Microencapsulation of a Flaxseed and Avocado Oil Blend: Influence of Octenyl Succinic Anhydride (OSA)-Modified Starch and Rice and Pea Proteins on Powder Characterization and Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Vegetable Proteins and OSA-Modified Starch
2.2. Emulsions Production and Characterization
2.3. Freeze-Drying Process
2.4. Characterization of the Powders
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Oxidative Stability
2.7. Statistical Analysis
3. Results
3.1. Characterization of Vegetable Proteins and OSA-Modified Starch
3.2. Emulsions Characterization
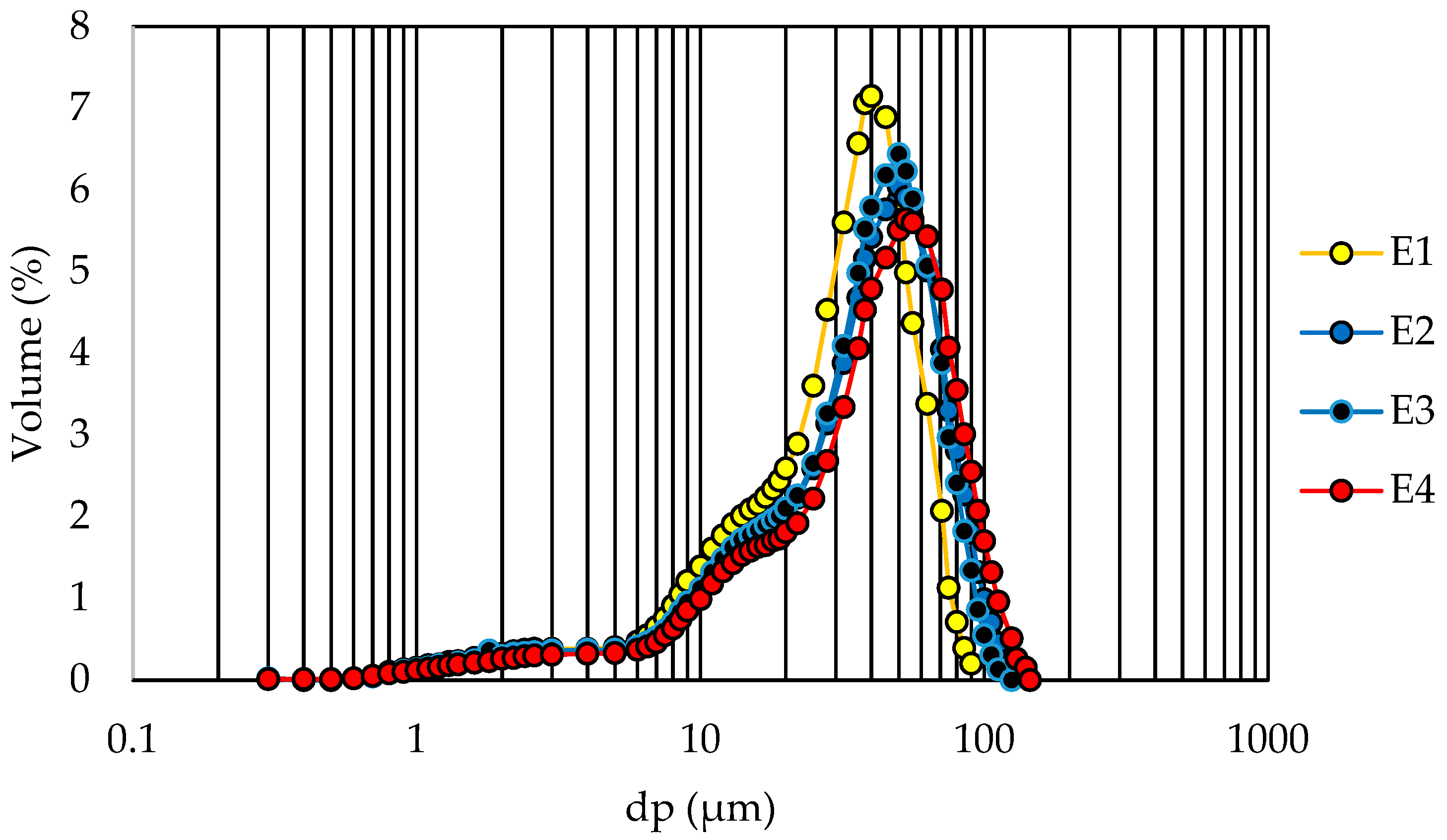
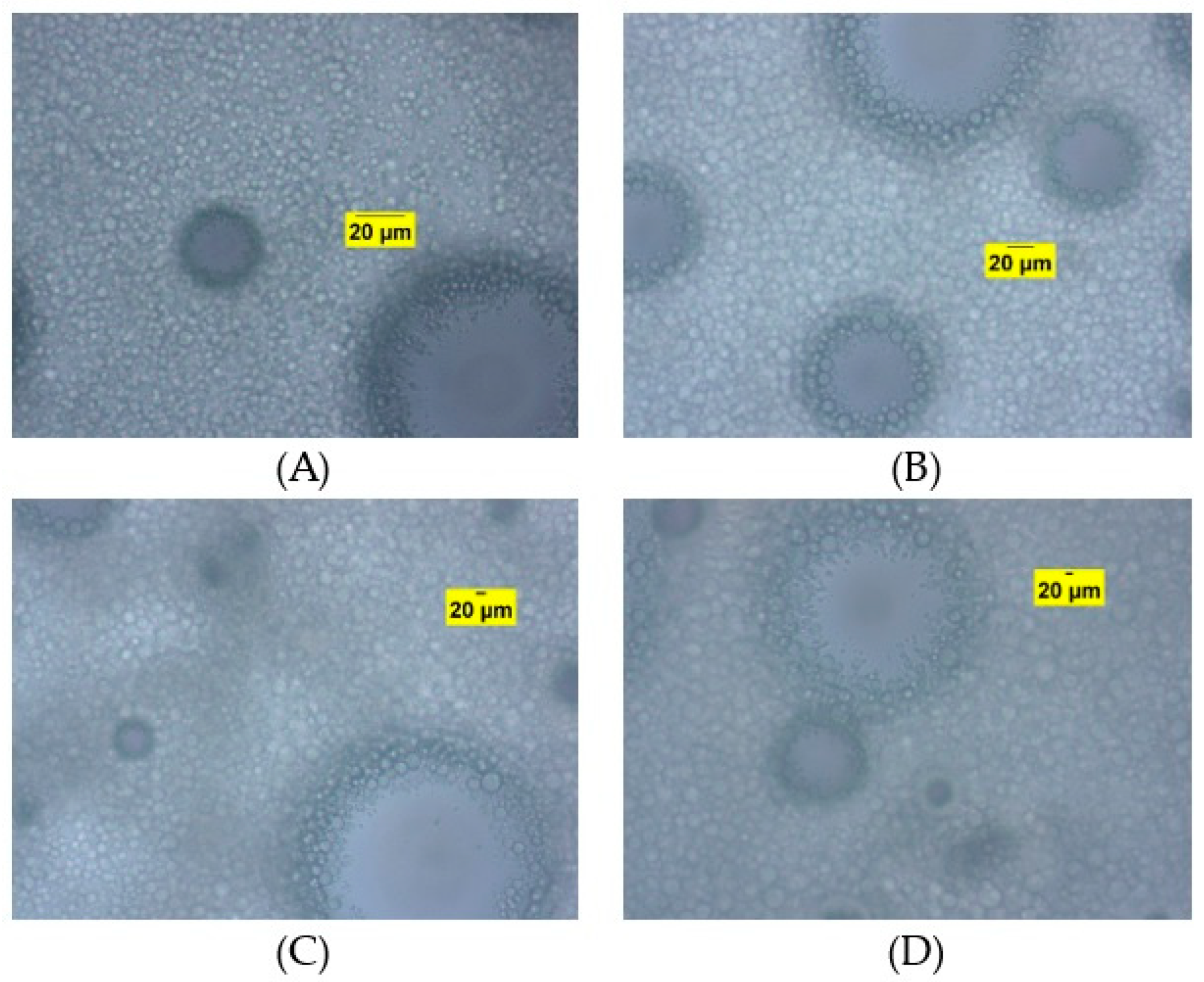
3.3. Powders Characterization

3.4. Oxidative Stability
3.5. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kouamé, K.J.E.-P.; Bora, A.F.M.; Li, X.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Wang, Y.; Ghosh, S.; Nickerson, M.T. Microencapsulation of flaxseed oil by lentil protein isolate-κ-carrageenan and -ι-carrageenan based wall materials through spray and freeze drying. Molecules 2022, 27, 3195. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Hussain, A.; Ashraf, S.; Fatima, G.; Ambreen; Javaria, S.; Abideen, Z.U.; Kabir, K.; Yaqub, S.; Akram, S.; et al. Flaxseed (Linum usitatissimum); phytochemistry, pharmacological characteristics and functional food applications. Food Chem. Adv. 2024, 4, 100573. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, D.; Ghosh, M. Antioxidant activity and oxidative stability of some binary and ternary blended vegetable oil. Int. Res. J. Eng. Technol. 2019, 6, 2068–2077. [Google Scholar]
- Choo, W.-S.; Birch, J.; Dufour, J.-P. Physicochemical and quality characteristics of cold-pressed flaxseed oils. J. Food Compos. Anal. 2007, 20, 202–211. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Morales-Hernández, N.; García-Márquez, E.; Rodríguez-Rodríguez, R. Development of fish oil microcapsules by spray drying using mesquite gum and chitosan as wall materials: Physicochemical properties, microstructure, and lipid hydroperoxide concentration. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 646–655. [Google Scholar] [CrossRef]
- Pattnaik, M.; Mishra, H.N. Amelioration of the stability of polyunsaturated fatty acids and bioactive enriched vegetable oil: Blending, encapsulation, and its application. Crit. Rev. Food Sci. Nutr. 2022, 62, 6253–6276. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.d.S.; da Silva, C.A.M.; Braga, M.B. Flaxseed and avocado oil blends: Physical and physicochemical characterization, nutritional quality and oxidative stability. Appl. Food Res. 2023, 3, 100370. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 714. [Google Scholar]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of vitamins A and E as spray-dried additives for the feed industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Can Karaca, A.; Low, N.; Nickerson, M. Encapsulation of flaxseed oil using a benchtop spray dryer for legume protein–maltodextrin microcapsule preparation. J. Agric. Food Chem. 2013, 61, 5148–5155. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Perrechil, F.; Louzi, V.C.; Alves da Silva Paiva, L.; Valentin Natal, G.S.; Braga, M.B. Evaluation of modified starch and rice protein concentrate as wall materials on the microencapsulation of flaxseed oil by freeze-drying. LWT 2021, 140, 110760. [Google Scholar] [CrossRef]
- Perrechil, F.; Natal, G.S.V.; Paiva, L.A.d.S.; Braga, M.B. Mixtures of modified starch and rice and pea protein concentrate as wall material in the microencapsulation of flaxseed Oil. Powders 2023, 2, 323–337. [Google Scholar] [CrossRef]
- Chen, X.D.; Mujumdar, A.S. Drying Technologies in Food Processing, 1st ed.; Blackwell Publishing: Singapore, 2008; p. 352. [Google Scholar]
- Mota da Silva, A.M.; Souza Almeida, F.; Kawazoe Sato, A.C. Functional characterization of commercial plant proteins and their application on stabilization of emulsions. J. Food Eng. 2021, 292, 110277. [Google Scholar] [CrossRef]
- Helm, R.M.; Burks, A.W. Hypoallergenicity of rice protein. Cereal Foods World 1996, 41, 839–843. [Google Scholar]
- Andreola, K.; Silva, C.A.M.; Taranto, O.P. Agglomeration process of rice protein concentrate using glucomannan as binder: In-line monitoring of particle size. Chem. Eng. Res. Des. 2018, 135, 37–51. [Google Scholar] [CrossRef]
- Zhu, S.M.; Lin, S.L.; Ramaswamy, H.S.; Yu, Y.; Zhang, Q.T. Enhancement of functional properties of rice bran proteins by high pressure treatment and their correlation with surface hydrophobicity. Food Bioprocess Technol. 2017, 10, 317–327. [Google Scholar] [CrossRef]
- Wu, D.; Lin, Q.; Singh, H.; Ye, A. Complexation between whey protein and octenyl succinic anhydride (OSA)-modified starch: Formation and characteristics of soluble complexes. Food Res. Int. 2020, 136, 109350. [Google Scholar] [CrossRef]
- Sosulski, F.; Garratt, M.D.; Slimkard, A.E. Functional properties of ten legume flours. Can. Inst. Food Sci. Technol. J. 1976, 9, 66–69. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Van Buren, J.P.; Killara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative study to develop a standardized food protein solubility procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Physicochemical and functional properties of protein concentrate from by-product of coconut processing. Food Chem. 2018, 241, 364–371. [Google Scholar] [CrossRef]
- Kiralan, M.; Bayrak, A.; Ozkaya, M.T. Oxidation stability of virgin olive oils from some important cultivars in East Mediterranean area in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 247–252. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, F.; Ping, L.; Song, J.; Ravee, Y.; Jin, L.; Xue, Y.; Xu, J.; Li, G.; et al. Production of octenyl succinic anhydride-modified waxy corn starch and its characterization. J. Agric. Food Chem. 2008, 56, 11499–11506. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N. Spray-drying of green or gold kiwifruit juice–milk mixtures; novel formulations and processes to retain natural fruit colour and antioxidants. Food Bioprocess Technol. 2015, 8, 191–207. [Google Scholar] [CrossRef]
- AOCS. A.O.C.S. Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 2004. [Google Scholar]
- Yousif, E.-S.I.; Kishk, Y.F.M.; El-Sheshetawy, H.E.; El-Makarem, M.A. Functional and nutritional characteristics of prepared rice bran protein products. World J. Dairy Food Sci. 2016, 11, 100–108. [Google Scholar]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef]
- Shevkani, K.; Kaur, A.; Kumar, S.; Singh, N. Cowpea protein isolates: Functional properties and application in gluten-free rice muffins. LWT 2015, 63, 927–933. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Rani, S.; Pooja, K.; Pal, G.K. Exploration of rice protein hydrolysates and peptides with special reference to antioxidant potential: Computational derived approaches for bio-activity determination. Trends Food Sci. Technol. 2018, 80, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Cheng, H.; Zhang, J.; Zhang, S. Investigating structure, biological activity, peptide composition and emulsifying properties of pea protein hydrolysates obtained by cell envelope proteinase from Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Biol. Macromol. 2023, 245, 125375. [Google Scholar] [CrossRef]
- Singh, T.P.; Siddiqi, R.A.; Sogi, D.S. Enzymatic modification of rice bran protein: Impact on structural, antioxidant and functional properties. LWT 2021, 138, 110648. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Jiang, B.; Hamaker, B.R.; Jin, Z.; Zhang, T. Characterizations of oil-in-water emulsion stabilized by different hydrophobic maize starches. Carbohydr. Polym. 2017, 166, 195–201. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Gomes, M.H.G.; Kurozawa, L.E. Improvement of the functional and antioxidant properties of rice protein by enzymatic hydrolysis for the microencapsulation of linseed oil. J. Food Eng. 2020, 267, 109761. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; McClements, D.J.; Meng, C.; Zhang, M.; Chen, H.; Deng, Q. Recent advances in understanding the interfacial activity of antioxidants in association colloids in bulk oil. Adv. Colloid Interface Sci. 2024, 325, 103117. [Google Scholar] [CrossRef]
- Da Silva Moura, M.; da Costa, B.d.S.G.; Giaconia, M.A.; da Andrade, R.R.; Braga, A.R.C.; Braga, M.B. Jaboticaba powders production by freeze-drying: Influence of octenyl succinic anhydride-modified starch concentrations over anthocyanins and physical properties. J. Food Process Eng. 2023, 46, e14256. [Google Scholar] [CrossRef]
- Ito, V.C.; Schnitzler, E.; Demiate, I.M.; Eusébio, M.E.S.; Lacerda, L.G.; Castro, R.A.E. Physicochemical, thermal, crystallographic, and morphological properties of biodynamic black rice starch, and of residual fractions from aqueous extraction. Starch-Stärke 2018, 70, 1700348. [Google Scholar] [CrossRef]
- Lao, Y.; Ye, Q.; Wang, Y.; Vongsvivut, J.; Selomulya, C. Quantifying the effects of pre-roasting on structural and functional properties of yellow pea proteins. Food Res. Int. 2023, 172, 113180. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Paul, U.C.; Athanassiou, A. Blending of polysaccharide-based carrot pomace with vegetable proteins for biocomposites with optimized performance for food packaging applications. Food Hydrocoll. 2024, 152, 109903. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Arpi, N.; Satriana; Mustapha, W.A.W.; Syamsuddin, Y.; Putra, T.W.; Supardan, M.D. Effect of cooking pre-treatment on the properties of dried avocado flesh and its oil extract. S. Afr. J. Chem. Eng. 2023, 43, 1–8. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization model for ultrasonic-assisted and scale-up extraction of Anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef]





| Analysis | RPC | PPC |
|---|---|---|
| EAI (m2/g) | 2.84 a ± 0.05 | 2.94 a ± 0.07 |
| ES (min) | 43.33 b ± 2.80 | 76.95 a ± 8.93 |
| AC (%) | 30.62 a ± 0.82 | 29.88 a ± 0.89 |
| S (%) pH = 3.5 | 2.09 b ± 0.10 | 12.66 a ± 0.27 |
| S (%) pH = 5.0 | 1.83 b ± 0.02 | 8.75 a ± 0.08 |
| S (%) pH = 7.0 | 1.94 b ± 0.01 | 15.87 a ± 0.14 |
| WRC (g/g) | 3.72 b ± 0.23 | 4.79 a ± 0.27 |
| Analysis | E1 | E2 | E3 | E4 |
|---|---|---|---|---|
| pH | 3.04 d ± 0.02 | 4.24 c ± 0.00 | 4.74 b ± 0.02 | 5.15 a ± 0.01 |
| ICr (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| dp (μm) 10% | 1.47 a ± 0.00 | 1.44 a ± 0.00 | 0.59 a ± 0.28 | 1.89 a ± 0.00 |
| dp (μm) 50% | 4.04 b ± 0.13 | 3.64 c ± 0.04 | 3.71 c ± 0,00 | 9.43 a ± 0.09 |
| dp (μm) 90% | 8.98 c ± 0.32 | 10.08 c ± 0.08 | 36.86 b ± 0.16 | 53.06 a ± 1.60 |
| Span | 1.86 d ± 0.02 | 2.37 c ± 0.01 | 9.79 a ± 0.04 | 5.43 b ± 0.12 |
| D[4,3] (μm) | 4.71 c ± 0.14 | 4.78 c ± 0.04 | 10.64 b ± 0.09 | 19.36 a ± 0.45 |
| k (Pa·sn) | 0.0825 a ± 0.0029 | 0.0843 a ± 0.0011 | 0.0729 b ± 0.0002 | 0.0680 b ± 0.0010 |
| n (-) | 0.9780 a ± 0.0009 | 0.9747 a ± 0.0003 | 0.9749 a ± 0.0000 | 0.9733 a ± 0.0014 |
| η (mPa·s) 100 s−1 | 75.45 a ± 2.42 | 71.42 a,b ± 2.54 | 65.66 b,c ± 0.23 | 61.40 c ± 1.28 |
| Analysis | E1 | E2 | E3 | E4 |
|---|---|---|---|---|
| Є (%) | 60.32 | 57.63 | 52.25 | 51.33 |
| U (%) | 3.62 a ± 0.08 | 3.18 b ± 0.14 | 3.06 b ± 0.10 | 3.02 b ± 0.13 |
| H (kg/kg) | 0.0934 a ± 0.0015 | 0.0921 a ± 0.0031 | 0.0934 a ± 0.0030 | 0.0809 b ± 0.0012 |
| dp (μm) 10% | 8.11 b ± 0.09 | 8.98 a ± 0.12 | 8.60 a,b ± 0.13 | 8.97 a ± 0.28 |
| dp (μm) 50% | 30.44 c ± 0.46 | 35.74 a,b ± 0.98 | 34.97 b ± 0.91 | 37.53 a ± 1.87 |
| dp (μm) 90% | 52.16 b ± 1.75 | 66.16 a ± 2.88 | 62.87 a,b ± 2.40 | 69.06 a ± 5.78 |
| Span | 1.45 b ± 0.04 | 1.60 a ± 0.03 | 1.55 a,b ± 0.02 | 1.60 a ± 0.06 |
| D[4,3] (μm) | 30.42 b ± 0.63 | 36.89 a ± 1.29 | 35.52 a,b ± 1.06 | 38.49 a ± 2.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junior, P.C.G.; Bertagnolli, C.; da Silva, C.A.M.; Braga, M.B. Microencapsulation of a Flaxseed and Avocado Oil Blend: Influence of Octenyl Succinic Anhydride (OSA)-Modified Starch and Rice and Pea Proteins on Powder Characterization and Oxidative Stability. Processes 2024, 12, 2230. https://doi.org/10.3390/pr12102230
Junior PCG, Bertagnolli C, da Silva CAM, Braga MB. Microencapsulation of a Flaxseed and Avocado Oil Blend: Influence of Octenyl Succinic Anhydride (OSA)-Modified Starch and Rice and Pea Proteins on Powder Characterization and Oxidative Stability. Processes. 2024; 12(10):2230. https://doi.org/10.3390/pr12102230
Chicago/Turabian StyleJunior, Paulo Cesar Gonçalves, Caroline Bertagnolli, Carlos Alexandre Moreira da Silva, and Matheus Boeira Braga. 2024. "Microencapsulation of a Flaxseed and Avocado Oil Blend: Influence of Octenyl Succinic Anhydride (OSA)-Modified Starch and Rice and Pea Proteins on Powder Characterization and Oxidative Stability" Processes 12, no. 10: 2230. https://doi.org/10.3390/pr12102230
APA StyleJunior, P. C. G., Bertagnolli, C., da Silva, C. A. M., & Braga, M. B. (2024). Microencapsulation of a Flaxseed and Avocado Oil Blend: Influence of Octenyl Succinic Anhydride (OSA)-Modified Starch and Rice and Pea Proteins on Powder Characterization and Oxidative Stability. Processes, 12(10), 2230. https://doi.org/10.3390/pr12102230






