Synthesis of ZnO Nanorods at Very Low Temperatures Using Ultrasonically Pre-Treated Growth Solution
Abstract
1. Introduction
2. Materials and Methods
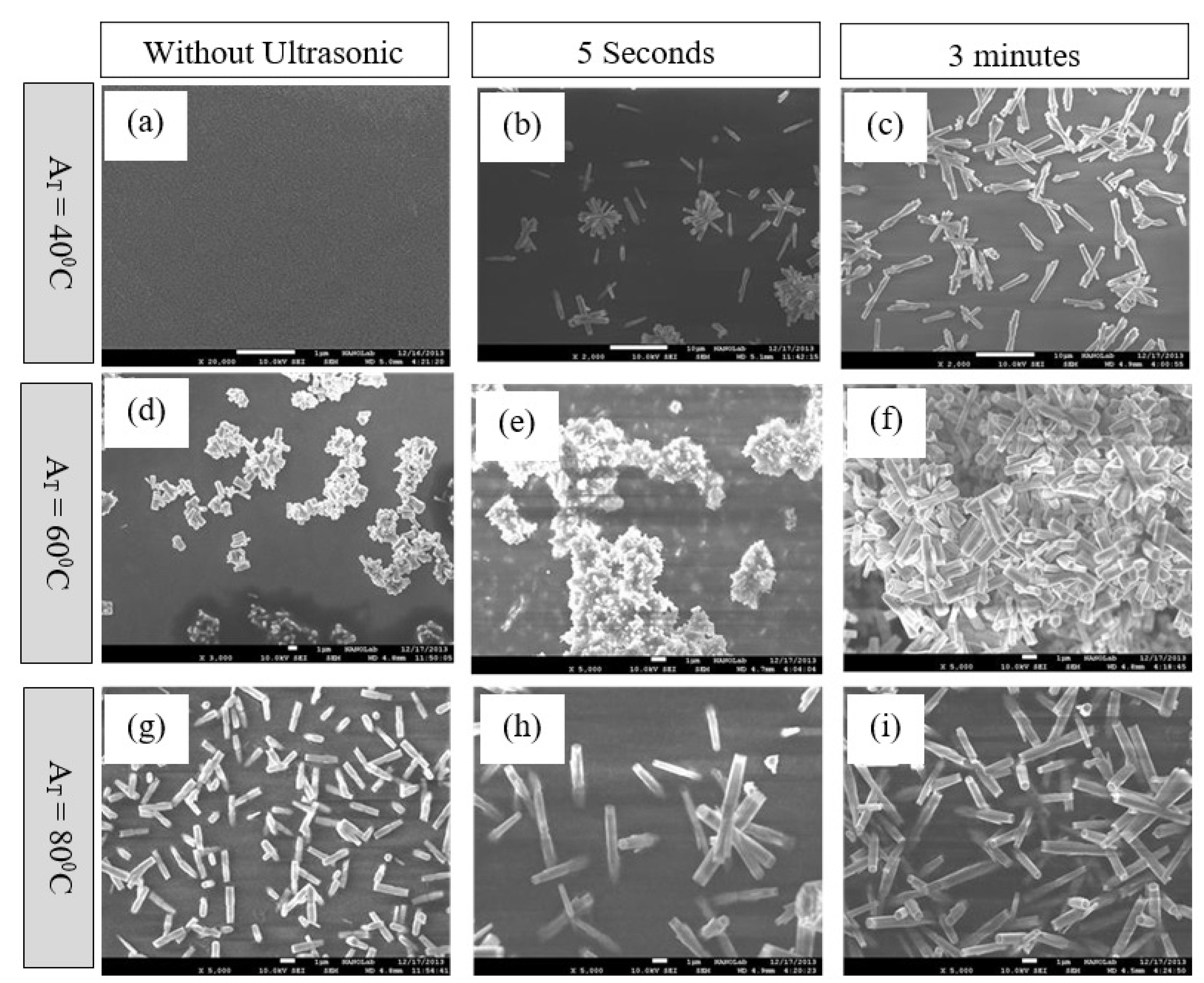
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.L. Zinc nanostructures: Growth, properties and applications oxide. J. Phys. 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Bykkam, S.; Kalagadda, V.R.; Kalagadda, B.; Selvam, K.P.; Hayashi, Y. Ultrasonic-assisted synthesis of ZnO nanoparticles decked with few layered graphene nanocomposite as photoanode in dye-sensitised solar cell. J. Mater. Sci. Mater. Electron. 2017, 28, 6217–6225. [Google Scholar] [CrossRef]
- Chu, F.H.; Huang, C.W.; Hsin, C.L.; Wang, C.W.; Yu, S.Y.; Yeh, P.H.; Wu, W.W. Well aligned ZnO nanowires with excellent field emission and photocatalytic properties. Nanoscale 2012, 4, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, P.C.; Ryu, K.; Zhou, C. Devices and chemical sensing applications of metal oxide nanowires. J. Mater. Chem. 2009, 19, 828–839. [Google Scholar] [CrossRef]
- Chang, S.J.; Weng, W.Y.; Hsu, C.L.; Hsueh, T.J. High sensitivity of a ZnO nanowire-based ammonia gas sensor with Pt nano particles. Nano Commun. Netw. 2010, 1, 283–288. [Google Scholar] [CrossRef]
- Lu, M.P.; Song, J.; Lu, M.Y.; Chen, M.T.; Gao, Y.; Chen, L.J.; Wang, Z.L. Piezoelectric nanogenerator using p-type ZnO nanowires arrays. Nano Lett. 2009, 9, 1223–1227. [Google Scholar] [CrossRef]
- Mohamed, M.; Sedky, A.; Kassem, M.A. Gradual growth of ZnO nanoparticles from globules-like to nanorods-like shapes: Effect of annealing temperature. Optik 2022, 265, 169559. [Google Scholar] [CrossRef]
- Kaushik, V.K.; Mukherjee, C.; Sen, P.K. ZnO based transparent thin film transistor grown by aerosol assisted CVD. J. Mater. Sci. Mater. Electron. 2018, 29, 15156–15162. [Google Scholar] [CrossRef]
- Elias, J.; Tena-Zaera, R.; Lévy-Clément, C. Electrochemical deposition of ZNO nanowire arrays with tailored dimension. J. Electroanal. Chem. 2008, 621, 171–177. [Google Scholar] [CrossRef]
- Chiou, W.T.; Wu, W.Y.; Ting, J.M. Growth of single crystal ZnO nanowires using sputter deposition. Diam. Relat. Mater. 2003, 12, 1841–1844. [Google Scholar] [CrossRef]
- Son, H.J.; Jeon, K.A.; Kim, C.E.; Kim, J.H.; Yoo, K.H.; Lee, S.Y. Synthesis of ZnO nanowires by pulsed laser deposition in furnace. Appl. Surf. Sci. 2007, 253, 7848–7850. [Google Scholar] [CrossRef]
- Choi, H.S.; Vaseem, M.; Kim, S.G.; Im, Y.H.; Hahn, Y.B. Growth of high aspect ratio ZnO nanorods by solution process: Effect of polyethyleneimine. J. Solid State Chem. 2012, 189, 25–31. [Google Scholar] [CrossRef]
- Wahid, K.A.; Lee, W.Y.; Lee, H.W.; Teh, A.S.; Bien, D.C.; Abd Azid, I. Effect of seed annealing temperature and growth duration on hydrothermal nanorod structures and their electrical characteristics. Appl. Surf. Sci. 2013, 283, 629–635. [Google Scholar] [CrossRef]
- Xu, S.; Wei, Y.; Kirkham, M.; Liu, J.; Mai, W.; Davidovic, D.; Snyder, R.L.; Wang, Z.L. Patterned growth of vertically ZnO nanowire arrays on inorganic substrates at low temperature without catalyst. J. Am. Chem. Soc. 2008, 130, 14958–14959. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Effect of seeded substrates in hydrothermally grown ZnO nanorods. J. Sol-Gel. Sci. Technol. 2009, 50, 456–464. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Yan, K.; Duan, M. Growth mechanism of different morphologies of ZnO crystals prepared by hydrothermal method. J. Mater. Sci. Technol. 2011, 27, 153–158. [Google Scholar] [CrossRef]
- Guo, M.; Diao, P.; Cai, S. Hydrothermal growth of well-aligned ZnO nanorod arrays: Dependance of morphology and alignment ordering upon preparing conditions. J. Solid State Chem. 2005, 178, 1864–1873. [Google Scholar] [CrossRef]
- Ho, G.W.; Wong, A.S.W. One step solution synthesis towards ultra-thin and uniform single-crystalline ZnO nanowires. Appl. Phys. 2007, 86, 457–462. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Mohan, R.; Kim, S.J. Facile synthesis of grapheme/ZnO nanocomposites by low temperature hydrothermal method. Mater. Res. Bull. 2013, 48, 878–883. [Google Scholar] [CrossRef]
- Liu, J.P.; Xu, C.X.; Zhu, G.P.; Li, X.; Cui, Y.P.; Yang, Y.; Sun, X.W. Hydrothermally grown ZnO nanorods on self-source substrate and their field emission. J. Phys. D Appl. Phys. 2007, 40, 1906–1909. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.; Zhang, N.; Liu, C. Hydrothermally synthesis and characterisation of tube-structured ZnO needles. Mater. Sci.-Pol. 2009, 27, 551–557. [Google Scholar]
- Lu, C.; Qi, L.; Yang, J.; Tang, L.; Zhang, D.; Ma, J. Hydrothermal growth of large scale micropatterned arrays of ultralong ZnO nanowires and nanobelts on zinc substrate. Chem. Commun. 2006, 33, 3551–3553. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Shin, N.; Kim, N.H.; Lee, K.H.; Jeong, S.H. A sonochemical approach to the fabrication of laterally aligned ZnO nanorods field emitter arrays on a planar substrate. IEEE Trans. Nanotechnol. 2011, 10, 319–324. [Google Scholar] [CrossRef]
- Askarinejad, A.; Alavi, M.A.; Morsali, A. Sonochemically assisted synthesis of ZnO nanoparticles: A novel direct method. Iran J. Chem. Chem. Eng. 2011, 30, 75–81. [Google Scholar]
- Geng, J.; Song, G.H.; Zhu, J.J. Sonochemical synthesis of Er3+-doped ZnO nanospheres with enhanced upconversion photoluminescence. J. Nanomater. 2012, 64, 3. [Google Scholar]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to material chemistry. Annu. Rev. Mater. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, S.; Li, X. Acoustic emission monitoring for ultrasonic cavitation based dispersion process. J. Manuf. Sci. Eng. 2013, 135. [Google Scholar] [CrossRef]
- Ondo-Ndong, R.; Ferblantier, G.; Al Kalfioui, M.; Boyer, A.; Foucaran, A. Properties of RF magnetron sputtered zinc oxide thin films. J. Cryst. Growth 2003, 255, 130–135. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Abdul Razak, K.; Lockman, Z.; Abdul Aziz, A. Structural and morphology of ZnO nanorods synthesised using ZnO seeded growth hydrothermal method and its properties as UV sensing. PLoS ONE 2012, 7, e50405. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chang, H.; Kim, M.S.; Leem, J.Y.; Ballato, J.; Kim, S.O. Low temperature growth of multiple stack high density ZnO nanoflowers/nanorods on plastic substrates. Nanotechnology 2012, 23, 485606. [Google Scholar] [CrossRef]
- Kim, K.H.; Utashiro, K.; Abe, Y.; Kawamura, M. Growth of Zinc Oxide Nanorods using various seed layer annealing temperature and substrate materials. Int. J. Electrochem. Sci. 2014, 9, 2080–2089. [Google Scholar]
- Oxtoby David, W.; Pat Gillis, H.; Laurie, B. Principles of Model Chemistry, 6th ed.; Thomson: Stamford, CT, USA, 2008. [Google Scholar]
- Wahab, R.; Kim, Y.S.; Shin, H.S. Synthesis, characterisation and effect of pH variation on Zinc Oxide nanostructures. Mater. Trans. 2009, 50, 2092–2097. [Google Scholar] [CrossRef]



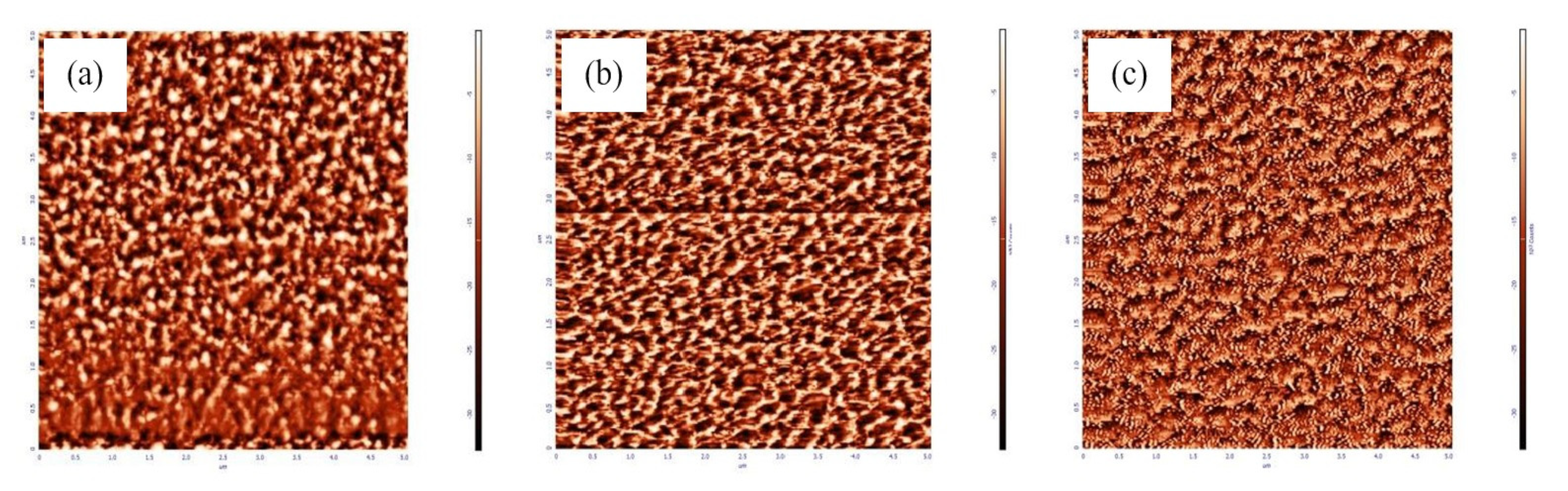

| Annealed at 40 °C | Annealed at 60 °C | Annealed at 80 °C | |
|---|---|---|---|
| Amount of sampling | 65,536 | 65,536 | 65,536 |
| Peak to peak | 293.005 nm | 410.465 nm | 1559.85 nm |
| Average roughness | 48.4501 nm | 67.8159 nm | 221.648 nm |
| Root mean square | 59.2803 nm | 83.4649 nm | 263.181 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahid, K.A.; Rahim, I.A.; Safri, S.N.A.; Ariffin, A.H. Synthesis of ZnO Nanorods at Very Low Temperatures Using Ultrasonically Pre-Treated Growth Solution. Processes 2023, 11, 708. https://doi.org/10.3390/pr11030708
Wahid KA, Rahim IA, Safri SNA, Ariffin AH. Synthesis of ZnO Nanorods at Very Low Temperatures Using Ultrasonically Pre-Treated Growth Solution. Processes. 2023; 11(3):708. https://doi.org/10.3390/pr11030708
Chicago/Turabian StyleWahid, Khairul Anuar, Irfan Abdul Rahim, Syafiqah Nur Azrie Safri, and Ahmad Hamdan Ariffin. 2023. "Synthesis of ZnO Nanorods at Very Low Temperatures Using Ultrasonically Pre-Treated Growth Solution" Processes 11, no. 3: 708. https://doi.org/10.3390/pr11030708
APA StyleWahid, K. A., Rahim, I. A., Safri, S. N. A., & Ariffin, A. H. (2023). Synthesis of ZnO Nanorods at Very Low Temperatures Using Ultrasonically Pre-Treated Growth Solution. Processes, 11(3), 708. https://doi.org/10.3390/pr11030708









