 ± 0.000 d |
| Monogalloyl glucoside | 0.524 ± 0.001 a | 0.532 ± 0.007 a | 0.390 ± 0.003 b | 0.382 ± 0.032 b | 0.312 ± 0021 c | Tr |
| Vanillic acid | Nd | Nd | Nd | Nd | Nd | 0.072 ± 0.001 |
| Ellagic acid | 0.016 ± 0.002 a | 0.015 ± 0.000 a,b | 0.013 ± 0.001 b,c | 0.012 ± 0.000 c | 0.007 ± 0.000 d | Nd |
| Protocatechuic aldehyde | 0.022 ± 0.001 a | 0.019 ± 0.002 a | Nd | Nd | Nd | Nd |
| Hydroxycinnamic acids | ||||||
| Caftaric acid | 0.029 ± 0.000 a | 0.025 ± 0.001 b | 0.023 ± 0.000 c | 0.021 ± 0.001 d | 0.021 ± 0.000 d | Nd |
| p-Coumaric acid | 0.040 ± 0.000 a | 0.036 ± 0.001 b | 0.031 ± 0.001 d | 0.033 ± 0.001 c | 0.021 ± 0.000 e | 0.014 ± 0.000 f |
| 3-Coumaric acid | 0.025 ± 0.000 a | 0.025 ± 0.001 a | 0.014 ± 0.000 b | 0.005 ± 0.000 d | 0.018 ± 0.001 c | Nd |
| Flavan-3-ols | ||||||
| Catechin | 32.405 ± 0.755 a | 31.643 ± 0.049 a | 21.349 ± 0.496 b | 18.028 ± 0.226 c | 13.160 ± 0.383 d | 3.315 ± 0.071 e |
| Epicatechin | 44.217 ± 0.089 a | 41.786 ± 0.737 b | 22.143 ± 0.044 c | 16.782 ± 0.106 d | 13.673 ± 1.037 e | 3.671 ± 0.087 f |
| Catechin gallate | 0.034 ± 0.004 a | 0.036 ± 0.002 a | 0.008 ± 0.001 c | 0.013 ± 0.001 b | 0.004 ± 0.001 d | 0.001 ± 0.000 e |
| Epicatechin gallate | 3.927 ± 0.019 a | 3.427 ± 0.031 b | 1.510 ± 0.003 d | 1.588 ± 0.002 c | 0.704 ± 0.081 e | 0.191 ± 0.001 f |
| Dimer B1 | 7.761 ± 0.017 b | 7.951 ± 0.132 a | 7.730 ± 0.021 b | 2.874 ± 0.077 c | 3.013 ± 0.029 c | 0.080 ± 0.003 d |
| Dimer B2 | 11.856 ± 0.015 a | 11.149 ± 0.205 b | 6.848 ± 0.008 c | 6.470 ± 0.082 d | 5.849 ± 0.073 e | 0.253 ± 0.002 f |
| Dimer A2 | 0.440 ± 0.018 a | 0.473 ± 0.021 a | 0.239 ± 0.006 b | 0.075 ± 0.028 c | 0.081 ± 0.007 c | Nd |
| Flavonols | ||||||
| Quercetin-3-O-glucuronide | 0.013 ± 0.000 a | 0.010 ± 0.000 b | 0.002 ± 0.001 c | 0.010 ± 0.001 b | 0.012 ± 0.001 a | Nd |
| Quercetin-3-O-glucoside | 0.101 ± 0.000 a | 0.094 ± 0.001 a | 0.063 ± 0.006 b | 0.067 ± 0.001 c | 0.039 ± 0.002 d | 0.023 ± 0.000 e |
| Quercetin | Tr | Tr | Nd | Nd | Nd | Nd |
| Σ Phenolic compounds | 102.77 ± 0.054 a | 98.494 ± 0.071 a | 61.652 ± 0.038 b | 47.584 ± 0.035 c | 37.779 ± 0.103 d | 7.674 ± 0.015 e |
| Initial | Oral | Stomach | Intestine | 0.45 µm | Apical | Basolateral | |
|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g extract) | 394.883 ± 30.543 a | 389.808 ± 20.648 a | 303.113 ± 8.254 b | 262.088 ± 6.256 c | 182.050 ± 7.507 d | 157.487 ± 21.386 e | 11.416 ± 1.260 f |
| DDPH (mmol Trolox/g extract) | 4.089 ± 0.236 a | 3.999 ± 0.046 a | 3.057 ± 0.053 b | 2.888 ± 0.147 b | 2.114 ± 0.016 c | 1.682 ± 0.007 d | 0.061 ± 0.000 e |
| ORAC (mmol Trolox/g extract) | 4.050 ± 0.003 a | 4.290 ± 0.740 a | 2.748 ± 0.175 b | 2.536 ± 0.239 b | 1.915 ± 0.086 c | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, J.A.; Santoyo, S.; de Sá, M.; Baoshan, S.; Reglero, G.; Jaime, L. Comprehensive Study of Sustainable Pressurized Liquid Extractions to Obtain Bioavailable Antioxidant Phenolic Compounds from Grape Seed By-Products. Processes 2024, 12, 2308. https://doi.org/10.3390/pr12112308
Nieto JA, Santoyo S, de Sá M, Baoshan S, Reglero G, Jaime L. Comprehensive Study of Sustainable Pressurized Liquid Extractions to Obtain Bioavailable Antioxidant Phenolic Compounds from Grape Seed By-Products. Processes. 2024; 12(11):2308. https://doi.org/10.3390/pr12112308
Chicago/Turabian StyleNieto, Juan Antonio, Susana Santoyo, Marta de Sá, Sun Baoshan, Guillermo Reglero, and Laura Jaime. 2024. "Comprehensive Study of Sustainable Pressurized Liquid Extractions to Obtain Bioavailable Antioxidant Phenolic Compounds from Grape Seed By-Products" Processes 12, no. 11: 2308. https://doi.org/10.3390/pr12112308
APA StyleNieto, J. A., Santoyo, S., de Sá, M., Baoshan, S., Reglero, G., & Jaime, L. (2024). Comprehensive Study of Sustainable Pressurized Liquid Extractions to Obtain Bioavailable Antioxidant Phenolic Compounds from Grape Seed By-Products. Processes, 12(11), 2308. https://doi.org/10.3390/pr12112308








