The Influence of Oxidizing and Non-Oxidizing Biocides on Enzymatic and Microbial Activity in Sugarcane Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sugarcane Juice, Enzymes, and Biocides
2.2. Bacterial Isolations
2.3. Susceptibility Testing of Bacterial Isolates
2.4. Enzyme Activity Measurement with Biocides
3. Results
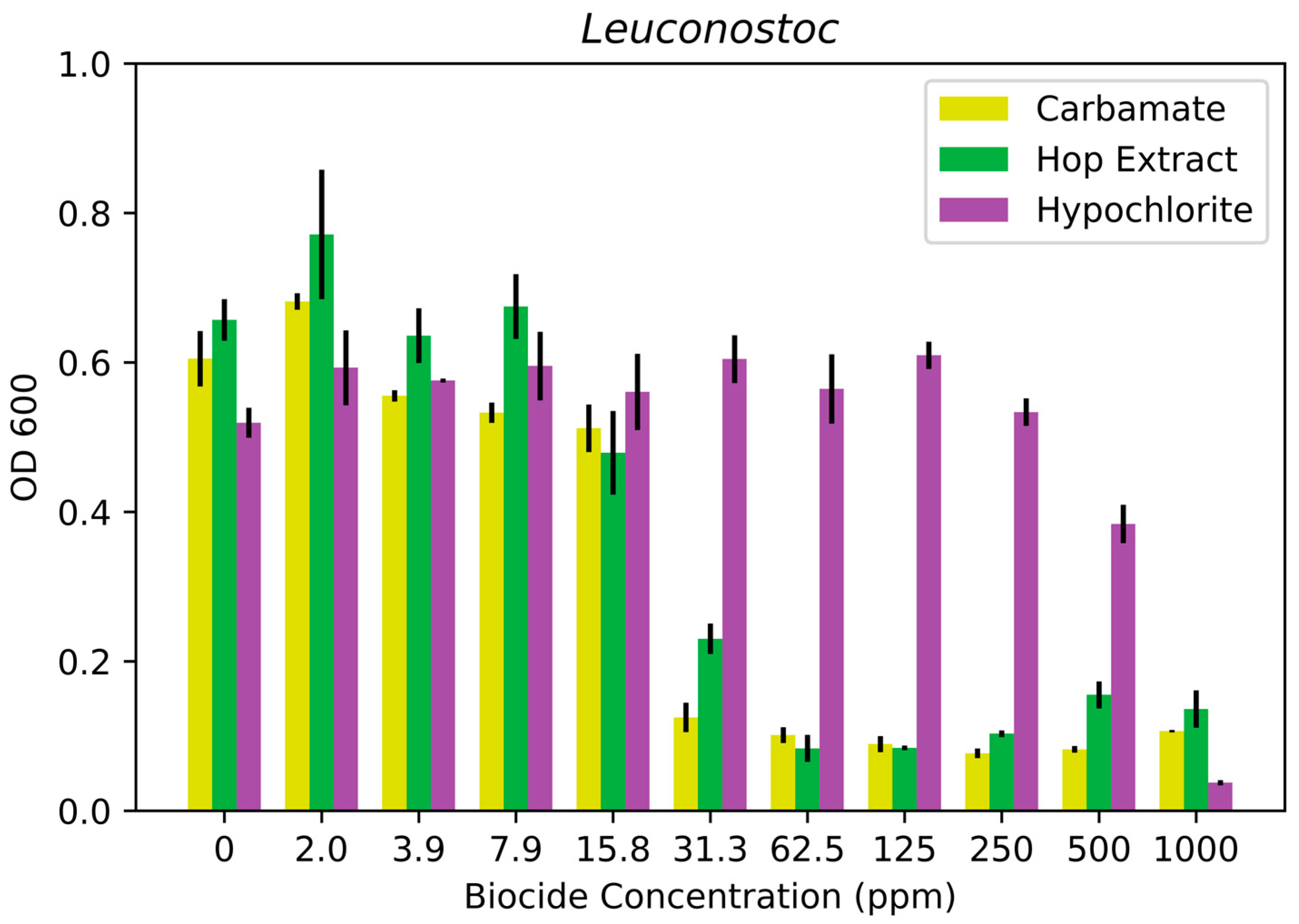
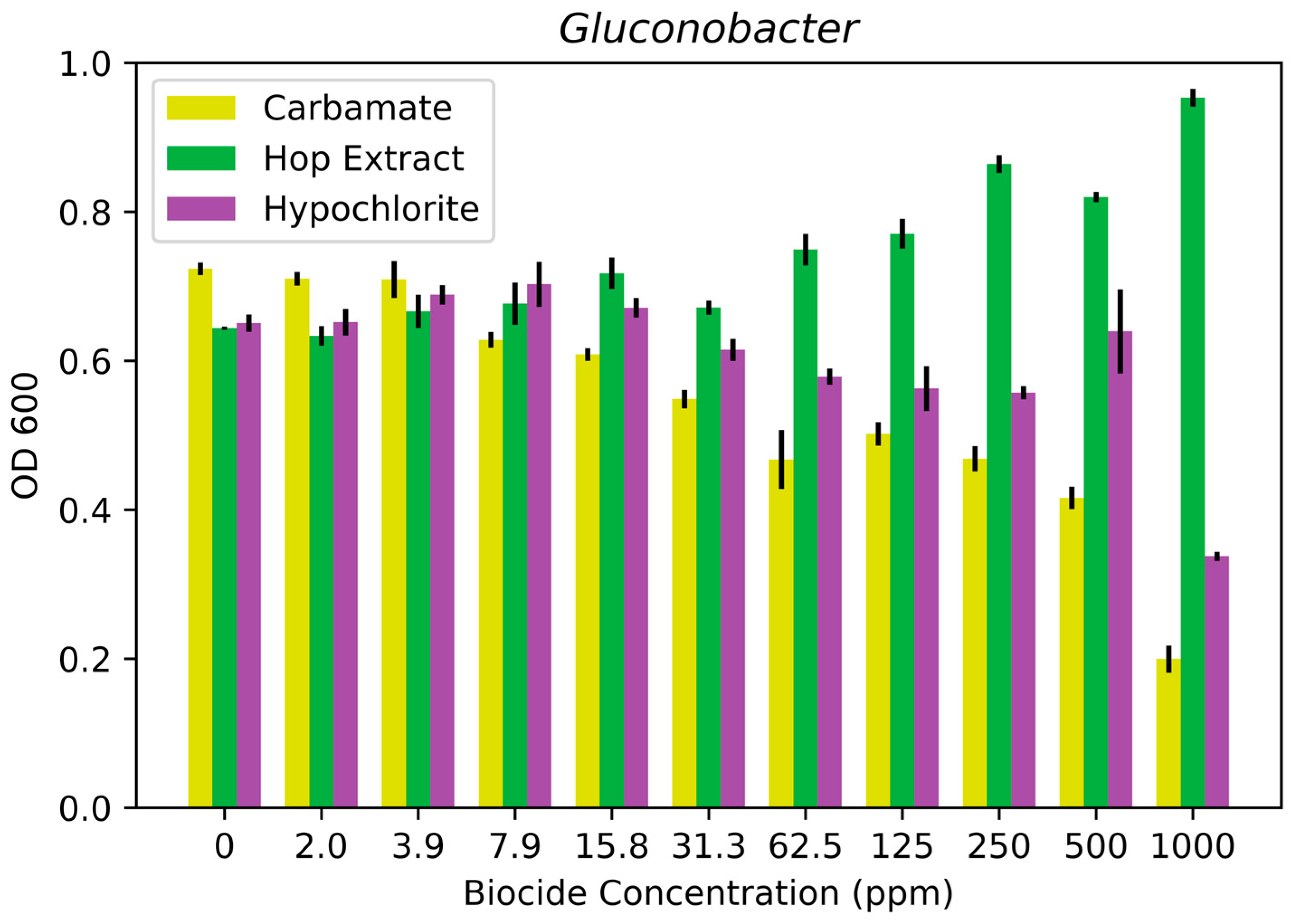
3.1. Biocide Susceptibility Results
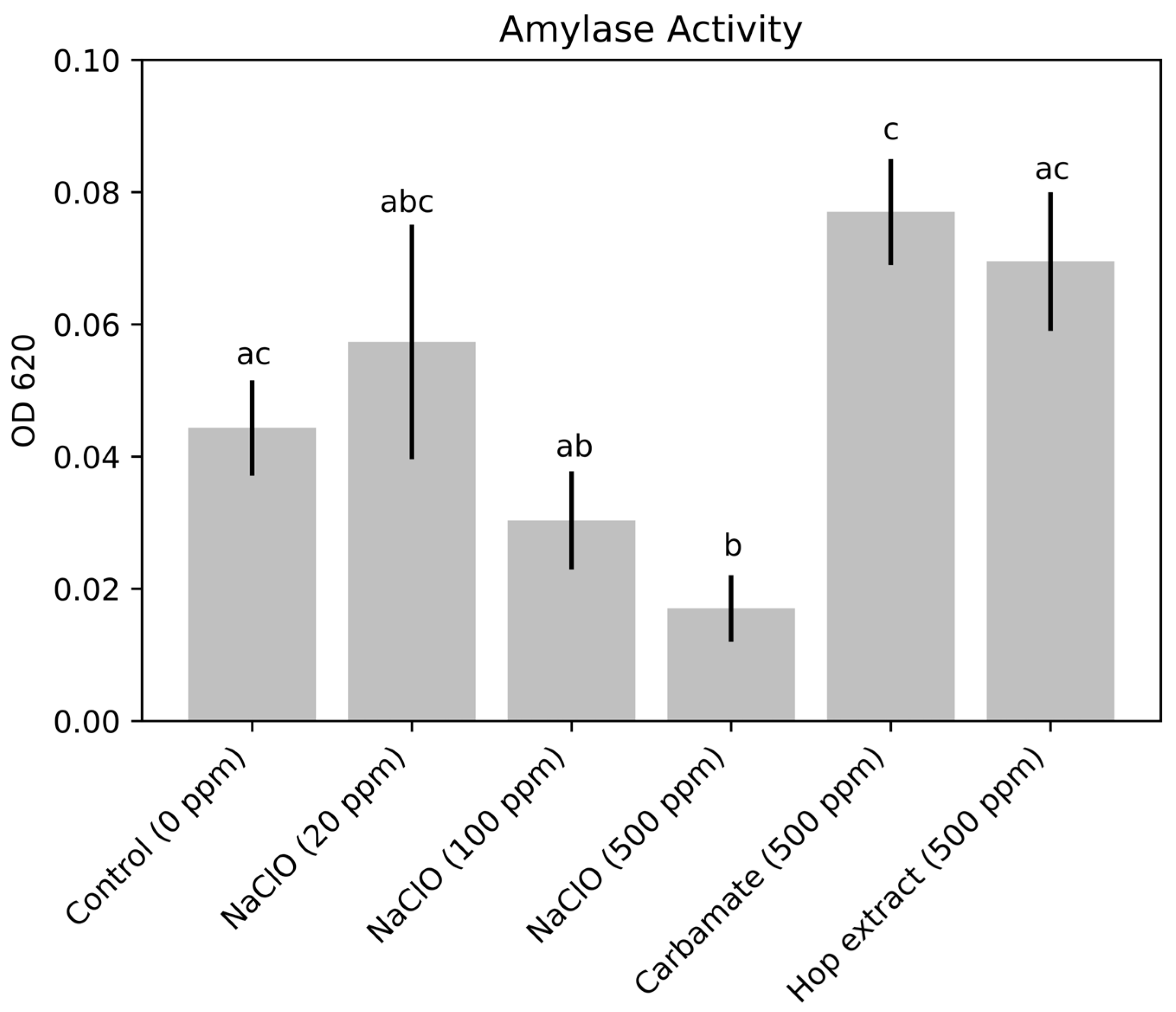
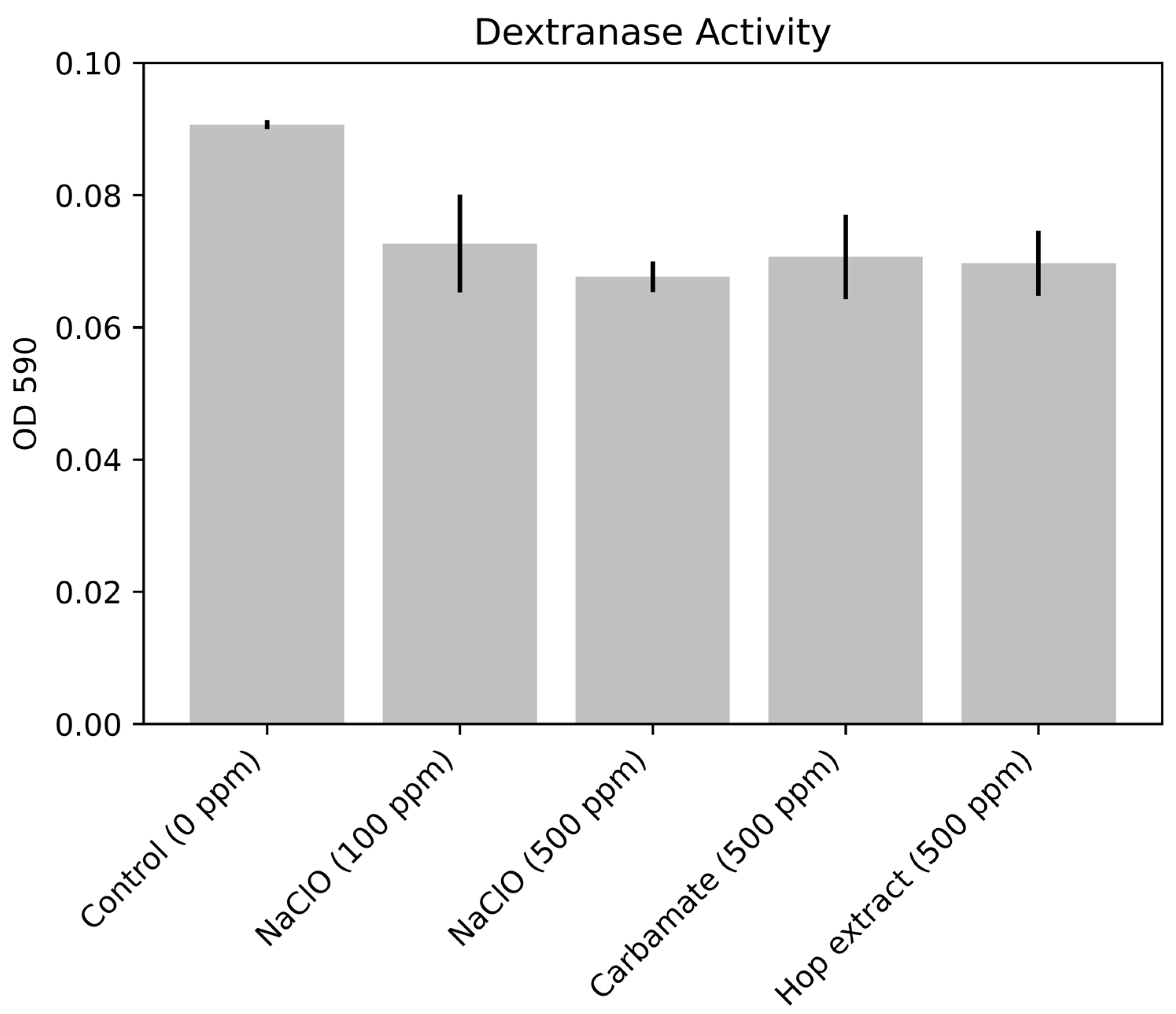
3.2. Enzyme Activity Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, J.D.; Clarke, M.L.; Jansson, P.E.; McNeil, K.E. Fructan from Erwinia herbicola. J. Bacteriol. 1982, 151, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Legaz, M.E.; Martin, L.; Pedrosa, M.M.; Vicente, C.; de Armas, R.; Martinez, M.; Medina, I.; Rodriguez, C.W. Purification and Partial Characterization of a Fructanase which Hydrolyzes Natural Polysaccharides from Sugarcane Juice. Plant Physiol. 1990, 92, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Menendez, C.; Chacon, O.; Fuentes, A.D.; Borges, D.; Sobrino, A.; Ramirez, R.; Perez, E.R.; Hernandez, L. Removal of bacterial dextran in sugarcane juice by Talaromyces minioluteus dextranase expressed constitutively in Pichia pastoris. J. Biotechnol. 2021, 333, 10–20. [Google Scholar] [CrossRef]
- Milintawisamai, N.; Niamsanit, S.; Ngasan, C.; Pliansinchai, U.; Weerathaworn, P. Dextran producing microorganisms from Mitr Phuveing Sugar Factory, Thailand. Sugar Tech 2009, 11, 196–199. [Google Scholar] [CrossRef]
- Solomon, S. Post-harvest deterioration of sugarcane. Sugar Tech 2009, 11, 109–123. [Google Scholar] [CrossRef]
- Cole, M.R.; Rose, I.; Chung, Y.J.; Eggleston, G. A structured approach to target starch solubilisation and hydrolysis for the sugarcane industry. Food Chem. 2015, 166, 165–172. [Google Scholar] [CrossRef]
- Figueira, J.d.A.; Carvalho, P.H.; Sato, H.H. Sugarcane starch: Quantitative determination and characterization. Ciên. Tecnol. Aliment. 2011, 31, 806–815. [Google Scholar] [CrossRef]
- Eggleston, G.; Legendre, B.; Tew, T. Indicators of freeze-damaged sugarcane varieties which can predict processing problems. Food Chem. 2004, 87, 119–133. [Google Scholar] [CrossRef]
- Eggleston, G.; Viator, R.; Gateuil, A.; Fenger, J.-A.; White, P.; Jackson, W.; Waguespack, H., Jr.; Blackwelder, N. Effects of seasonal variations of sugarcane stalk and extraneous matter quantity and quality as they affect recoverable sugar, starch and fiber: Part I. Int. Sugar J. 2013, 115, 477–487. [Google Scholar]
- Nel, S.; Davis, S.B.; Endo, A.; Dicks, L.M.T. Microbial Diversity Profiling of Polysaccharide (gum)-Producing Bacteria Isolated from a South African Sugarcane Processing Factory. Curr. Microbiol. 2019, 76, 527–535. [Google Scholar] [CrossRef]
- Bhatia, S.; Jyoti; Uppal, S.K.; Thind, K.S.; Batta, S.K. Post harvest quality deterioration in sugarcane under different environmental conditions. Sugar Tech 2009, 11, 154–160. [Google Scholar] [CrossRef]
- Boone, S.; Klasson, K.T.; St Cyr, E.; Montes, B.; Pontiff, K.; Legendre, D.; Wright, M. Limiting sucrose loss in Louisiana raw sugar factories: Are biocides necessary? Int. Sugar J. 2017, 119, 288–293. [Google Scholar]
- Hector, S.; Willard, K.; Bauer, R.; Mulako, I.; Slabbert, E.; Kossmann, J.; George, G.M. Diverse Exopolysaccharide Producing Bacteria Isolated from Milled Sugarcane: Implications for Cane Spoilage and Sucrose Yield. PLoS ONE 2015, 10, e0145487. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Day, D.F. Dextran analysis: A modified method. J. ASSCT 1986, 6, 102–107. [Google Scholar]
- de Souza, P.M.; de Oliveira Magalhaes, P. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.R. Dextranase in sugar industry: A review. Sugar Tech 2009, 11, 124–134. [Google Scholar] [CrossRef]
- Prakash, B.; Vidyasagar, M.; Madhukumar, M.S.; Muralikrishna, G.; Sreeramulu, K. Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem. 2009, 44, 210–215. [Google Scholar] [CrossRef]
- Hutcheon, G.W.; Vasisht, N.; Bolhuis, A. Characterisation of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 2005, 9, 487–495. [Google Scholar] [CrossRef]
- Sodhi, H.K.; Sharma, K.; Gupta, J.K.; Soni, S.K. Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005, 40, 525–534. [Google Scholar] [CrossRef]
- Asoodeh, A.; Chamani, J.; Lagzian, M. A novel thermostable, acidophilic α-amylase from a new thermophilic “Bacillus sp. Ferdowsicous” isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int. J. Biol. Macromol. 2010, 46, 289–297. [Google Scholar] [CrossRef]
- Sajedi, R.H.; Naderi-Manesh, H.; Khajeh, K.; Ahmadvand, R.; Ranjbar, B.; Asoodeh, A.; Moradian, F. A Ca-independent α-amylase that is active and stable at low pH from the Bacillus sp. KR-8104. Enzym. Microb. Technol. 2005, 36, 666–671. [Google Scholar] [CrossRef]
- Deutch, C.E. Characterization of a salt-tolerant extracellular a-amylase from Bacillus dipsosauri. Lett. Appl. Microbiol. 2002, 35, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G.; Morlon-Guyot, J.; Trejo-Aguilar, B.; Guyot, J.P. Purification and characterization of an extracellular α-amylase produced by Lactobacillus manihotivorans LMG 18010T, an amylolytic lactic acid bacterium. Enzym. Microb. Technol. 2000, 27, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Ito, A.; Yamaguchi, T. Studies on dextranase. II. New exo-dextranase from Brevibacterium fuscum var. Dextranlyticum. Biochim. Biophys. Acta (BBA) Enzymol. 1974, 350, 61–70. [Google Scholar] [CrossRef]
- Ellis, D.W.; Miller, C.H. Extracellular Dextran Hydrolase from Streptococcus mutans Strain 6715. J. Dent. Res. 1977, 56, 57–69. [Google Scholar] [CrossRef]
- Decker, S.R.; Adney, W.S.; Vinzant, T.B.; Himmel, M.E. Alkaline Tolerant Dextranase from Streptomyces anulatus. U.S. Patent 6,509,184 B1, 21 January 2003. [Google Scholar]
- Kobayashi, M.; Takagi, S.; Shiota, M.; Mitsuishi, Y.; Matsuda, K. An Isomaltotriose-producing Dextranase from Flavobacterium sp. M-73: Purification and Properties. Agric. Biol. Chem. 1983, 47, 2585–2593. [Google Scholar] [CrossRef]
- Wynter, C.; Patel, B.K.; Bain, P.; de Jersey, J.; Hamilton, S.; Inkerman, P.A. A novel thermostable dextranase from a Thermoanaerobacter species cultured from the geothermal waters of the Great Artesian Basin of Australia. FEMS Microbiol. Lett. 1996, 140, 271–276. [Google Scholar] [CrossRef][Green Version]
- Wynter, C.V.A.; Chang, M.; De Jersey, J.; Patel, B.; Inkerman, P.A.; Hamilton, S. Isolation and characterization of a thermostable dextranase. Enzym. Microb. Technol. 1997, 20, 242–247. [Google Scholar] [CrossRef]
- Hoster, F.; Daniel, R.; Gottschalk, G. Isolation of a new Thermoanaerobacterium thermosaccharolyticum strain (FH1) producing a thermostable dextranase. J. Gen. Appl. Microbiol. 2001, 47, 187–192. [Google Scholar] [CrossRef]
- Eggleston, G.; Monge, A. Optimization of sugarcane factory application of commercial dextranases. Process Biochem. 2005, 40, 1881–1894. [Google Scholar] [CrossRef]
- Eggleston, G.; Monge, A.; Montes, B.; Stewart, D. Application of dextranases in sugarcane factory: Overcoming practical problems. Sugar Tech 2009, 11, 135–141. [Google Scholar] [CrossRef]
- de Carvalho, R.V.; Côrrea, T.L.R.; da Silva, J.C.M.; de Oliveira Mansur, L.R.C.; Martins, M.L.L. Properties of an amylase from thermophilic Bacillus sp. Braz. J. Microbiol. 2008, 39, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Imbachi-Ordonez, S.; Eggleston, G.; Triplett, A.; Goudeau, S.; Gaston, P. Control of fructans, dextran, and mannitol at the sugarcane factory with commercial biocides. Part I: Juice deterioration studies. Int. Sugar J. 2022, 124, 46–55. [Google Scholar]
- Shahid, M.; Manoharadas, S.; Chakdar, H.; Alrefaei, A.F.; Albeshr, M.F.; Almutairi, M.H. Biological toxicity assessment of carbamate pesticides using bacterial and plant bioassays: An in-vitro approach. Chemosphere 2021, 278, 130372. [Google Scholar] [CrossRef]
- Nel, S. Isolation and Identification of Polysaccharide (gum)-Producing Bacteria from a Sugarcane Factory and Strategies to Prevent Their Growth. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Behr, J.; Vogel, R.F. Mechanisms of hop inhibition: Hop ionophores. J. Agric. Food Chem. 2009, 57, 6074–6081. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.; Ihli, S.; Hartsough, D.; Hernandez, L.; Sanders, J.; Klasson, K.T.; Lima, I.M. Application of permanganate to reduce microbial contamination and sugar loss in raw-sugar production in Louisiana, USA. Int. Sugar J. 2020, 122, 205–211. [Google Scholar]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef]
- Muthaiyan, A.; Limayem, A.; Ricke, S.C. Antimicrobial strategies for limiting bacterial contaminants in fuel bioethanol fermentations. Prog. Energy Combust. Sci. 2011, 37, 351–370. [Google Scholar] [CrossRef]
- Qi, Y.; Bruni, G.O.; Klasson, K.T. Microbiome Analysis of Sugarcane Juices and Biofilms from Louisiana Raw Sugar Factories. Microbiol. Spectr. 2023, 11, e04345-22. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y.J. Structural characteristics and physicochemical properties of oxidized corn starches varying in amylose content. Carbohydr. Res. 2006, 341, 1896–1915. [Google Scholar] [CrossRef]
- Asadi, M. Beet-Sugar Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bruni, G.O.; Qi, Y.; Klasson, K.T.; Lima, I.M.; Terrell, E. Isolation and analysis of microbial contaminants from Louisiana raw sugarcane factories. Int. Sugar J. 2022, 124, 530–538. [Google Scholar]
- Qi, Y.; Bruni, G. Draft genomes of 17 bacterial isolates from Louisiana raw sugarcane factory juices and biofilms. Microbiol. Resour. Announc. 2023, e00416-23. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2018. [Google Scholar]
- Haynes, W.C.; Wickerham, L.J.; Hesseltine, C.W. Maintenance of cultures of industrially important microorganisms. Appl. Microbiol. 1955, 3, 361–368. [Google Scholar] [CrossRef]
- Phadebas. Amylase Test Products. Available online: https://www.phadebas.com/products/amylase-test-products/ (accessed on 1 February 2023).
- Megazyme. α-Dextrazyme Tablets. Available online: https://www.megazyme.com/alpha-dextrazyme-tablets (accessed on 1 February 2023).
- Saxena, R.K.; Dutt, K.; Agarwal, L.; Nayyar, P. A highly thermostable and alkaline amylase from a Bacillus sp. PN5. Bioresour. Technol. 2007, 98, 260–265. [Google Scholar] [CrossRef]
- Nair, H.P.; Bhat, S.G. Arabian Sea metagenome derived-α-amylase P109 and its potential applications. Ecol. Genet. Genom. 2020, 16, 100060. [Google Scholar] [CrossRef]
- Chakraborty, S.; Khopade, A.; Kokare, C.; Mahadik, K.; Chopade, B. Isolation and characterization of novel α-amylase from marine Streptomyces sp. D1. J. Mol. Catal. B Enzym. 2009, 58, 17–23. [Google Scholar] [CrossRef]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial dextran-hydrolyzing enzymes: Fundamentals and applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, L.K.; Gupta, J.K.; Banerjee, U.C. Levanases for control of slime in paper manufacture. Biotechnol. Adv. 1998, 16, 899–912. [Google Scholar] [CrossRef]
- Pereira, M.O.; Vieira, M.J.; Beleza, V.M.; Melo, L.F. Comparison of two biocides--carbamate and glutaraldehyde--in the control of fouling in pulp and paper industry. Environ. Technol. 2001, 22, 781–790. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, L.K.; Gupta, J.K.; Banerjee, U.C. Studies on slime-forming organisms of a paper mill—Slime production and its control. J. Ind. Microbiol. Biotechnol. 1997, 18, 348–352. [Google Scholar] [CrossRef]
- Goetz, C.; Larouche, J.; Velez Aristizabal, M.; Niboucha, N.; Jean, J. Efficacy of Organic Peroxyacids for Eliminating Biofilm Preformed by Microorganisms Isolated from Dairy Processing Plants. Appl. Environ. Microbiol. 2022, 88, e0188921. [Google Scholar] [CrossRef]
- Anand, S.; Singh, D.; Avadhanula, M.; Marka, S. Development and Control of Bacterial Biofilms on Dairy Processing Membranes. Compr. Rev. Food Sci. Food Saf. 2014, 13, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; Zavareze, E.d.R. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2003, 52, 207–217. [Google Scholar] [CrossRef]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Q.; Zhang, H.; Chen, Q.; Kong, B. Potato starch oxidation induced by sodium hypochlorite and its effect on functional properties and digestibility. Int. J. Biol. Macromol. 2016, 84, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Yousef, M.M.; Zohri, A.A.; Darwish, A.M.G.; Shamseldin, A.; Kabeil, S.A.; Abdelkhalek, A.; Binsuwaidan, R.; Jaremko, M.; Alshwyeh, H.A.; Hafez, E.E.; et al. Exploring the antibacterial potential of plant extracts and essential oils against Bacillus thermophilus in beet sugar for enhanced sucrose retention: A comparative assessment and implications. Front. Microbiol. 2023, 14, 1219823. [Google Scholar] [CrossRef]

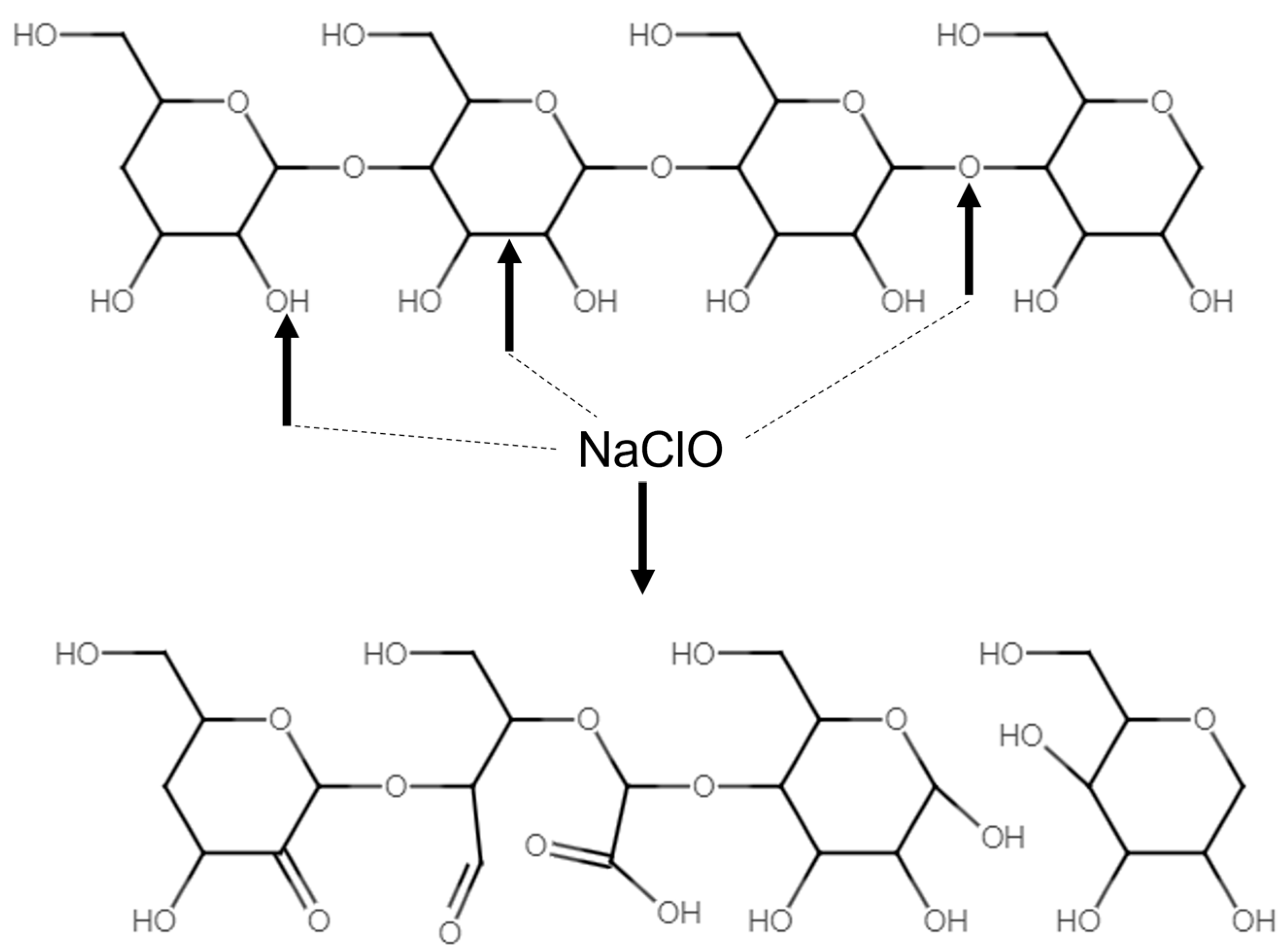
| Juice Starch Content (ppm/Brix) 1 | Cane Variety for Starch Measured in Juice (Brazil) | Juice Dextran Content (ppm/Brix) 2 | Cane Variety for Dextran Measured in Juice (Louisiana) |
|---|---|---|---|
| 2628 | RB 86-7515 | 112 | CP 70-321 |
| 1679 | SO 83-2847 | 135 | CP 79-318 |
| 1896 | RB 72-454 | 133 | HoCP 85-845 |
| 1740 | SP 80-3280 | 126 | HoCP 91-555 |
| 1798 | RB 85-5536 | 195 | HoCP 96-540 |
| Enzyme | Source | Opt. pH | Opt. Temp. (°C) | Enzyme mol. wt. (kDa) | Ref. |
|---|---|---|---|---|---|
| Amylase | Chromohalobacter sp. TVSP 101 | 7.0–9.0 | 65 | 72 | [17] |
| Amylase | Haloarcula hispanica | 6.5 | 50 | 43 | [18] |
| Amylase | Bacillus sp. PS-7 | 6.5 | 60 | 71 | [19] |
| Amylase | Bacillus sp. Ferdowsicous | 4.5 | 70 | 53 | [20] |
| Amylase | Bacillus sp. KR-8104 | 4.0–6.0 | 70–75 | 59 | [21] |
| Amylase | Bacillus dipsosauri DD1 | 6.1 | 60 | 80 | [22] |
| Amylase | Lactobacillus manihotivorans LMG 18010T | 5.5 | 55 | 135 | [23] |
| Dextranase | Brevibacterium fuscum | 7.0–7.5 | -- 1 | -- 1 | [24] |
| Dextranase | Streptococcus mutans | 5.5 | 37 | -- 1 | [25] |
| Dextranase | Streptomyces anulatus (two strains) | 5.0–9.5 | 40 and 50 | 63 and 82 | [26] |
| Dextranase | Flavobacterium sp. M-73 | 7.0 | 35 | 114 | [27] |
| Dextranase | Thermoanaerobacter wiegelii | 5.5 | 70 | -- 1 | [28] |
| Dextranase | Thermoanaerobacter strain | 4.5–5.5 | 80 | 150 | [29] |
| Dextranase | Thermoanaerobacterium thermosaccharolyticum | 5.5 | 65–70 | 200 | [30] |
| Isolate | NaClO MIC | Carbamate MIC | Hop Extract MIC |
|---|---|---|---|
| Leuconostoc suionicum strain LASM7 | >500 ppm | 60 ppm | 60 ppm |
| Gluconobacter japonicus strain LASM12 | >1000 ppm | >1000 ppm | No effect |
| Pantoea dispersa strain LASM22 | >1000 ppm | 125–250 ppm | No effect |
| Parameter | Juice in This Work (Samples Stored Frozen, from Fall 2020) | Juice Data from 2022 to 2023 Season (Louisiana Factory, First Six Weeks) |
|---|---|---|
| pH | 5.38 ± 0.15 | 5.37 ± 0.03 |
| Brix | 15.4 ± 0.8 | 15.6 ± 0.1 |
| Pol | 13.1 ± 0.9 | 13.1 ± 1.0 |
| Purity (%) | 84.6 ± 2.0 | 84.3 ± 0.3 |
| Enzyme | Summary | Ref. |
|---|---|---|
| Amylase from thermophilic Bacillus sp. | Residual activity is reported for amylase with the following inhibitors: sodium dodecylsulfate (SDS), EDTA, NaClO, H2O2 at concentrations of 5 mM. For EDTA and NaClO specifically, a time of 20 min showed between 40 and 50% activity, and a time of 60 min showed between 10 and 20% activity. | [33] |
| Amylase from Bacillus sp. PN5 | Residual activity is reported for amylase with the following inhibitors: sodium dodecylsulfate (SDS), EDTA, NaClO, H2O2 at concentrations of 5 mM. For EDTA and NaClO specifically, a time of 20 min showed between 40 and 50% activity, and a time of 60 min showed between 10 and 20% activity. | [51] |
| Metagenome-derived Amylase P109 | Residual activity was tested for oxidizing agents H2O2 and NaClO at concentrations of 5, 10, 15, 20, and 25 mM and the reducing agent β-mercaptoethanol (BME). All three reagents showed dosage-dependent increasing levels of inhibition, with BME having the greatest residual activities and NaClO having the least (between 50 and 60%). | [52] |
| Amylase from marine Streptomyces sp. D1 | Residual activity of approximately 80% is reported for both NaClO and H2O2 at concentrations of 1% w/v. A gradual, dosage-depended decline in residual activity is shown for both oxidizers, although concentrations of 0.2% show nearly 100% residual activity. | [53] |
| Glucan 1,6-α-isomaltosidase from Arthrobacter globiformis | Enzyme with reported substrate specificity for several native NRRL dextrans. Stable at pH between 3.0 and 8.0, with 90% residual activity following heating to 60 °C. Reported inhibitors include some metal ions (Ag2+, Hg2+, Fe3+) and potassium permanganate oxidizer. | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrell, E.; Qi, Y.; Bruni, G.O.; Heck, E. The Influence of Oxidizing and Non-Oxidizing Biocides on Enzymatic and Microbial Activity in Sugarcane Processing. Processes 2023, 11, 2693. https://doi.org/10.3390/pr11092693
Terrell E, Qi Y, Bruni GO, Heck E. The Influence of Oxidizing and Non-Oxidizing Biocides on Enzymatic and Microbial Activity in Sugarcane Processing. Processes. 2023; 11(9):2693. https://doi.org/10.3390/pr11092693
Chicago/Turabian StyleTerrell, Evan, Yunci Qi, Gillian O. Bruni, and Emily Heck. 2023. "The Influence of Oxidizing and Non-Oxidizing Biocides on Enzymatic and Microbial Activity in Sugarcane Processing" Processes 11, no. 9: 2693. https://doi.org/10.3390/pr11092693
APA StyleTerrell, E., Qi, Y., Bruni, G. O., & Heck, E. (2023). The Influence of Oxidizing and Non-Oxidizing Biocides on Enzymatic and Microbial Activity in Sugarcane Processing. Processes, 11(9), 2693. https://doi.org/10.3390/pr11092693









