3.1. Thermochemical Redox Performance of La0.7Sr0.3 Mn1−yZyO3 (Z = Ni, Co, and Mg)
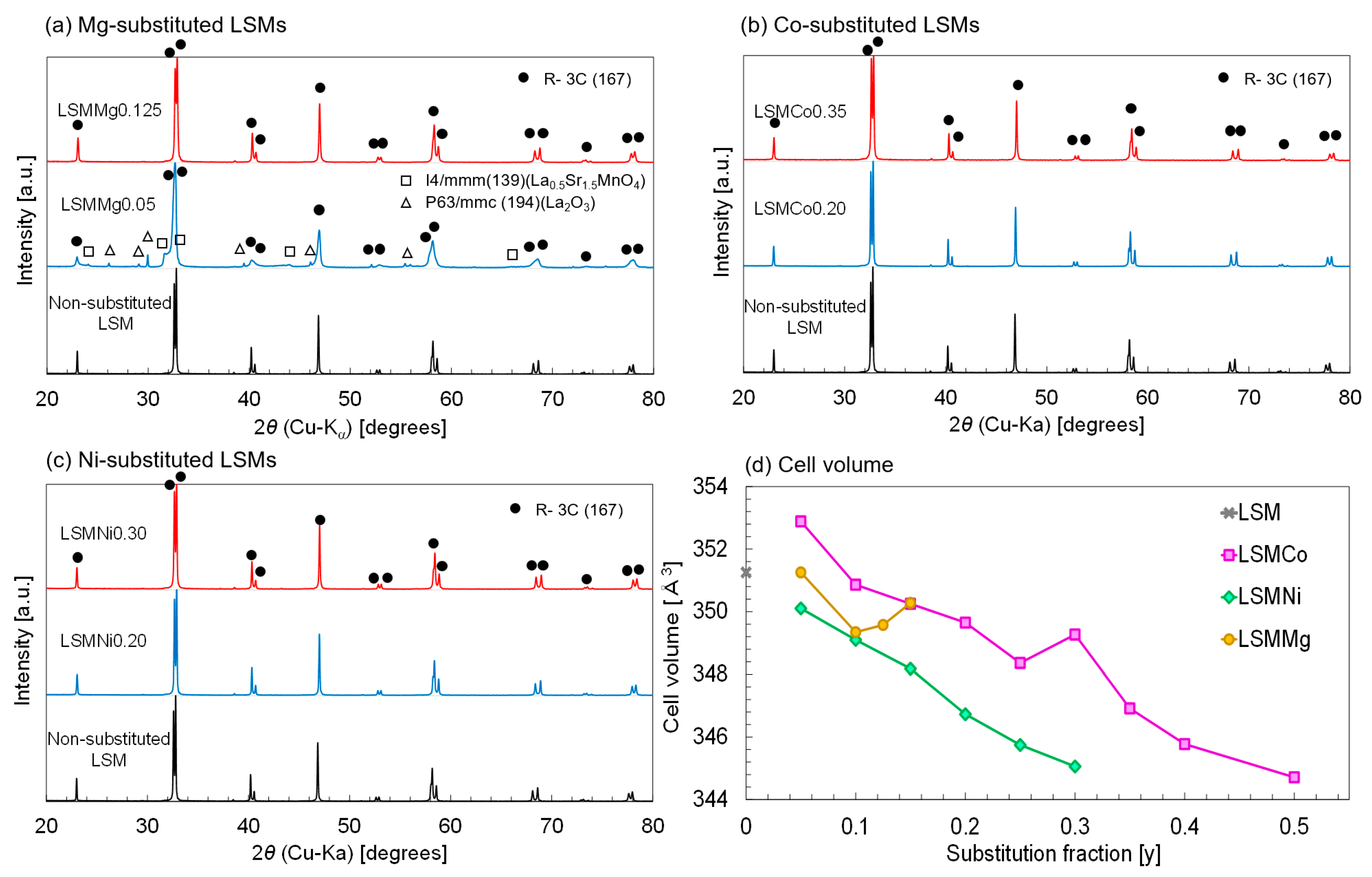
The chemical compositions, lattice parameters, space groups, and crystal systems of all prepared samples of La
1−xSr
xMn
1−yZ
yO
3 (LS
xMZ
y) are listed in
Table 2. Almost all the prepared samples comprised a single solid phase indexed to the trigonal unit cell (space group R-3c (167)), whereas the LSMMg0.05 sample was composed of three solid phases, including a main phase with space group R-3c (167).
Figure 1 shows the XRD patterns of (a) Mg-, (b) Co-, and (c) Ni-substituted LSMs at room temperature in the evaluated 2θ range. As shown in
Figure 1a, the XRD pattern of LSMMg0.125 is identical to that of the crystalline phase, and the peaks are indexed to the reference La
0.7Sr
0.3Mn
0.85Mg
0.15O
3 (ICSD code: 258933). This result indicates that Mg ions were incorporated into the B site of the crystal structure and formed a solid solution without byproducts. However, the pattern of LSMMg0.05 showed that some Mg ions were substituted at the A site of the crystal structure (La
0.67Sr
0.28Mg
0.05MnO
3 (ICSD code:254217)), resulting in the observation of the La
2O
3 phase (COD data number:2002286) and La
0.5Sr
1.5MnO
4 (COD data number:1008251). As shown in
Figure 1b,c, the patterns of the Co and Ni-substituted LSMs are in good agreement with those of the nonsubstituted LSM. These results indicate that Co and Ni ions were substituted into the B site of the crystal structure as a solid solution within the substitution fraction range. To verify the formation of a solid solution in the substituted LSM samples, the cell parameters of all prepared samples were determined by Rietveld refinement, and the cell volume of the samples was calculated based on the crystal structure.
Figure 1d shows the relationship between the estimated cell volume and the substitution fraction (
y) of La
0.7Sr
0.3Mn
1-yZ
yO
3 (Z = Mg, Ni, and Co). In the series of Ni-substituted LSMs, the cell volume decreased as the substitution fraction increased, resulting in an almost linear downward trend. One primary reason for this downtrend is the intermediate value of the ionic radius of trivalent Mn (Mn
3+:0.65 Å, six coordination by O
2− ion at the high spin) between the ionic radius of divalent and trivalent Ni (Ni
2+:0.70 Å, Ni
3+:0.60 Å, six coordination by O
2− ion in the high spin state) [
57] in the B site of the crystal structure. These results indicate that trivalent Ni ions, together with bivalent Ni ions, were incorporated into the B site of the Ni-substituted LSMs as the substitution fraction increased. Another possibility is that the average valence of the Mn ions in the B site increased as the fraction of Ni substitution increased, inducing a decrease in the cell volume. For the Co-substituted LSMs series (
Figure 1d), the cell volume of the LSMCo0.05 sample was higher than that of the nonsubstituted LSM. These results indicated that divalent Co ions (ionic radius of Co
2: 0.735 Å, six coordinated by O
2− ions in the high-spin state) [
57] were incorporated into the B site of the Co-substituted LSMs. As the fraction of Co substitution increased, the cell volume decreased in the fraction range of
y = 0.1–0.5. The resulting downtrend indicates that divalent, trivalent, or tetravalent Co ions (ionic radius of Co
3+: 0.61 Å, Co
4+: 0.53 Å, six coordination by O
2− ion in the high spin state) [
57] are incorporated into the B site instead of Mn ions. The different tendency at
y = 0.3 may be owing to the irregular change in the fraction of multivalent ions (Co
2+/Co
3/Co
4) in the LSMCo0.30 sample. For the Mg-substituted LSMs series (
Figure 1d), the cell volume of the LSMMg0.10 sample was smaller than that of the nonsubstituted LSM. Because the ionic radius of divalent Mg ions (Mg
2+: 0.720 Å, six coordination by O
2− ion) [
57] is larger than that of Mn
3+ ions, the average valence of the Mn ions at the B site increases at
y = 0.10. When the substitution fraction in the Mg-substituted LSM series (
y = 0.10–0.15) was increased, the cell volume monotonically increased. The results indicate that the average valence of the Mn ions decreased at the B site of the Mg-substituted LSMs. Therefore, maintaining charge valence in the crystal structure is difficult with Mg substitution at
y > 0.15. These results indicate that all the samples, except LSMMg0.05, were successfully prepared as a solid solution of the single-crystalline phase of La
0.7Sr
0.3Mn
1−yZ
yO
3 without impurities.
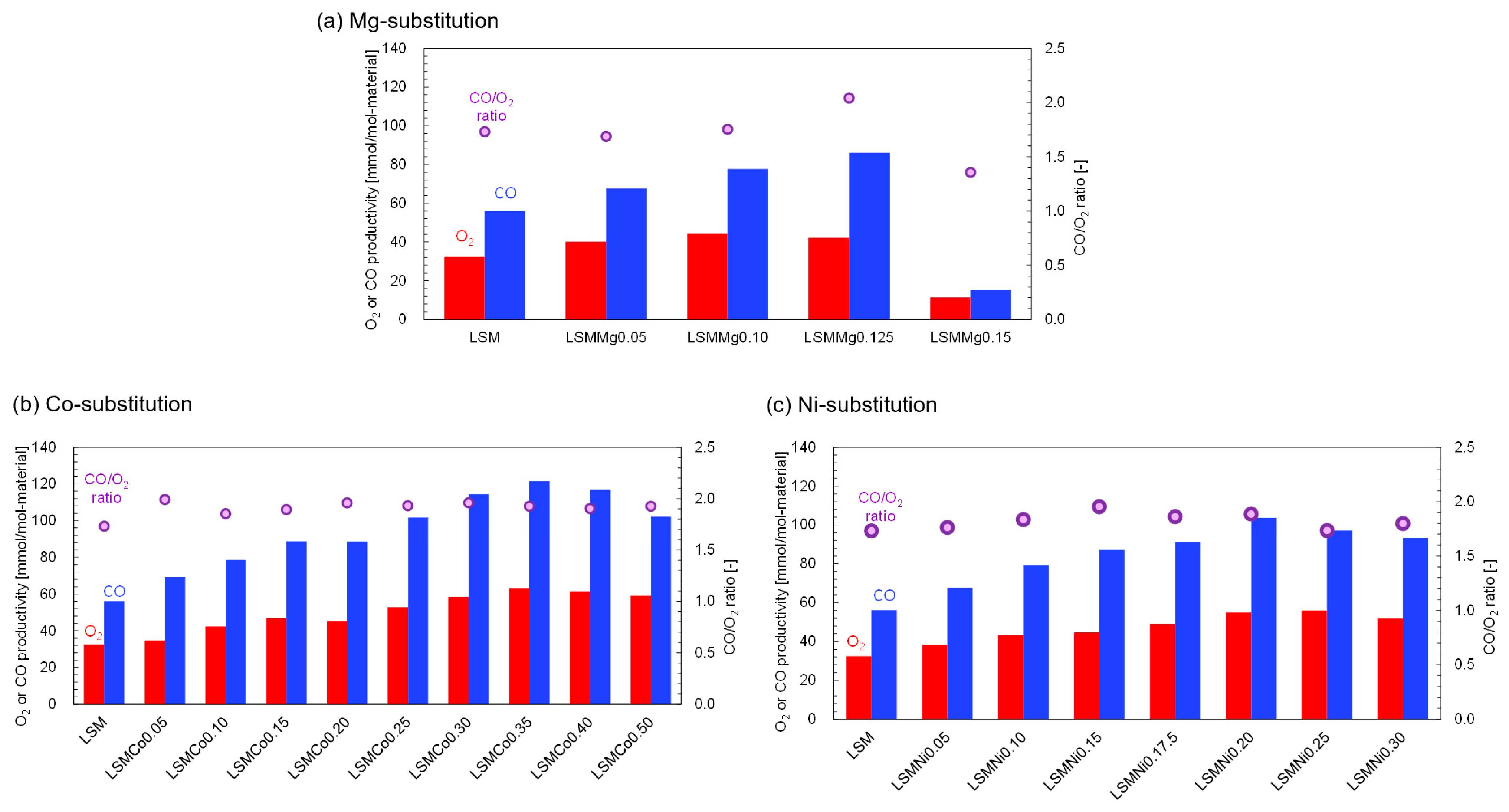
Figure 2 shows the average O
2 and CO productivities of Ni-, Co-, and Mg-substituted LSMs. Each productivity value was estimated based on the extent of the weight change. The average productivity was calculated from the second and third runs of the two-step thermochemical CO
2 splitting cycling. The value from the first run was excluded from the calculation because the data may have been affected by the sample preparation process. For comparison, nonsubstituted LSM was also evaluated to verify the impact of partial substitution at the B site. As shown in
Figure 2a, the O
2 productivity of the Mg-substituted LSMs increased with increasing substitution fraction (
y) and reached a maximum value of 44.3 mmol/mol-material at
y = 0.10 (LSMMg0.10). The CO productivity also increased with the substitution fraction and was maximal (86.1 mmol/mol-material) at
y = 0.125 (LSMMg0.125). These results indicate that the partial substitution of Mg at the B site of LSM enhances the O
2 and CO productivities. The CO/O
2 (CO/O
2 = 2.04) value at
y = 0.125 is approximately equal to the stoichiometric ratio. The results in
Figure 2a indicate that, from the viewpoint of productivity, LSMMg0.125 can provide stable and reproducible O
2 and CO production with maximal redox performance. The results obtained for the Co-substituted LSM perovskites are shown in
Figure 2b. Maximum O
2 and CO productivities were obtained at
y = 0.350 and CO/O
2 = 1.93. For the Ni-substituted LSM perovskites (
Figure 2c), the O
2 and CO productivities were maximized at
y = 0.200, with CO/O
2 = 1.89. These results indicate that the partial substitution of the B site for all ion species (Mg, Co, and Ni) improved the O
2 and CO productivities while maintaining stoichiometric reactivity. The most suitable chemical composition depends on the substitution species used.
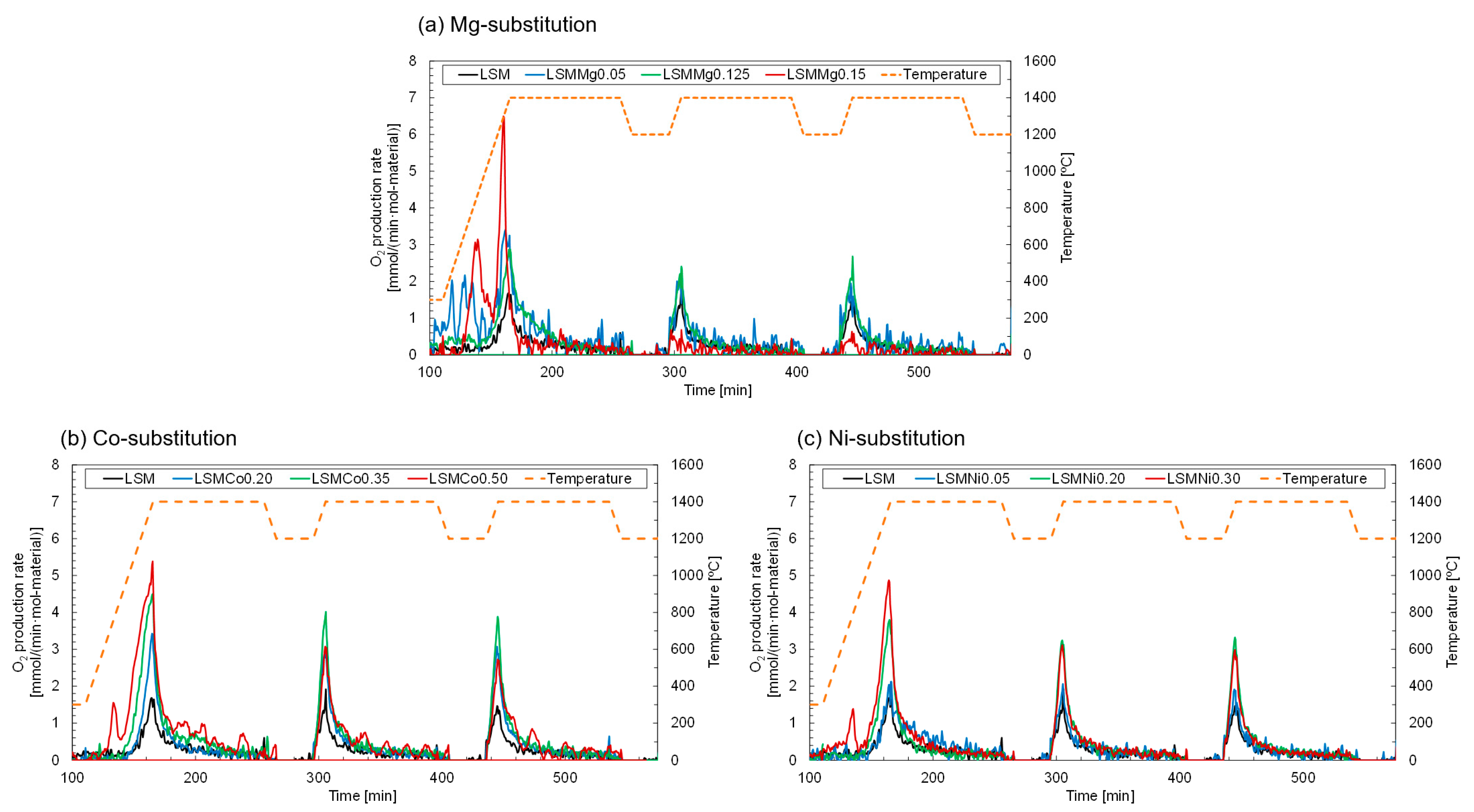
Figure 3 shows the O
2 production profiles of the Ni, Co, and Mg-substituted LSMs during the TR step in the first to third runs. As a reference, the results obtained with the non-substituted LSM are shown in the figure. In all runs, the production rate of O
2 reached a maximum value when the sample was heated at a temperature of 1400 °C.
Figure 3a shows that LSMMg0.125 (green curve) afforded stable and repeatable O
2 production without degradation during thermochemical cycling, unlike the other Mg-substituted samples. The O
2 production of LSMMg0.150 (red curve) was poor and lower than that of the nonsubstituted LSM.
Figure 3b shows the O
2 production profiles of LSMCo0.20 (blue curve), LSMCo0.35 (green curve), and LSMCo0.50 (red curve). LSMCo0.35 exhibited the most stable and highest O
2 production among the Co-substituted LSMs, whereas, with LSMCo0.50, the peak rate of O
2 production declined with an increase in the number of runs of thermochemical cycling. The O
2 production profiles of the Ni-substituted LSM are shown in
Figure 3c. LSMNi 0.20 (green curve) demonstrated a stable and reproducible O
2 production profile throughout the second and third runs and exhibited the highest peak rates of O
2 production.
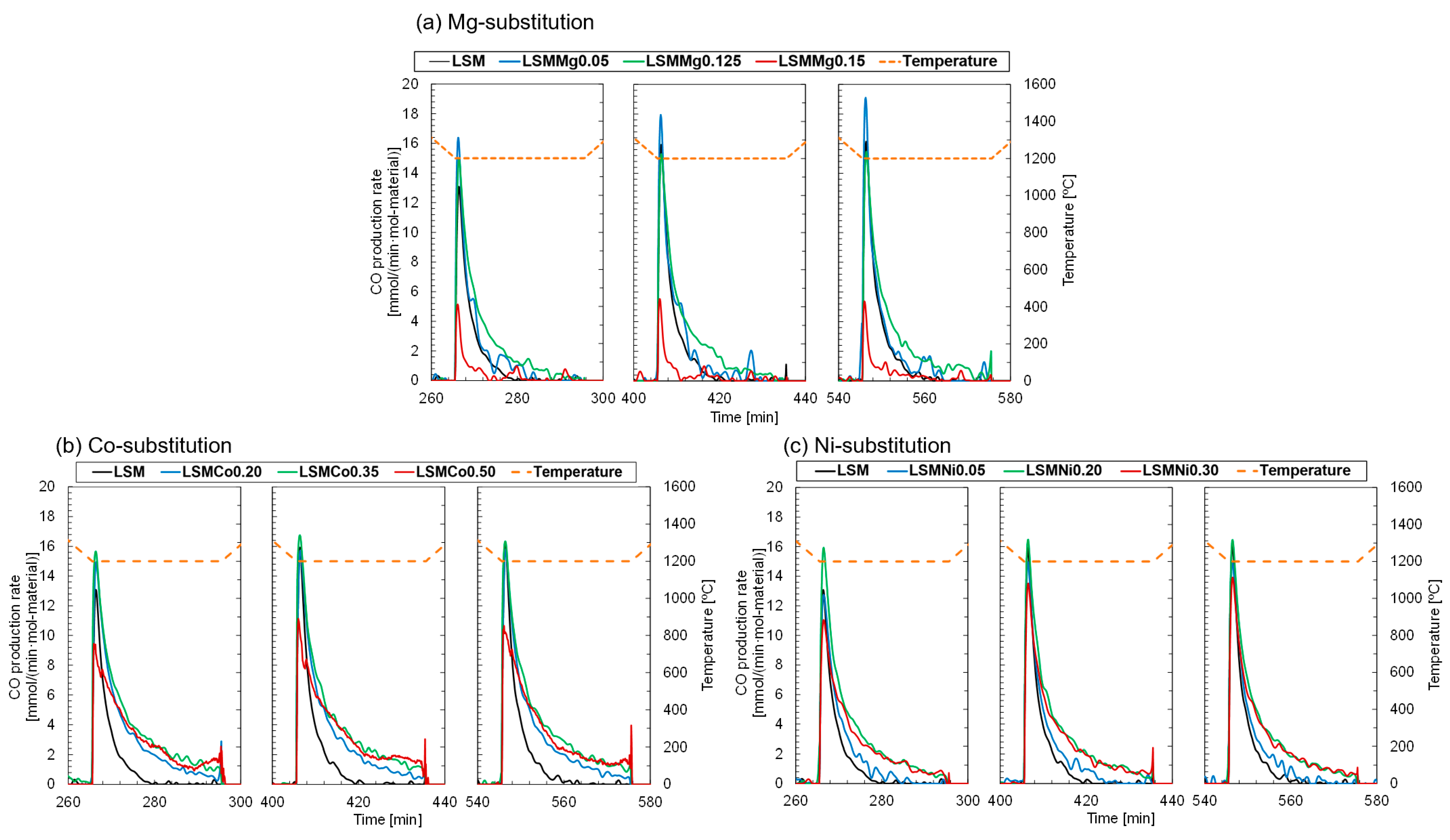
Figure 4a shows the CO production profiles of the Mg-substituted LSMs series during the subsequent CS steps in the first to third runs. For all the Mg-substituted samples, CO production was immediately initiated at the target temperature under a gas mixture of N
2 and CO
2 flow. The peak rate of CO production for LSMMg0.05 was higher in all runs compared to that of the nonsubstituted LSM but decreased for LSMMg0.15. The results for the Co-substituted LSMs series are shown in
Figure 4b. The peak rate of CO production for LSMCo0.35 reached the maximum value but decreased for the samples with 40–50% substitution. One main reason for the improvement in the CO productivity with Co-substitution (
Figure 2b) is that the duration of CO production is extended for all substitution levels compared to the case with nonsubstitution. For the Ni-substituted LSMs series (
Figure 4c), the peak CO production rate was higher with 5–20% substitution but decreased with 25–35% substitution. Prolonged duration of CO production was observed for all the Ni-substituted LSMs series. These results indicate that the Co- and Ni-substituted LSMs demonstrate higher O
2 and CO productivities and superior gas releasing profiles compared to the nonsubstituted LSM. Notably, from the viewpoint of the release behavior and productivity of O
2 and CO in the thermochemical cycle, the LSMCo0.35 and LSMNi0.20 samples are promising materials among the substituted LSMs series investigated in this study.
The superior CO release behavior of Co- and Ni-substituted LSMs enables an increase in solar-to-fuel efficiency under the same reaction conditions due to the enhancement of cycle-averaged and time-averaged CO productivity. As another approach for improvement in solar-to-fuel efficiency, a reticulated porous ceramic structure [
23] or nanostructuring methods [
24] with higher specific surface area were investigated in the literature.
3.2. Repeatability and Thermal Durability of LSMCo0.35 and LSMNi0.20 in Continuous Multiple Cycling of Thermochemical Two-Step CO2 Splitting
In this section, the repeatability and thermal durability of the promising LSMCo0.35 and LSMNi0.20 samples are examined and evaluated.
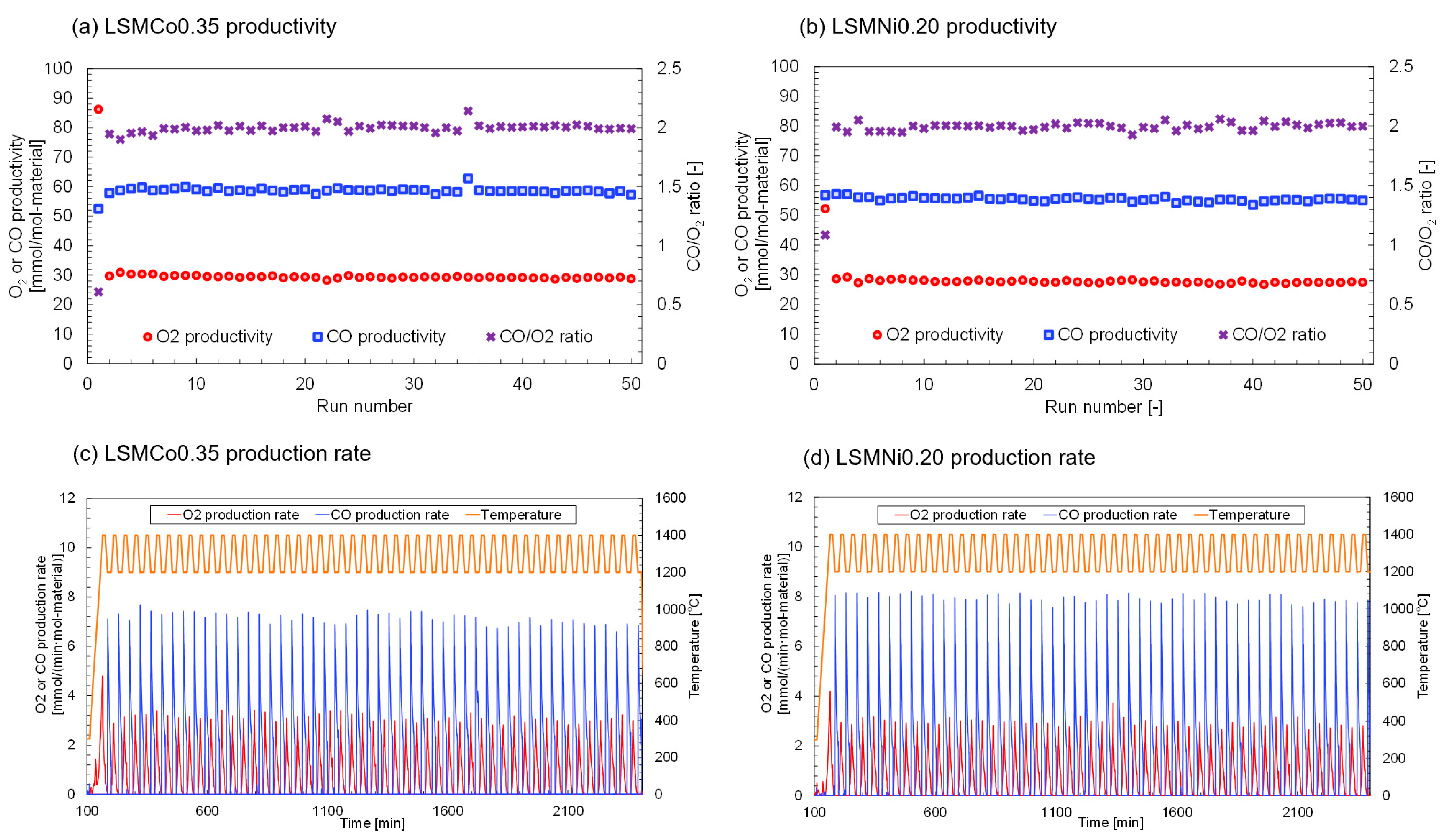
Figure 5 shows the O
2 and CO productivities in the long-term continuous test for the thermochemical two-step CO
2 splitting cycling using LSMCo0.35 and LSMNi0.20. To monitor and evaluate the stoichiometric reactivity of the samples in each run, the CO/O
2 ratio was calculated and plotted in the figure. The results of the first run may include nonreproducible effects of the sample and reactor preparations (adsorption of moisture and gaseous species on the surface of the sample and crucible in the thermogravimetric reactor, and volatile/combustible contaminations). Thus, the results of the second–fiftieth runs were evaluated to estimate thermal durability and stability. As shown in
Figure 5a, the decline fraction of CO productivity during the continuous thermochemical cycling was 1.0%. Thus, O
2 and CO production were maintained at the same level without degradation, significant effusion, or vaporization of the sample over 50 continuous runs. These results indicate that LSMCo0.35 is thermally durable during long-term thermochemical cycling at high temperatures and undergoes reproducible redox reactions. In the case of LSMNi0.20 (
Figure 5b), the decline fraction of CO productivity was estimated to be 3.7%. The LSMNi0.20 and LSMCo0.35 samples showed stable and reproducible O
2 and CO productivity over multiple continuous cycles while maintaining stoichiometric reactivity. The production rates of O
2 and CO were determined for the LSMCo0.35 (
Figure 5c) and LSMNi0.20 (
Figure 5d) samples. Both the samples exhibited stable and reproducible O
2 and CO release profiles during continuous thermochemical cycling. The crystallographic phases of LSMCo0.35 and LSMNi0.20 samples were observed and compared to analyze the crystal structure and morphology.
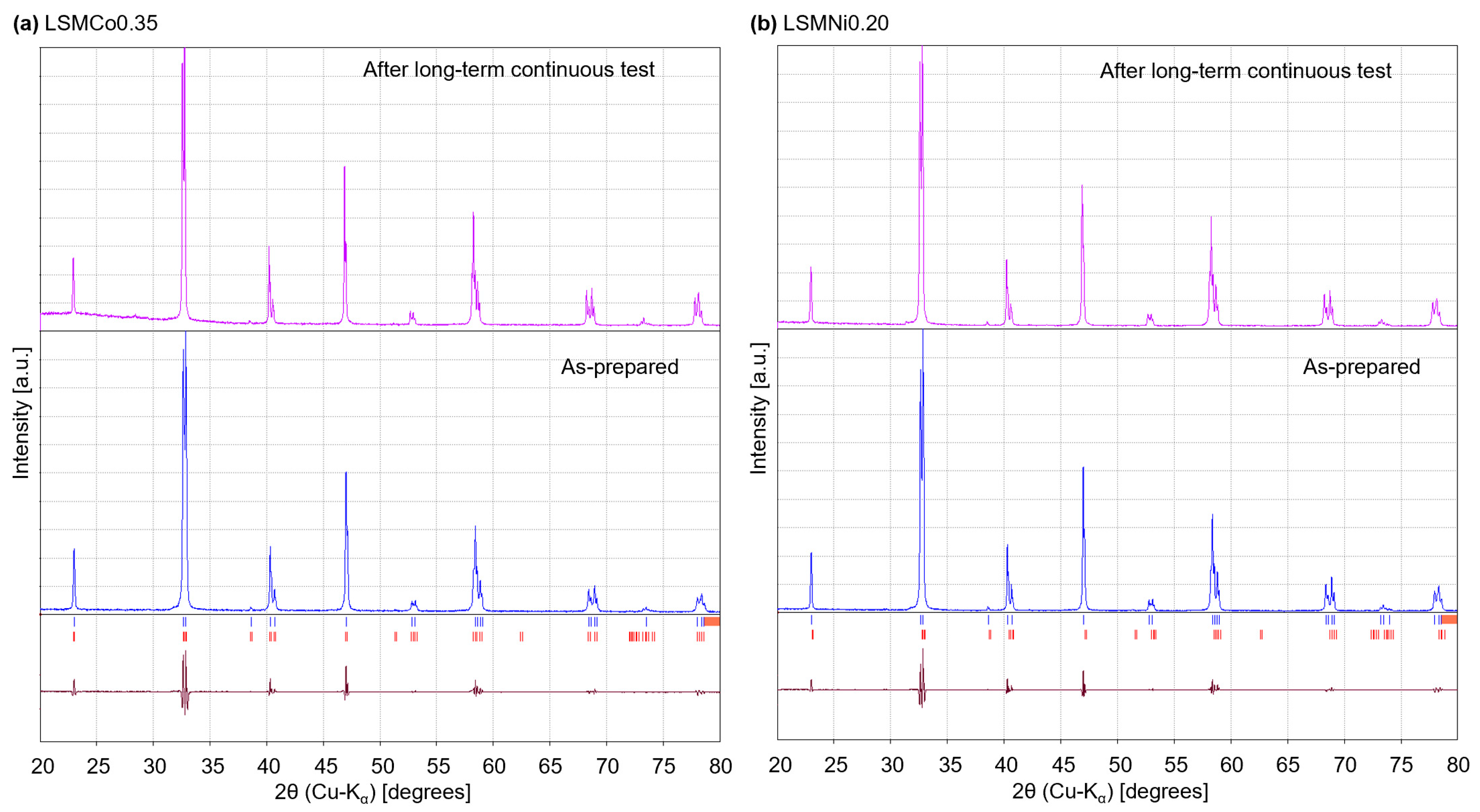
Figure 6 shows the XRD patterns of the samples obtained before and after continuous testing. The peak positions of the sample after the test were the same as those of the as-prepared sample and corresponded to the same crystalline phases (red vertical bars below the XRD pattern). No secondary phases or phase separation were observed in either pattern, indicating that the LSMCo0.35 and LSMNi0.20 samples underwent repeatable and thermally durable thermochemical cycling without crystal structure degradation.
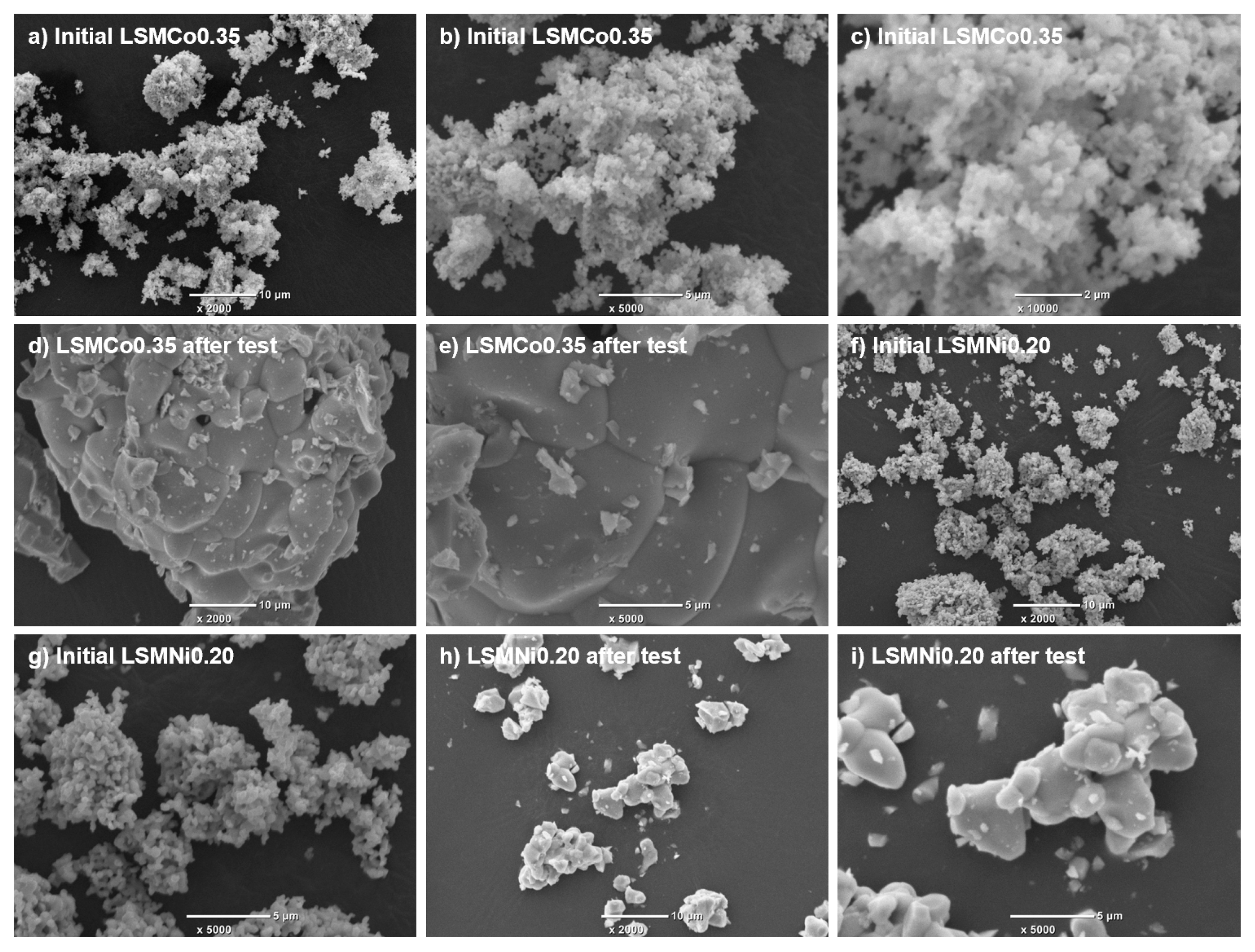
Figure 7 shows secondary electron image (SEI) micrographs of LSMCo0.35 (
Figure 7a–e) and LSMNi0.20 (
Figure 7f–i). For the as-prepared LSMCo0.35 sample (
Figure 7a–c), the particle size was 0.1–0.2 µm, and agglomerations of the non-porous fine particles appeared. After the long-term test (
Figure 7d,e), a coagulated mass composed of coarse particles with sizes of 2–10 µm was observed. Thus, the as-prepared samples were severely sintered at high temperatures during the continuous testing. For the as-prepared LSMNi0.20 sample (
Figure 7f,g), the particle size was 0.2–0.5 µm, and agglomerations of particles were apparent. After the long-term test (
Figure 7h,i), the observed coagulated mass was smaller than that of LSMCo0.35. These results indicate that Ni substitution may reduce the agglomeration of the LSM particles subjected to high temperatures during continuous testing. Both LSMCo0.35 and LSMNi0.20 were sintered at high temperatures during the continuous testing. However, the decline fractions remained at low values, and a multivalence state of Co and Ni existed in the crystal lattice of LSMCo0.35 and LSMNi0.20. Thus, it may be caused by the enhancement of oxygen transfer in the crystal lattice by Co and Ni substitution.
3.3. Impact of La/Sr Substitution Fraction in a Site on the Redox Activity of Substituted LSM
In this section, we evaluate the impact of the La/Sr substitution fraction (x) in the A site of the substituted LSM on the O
2 and CO productivities during thermochemical cycling. LSMNi0.20, in which the chemical composition of the B site was fixed, was selected as the representative LSM sample. The A-site-substituted samples (La
1−xSr
xMn
0.8Ni
0.2O
3) were subjected to the TR step at 1400 °C for 90 min. Subsequently, the CS step was performed at 1000 °C for 30 min.
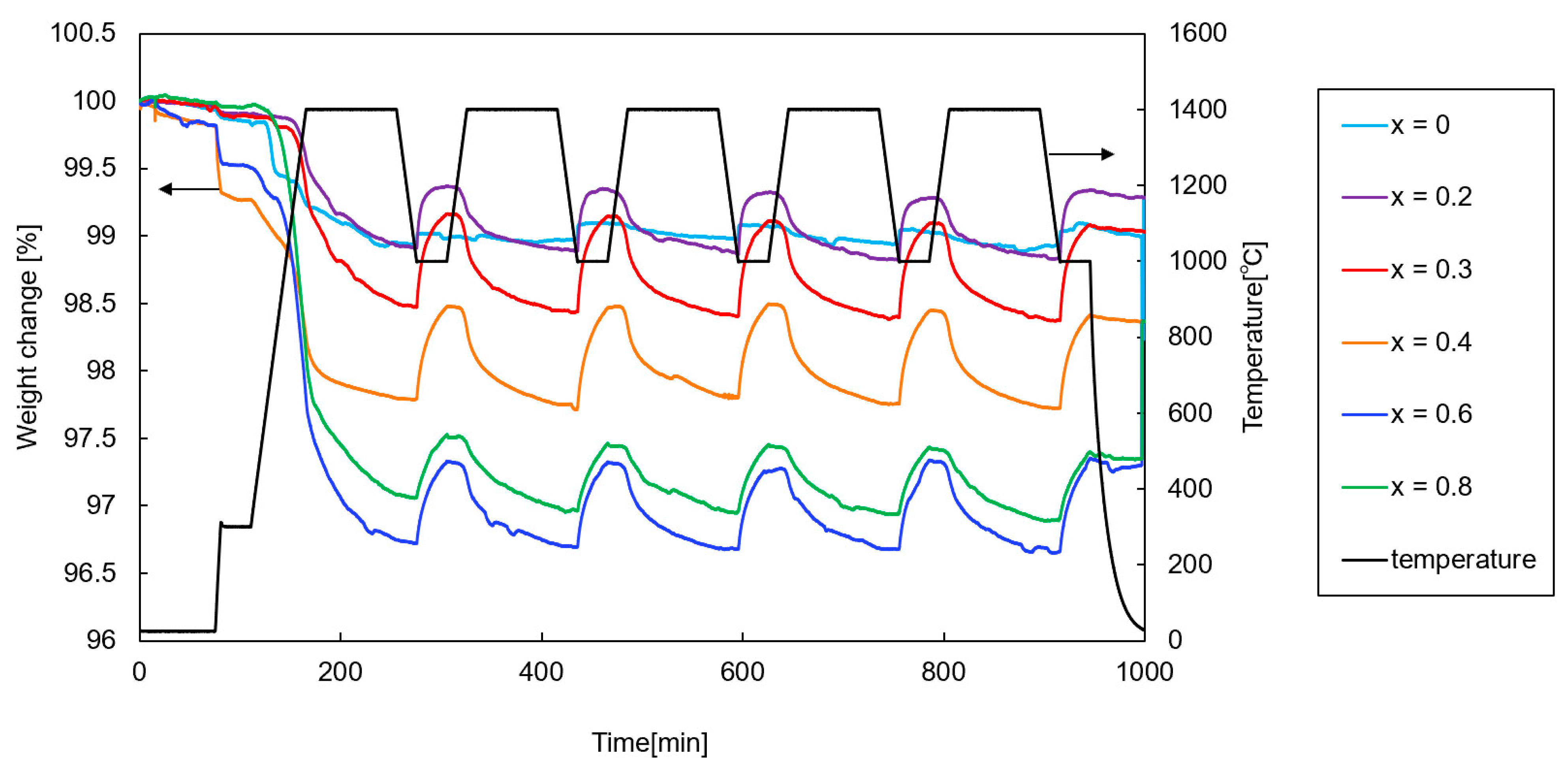
Figure 8 shows the weight change profiles of the samples (x = 0–0.8) during the thermochemical cycling (first–fifth runs). Except for the first run, repeatable and reproducible profiles were observed for all the samples.
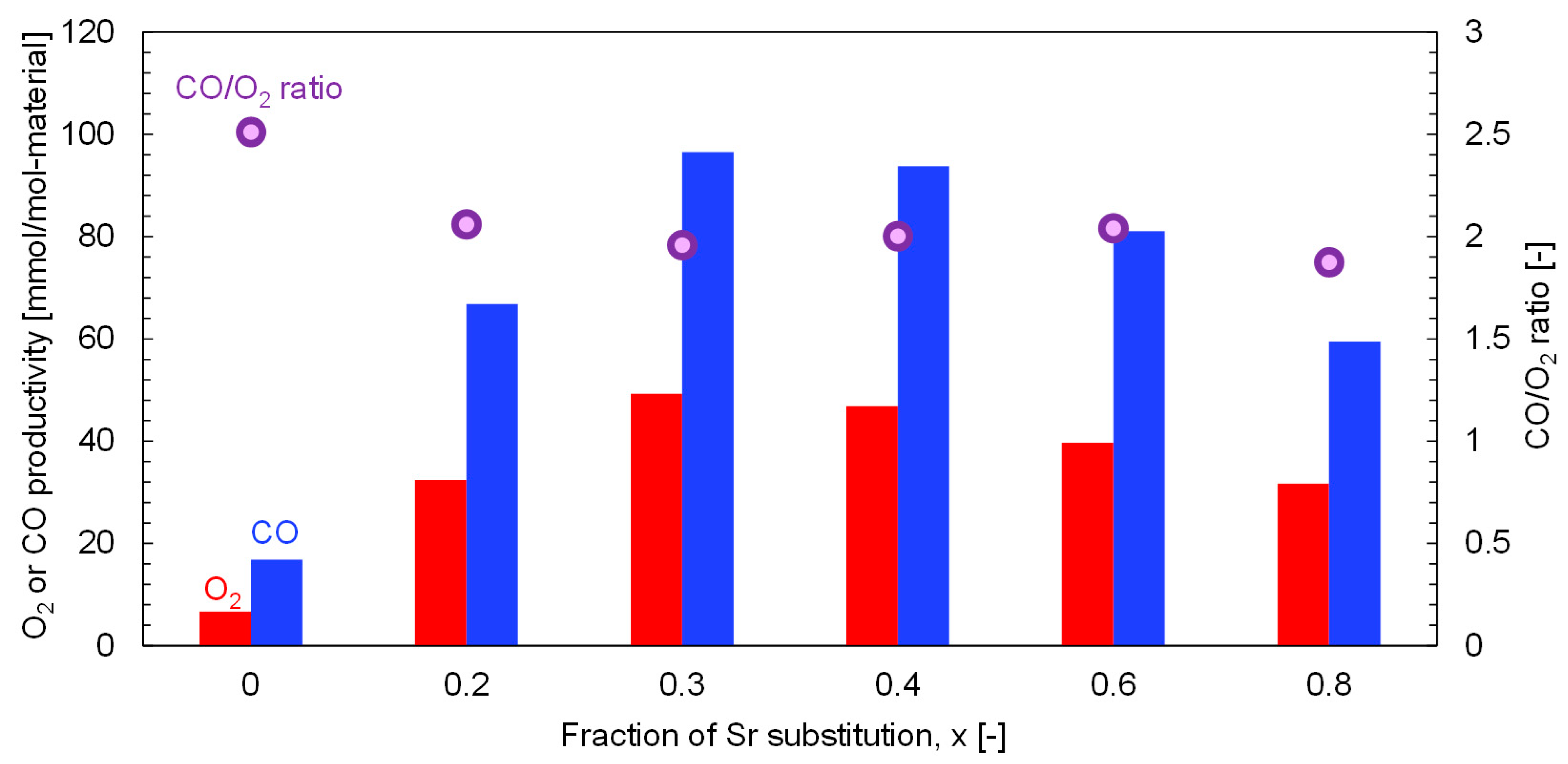
Figure 9 shows the O
2 and CO productivities and the CO/O
2 ratios for various x values. The O
2 and CO productivities reached their highest values of 49.3 and 96.5 mmol/mol-material at x = 0.3. In addition, the O
2/CO ratio of 1.95 at x = 0.3 means that the two-step thermochemical CO
2 splitting proceeds stoichiometrically, retaining the greatest productivity among all x fractions. To optimize the chemical composition of the A site and maximize the redox reactivity, La
0.7Sr
0.3Mn
0.8Ni
0.2O
3 is a feasible chemical composition for the thermochemical two-step CO
2 splitting cycle.
The impact of the La/Sr fraction on the O
2 and CO productivities of the Ni-substituted LSM indicates the following two possibilities: (1) an initial increase in the substitution fraction (x = 0–0.4) leads to an increase in the number of lattice defects (vacancies) in the crystal structure. The results indicated that the O
2 productivity was enhanced owing to the improved oxygen mobility in the crystal structure at high temperatures in both steps. However, (2) not all vacancies induced by Sr substitution are available for the redox reaction in the thermochemical cycle (x = 0.4–0.8). The XRD patterns of the as-prepared samples (La
1−xSr
xMn
0.8Ni
0.2O
3) are shown in
Figures S1–S6 in the Supplementary Information. The space group and crystal structure of the identified phases are presented in
Table 3. The experimental findings revealed that the crystal structure (cubic system) of the primary phase in samples with a high degree of substitution (x = 0.6 and 0.8) was different from that of a trigonal system with less substitution (x = 0–0.4). This result indicates that the excess vacancies are accommodated in the trigonal system as x increases (x = 0–0.4). However, the phase transition stabilizes the crystal structure by accommodating excess vacancies when the value of x increases further (x = 0.6–0.8). Consequently, the acceptable number of vacancies in the cubic system may decrease compared to that in the trigonal system. The space groups and crystal structures of the samples obtained after the redox tests are listed in
Table 4. Phase separation was observed at chemical compositions of x = 0.6 and 0.8. These results indicated that they were thermally or chemically unstable during thermochemical cycling. The threshold value of a stable crystal structure without phase transition and separation before and after thermochemical cycling was approximately x = 0.5.
3.4. XPS Analysis of Nonsubstituted-, Ni-, and Co-Substituted LSMs
The changes in the valence states of the constituent ionic species in the substituted LSMs were analyzed and evaluated to elucidate the functions of each ionic species in each step of the two-step thermochemical CO
2 splitting. LSMNi0.20 and LSMCo0.35 were selected as the samples with the highest reactivity among the substituted LSMs series tested in the present study. Nonsubstituted LSM was analyzed and compared.
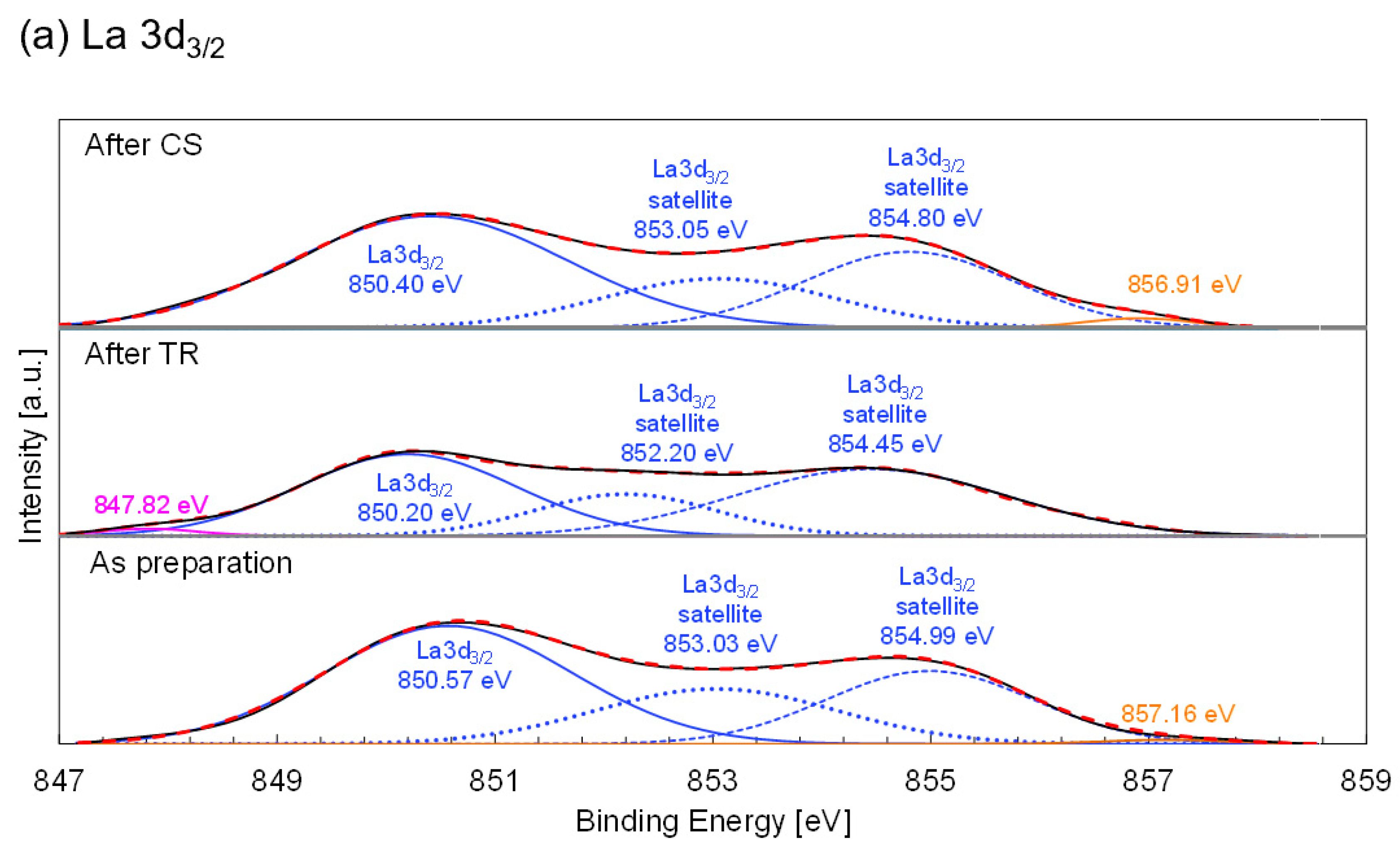
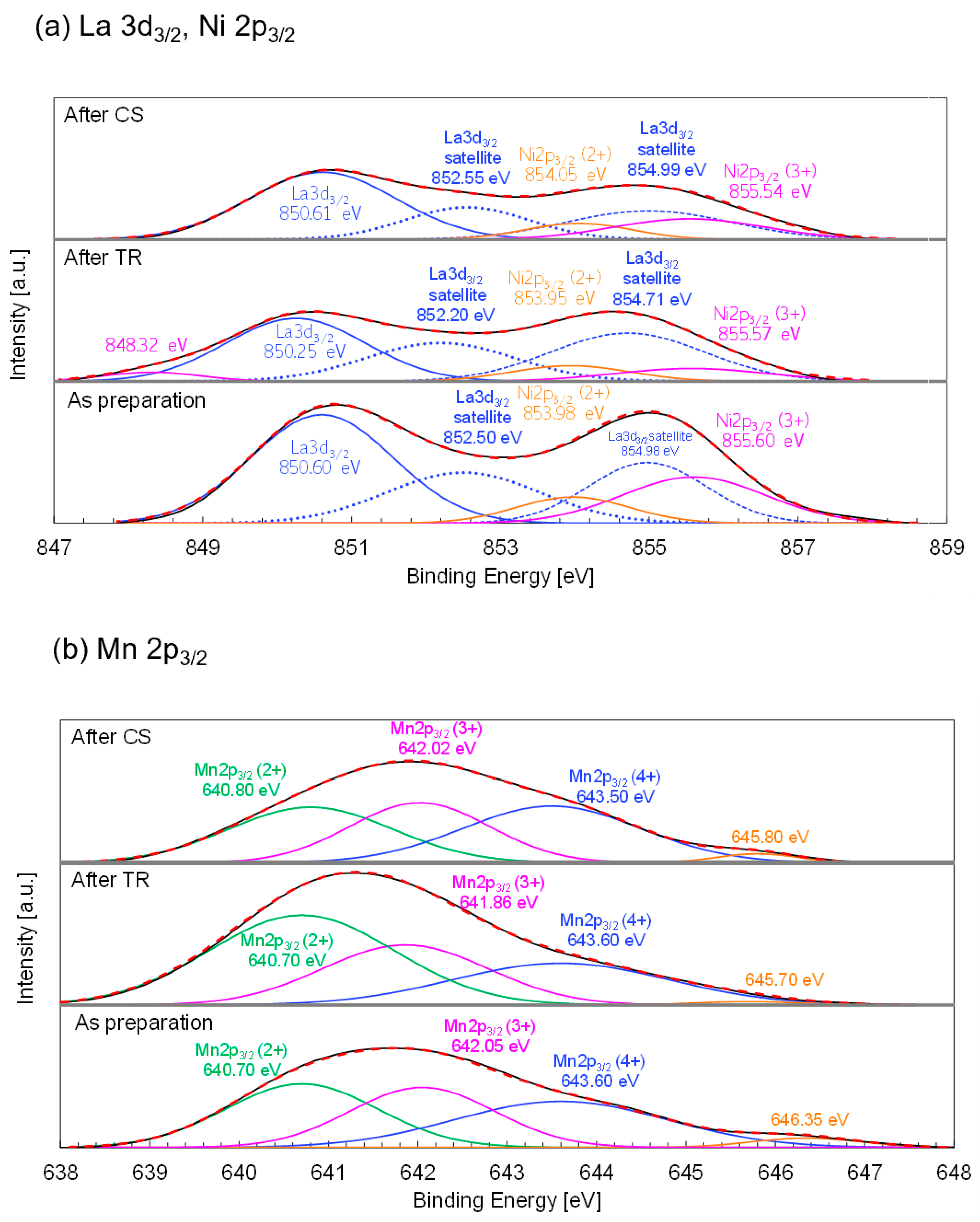
Figure 10a shows the La 3d
3/2 X-ray photoelectron spectra with peak deconvolutions for nonsubstituted LSM. Triplet peaks centered at 850.57, 853.03, and 854.99 eV are observed for the as-prepared sample. No evident changes in the peak positions and areas were observed for the samples obtained after the TR and subsequent CS steps in the two-step thermochemical cycle. The results indicate that the La valence in the nonsubstituted LSM did not vary at each step of the thermochemical cycle.
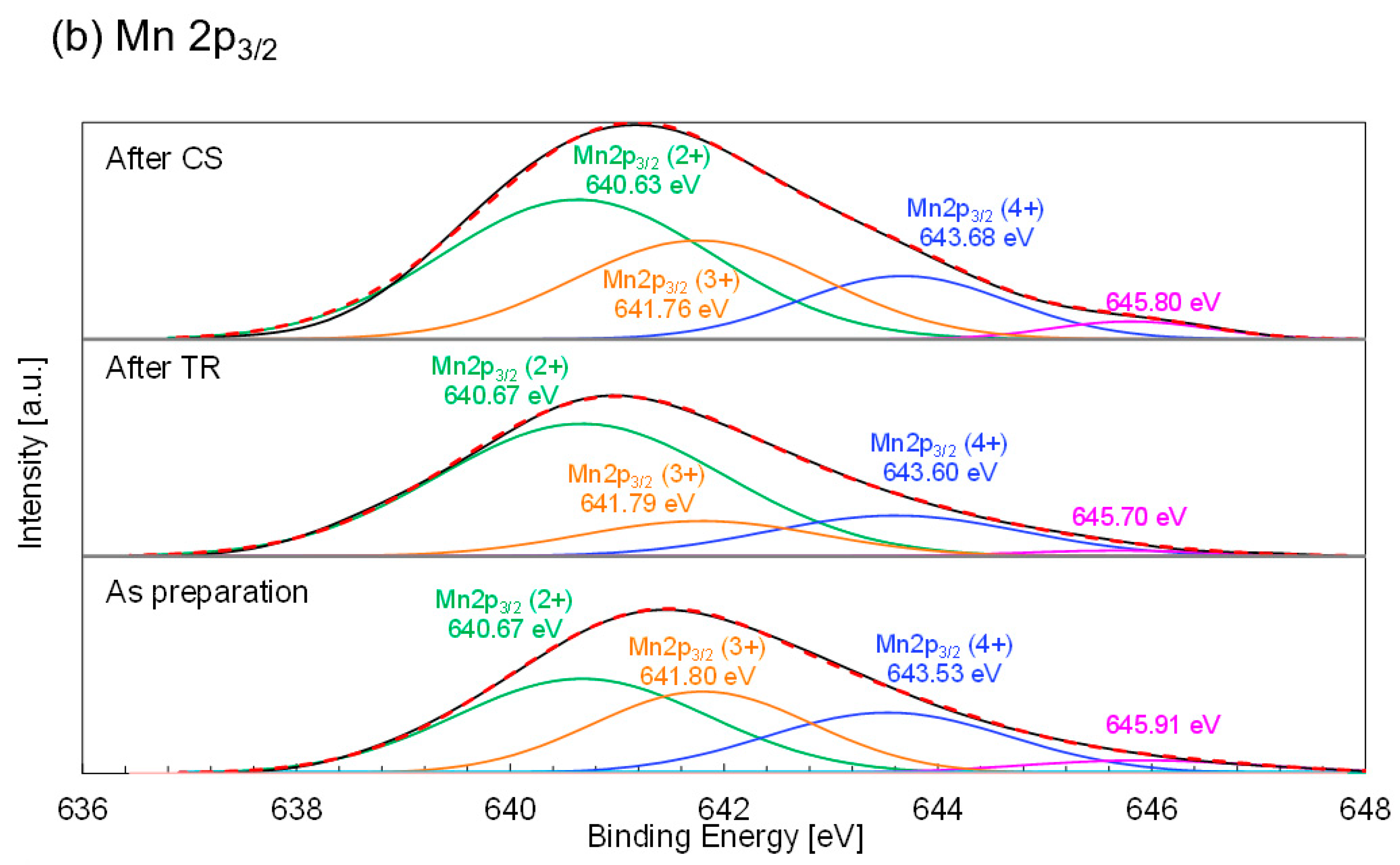
Figure 10b shows the Mn 2p
3/2 spectrum with peak deconvolution for nonsubstituted LSM. For the as-prepared sample, the Mn 2p
3/2 spectrum was decomposed into triplet peaks (Mn
2+ = 640.67 eV, Mn
3+ = 641.80 eV, and Mn
4+ 643.53 eV). The average Mn valences were estimated from the peak areas corresponding to the Mn
2+/Mn
3+/Mn
4+ species. The estimated average valences of Mn in the nonsubstituted LSM are listed in
Table 5. The variation in the average valence of Mn indicates that the Mn ions at the B site of the nonsubstituted LSM are responsible for the redox reaction in the thermochemical cycle.
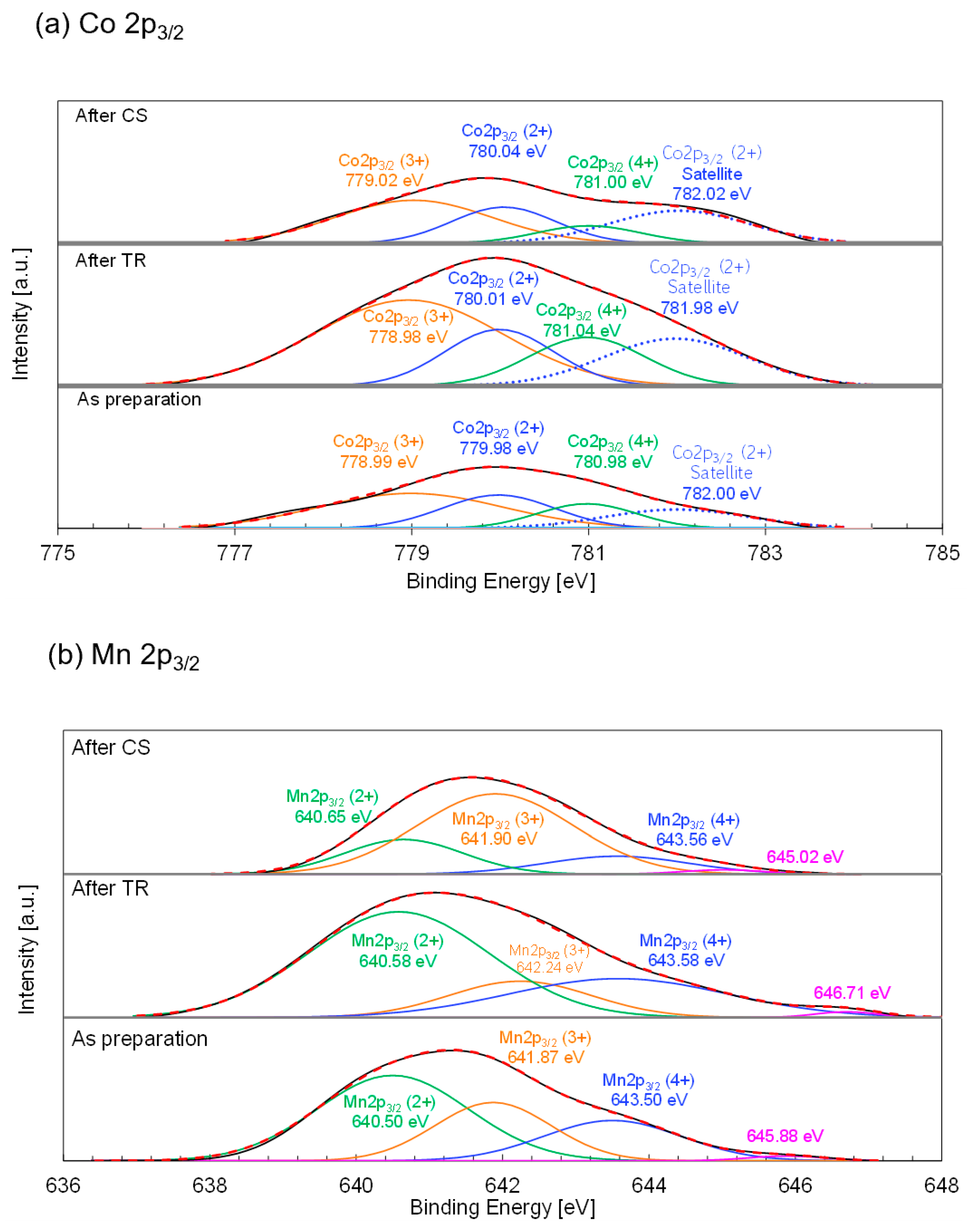
The deconvoluted Co 2p
3/2 and Mn 2p
3/2 XPS profiles of the LSMCo0.35 sample are shown in
Figure 11. The Co 2p
3/2 XPS profile of the as-prepared sample fitted the quadruplet peaks centered at 778.99, 779.98, 780.98, and 782.00 eV, respectively (
Figure 11a). Each peak centered at 778.99 and 779.98 eV corresponded to the Co
3+ and Co
2+ signal, respectively. According to the literature [
58], the XPS profile of Co
3O
4 shows a peak centered at 779.6 eV, assigned to trivalent cobalt, whereas the peaks centered at 780.1 (strongest), 782.1, 785.5, and 786.5 eV were attributed to bivalent cobalt. The XPS results of LiCoO
2 showed a peak centered at 779.6 eV in the Co 2p
3/2 spectrum, attributed to trivalent cobalt. This value agrees with the previously reported data [
59], in which the peak centered at 781 eV in the Co 2p
3/2 spectrum of Li
0.66CoO
2 was attributed to tetravalent cobalt. This value corresponds to the current results (tetravalent cobalt). Finally, it was proposed that the other peak-centered redox reaction occurred in LSMCo0.35 during the thermochemical two-step process. In contrast, 782.00 eV corresponded to the satellite peak of the Co
2+ signal.
Figure 11b shows the deconvoluted Mn 2p
3/2 XPS profile of LSMCo0.35. The triplet peaks at 640.50, 641.87, and 643.50 eV for the as-prepared sample were attributed to Mn
2+, Mn
3+, and Mn
4+, respectively. The binding energy peaks of the as-prepared samples agreed with those reported in the literature [
60,
61,
62]. The valence states of the Mn and Co ions in the LSMCo0.35 sample were calculated and estimated from the peak positions and areas of the signals for the Mn and Co ions. As shown in
Table 6, the valence states of Mn decreased after the TR step, indicating the release of oxygen from the sample, and increased after the subsequent CS step owing to oxygen absorption via CO
2 splitting. The Mn 2p
3/2 XPS data indicate that the valence states of the Mn ions contributed to the redox reaction in LSMCo0.35 during the thermochemical two-step process. In contrast to the variation in the valence of Mn, the valence states of Co (
Table 7) either did not change in either step or changed negligibly, the change having no relation to the redox reaction. These results indicate that the Co ions at the B site of the LSMCo0.35 sample may promote the redox reaction of Mn ions in the thermochemical cycle. Compared to the valence change of the Mn ions in the nonsubstituted sample, the valence change increased in the LSMCo0.35 sample. The result of the LSMCo0.35 sample agreed well with the result of the 10%Mg-substituted sample without a valence change in Mg in a previous study [
53].
Figure 12a shows the deconvoluted La 3d
3/2 and Ni 2P
3/2 X-ray photoelectron spectra of the as-prepared LSMNi0.20 after TR and after subsequent CS steps in the thermochemical cycle. For the as-prepared LSMNi0.20, the triplet peaks centered at 850.60, 852.50, and 854.98 eV can be assigned to the La 3d
3/2 main and satellite peaks. Triplet La 3d
3/2 peaks have previously been reported for LaFe
xO
3-δ (0.7 ≤ x ≤ 1.3) perovskites [
63]. According to the literature, the main peak in the La 3d
3/2 spectrum appears at a binding energy of ~850 eV, whereas satellite peaks are present between 850 and 855 eV. These results are in agreement with those of the current study. Thus, the La 3d
3/2 XPS data indicate that lanthanum ions were present in trivalent form in the as-prepared LSMNi0.20. The binding energies of the main and satellite peaks in the La 3d
3/2 region were not significantly different for the as-prepared LSMNi0.20 and samples obtained after the TR and subsequent CS steps. These results indicate that the valence state of La did not change during either step of the thermochemical cycle. For the double perovskite Nd
2NiMnO
6, the Ni 2p XPS profile shows the most prominent peak in the vicinity of 850–860 eV [
64]. The Ni 2p
3/2 peak appeared at lower binding energies and higher intensities than the Ni 2p
1/2 peak [
65]. The Ni 2p
3/2 peaks for the La
0.8Sr
0.2Mn
1−xNi
xO
3 nanoparticles were observed together with the La 3d
3/2 peaks, and the former were separated into doublet peaks centered at ~854 and 855.5 eV. The low and high binding energies correspond to the divalent and trivalent ions. The peak position of the doublet did not change with Ni substitution levels of 20% or 40% [
66]. In the case of the porous Ni (II) hydroxide nanosheets supported on carbon paper [
65], doublet peaks due to divalent and trivalent Ni appeared at low and high binding energies, respectively, in the Ni 2p
3/2 spectra. As shown in
Figure 12a, the Ni 2p
3/2 XPS profile of the as-prepared LSMNi0.20 shows doublet peaks centered at 853.98 and 855.60 eV. The low and high binding energies correspond to the divalent and trivalent ions. These peak positions are in good agreement with those reported previously [
66]. The results in
Figure 12a indicate that the as-prepared LSMNi0.20 contained trivalent and divalent Ni ionic species at the B site of the perovskite. For the samples obtained after the TR and subsequent CS steps, negligible differences (853.95–854.05 eV for Ni
2 and 855.54–855.60 eV for Ni
3+) in the binding energies were observed. The corresponding Ni valences were calculated from the peak areas of the Ni
2+ and Ni
3+ curves. The results are summarized in
Table 8. The estimated average values of Ni valence were 2.70, 2.51, and 2.40 for the as-prepared sample and samples obtained after the TR and CS steps. The variations in the Ni valences did not change for either step or remained limited without being related to the redox reaction.
Figure 12b shows the Mn 2p
3/2 spectra with peak deconvolutions of the as-prepared samples and those obtained after each step. The peak positions obtained for the as-prepared samples were within the range of previously reported peak position variations and were in good agreement with the data for the La
0.8Sr
0.2Mn
1-xNi
xO
3 perovskite [
66] with similar crystal structures and chemical compositions. The positions and areas of the peaks corresponding to the Mn
2+/Mn
3+/Mn
4+ species are summarized in
Table 9. The corresponding Mn valences were estimated based on the peak areas. The estimated Mn
2+, Mn
3+, and Mn
4+ fractions for the as-prepared samples were 34.0%, 31.7%, and 34.3%, respectively, and the average Mn valence was identical to the stoichiometric value of 3.00+. As shown in
Table 9, the average Mn valences of the samples obtained after the TR and CS steps were 2.73+ and 3.06+, respectively. The variations in Mn valences can be used to describe the redox reaction in two-step thermochemical cycling. Notably, the average Mn valence returns to its initial value, suggesting that the sample undergoes continuous stoichiometric cycling without degradation.
3.5. Comparison of Mg, Ni, and Co-Substituted LSMs with Previous Literature
The CO
2-to-CO conversions and CO productivities of LSMNi0.20, LSMCo0.35, and LSMMg0.125 were compared with those of cerium oxide and previously reported congeners. The weight change profiles of cerium oxide during thermochemical cycling are shown in
Figure S7 of the Supplementary Information. The O
2 and CO productivities are summarized in
Table S1.
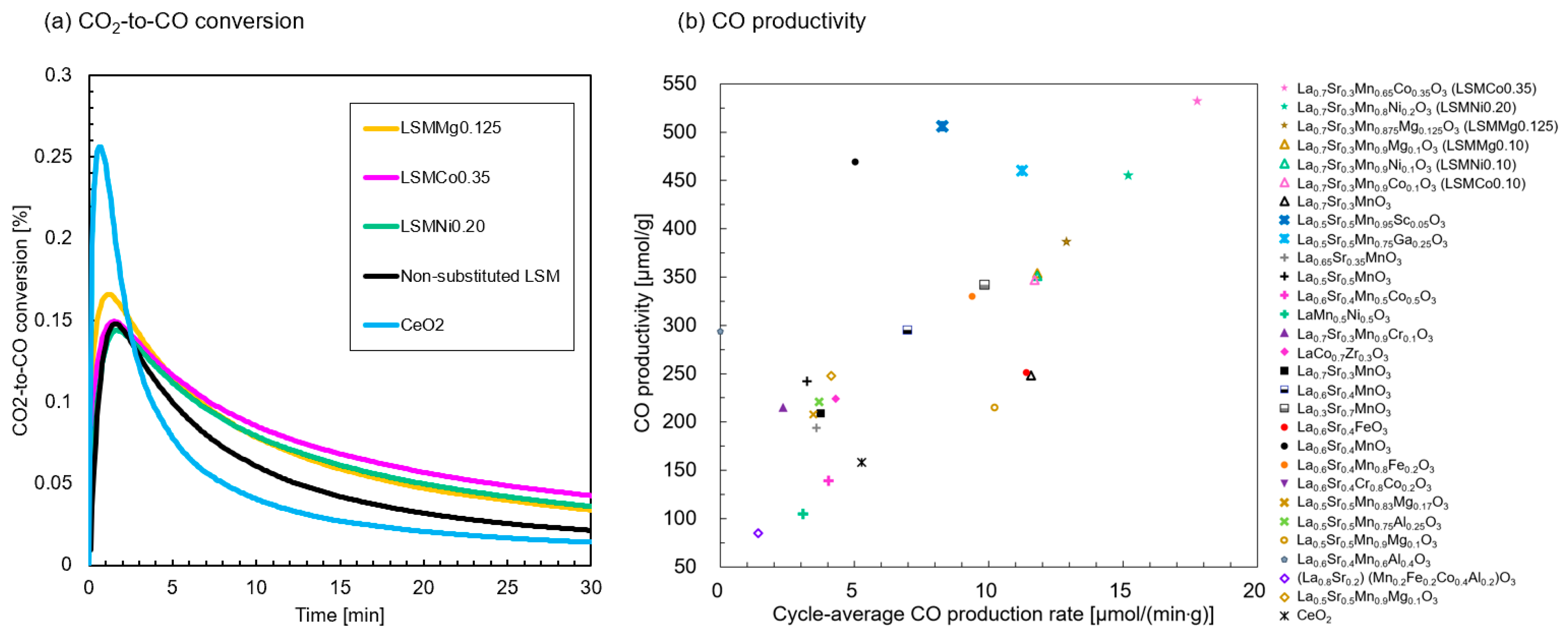
Figure 13a shows the time variations in CO
2-to-CO conversion (
) of the tested samples. The results for the nonsubstituted sample and cerium oxide are also shown for comparison. The peak value of
for the LSMMg0.125 sample was ~0.17%, which was higher than that of the nonsubstituted sample but less than that of cerium oxide. However, the values of
after 30 min of the CS step for the three samples were enhanced compared with those of the nonsubstituted sample and cerium oxide. This is owing to the extended duration of CO production.
Figure 13b shows the CO productivities per gram of material and average value per duration. Previous data on the thermochemical two-step CO
2 splitting using LSM-based perovskites in a thermogravimetric reactor have been cited from the literature [
29,
31,
32,
33,
36,
39,
40,
41,
45,
47,
53,
67]. The best-performing samples in this study ranked at the highest level of both CO productivities compared to the reported substituted LSMs, where LSMCo0.35 exhibited the maximum CO productivity (533 μmol/g material) among the reported LSM perovskites. Compared to the authors’ previous results marked with a triangle (LSMMg0.10, LSMCo0.10, and LSMNi0.10) [
53], obtained under the same test conditions using the same methods and devices, the CO productivity of LSMCo0.35 was ~1.5 times higher. Other perovskite materials that are not LSM series, Sm
0.6Ca
0.4Mn
0.8Al
0.20O
3 [
68], Sr
0.6Ce
0.4Mn
0.8Al
0.2O
3 [
69], and Sm
0.6Sr
0.4MnO
3 [
70], were studied for the thermochemical two-step CO
2 splitting cycle. A comparison with previous literature data can be biased by the test equipment, test conditions (reaction time, temperature in both steps, gas flow rates, and concentrations), morphological features of the samples, configuration of the samples, passing gases (inert gas and CO
2), and whether the gas flow directly contacts the surface of the sample or indirectly reacts with the sample mounted in the crucible. The thermogravimetric reactor measures the weight change of the sample under various atmospheres during the thermochemical two-step CO
2 splitting cycle and estimates the O
2 and CO productivities. However, some reactors directly measure O
2 and CO gases using gas chromatography and gas analysis [
34,
45]. When the test results from the thermogravimetric reactor contain anything other than oxygen release/absorption based on the reaction mechanism (Equations (1) and (2)), the results should be evaluated and compared with literature data.