Abstract
The production of fermented sausages from poultry meat using traditional technologies and natural maturation conditions is a major challenge. The aim of this study was to identify indigenous microbiota with antilisterial activity from an innovative, additive-free, traditionally fermented chicken sausage. Isolates (n = 88) of lactic acid bacteria (LAB) were collected during maturation and subjected to MALDI-TOF mass spectrometry identification. The capacity to combat Listeria was screened against five strains using the agar well diffusion method in 63 selected LAB isolates. MALDI-TOF mass spectrometry identified four different LAB genera, namely Enterococcus, Lactococcus, Leuconostoc and Lactobacillus, the proportions of which differed significantly during the production phases (p < 0.001). Enterococcus faecalis was the most prevalent LAB species in the initial sausage dough. The presence of lactococci (Lactococcus lactis) and enterococci was detected during the 14- and 30-day ripening period and was gradually displaced by leuconostocs and lactobacilli. Lactobacilli appeared to be abundant during the central and late maturation phases, and consisted of only two species—Latilactobacillus sakei and Latilactobacillus curvatus. In total, 38 LAB isolates (60%) showed antilisterial activity toward at least one Listeria indicator strain. The proportions of antilisterial LAB differed significantly during sausage maturation. Inhibitory activity against all indicator Listeria was detected in the neutralized cell-free supernatants of five strains of Enterococcus faecalis, two L. sakei strains and one Leuconostoc mesenteroides strain. The antilisterial activity observed in the indigenous LAB revealed the possible role of L. sakei as a bioprotective culture, as well as the role of Ln. mesenteroides and E. faecalis as bacteriocin producers, for practical applications.
1. Introduction
Traditionally, poultry was consumed immediately after slaughter, as its size compared to red meat meant that the whole bird could be consumed in the home. As a result, traditional fermented meat products were developed primarily from pork and beef, but not poultry [1]. There is a growing interest in the use of poultry meat in sausage recipes, either with other meats and additives [2], with chicken meat and pork fat [3], with chicken meat and skin with the addition of corn starch as a binder [4] or with chicken meat and chicken fat without skin [5] to develop a low-fat product. Pereira et al. [6] classified chicken and turkey sausages as nutritionally more beneficial than conventional sausages due to their fat, protein, cholesterol, and fatty acid content, as well as their favorable ratio of polyunsaturated fatty acids to saturated fatty acids. Accordingly, poultry meat is considered a suitable raw material for such products due to its chemical composition and nutritional value. It is known that poultry meat is rich in protein, and lower in fat, cholesterol and connective tissue compared to red meat, and is also a source of all essential amino acids [7]. Particularly noteworthy is the fact that the consumption of poultry meat is not culturally and religiously restricted, as is the case with pork or beef [8]. In practice, attempts are usually made to compensate for the complexity of production due to the high water content, slow drying, low fat content and poor binding of the sausage compounds by adding solid pork fat, starter cultures and other additives [3]. Fermented poultry sausages prepared exclusively from poultry have rarely been studied, making them a novel product [9]. Our recently developed product—naturally fermented sausages made from chicken meat from heavy hybrids (Cobb 500, parent flocks at the end of production) without added fat and additives—has favorable and health-promoting chemical properties. As previously reported, the protein content was over 46% and the fat content was only 11%, while the monounsaturated fatty acid and polyunsaturated fatty acid content was 53% and 12.4%, respectively [9,10]. In addition, the safety of products was confirmed by the absence of Salmonella and Listeria monocytogenes [10].
From a microbiological point of view, dry-fermented sausages are stable and safe meat products due to the known sequence of physicochemical changes during maturation. Antimicrobial hurdles include preservatives, increases in acidity, decreases in redox potential (Eh), increases in salt content, succession of competing microbiota and decreases in water activity [11]. The same process would be expected in naturally fermented poultry sausages, but very limited research has been done on the natural microbiota in terms of its composition or antimicrobial capabilities. The microbiological succession of lactic acid bacteria (LAB) was reported by Zinina et al. [3] to be 6 log10 CFU/g in fermented poultry sausages. In fermented sausages made from Croatian chicken meat, the LAB count reached 7 log10 CFU/g but was stable during the slow maturation process of 90 days [10]. Traditionally fermented poultry sausages are rarely described in the literature, so there is a lack of studies on the indigenous microbiota of this type of product. Therefore, this preliminary study is one of the first reports on the identification of LAB in spontaneously fermented sausages made from poultry meat.
In recent decades, the antimicrobial capacity of natural LAB strains isolated from fermented sausages has been extensively studied against a number of different foodborne pathogens, in particular Listeria monocytogenes [12,13,14]. However, as far as we know, these types of studies have not yet been conducted on the microbiota of fermented poultry sausages. In the LAB population in poultry carcasses, the presence of LAB strains that are active against Listeria and can be used as protective cultures in poultry meat products was detected [15]. The selection of strains with antilisterial activity is indeed the starting point for the development of protective cultures for practical application in fermented sausage production. Naturally existing strains that are well adapted to the poultry meat environment would be the best candidates for this purpose.
This study aims to characterize the natural LAB populations present during the fermentation and drying of chicken sausages to identify them at the species level and to evaluate their antimicrobial activity against various Listeria strains in vitro.
2. Materials and Methods
2.1. Dry-Fermented Chicken Sausage Production
Meat was obtained from COBB 500 breeding hens aged 62–65 weeks, after their production cycle. The process of the sausages’ production was previously described [10]. Briefly, the meat (breast and thigh muscles) was minced through an 8 mm diameter grinder, and mixed with 2% NaCl, garlic and paprika. The mixture was stuffed into collagen casings (diameter 45 mm). The sausages were hung in a dark room at 15 °C for two days to activate the LAB population and initiate fermentation. During the 10-day period, the products were traditionally cold-smoked (approx. 20 °C) (every second day). The process consisted of the slow smoldering of beech and hornbeam wood, and mild smoking. The drying and maturing of the sausages lasted 90 days, during the winter season, from December to March. The temperature and humidity during sausage production were measured in the smokehouse and maturation chamber daily. The average values of temperature and humidity were 5.88 ± 1.73 °C and 71.25 ± 9.04% in December, 5.32 ± 1.27 °C and 63.41 ± 1.70% in January, 7.06 ± 0.70 °C and 66.65 ± 0.97% in February and 8.40 ± 0.54 °C and 66.80 ± 1.09% in March (production ended on March 5).
2.2. Microbiological Analyses
Three samples were taken from two batches on days 0, 7, 14, 30, 60 and 90 of maturation. Ten grams of the sample were aseptically collected, added to 90 mL buffered peptone water (BioRad, Hercules, CA, USA) and homogenized in a Stomacher 400 Circulator (Seward, Worthing, UK). The initial suspension was serially diluted (1:9) and 0.1 mL of the appropriate dilution was spread onto MRS agar plates (Merck, Darmstadt, Germany) and M17 agar (Merck, Darmstadt, Germany), and incubated anaerobically at 30 °C for 48 h (Anaerocult, Merck, Darmstadt, Germany). The enterococcal count was determined by Compass Enterococcus agar (BioRad, Hercules, CA, USA) incubated at 44 °C for 24 h. Bacterial populations were enumerated using the automated SCAN1200 device (Interscience, Saint Nom la Bretêche, France). The counts were expressed in log10 CFU/g as arithmetic means with standard deviations of six sausage samples from two batches.
2.3. Identification of LAB
Fifteen colonies (combined from two batches) from the MRS plates with the highest dilutions were randomly selected per sampling day, examined morphologically (Gram staining) and subjected to identification. The isolates were determined by matrix-assisted laser desorption ionisation and time-of-flight mass spectrometry (MALDI-TOF MS). The isolated colonies were spotted onto a MALDI plate using a sterile toothpick, and 1 μL of 70% formic acid (v/v) (Fisher Chemical, Barcelona, Spain) was added. The sample was dried at room temperature and overlaid with 1 μL of MALDI matrix (saturated solution of α-cyano-4-hydroxycinnamic acid (HCCA, Bruker Daltonik, Bremen, Germany)) in 50% acetonitrile and 2.5% trifluoroacetic acid (Sigma–Aldrich, Steinheim, Germany) and dried again at room temperature. Characteristic mass spectra in the range of 2000 to 20,000 Da were recorded for each sample using the microflex LT mass spectrometer (Bruker Daltonik, Bremen, Germany). The recorded mass spectra were processed using MALDI Biotyper 3.0 software (Bruker Daltonik, Bremen, Germany). The results of the MALDI Biotyper are expressed as a logarithmic value in the range of 0–3.0, which indicates the probability of the correct identification of the sample by comparing the protein profiles of a still-unknown sample with the reference spectrum in the database.
2.4. Screening of the Antilisterial Activity of LAB Isolates
2.4.1. Preparation of Strains
The LAB strains were stored at −80 °C (ULUF4502M, Arctiko, Esbjerg, Denmark) in MRS broth (Merck, Darmstadt, Germany) with glycerol and in the Microbank system (Pro-Lab Diagnostics, Bromborough, UK). The strains were propagated in 10 mL of MRS broth (Merck, Germany) and then incubated at 30 °C for 48–72 h. These revived cultures were then cultured in 100 mL of MRS broth at 30 °C for 24 h. After secondary culturing, each sample was centrifuged (Centrifuge 5804 R, Eppendorf, Hamburg, Germany) at 6.780× g for 10 min at 4 °C. The supernatant was transferred to two new sterile tubes, the pH was measured (XS Instruments, pH 50 VioLab, Carpi MO, Italy) and half of the supernatant was neutralized with 1 M NaOH until a pH of 7 was reached. The remaining cells were resuspended with MRS broth mixed with 20% glycerol and stored at −80 °C.
2.4.2. Indicator Listeria Strains
The inhibitory effects of the 63 selected indigenous LAB strains were tested on the following indicator bacteria: Listeria innocua ATCC 33090, Listeria monocytogenes ATCC 13932 and three Listeria monocytogenes strains from the laboratory’s collection, which we refer to as 1411, 1414 and 1416 and which were previously isolated from fermented pork sausages. All isolates were always freshly cultured in Fraser broth (Biolife, Milano, Italy) at 37 °C for 24 h prior to the experiment.
2.4.3. Agar Well Diffusion Assay
Plates with brain–heart infusion agar (BHI, Biolife, Milano, Italy) were covered with 8 mL of soft BHI agar (0.7% agar in BHI broth) containing a Listeria culture (0.1 mL of active culture, 5 log10 CFU/mL). Four wells per plate were prepared with a sterile 200 µL pipette tip, and the bottom of the well was covered with soft BHI agar. Both culture supernatants and neutralized culture supernatants (pH 7) were tested for inhibition of Listeria growth. Each sample (100 µL) was pipetted into the appropriate well. The plates were pre-incubated for 1 h at 5 °C in the refrigerator and then incubated at 37 °C for 24 h. After incubation, the appearance of inhibition zones was recorded. The bacteriocin-producing strain Leuconostoc mesenteroides E131 was used as a positive control.
2.5. Statistical Analysis
Results of the microbiological succession during sausage maturation were analyzed using descriptive statistics methods (Statistica 13.5, TIBCO Software, Palo Alto, CA, USA). The counts of lactic acid bacteria determined on MRS, M17 and Enterococcus agar were expressed as arithmetic means with standard deviations (x ± SD). A one-way ANOVA was used with a probability level of 0.001 to determine statistically significant differences between the numbers of lactic acid bacteria groups (on MRS, M17 and Enterococcus agar) by sampling days. The post-hoc analysis was applied to determine differences between LAB counts on the same day of sampling and pouring on different culture media.
Differences in the proportion of LAB genera and the proportion of antilisterial strains between sampling days were tested using the χ2 test.
3. Results
The succession of LAB populations, including enterococci, determined during the maturation of the sausages on different culture media is shown in Table 1. The initial LAB count on MRS agar (a medium more selective for lactobacilli) was significantly different from the other sampling days (p < 0.001), showing a 3-fold increase on day 7. The same trend was observed for the M17 population (more selective for lactococci/streptococci), with stable populations after day 14. The increase in the enterococci population was significant after day 14 (p < 0.001), while in the subsequent days, the population was reduced to the initial numbers (p > 0.001). The differences between the LAB populations on the individual sampling days were observed on days 14, 30 and 60. In the final product, the MRS and M17 counts were at the same level (7.8 log10 CFU/g).

Table 1.
Lactic acid bacteria counts (log10 CFU/g, X ± SD) obtained from different culture media.
The isolates from the MRS plates (n = 88) were identified by MALDI-TOF MS. They were from different stages of sausage maturation (0–90 days) and had log scores above 2.0 (high confidence identification). The abundance of LAB genera at different sampling time points is shown in Figure 1.
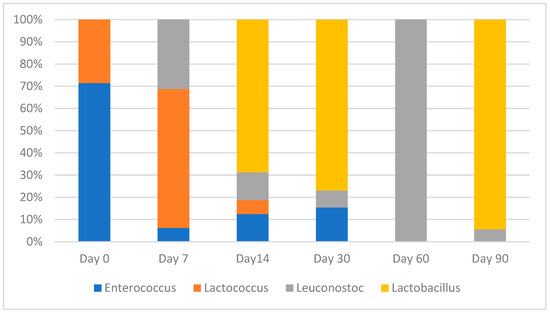
Figure 1.
Abundance of LAB genera during the maturation of dry-fermented chicken sausages.
MALDI-TOF mass spectrometry identified four different LAB genera, namely Enterococcus, Lactococcus, Leuconostoc and Lactobacillus, the proportions of which differed significantly during the production phases (p < 0.001). Enterococci, particularly E. faecalis, were predominant in the initial sausage mixture. The presence of lactococci (Lc. lactis) was detected during the 14-day ripening period and was gradually suppressed by leuconostocs and lactobacilli. The same was true for enterococci up to day 30. Lactobacilli were abundant throughout fermentation and maturation, comprising only two species: Latilactobacillus sakei and Latilactobacillus curvatus. The genus Leuconostoc was represented by Ln. mesenteroides.
After identification, the LAB isolates were tested for their ability to inhibit the growth of the major pathogen in ready-to-eat (RTE) food Listeria monocytogenes (and a non-pathogenic L. innocua). Of the 63 isolates tested by the agar well diffusion method, 38 (60%) of the cell-free supernatants had antilisterial activity against at least one Listeria indicator strain (Table 2). The pH of the supernatants ranged from 3.80 to 5.67, but the pH had no effect on the width of the inhibition zones (4 mm). In addition, our aim was to identify those strains that inhibited Listeria growth when acid inhibition was excluded. This was the case for eight of the LAB strains, which showed inhibitory activity against all five Listeria indicators, including the species E. faecalis, L. sakei and Ln. mesenteroides.

Table 2.
Profiles of the antilisterial activities of the LAB strains (n = 38) isolated during the maturation phases of dry-fermented chicken sausages.
4. Discussion
The microbial diversity of naturally fermented sausages is generally very variable and consists mainly of lactic acid bacteria and coagulase-negative staphylococci and, in some specific products, molds [16]. Microbiological succession is driven by intrinsic (e.g., acidity, salinity, microaerophilic conditions, water activity) and extrinsic conditions (humidity, temperature, air flow, casings). Intrinsic factors suppress Gram-negative and aerobic spoilage bacteria or even pathogens (such as Pseudomonas, Enterobacteriaceae, E. coli) and favor osmotolerant, halotolerant, acid-resistant and microaerophilic lactic acid bacteria [13]. The composition of the indigenous LAB population in dry-fermented sausages made from poultry meat has been little studied. Microbiological succession in this type of product has been evaluated, but focused mainly on the application of starter or protective cultures [5,17,18,19,20]. These studies were conducted on rapidly fermented poultry sausages (<30 days of process time), while our product slowly matured at a low temperature. The comparison of our results with other studies is hardly possible due to the lack of similar design and methods.
As far as enterococci are concerned, their presence in naturally fermented sausages is generally accepted as a common finding due to their wide distribution in the environment and their persistence, fermentative capacity and competitiveness within the fermentative microbiome [21]. Some species of enterococci may pose a potential risk to food safety due to their virulence, antimicrobial resistance or biogenic amine synthesis [22]. Our results showed the low competitive ability of enterococci in the fermentation of poultry meat, which could be of importance for food safety, assuming the presence of pathogenic strains in the sausage mixture. Their population can be suppressed during fermentation by competing and dominant lactobacilli with antimicrobial properties (confirmed against Listeria). The low acidity of the product (pH 5) favors the overgrowth of lactobacilli compared to enterococci [21]. Leuconostoc mesenteroides is found sporadically in various fermented sausages [23,24,25], but its presence as a gas-producing heterofermentative LAB species can affect sausage quality. The dominant species in the final product was L. sakei, a species known to be well adapted to the meat substrate and a favorable homofermentative species in meat fermentation [24].
The antimicrobial mechanisms of LAB have been extensively studied, but data on dry-fermented poultry sausages remain scarce. In general, the antimicrobial activity of LAB species is known due to the production of organic acids against foodborne pathogens, mainly Gram-negative bacteria [26,27,28]. Sensitivity to acidic conditions varies among L. monocytogenes strains, but in general, a pH above 4.2 is considered suitable for their growth [29]. Another known antimicrobial mechanism is the synthesis of bacteriocins, which are usually effective against closely related bacteria in the Gram-positive group [30]. As mentioned above, the neutralized supernatants of eight LAB strains were active against all five Listeria indicators, including species of E. faecalis, L. sakei and Ln. mesenteroides. Interestingly, the antimicrobial capacity of these species has already been demonstrated in vitro and in fermented pork sausage in our previous research, where we focused on the efficacy of their bacteriocins [10,31]. Fermented sausages are a source of LAB with inhibitory activities against L. monocytogenes, one of the major foodborne pathogens associated with this type of meat product [30]. Antilisterial activity may be present in most LAB strains isolated from specific fermented sausages [32,33], but only a limited portion of these populations harbor bacteriocin-encoding genes [32]. Milani et al. [32] found bacteriocin-encoding genes only in strains of L. sakei and L. curvatus among an LAB population in fermented sausages. The use of bacteriocinogenic protective cultures of L. sakei or L. curvatus has been shown to be a successful antilisterial strategy in the production of fermented sausage, resulting in a two or more log reductions of L. monocytogenes [34,35,36,37].
Based on the negative properties of enterococci or leuconostocs mentioned above, the two strains of L. sakei with antilisterial activity and potential bacteriocinogenic capacity that we found in our study can be considered for further studies as possible protective cultures in poultry meat processing. The ability of L. sakei to compete with other bacteria in the sausage matrix, as well as to combine with the metabolomic pathways of staphylococcal starter cultures, has only been studied in fermented pork and beef sausages [38]. We do not suggest using selected L. sakei strains as starter cultures in traditional production, but their adaptability and activity need to be investigated under controlled conditions on an industrial scale in fermented poultry products. The microbial diversity of the unique fermented chicken sausage should be preserved, and can be used for potential applications in industry, based on similar practices in meat fermentation [39].
5. Conclusions
This study provides the first insight into microbial succession during the natural fermentation of chicken dry sausage, with a focus on lactic acid bacteria. Instead of metagenomics tools, the traditional approach was chosen to identify active and viable strains for further characterization. The fermentation of chicken sausage was driven by a mixed, diverse microbiota dominated by enterococci—the benefits of which are controversial—in the initial stages of fermentation, which were subsequently suppressed by lactobacilli. Although MRS medium is more selective for lactobacilli, it promoted the growth of other LAB genera in the early stages of fermentation, when intensive microbial interactions took place, allowing us to select strains with strong antilisterial capacities. The intensity of antilisterial activity observed in indigenous LAB revealed a possible role of L. sakei as a bioprotective culture, or of Ln. mesenteroides and E. faecalis as bacteriocin producers, for practical applications.
Author Contributions
Conceptualization, N.Z. and S.K.; methodology, M.K.; formal analysis, M.K., M.V. and S.K.; investigation, I.B., H.M.; resources, N.Z.; data curation, S.K., M.V.; writing—original draft preparation, N.Z., M.V.; writing—review and editing, H.M., N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by the family business OPG Milan Varešak, who provided the raw materials, ingredients, equipment and facilities for the preparation and production of the sausages.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Santchurn, S.J.; Collignan, A. Fermented poultry sausages. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; Wiley: Ames, IA, USA, 2007; pp. 361–368. [Google Scholar]
- Yılmaz, İ.; Şimşek, O.; Işıklı, M. Fatty acid composition and quality characteristics of low-fat cooked sausages made with beef and chicken meat, tomato juice and sunflower oil. Meat Sci. 2002, 62, 253–258. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Soloveva, A.; Savostina, T.; Sayfulmulyukov, E.; Lykasova, I.; Mizhevikina, A. The effect of starter cultures on the qualitative indicators of dry fermented sausages made from poultry meat. Agron. Res. 2018, 16, 2265–2281. [Google Scholar] [CrossRef]
- Raleng, A.; Thangjam, T.; Devi, M.P.; Bandana, S.; Behera, S.K. Enhancing Chicken Sausage Quality: Investigating the impact of chicken skin fat and corn starch powder on physicochemical attributes, textural properties, and storage stability. Indian J. Hill Farming 2023, 36, 31–39. [Google Scholar] [CrossRef]
- El Adab, S.; Essid, I.; Hassouna, M. Microbiological, biochemical and textural characteristics of a Tunisian dry fermented poultry eat sausage inoculated with selected starter cultures. J. Food Saf. 2015, 35, 75–85. [Google Scholar] [CrossRef]
- Pereira, N.R.; Tarley, C.R.T.; Matsushita, M.; de Souza, N.E. Proximate composition and fatty acid profile in Brazilian poultry sausages. J. Food Compos. Anal. 2000, 13, 915–920. [Google Scholar] [CrossRef]
- Menegas, L.Z.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Dry-fermented chicken sausage produced with inulin and corn oil: Physicochemical, microbiological, and textural characteristics and acceptability during storage. Meat Sci. 2013, 93, 501–506. [Google Scholar] [CrossRef]
- Fessler, D.M.T.; Navarrete, C.D. Meat is good to taboo: Dietary proscriptions as a product of the interaction of psychological mechanisms and social processes. J. Cogn. Cult. 2003, 3, 1–40. [Google Scholar] [CrossRef]
- Zdolec, N.; Mikuš, T.; Kiš, M. Lactic acid bacteria in meat fermentation: Dry sausage safety and quality. In Lactic Acid Bacteria in Food Biotechnology Innovations and Functional Aspects Applied Biotechnology Reviews; Ray, R., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–159. ISBN 9780323898751. [Google Scholar]
- Zdolec, N.; Kiš, M.; Cvrtila, Ž.; Mikuš, T.; Kazazić, S.; Pleadin, J.; Lešić, T.; Kozačinski, L.; Dobranić, V.; Mazija, H. Microbiological and phisico-chemical properties of traditional dry-fermented hen meat sausages. Meso 2020, 22, 368–377. [Google Scholar] [CrossRef]
- Leistner, L.; Gould, G.W. The hurdle concept. In Hurdle Technologies; Food engineering series; Springer: Boston, MA, USA, 2002; pp. 17–28. [Google Scholar]
- Siddi, G.; Piras, F.; Spanu, V.; Meloni, M.P.; Sanna, R.; Carta, N.; Errico, M.; Cuccu, M.; De Santis, E.P.L.; Scarano, C. Selection of commercial protective cultures to be added in Sardinian fermented sausage to control Listeria monocytogenes. Ital. J. Food Saf. 2022, 11, 10368. [Google Scholar] [CrossRef]
- Zdolec, N. Fermented Meat Products: Health Aspects; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 9781498733045. [Google Scholar]
- Mangia, N.P.; Cottu, M.; Mura, M.E.; Murgia, M.A.; Blaiotta, G. Technological parameters, anti-Listeria activity, biogenic amines formation and degradation ability of L. plantarum strains isolated from sheep-fermented sausage. Microorganisms 2021, 9, 1895. [Google Scholar] [CrossRef]
- Sakaridis, I.; Soultos, N.; Dovas, C.I.; Papavergou, E.; Ambrosiadis, I.; Koidis, P. Lactic acid bacteria from chicken carcasses with inhibitory activity against Salmonella spp. and Listeria monocytogenes. Anaerobe 2012, 18, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Miličević, B.; Danilović, B.; Zdolec, N.; Kozačinski, L.; Dobranić, V.; Savić, D. Microbiota of the fermented sausages: Influence to product quality and safety. Bulg. J. Agric. Sci. 2014, 20, 1061–1078. [Google Scholar]
- Deumier, F.; Collignan, A. The effects of sodium lactate and starter cultures on pH, lactic acid bacteria, Listeria monocytogenes and Salmonella spp. levels in pure chicken dry fermented sausage. Meat Sci. 2003, 65, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Geeta; Yadav, A.S. Antioxidant and antimicrobial profile of chicken sausages prepared after fermentation of minced chicken meat with Lactobacillus plantarum and with additional dextrose and starch. LWT 2017, 77, 249–258. [Google Scholar] [CrossRef]
- Menegas, L.Z.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Effect of adding inulin as a partial substitute for corn oil on the physicochemical and microbiological characteristics during processing of dry-fermented chicken sausage. J. Food Process. Preserv. 2017, 41, 13166. [Google Scholar] [CrossRef]
- Austrich-Comas, A.; Serra-Castelló, C.; Jofré, A.; Gou, P.; Bover-Cid, S. Control of Listeria monocytogenes in chicken dry-fermented sausages with bioprotective starter culture and high-pressure processing. Front. Microbiol. 2022, 13, 983265. [Google Scholar] [CrossRef]
- Hugas, M.; Garriga, M.; Aymerich, M.T. Functionality of enterococci in meat products. Int. J. Food Microbiol. 2003, 88, 223–233. [Google Scholar] [CrossRef]
- Jiménez, E.; Ladero, V.; Chico, I.; Maldonado-Barragán, A.; López, M.; Martín, V.; Fernández, L.; Fernández, M.; Álvarez, M.A.; Torres, C.; et al. Antibiotic resistance, virulence determinants and production of biogenic amines among enterococci from ovine, feline, canine, porcine and human milk. BMC Microbiol. 2013, 13, 288. [Google Scholar] [CrossRef]
- Mataragas, M.; Metaxopoulos, J.; Drosinos, E.H. Characterization of two bacteriocins produced by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442, isolated from dry fermented sausages. World J. Microbiol. Biotechnol. 2002, 18, 847–856. [Google Scholar] [CrossRef]
- Aymerich, T.; Martín, B.; Garriga, M.; Vidal-Carou, M.C.; Bover-Cid, S.; Hugas, M. Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J. Appl. Microbiol. 2006, 100, 40–49. [Google Scholar] [CrossRef]
- Vesković Moračanin, S.; Turubatović, L.; Škrinjar, M.; Obradović, D. Antilisterial activity of bacteriocin isolated from Leuconostoc mesenteroides ssp. mesenteroides IMAU:10231 in the production of Sremska sausages: Lactic acid bacteria isolation, bacteriocin identification and meat application experiments. Food Technol. Biotechnol. 2013, 51, 247–256. [Google Scholar]
- Helander, I.M.; von Wright, A.; Mattila-Sandholm, T.-M. Potential of lactic acid bacteria and novel anti-microbials against Gram-negative bacteria. Trends Food Sci. Technol. 1997, 8, 146–150. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Volzing, K.; Borrero, J.; Sadowsky, M.J.; Kaznessis, Y.N. Antimicrobial peptides targeting Gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth. Biol. 2013, 2, 643–650. [Google Scholar] [CrossRef]
- Shabala, L.; Lee, S.H.; Cannesson, P.; Ross, T. Acid and NaCl limits to growth of Listeria monocytogenes and influence of sequence of inimical acid and NaCl levels on inactivation kinetics. J. Food Prot. 2008, 71, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Nychas, G.J.E.; Luo, X.; Zhu, L.; Mao, Y.; Dong, P.; Zhang, Y. Utilizing lactic acid bacteria and their metabolites for controlling Listeria monocytogenes in meat products: Applications, limitations, and future perspectives. Trends Food Sci. Technol. 2024, 152, 104699. [Google Scholar] [CrossRef]
- Zdolec, N.; Hadžiosmanović, M.; Kozačinski, L.; Cvrtila, Ž.; Filipović, I.; Škrivanko, M.; Leskovar, K. Microbial and physicochemical succession in fermented sausages produced with bacteriocinogenic culture of Lactobacillus sakei and semi-purified bateriocin mesenterocin Y. Meat Sci. 2008, 80, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Tabanelli, G.; Barbieri, F.; Montanari, C.; Gardini, F.; Belloso Daza, M.V.; Castellone, V.; Bozzetti, M.; Cocconcelli, P.S.; Bassi, D. Technological traits and mitigation activity of autochthonous lactic acid bacteria from mediterranean fermented meat-products. LWT 2024, 196, 115861. [Google Scholar] [CrossRef]
- Tönz, A.; Freimüller Leischtfeld, S.; Stevens, M.J.A.; Glinski-Häfeli, D.; Ladner, V.; Gantenbein-Demarchi, C.; Miescher Schwenninger, S. Growth control of Listeria monocytogenes in raw sausage via bacteriocin-producing Leuconostoc carnosum DH25. Foods 2024, 13, 298. [Google Scholar] [CrossRef]
- Tadić, V.; Djordjcvić, J.; Bosković, M.; Baltic, M.; Lakicevic, B.; Vasilev, D.; Dimitrijević, M. Two different starter cultures as potential inhibitors of L. monocytogenes in fermented sausages. Fleischwirtschaft 2018, 98, 93–97. [Google Scholar]
- Giello, M.; La Storia, A.; De Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus curvatus 54M16 on microbiota composition and growth of Listeria monocytogenes in fermented sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “Salchichón” processing with a selected Lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Bustins, N.; Costa, J.C.C.P.; Pérez-Rodríguez, F.; Martín, B.; Bover-Cid, S.; Jofré, A. The antilisterial effect of Latilactobacillus sakei CTC494 in relation to dry fermented sausage ingredients and temperature in meat simulation media. Fermentation 2024, 10, 326. [Google Scholar] [CrossRef]
- Li, X.; Zhao, G.; Zheng, Y.; Wang, Y.; Bai, X.; Li, F.; Gu, Y.; Zhu, C. Effects of single fermentation of Lactobacillus sakei and compound fermentation with Staphylococcus carnosus on the metabolomics of beef sausages. Food Chem. 2025, 464, 141728. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Angelucci, C.; Gardini, F.; Tabanelli, G. Use of indigenous lactic acid bacteria for industrial fermented sausage production: Microbiological, chemico-physical and sensory features and biogenic amine content. Fermentation 2024, 10, 507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).