Use of Lactulose as Prebiotic and Chitosan Coating for Improvement the Viability of Lactobacillus sp. FM4.C1.2 Microencapsulate with Alginate
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Culture and Identification of the Probiotic Strain
2.3. Microencapsulation
2.3.1. Extrusion
2.3.2. Emulsion
2.4. Encapsulation Efficiency
2.5. Characterization of Microcapsules
2.5.1. Microcapsule Moisture
2.5.2. Size
2.6. Coating with Chitosan
2.7. Viability of Chitosan-Coated Microencapsulated LAB
2.7.1. Resistance to pH 2.5
2.7.2. Resistance to Bile Salts
2.7.3. Storage at 4 °C
2.8. Data Analysis
3. Results and Discussion
3.1. Characterization of Microcapsules
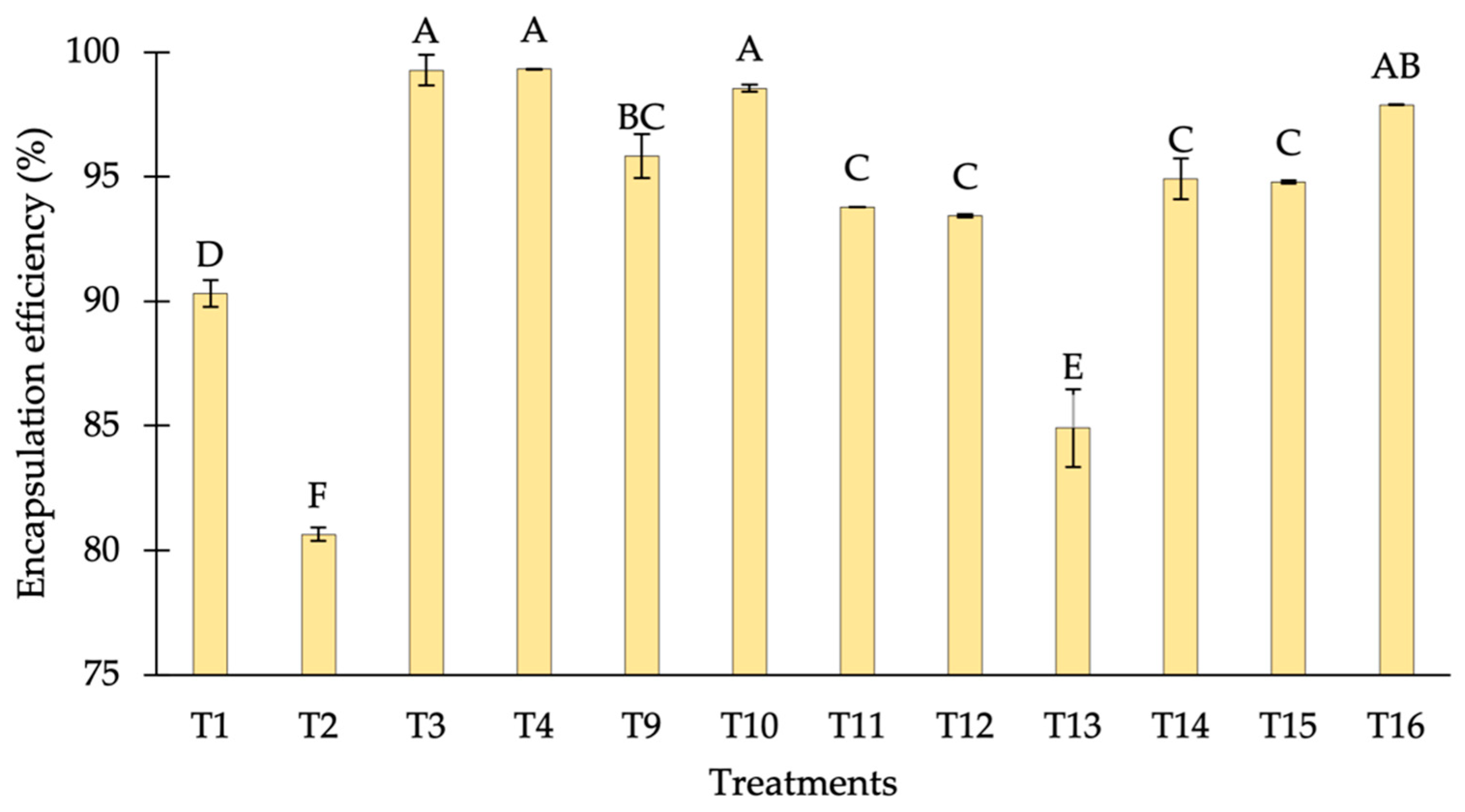
3.1.1. Encapsulation Efficiency
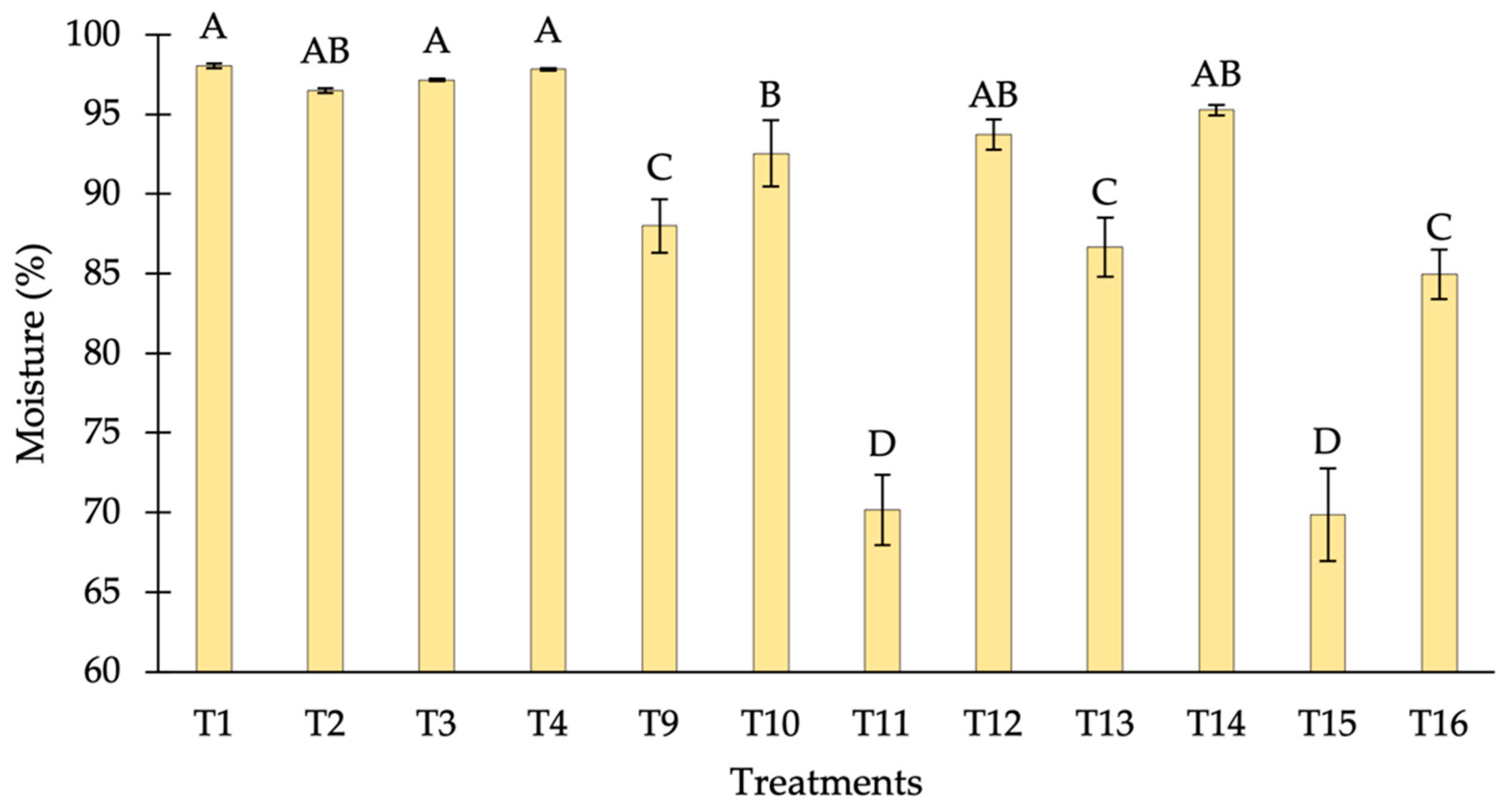
3.1.2. Moisture
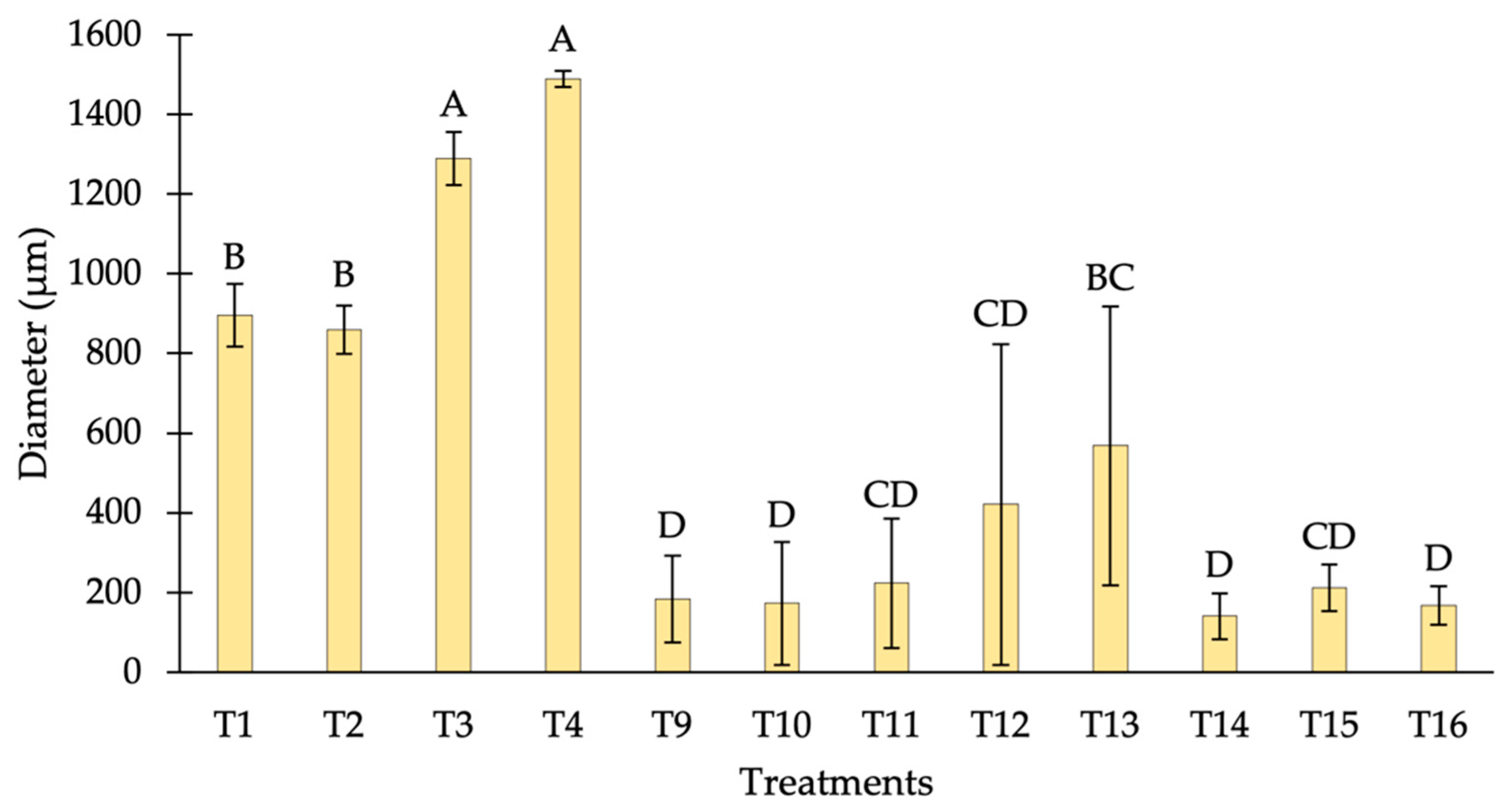
3.1.3. Size
3.2. Cluster Analysis
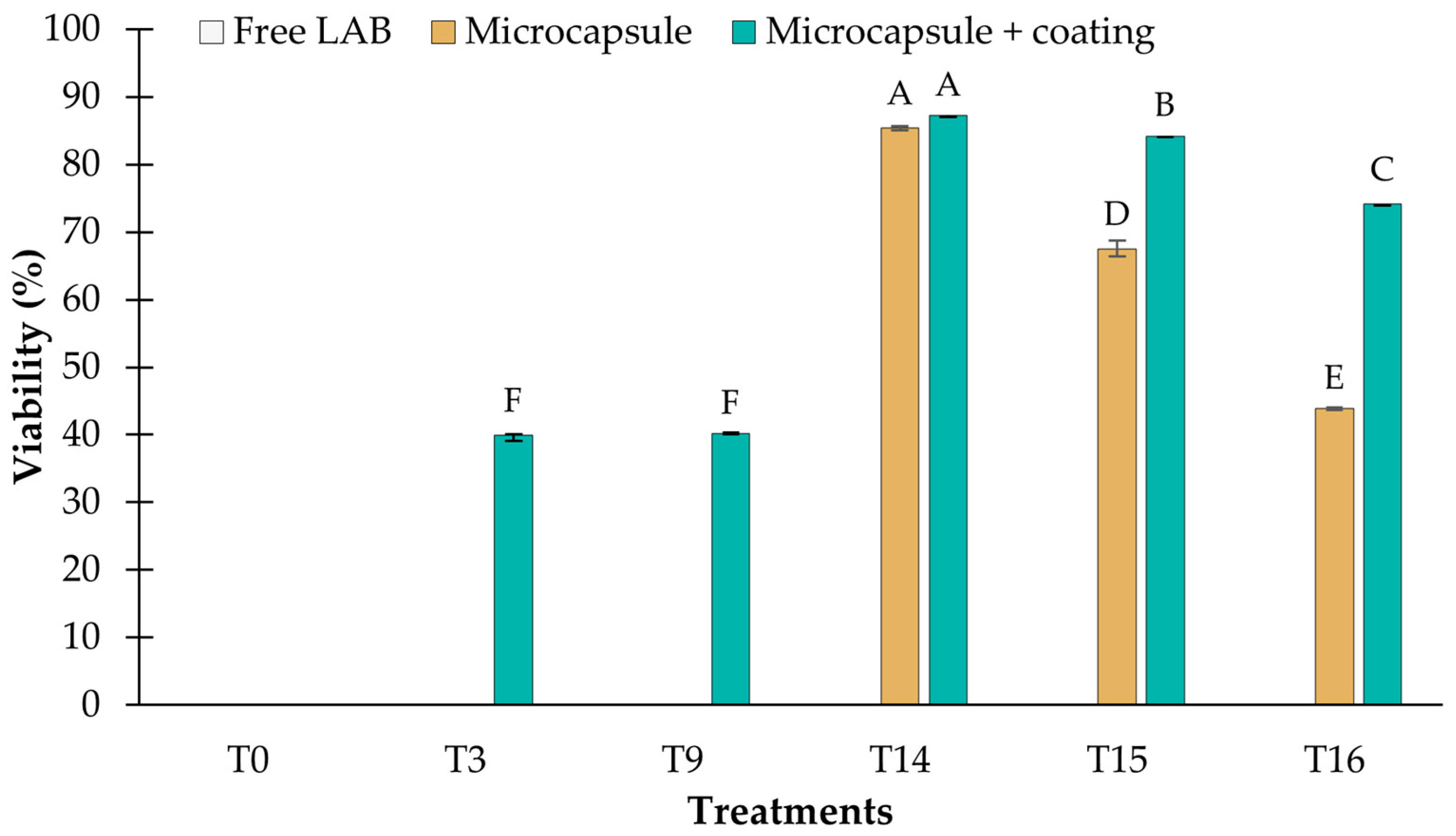
3.3. Viability of LAB Microencapsulated and Coated with Chitosan
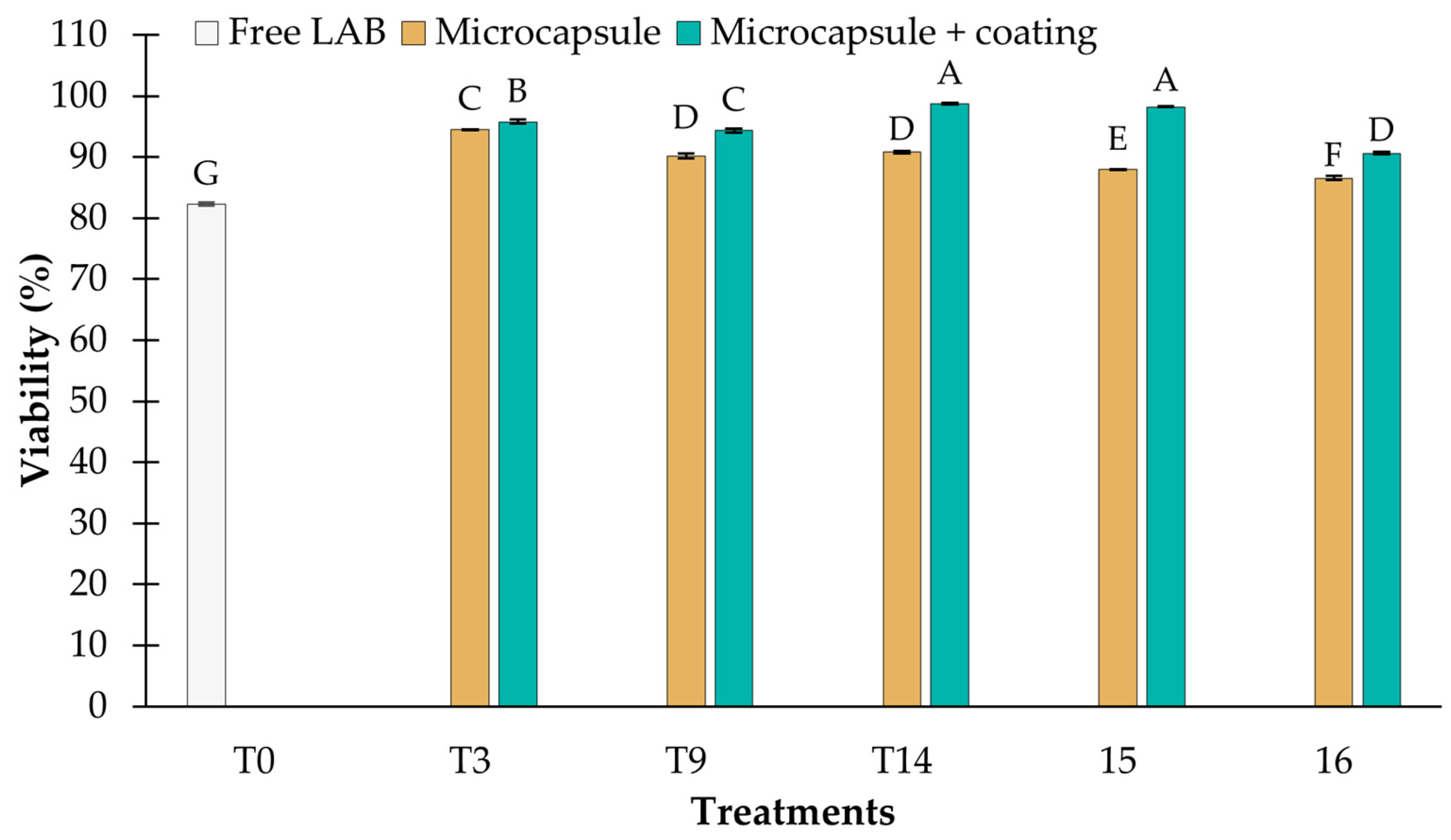
3.3.1. Resistance to pH 2.5
3.3.2. Resistance to Bile Salts
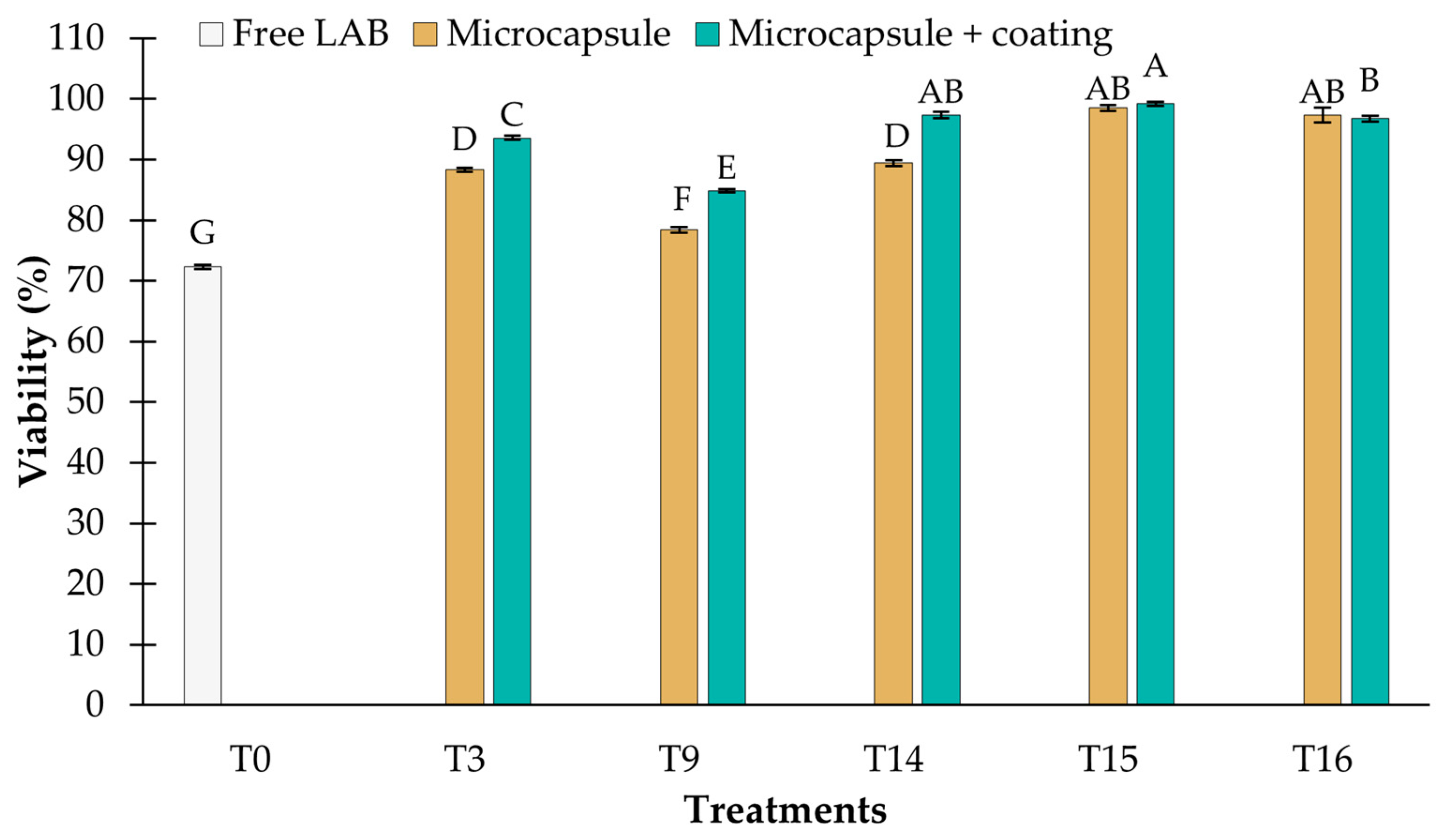
3.3.3. Storage at 4 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kiran, F.; Mokrani, M.; Osmanagaoglu, O. Effect of encapsulation on viability of Pediococcus pentosaceus OZF during its passage through the gastrointestinal tract model. Curr. Microbiol. 2015, 71, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Recent advances in probiotic encapsulation to improve viability under storage and gastrointestinal conditions and their impact on functional food formulation. Food Rev. Internat. 2021, 39, 992–1013. [Google Scholar] [CrossRef]
- Mirzaei, R.; Attar, A.; Papizadeh, S.; Jeda, A.S.; Hosseini-Fard, S.R.; Jamasbi, E.; Kazemi, S.; Amerkani, S.; Talei, G.R.; Moradi, P.; et al. The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Arch. Virol. 2021, 166, 1819–1840. [Google Scholar] [CrossRef] [PubMed]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar]
- De Ameida-Brasiel, P.G.; Luquetti, S.C.P.D.; Peluzio, M.D.C.G.; Novaes, R.D.; Gonçalves, R.V. Preclinical evidence of probiotics in colorectal carcinogenesis: A systematic review. Dig. Dis. Sci. 2020, 65, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-moisture food matrices as probiotic carriers. FEMS Microbiol. Lett. 2019, 366, fnz006. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Wang, J. Extending viability of Bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Popović, M.; Stojanović, M.; Veličković, Z.; Kovačević, A.; Miljković, R.; Mirković, N.; Marinković, A. Characterization of potential probiotic strain, L. reuteri B2, and its microencapsulation using alginate-based biopolymers. Int. J. Biol. Macromol. 2021, 183, 423–434. [Google Scholar]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 61, 1515–1536. [Google Scholar] [CrossRef]
- Erdélyi, L.; Fenyvesi, F.; Gál, B.; Haimhoffer, Á.; Vasvári, G.; Budai, I.; Remenyik, J.; Bereczki, I.; Fehér, P.; Ujhelyi, Z.; et al. Investigation of the role and effectiveness of chitosan coating on probiotic microcapsules. Polymers 2022, 14, 1664. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Socaciu, C. Selenium enriched green tea increase stability of Lactobacillus casei and Lactobacillus plantarum in chitosan coated alginate microcapsules during exposure to simulated gastrointestinal and refrigerated conditions. LWT Food Sci. Technol. 2014, 57, 406–411. [Google Scholar] [CrossRef]
- Bepeyeva, A.; de Barros, J.M.; Albadran, H.; Kakimov, A.K.; Kakimova, Z.K.; Charalampopoulos, D.; Khutoryanskiy, V.V. Encapsulation of Lactobacillus casei into calcium pectinate-chitosan beads for enteric delivery. J. Food Sci. 2017, 82, 2954–2959. [Google Scholar] [CrossRef]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About functional foods: The probiotics and prebiotics state of art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Rodríguez-Barona, S.; Giraldo, G.I.; Montes, L.M. Encapsulation of probiotic foods by freeze-drying in the presence of prebiotics. Inf. Tecnol. 2016, 27, 135–144. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications–A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-dose lactulose as a prebiotic for improved gut health and enhanced mineral absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Khamrui, K.; Singh, K. Enhancement of survival of alginate-encapsulated Lactobacillus casei NCDC 298. J. Sci. Food Agric. 2014, 94, 1994–2001. [Google Scholar] [CrossRef]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of alginate-whey protein isolate microcapsules for survivability and release behavior of probiotic bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.J.; Cho, Y. Microencapsulation of probiotic Lactobacillus acidophilus kbl409 by extrusion technology to enhance survival under simulated intestinal and freeze-drying conditions. J. Microb. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Medina, A.; Mejía-Reyes, D.; Ruiz-González, S.; De Gyves-Córdova, G.; Vázquez-Ovando, A. Isolation of antimicro-bial lactic acid bacteria with potential use as a protective culture for ‘Queso Fresco’. J. Microbiol. Biotechnol. Food Sci. 2023; submitted. [Google Scholar]
- Vázquez-Ortiz, A.A.; Vázquez-Ovando, A.; Ruiz-González, S.; De Gyves-Córdova, M.G.; Mejía-Reyes, J.D. Isolation of lactic acid bacteria with the ability to inhibit the growth of pathogenic bacteria and evolution of their probiotic potential. IBCIENCIAS 2023, 5, 18–25. [Google Scholar]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-López, D.; Vázquez-Ovando, A. Antifungal lactic acid bacteria isolated from fermented beverages with activity against Colletotrichum gloeosporioides. Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- Qaziyani, S.D.; Pourfarzad, A.; Gheibi, S.; Nasiraie, L.R. Effect of encapsulation and wall material on the probiotic survival and physicochemical properties of synbiotic chewing gum: Study with univariate and multivariate analyses. Heliyon 2019, 5, e02144. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, N.; Mirhosseini, H.; Mustafa, S.; Abd Manap, M.Y. Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr. Polym. 2014, 111, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, Z.; Rangel-Vargas, E.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Cadena-Ramírez, A.; Acevedo-Sandoval, O.A.; Falfán-Cortés, R.N. Optimization of a spray-drying process for the production of maximally viable microencapsulated Lactobacillus pentosus using a mixture of starch-pulque as wall material. LWT 2018, 95, 216–222. [Google Scholar] [CrossRef]
- Yudiastuti, S.O.N.; Kastaman, R.; Sukarminah, E.; Mardawati, E. Value-added analysis of Lactobacillus acidophilus cell encapsulation using Eucheuma cottonii by freeze-drying and spray-drying. Open Agric. 2022, 7, 300–310. [Google Scholar] [CrossRef]
- Darjani, P.; Nezhad, M.H.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Gómez-Aldapa, C.A.; Chávez-Urbiola, E.A.; Hernández-Bautista, M.; Rodríguez-Marín, M.L.; Cabrera-Canales, Z.E.; Falfán-Cortés, R.N. Characterisation, storage viabilit, and application of microspheres with Lactobacillus paracasei obtained by the extrusion technique. Int. J. Food Sci. Technol. 2021, 56, 1809–1817. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Arshad, M.S.; Khan, M.A.; Saeed, M.; Anjum, F.M. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in ice cream. Food Sci. Nutr. 2020, 8, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT 2018, 89, 392–399. [Google Scholar] [CrossRef]
- Petraitytė, S.; Šipailienė, A. Enhancing encapsulation efficiency of alginate capsules containing lactic acid bacteria by using different divalent cross-linkers sources. LWT 2019, 110, 307–315. [Google Scholar] [CrossRef]
- Elvan, M.; Baysal, A.H.; Harsa, S. Microencapsulation of a potential probiotic Lactiplantibacillus pentosus and its impregnation onto table olives. LWT 2022, 156, 112975. [Google Scholar] [CrossRef]
- da Silva, S.Â.D.; Batista, L.D.S.P.; Diniz, D.S.; Nascimento, S.S.D.C.; Morais, N.S.; de Assis, C.F.; de Sousa Júnior, F.C. Microencapsulation of probiotics by oil-in-water emulsification technique improves cell viability under different storage conditions. Foods 2023, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Dervisoglu, M. Application of multicriteria decision technique to determine optimum sodium alginate concentration for microencapsulation of Lactobacillus casei Shirota by extrusion and emulsification. J. Food Process Eng. 2017, 40, e12481. [Google Scholar] [CrossRef]
- Poletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; de Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Zheng, Y.; Zi, Y.; Shi, C.; Gong, H.; Zhang, H.; Wang, X.; Zhong, J. Tween emulsifiers improved alginate-based dispersions and ionic crosslinked milli-sized capsules. npj Sci. Food 2023, 7, 33. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Frutos, M.J. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meenu, M.; Baojun, X. A narrative review on microencapsulation of obligate anaerobe probiotics Bifidobacterium, Akkermansia muciniphila, and Faecalibacterium prausnitzii. Food Rev. Int. 2022, 38 (Suppl. 1), 373–402. [Google Scholar] [CrossRef]
- Song, H.; Yu, W.; Gao, M.; Liu, X.; Ma, X. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr. Polym. 2013, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ashwar, B.A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Sánchez, M.; Carrasco-Navarro, U.; Juárez-Castelán, C.; Lozano-Aguirre Beltrán, L.; Pérez-Chabela, M.L.; Ponce-Alquicira, E. Probiotic properties and proteomic analysis of Pediococcus pentosaceus 1101. Foods 2023, 12, 46. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J. Food Sci. 2009, 74, M53–M61. [Google Scholar] [CrossRef]
- Jooyandeh, H.; Momenzadeh, S.; Alizadeh Behbahani, B.; Barzegar, H. Effect of Malva neglecta and lactulose on survival of Lactobacillus fermentum and textural properties of synbiotic stirred yogurt. J. Food Sci. Technol. 2023, 60, 1136–1143. [Google Scholar] [CrossRef]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Factor A | Factor B | Factor C | Factor D |
|---|---|---|---|---|
| 1 | Extrusion | 2% lactulose | Dia-0.3 | Dis-5 |
| 2 | Extrusion | 2% lactulose | Dia-0.3 | Dis-10 |
| 3 | Extrusion | 2% lactulose | Dia-0.6 | Dis-5 |
| 4 | Extrusion | 2% lactulose | Dia-0.6 | Dis-10 |
| 5 | Extrusion | 4% lactulose | Dia-0.3 | Dis-5 |
| 6 | Extrusion | 4% lactulose | Dia-0.3 | Dis-10 |
| 7 | Extrusion | 4% lactulose | Dia-0.6 | Dis-5 |
| 8 | Extrusion | 4% lactulose | Dia-0.6 | Dis-10 |
| 9 | Emulsion | 2% lactulose | S-300 | T-10 |
| 10 | Emulsion | 2% lactulose | S-300 | T-30 |
| 11 | Emulsion | 2% lactulose | S-600 | T-10 |
| 12 | Emulsion | 2% lactulose | S-600 | T-30 |
| 13 | Emulsion | 4% lactulose | S-300 | T-10 |
| 14 | Emulsion | 4% lactulose | S-300 | T-30 |
| 15 | Emulsion | 4% lactulose | S-600 | T-10 |
| 16 | Emulsion | 4% lactulose | S-600 | T-30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Vázquez, F.; Vázquez-Ovando, A.; Mejía-Reyes, D.; Gálvez-López, D.; Rosas-Quijano, R. Use of Lactulose as Prebiotic and Chitosan Coating for Improvement the Viability of Lactobacillus sp. FM4.C1.2 Microencapsulate with Alginate. Processes 2024, 12, 133. https://doi.org/10.3390/pr12010133
Rizo-Vázquez F, Vázquez-Ovando A, Mejía-Reyes D, Gálvez-López D, Rosas-Quijano R. Use of Lactulose as Prebiotic and Chitosan Coating for Improvement the Viability of Lactobacillus sp. FM4.C1.2 Microencapsulate with Alginate. Processes. 2024; 12(1):133. https://doi.org/10.3390/pr12010133
Chicago/Turabian StyleRizo-Vázquez, Fabiola, Alfredo Vázquez-Ovando, David Mejía-Reyes, Didiana Gálvez-López, and Raymundo Rosas-Quijano. 2024. "Use of Lactulose as Prebiotic and Chitosan Coating for Improvement the Viability of Lactobacillus sp. FM4.C1.2 Microencapsulate with Alginate" Processes 12, no. 1: 133. https://doi.org/10.3390/pr12010133
APA StyleRizo-Vázquez, F., Vázquez-Ovando, A., Mejía-Reyes, D., Gálvez-López, D., & Rosas-Quijano, R. (2024). Use of Lactulose as Prebiotic and Chitosan Coating for Improvement the Viability of Lactobacillus sp. FM4.C1.2 Microencapsulate with Alginate. Processes, 12(1), 133. https://doi.org/10.3390/pr12010133









