Abstract
Many applications in the life science and food industries require (semi-)crystalline oil-in-water (O/W) dispersions. Unfortunately, high supercooling and, thus, low temperatures are often needed to induce the crystallization of droplets. As low molecular weight emulsifiers (LMWEs) are able to act as nucleation templates, they might help to decrease the required level of supercooling. Furthermore, proteins and LMWEs are frequently co-formulated to improve the colloidal stability of emulsions and dispersions. Hence, choosing a suitable protein and LMWE mixture would allow for achieving specific product properties for controlling the solid fat content (SFC) and take advantage of the stabilization mechanisms of both emulsifiers. Therefore, this study focuses on the impact of the co-existence of β-lactoglobulin (β-lg) and phospholipids (PLs) LMWEs on the SFC of triglyceride (TAG) droplets at isothermal conditions using a thermo-optical method. When β-lg alone was used as an emulsifier, a maximum SFC of 80% was obtained at a supercooling of 32 K and 42 K for trilaurin and tripalmitin, respectively. The SFC could be increased to 100% using a PL containing saturated fatty acids (FAs) and a small hydrophilic headgroup. At the same supercooling, a PL containing saturated FAs and a large hydrophilic headgroup led to a maximum SFC of 80%. At lower supercooling, the SFC was reduced with this PL by 10% compared to β-lg alone. In addition, when the PLs had more time to adsorb and rearrange with ß-lg at the interface, even lower SFCs were observed compared to cooling directly after emulsification.
1. Introduction
Oil in water (O/W) emulsions are a common dispersed food system that may be used for many different applications. Emulsions are thermodynamically unstable systems, where stability is the resistance to physical changes [1]. They may, therefore, undergo coalescence, aggregation, Ostwald ripening, and creaming during storage and transport [2,3,4,5]. Dispersed droplets are kinetically stabilized by low molecular weight emulsifiers (LMWEs) and/or polymers and usually consist of complex vegetable or animal fats or oils (triglyceride mixes), pure triglycerides (TAGs), or more defined mixtures thereof. The fatty acid composition and their spatial distribution in TAGs influence taste, smell, and, especially, the melting and crystallization behavior of the fat [6]. Hence, the nature of the dispersed phase often results in (partially) crystalline O/W dispersions. In contrast to O/W emulsions containing liquid oil as the dispersed phase, O/W dispersions may contain both liquid oil and solid fat. For some applications, such as the whippability of cream, a specific crystallinity, i.e., solid fat content (SFC), is required. Those systems are typically produced via a two-step melt emulsification process. First, the droplet size distribution is set by emulsification above the melting temperature of the dispersed phase. Second, the emulsion is transferred into a dispersion with crystalline dispersed particles by cooling to temperatures below the crystallization temperature of the dispersed phase material [7]. Depending on temperature, time, and droplet size, the droplets may remain supercooled liquid or crystallize in mono- and multi-crystalline structures. In typical products, crystallization may differ from droplet to droplet. This is due to the individual and stochastic crystallization behavior of droplets in O/W emulsions, which occurs over a wide temperature range [7,8]. In general, higher supercooling is needed for droplet crystallization compared to bulk fat, and the necessary supercooling for crystallization onset increases with decreasing droplet size [9,10]. Additionally, the droplet crystallization behavior may be influenced by the polydispersity [8,11,12], cooling rate and supercooling [12,13], external forces [9,14], and additives, especially LMWEs [15,16,17].
LMWEs, like phospholipids (PLs), have been shown to act as crystallization templates that can trigger the crystallization of dispersed droplets [17,18]. The crystallization process itself is commonly divided into two sub-processes, i.e., nucleation and crystal growth. First, stable nuclei have to be formed via homogeneous or heterogeneous nucleation [19]. Homogeneous nucleation occurs in the absence of impurities in the melt or solution [20]. Nucleation due to the presence of impurities is referred to as heterogeneous nucleation [20,21]. The formation of nuclei in emulsified droplets can further be divided into volume and surface heterogeneous nucleation. LMWEs can promote surface heterogeneous nucleation, especially when their hydrophobic tail is similar in molecular structure to the nucleating oil [22,23]. Here, the LMWEs’ hydrophobic tails are oriented towards the oil phase and thereby confer a certain degree of order to neighboring oil molecules at the droplet surface [24]. The LMWEs then may act as “templates” for nuclei formation. This process has been proposed to increase the nucleation rate and shown to influence the temperature at which nucleation is first observed in O/W emulsions [23,25,26,27,28,29]. Volume heterogeneous nucleation occurs when catalytic impurities are present in the oil droplets, on which nuclei start to form throughout the volume of the oil [30]. Thus, oil-soluble LMWEs present within the oil droplets as either single molecules or assembled into inverse micelles, can induce volume heterogeneous nucleation.
It has been shown that the crystallization temperature of TAGs can be increased by the addition of partially unsaturated or fully saturated PLs [18]. In contrast, highly unsaturated PLs are less effective in increasing the crystallization temperature [18], and fully unsaturated PLs appear not to have any effect on the crystallization temperature [31]. This has been attributed to the solidification of the partially/fully saturated phospholipid chains, compared to unsaturated PLs. It is possible to use these phenomena to control the nucleation rate and crystal formation by the appropriate choice of emulsifiers stabilizing the lipid droplets. However, in many life science applications, and especially in the food industry, the choice of emulsifiers is limited. Commonly used emulsifiers are LMWEs like phospholipids (PLs) and proteins, e.g., whey protein. LMWEs and proteins may be specifically used in combination for a wide range of applications or co-occur due to their presence in specific ingredients. In general, the stabilization mechanisms of PLs and proteins differ due to their nature. Proteins are large polymers and, therefore, adsorb comparably slowly at droplet interfaces, whereas PLs can adsorb quickly due to their smaller size. Thus, PLs are very efficient in stabilizing newly created uncovered interfaces, and smaller droplets can be stabilized compared to proteins due to their high interfacial activity [32]. When proteins adsorb at the interface and are given enough time, they are believed to unfold at the interface. It was shown that they can generate a viscoelastic film through lateral interactions between adsorbed molecules, which can protect droplets against coalescence [33,34]. Also, steric stability is provided due to the thick interfacial layer [19]. When PLs and proteins are both present in O/W emulsions, it has been shown that PLs can displace proteins from interfaces and form mixed interfacial layers [34,35]. In some cases, this protein displacement with time only occurs when both PLs and proteins are present during emulsification [36]. The solubility of LMWEs also influences their ability to displace proteins, i.e., water-soluble LMWEs show stronger displacement of proteins than oil-soluble LMWEs [37,38]. Consequently, it is reasonable to assume that a more hydrophilic PL with a more pronounced interfacial affinity will also have a higher ability to displace proteins from the interface. However, a complete displacement of proteins has not been reported [39]. Furthermore, PLs and proteins can undergo complexation [40,41]. These complexes can display increased or decreased interfacial activity compared to the individual constituents [40,42,43]. As a consequence, proteins and LMWEs are frequently co-formulated to improve the colloidal stability of emulsions and dispersions.
Controlling the crystallinity of dispersions with a suitable choice of protein and LMWE mixtures would allow for achieving specific product properties and take advantage of the stabilization mechanisms of both emulsifiers. Therefore, this study focuses on the impact of the co-existence of β-lg and LMWEs on the crystallization behavior of TAG droplets. We compare the crystallization behavior of TAG droplets stabilized with solely β-lactoglobulin (β-lg) with the crystallization behavior of droplets stabilized by mixtures of β-lg and PLs. The crystallization of droplets stabilized only by PLs was not investigated, as numerous studies have already been conducted on this subject [16,18,29]. We expect a higher SFC, which is equivalent to a higher crystallinity, at similar supercooling through the co-existence of saturated PLs and β-lg as emulsifiers, compared to β-lg alone. We also presume that the addition of unsaturated PLs as emulsifiers will not have an increasing effect on the SFC at similar supercooling compared to β-lg alone. We investigate these hypotheses using two PLs with a similar hydrophilic headgroup and lipophilic chain length but different saturation levels.
As the template effect is supposed to be more effective when the hydrophobic tail of the LMWE is more similar to the molecular structure of the nucleating oil, we investigated this effect using two different dispersed phases, i.e., trilaurin (C12) and tripalmitin (C18). We expect a more pronounced template effect in tripalmitin dispersions because the lipophilic tails of the PLs used have a greater similarity to tripalmitin than to trilaurin. Hence, we presume a greater impact on the SFC in tripalmitin dispersions using the chosen saturated PLs compared to β-lg alone than in trilaurin dispersions.
Moreover, we expect that the hydrophilic headgroup of the PL will influence the TAG crystallization, i.e., the SFC. Therefore, we chose two PLs with the same hydrophobic tail phosphatidylcholine (PC) and phosphatidylethanolamine (PE) but with a larger hydrophilic headgroup in the case of PC compared to PE. PC is hydrophilic at all pH values [44] and is able to form well-ordered lamellar monolayers or bilayers around lipid droplets [45]. PE, on the other hand, is poorly hydratable at neutral pH and becomes more hydrophilic when the pH is increased into the alkali region [44]. In addition, PE tends to assemble into reversed hexagonal structures in an aqueous environment, which are more difficult to form around lipid droplets [46]. Furthermore, PCs are reported to form more compact interfacial monolayers when used as emulsifiers in O/W emulsions as compared to PE, which confers better stability and is related to the PC molecule being in a liquid condensed state as opposed to PEs being in a liquid expanded state. This leads us to the assumption that PC will be able to better adsorb at the TAG interface and, thus, have a more pronounced effect on the TAG crystallization compared to PE due to its more pronounced interfacial affinity and increased ability to displace proteins from the interface. Hence, the use of β-lg + PC might result in a higher SFC at similar supercooling compared to β-lg + PE. However, when PE is able to adsorb at the TAG interface, the smaller headgroup of PE might lead to a higher interfacial loading compared to PC, which might be preferable for the template effect. As the displacement of proteins from the interface is time-dependent, we expect higher SFCs when the PLs are given more time for this process. Thus, we investigated the SFC of a freshly produced emulsion and the SFC at similar supercooling of an emulsion, which was kept for two hours above the melting temperature of the dispersed phase.
The findings of our study will improve the understanding of droplet crystallization using co-emulsifiers and may then be used to control the nucleation rate and crystal formation in O/W emulsions via the product formulation. An optimized formulation and manufacturing process would allow businesses to achieve specific product properties, raising the appeal and competitiveness of the formulated products, saving resources, and preventing product waste. In addition, an optimized crystallization process might also help to reduce energy costs, as higher temperatures might suffice to induce crystallization. There is still some lack of knowledge of how PLs are able to act as nucleation templates in the presence of proteins and how the combination of both emulsifiers influences the SFC of emulsions at isothermal conditions over a certain time. Moreover, the influence of the hydrophilic head group on droplet crystallization has been less studied than the influence of the lipophilic moiety. Hence, we assess the SFC of dispersed TAGs at isothermal conditions using a thermo-optical method and show that the addition of certain PLs lead to an increase in SFC compared to β-lg alone, whereas others lead to a decrease in SFC. To be more precise, we attribute these differences in SFC to molecular differences of the hydrophilic and lipophilic parts of the PLs and associated properties.
2. Materials and Methods
2.1. Raw Materials
Glyceryl trilaurate (trilaurin, purity 98%, ϑmelt = 47 °C) and glyceryl tripalmitate (tripalmitin, purity 99%, ϑmelt = 65 °C) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Trilaurin consists of lauric acid and tripalmitin of palmitic acid, which are saturated FAs with a 12-carbon chain and 16-carbon chain, respectively. The PLs 1,2-Distearoyl-sn-glycero-3-phosphocholin (DSPC, ϑmelt = 55 °C), 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE, ϑmelt = 74 °C) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, ϑmelt = −16 °C) were purchased from Avanti Polar Lipids (Croda International, Birmingham, USA). DSPC and DSPE were chosen because they have a similar fatty acid (FA) composition (stearic acid, C18:0) but differ in their hydrophilic headgroup. Both PLs have a greater similarity to tripalmitin due to their 18-carbon chain. DSPC is a phosphatidylcholine, whereas DSPE is a phosphatidylethanolamine, which means the choline, or the ethanolamine head group, is esterified with FAs, respectively. Hence, DSPC contains a larger hydrophilic headgroup compared to DSPE. DOPE (oleic acid, C18:1) was chosen because it has the same headgroup as DSPE but differing FA composition. DOPE contains unsaturated FAs, whereas DSPE contains saturated FAs as a lipophilic tail. β-lactoglobulin (β-lg) and sodium hydroxide (NaOH) were purchased from TU Munich (Munich, Germany) and Carl Roth GmbH + Co. KG (Karlsruhe, Germany), respectively. Water was purified using a MicroPureTM water purification system by Thermo Fisher Scientific Inc. (Waltham, MA, USA).
2.2. Emulsion Composition
The continuous phase consisted of purified water and β-lg and was adjusted to a pH of 6.5 using 1 M NaOH to avoid protein precipitation at its isoelectric point. The dispersed phase consisted of either trilaurin or tripalmitin. Additionally, PLs were added in three samples as co-emulsifiers to β-lg. The composition of the samples is given in Table 1.

Table 1.
Sample names and their composition.
2.3. Emulsification Process
The emulsions were prepared by premix membrane emulsification. Premix membrane emulsification was carried out 10 K above the melting temperature of the dispersed phase (ϑmelt,DP). The tips of syringes were closed using a Luer-Lock syringe cap. The continuous phase was weighed in a syringe, and the TAG was weighed into another one. Both syringes were put in a water bath at a temperature 10 K above ϑmelt,DP to melt the TAG and heat the continuous phase to prevent early TAG crystallization upon contact. After the TAG was fully molten, the syringes were connected with a Luer-Lock adapter, and a pre-emulsion was produced by pushing the liquids twenty times back and forth between the syringes. Afterward, a hydrophilic syringe filter with a pore size of 10 µm (Pall Corporation, New York, NY, USA) was inserted between the syringes. Then, a controlled membrane melt emulsification (ϑ > ϑmelt,DP) was carried out by pushing the syringes pneumatically back and forth with an inlet pressure of 1.5 bar until an average droplet size of 12 µm and a narrow, monomodal size distribution was achieved. A passage number of 8 was generally needed. To obtain similar droplet sizes, higher passage numbers were needed for samples containing only β-lg compared to samples containing β-lg + PL. The droplet size was controlled by diffraction analysis using a HORIBA LA-950V2 (Retsch Technology, Haan, Germany). The detailed design of our customized apparatus is described in Reiner et al. [47]. After emulsification, all samples were kept 10 K above ϑmelt,DP until further analysis.
2.4. Thermo-Optical Microscopy
Droplet crystallization was observed using polarized microscopy (Eclipse LV100ND, Nikon, Shinagawa, Tokyo, Japan) equipped with an optically accessible temperature-controlled stage (LTS 420, Linkam Scientific, Tadworth, UK). Sample preparation was carried out according to Abramov et al. [7]. In short, 25 µL of emulsion was pipetted between two microscope cover glasses glued on a microscope object slide and covered with a third cover glass. The object slide and cover glasses were tempered to prevent crystallization prior to thermo-optical analysis. Polarized light microscopy allowed distinguishing between isotropic and anisotropic materials; thus, liquid oil appears non-colored and crystalline fat appears as bright colored structures, respectively.
First, trilaurin and tripalmitin dispersions stabilized with only β-lg were cooled at a constant rate of 0.5 K/min to determine the temperature at which the first droplet crystallized. For trilaurin and tripalmitin, this was the case at 15 °C (ΔT = 32 K) and 43 °C (ΔT = 22 K), respectively. Based on these preliminary tests, the three isothermal crystallization temperatures were chosen. First, the temperature at which the first trilaurin or tripalmitin droplet crystallized (ϑcryst,1). Second, 5 K below the temperature of the first droplet crystallization (ϑcryst,2). Third, 10 K below the temperature of the first droplet crystallization (ϑcryst,3). The crystallization temperatures and the supercooling to ϑmelt,DP of the bulk fat are listed in Table 2.

Table 2.
Crystallization temperatures (ϑcryst) and the respective supercooling (ΔTDP) related to ϑmelt,DP applied to trilaurin and tripalmitin dispersions for isothermal crystallization tests.
To investigate the crystallization behavior under isothermal conditions, the emulsions were cooled at a rate of 50 K/min to the respective ϑcryst. They were kept at this temperature for one hour, except for the highest supercooling, where a holding time of 30 min was chosen because the plateau was reached. Pictures were automatically taken at defined time intervals, i.e., every 30 s. The crystallization behavior was investigated directly after preparation, i.e., less than 5 min between preparation and experiment, and after two hours of storage. This procedure was performed for all samples (dispersed phases, emulsifiers) and all supercooling levels.
The pictures taken during the isothermal optical procedure were used to determine the number-based crystallization index according to Abramov et al. [7]. Exemplary sections of microscopic images of an emulsion during the isothermal optical procedure are shown in Figure 1. From left to right, the holding time increases, and more droplets crystallize. Polarized microscopy allows distinguishing liquid droplets, appearing as grey and transparent, and crystalline particles, appearing in bright yellow and green colors. The number of liquid droplets and crystalline particles was determined by counting and used to calculate CIN.
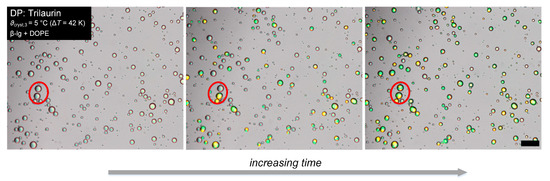
Figure 1.
Exemplary sections of microscopic images of Trilaurin droplets stabilized with β-lg + DOPE during isothermal crystallization at ϑcryst,3. From left to right, the holding time increases. The red circle accentuates droplets that are both liquid at first (left picture), the lower crystallizes first (middle picture), and then both are crystalline after some time (right picture). The length of the scale bar is 50 µm.
The CIN was determined as a function of time at the different supercooling levels and is defined as the relation of the number of crystallized droplets, named particles (Np), to the total number of droplets and particles (Nd + Np):
2.5. Statistical Analysis
All samples were prepared at least three times independently. All tests were performed at least three times if not stated otherwise. Uncertainty was calculated using Origin 2022b (OriginLab Corporation, Northampton, MA, USA) and is expressed as standard deviation.
3. Results and Discussion
3.1. Crystallization Behavior of TAG Droplets Stabilized with β-Lactoglobulin in Dependence of Crystallization Temperature
Before assessing the influence of PL addition on the crystallization behavior of dispersed TAGs, the crystallization behavior of the systems stabilized solely with β-lg was characterized as a function of the crystallization temperature. Hence, the crystallization index CIN over time of trilaurin and tripalmitin droplets stabilized with β-lg is depicted in Figure 2a,b, respectively. For each system, the curves for three different crystallization temperatures (ϑcryst,1, ϑcryst,2, and ϑcryst,3) and, thus, levels of supercooling are shown.
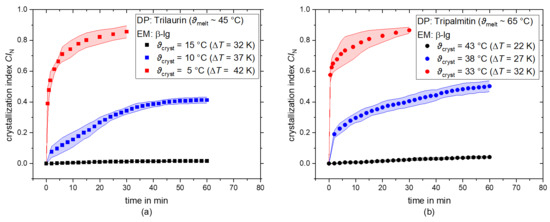
Figure 2.
Number-based crystallization index CIN as a function of time for trilaurin (a) and tripalmitin (b) as dispersed phase (DP). The droplets are stabilized with the emulsifier (EM) β-lg and the CIN is shown for three different crystallization temperatures each (ϑcryst,1, ϑcryst,2, ϑcryst,3). The supercooling to ϑmelt,DP (∆T) is stated for each ϑcryst.
In general, the CIN increases over time until it reaches a plateau. At the highest crystallization temperature (ϑcryst,1), i.e., lowest supercooling, extremely low CIN of 0.02 and 0.04 are achieved in trilaurin and tripalmitin samples, respectively. This means less than 5% of droplets are crystalline. As only a few isolated droplets crystallized at ϑcryst,1, it is highly likely that crystallization via heterogeneous nucleation took place. This temperature appears to provide insufficient supersaturation to induce homogeneous nucleation, and thus, only droplets containing impurities crystallize. As β-lg is almost insoluble in oil, it is more likely that for a volume heterogeneous nucleation, impurities are responsible for this nucleation and not the β-lg. Nevertheless, β-lg present at the droplet surface may act as an impurity,inducing surface heterogeneous nucleation [20,21]. However, a recent study revealed that whey protein isolate delayed the TAG crystal formation at the lipid–solution interface of a model dairy emulsion [48]. Hence, it is reasonable to assume that β-lg did not induce surface heterogeneous nucleation. Increasing the supercooling (ϑcryst,2 and ϑcryst,3), the plateau of CIN significantly increases, independent of the dispersed phase composition. This is because the supersaturation, and thus, the driving force for nucleation and crystal growth, increases with increasing supercooling [20]. We assume that homogeneous nucleation takes place at these temperatures. A CIN of 0.4–0.5 and 0.85–0.9 was achieved for ϑcryst,2 and ϑcryst,3, respectively [19]. To reach a CIN of over 0.8 s, supercooling of 32 K was needed for tripalmitin and 42 K for trilaurin. As we used pure TAG systems, we expected that similar supercooling would be needed to achieve similar CI values. However, we did not further purify the TAGs; hence, there might be differences in purity between tripalmitin and trilaurin, resulting in this behavior. In both cases, a crystallization of 100% of the droplets was not achieved. In general this is a high supercooling level compared, e.g., to alkanes in emulsions, where 12–15 K supercooling is needed for full crystallization [10]. As almost no droplet crystallization was observed with the lowest supercooling (ϑcryst,1), further experiments were carried out at ϑcryst,2 and ϑcryst,3.
3.2. Crystallization Behavior of Dispersed TAGs Stabilized by Mixtures of β-lg and PLs
The influence of using mixtures of PL and β-lg as emulsifiers on the TAG crystallization was compared to the TAG crystallization with only β-lg as an emulsifier present.
First, the general influence of the addition of saturated and unsaturated PLs on the SFC, i.e., CIN, is discussed. The crystallization index CIN over time of trilaurin droplets for the crystallization temperatures ϑcryst,2 = 10 °C and ϑcryst,3 = 5 °C is depicted in Figure 3a,b, respectively. The droplets are either stabilized solely with β-lg (blue squares), β-lg + DOPE (black squares), β-lg + DSPE (red squares), or β-lg + DSPC (green squares). The CIN increases over time in all cases, and a plateau value is reached in most cases in the investigated time frame. In all cases, the saturated PL DSPE leads to an increase in CIN compared to β-lg alone. At a crystallization temperature of 10 °C, a CIN of 0.45 is reached with β-lg + DSPE, and a CIN = 1 is achieved at a temperature of 5 °C, which means all droplets are crystalline. The use of β-lg alone only resulted in a CIN of 0.38 (ϑcryst,2) and 0.8 (ϑcryst,3). In addition, the samples stabilized with β-lg + DSPE reached their plateau value in the first 2.5–5 min, whereas 40–60 min were needed to reach a plateau value in samples stabilized with β-lg alone. DSPE not only increases the SFC but also accelerates the nucleation and crystallization at a certain supercooling. This result seems to indicate that the addition of a saturated PL increases the SFC compared to β-lg alone, as expected. However, the addition of the saturated PL DSPC resulted in the opposite behavior. The CIN at ϑcryst,2 obtained with β-lg + DSPC is ~0.27 lower than the CIN obtained with β-lg alone (CIN = 0.38). Hence, the combination of β-lg + DSPC seems to disturb nucleation and crystal growth, contrary to our expectations. At the highest supercooling (ϑcryst,3), no differences were observed between β-lg and β-lg + DSPC.
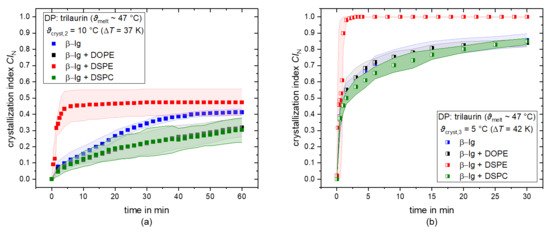
Figure 3.
Number-based crystallization index CIN as a function of time for trilaurin as dispersed phase (DP) for the crystallization temperatures ϑcryst,2 = 10 °C (a) and ϑcryst,3 = 5 °C (b). Droplets are stabilized with β-lg (blue), β-lg + DOPE (black), β-lg + DSPE (red), and β-lg + DSPC (green).
Similar behavior was observed with tripalmitin as dispersed phase. The CIN over time of tripalmitin samples in dependence of the used emulsifiers for the crystallization temperatures ϑcryst,2 = 38 °C and ϑcryst,3 = 33 °C is depicted in Figure 4a,b, respectively. At a crystallization temperature of 33 °C, a CIN = 1 is achieved with β-lg + DSPE (saturated), whereas CIN = 0.8 is achieved with β-lg alone. Moreover, the plateau value is reached in less than two minutes, which is significantly faster than the other emulsifier mixtures, where it took 30–60 min to reach a plateau. However, at a crystallization temperature of 38 °C, a high standard deviation of the mean CIN of β-lg + DSPE can be seen, and a clear conclusion cannot be drawn. A more distinct assertion can be made at a crystallization temperature of 33 °C, where the CIN of samples containing β-lg+ DSPE reaches one in the first 2 min, whereas it takes longer times for the other emulsifier mixtures. When using the saturated PL DSPC, a lower CIN compared to β-lg can be found for ϑcryst,2 = 38 °C, whereas the CIN values are similar for ϑcryst,3 = 33 °C.
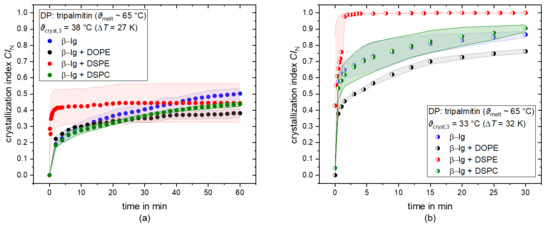
Figure 4.
Number-based crystallization index CIN as a function of time for tripalmitin as dispersed phase (DP) for the crystallization temperatures ϑcryst,2 = 38 C (a) and ϑcryst,3 = 33 °C (b). Droplets are stabilized with β-lg (blue), β-lg + DOPE (black), β-lg + DSPE (red), and β-lg + DSPC (green).
These results show that the addition of a saturated PL to β-lg as an emulsifier does not necessarily result in a higher SFC compared to β-lg alone, which is opposite to our assumption. In some cases, the proposed template effect seems to be in effect [24,26,29], and in some cases, processes that hinder TAG droplet crystallization seem to prevail. The addition of the unsaturated PL DOPE further promotes this assumption. We expected no influence of the unsaturated PL on the crystallization compared to β-lg due to the results of earlier studies [18,31]. However, lower CIN values were obtained with β-lg + DOPE compared to β-lg alone at ϑcryst,2. This is the case for both dispersed phases studied, i.e., trilaurin and tripalmitin. When tripalmitin was present as dispersed phase, this behavior was even observed at the lowest crystallization temperature at ϑcryst,3. In the case of DOPE, one reason could be the unsaturated FAs, which might act as defects hindering nucleation and crystal growth. In addition, due to the difference in FA saturation, the phase transition temperature of the PLs itself differs between DOPE (unsaturated) and DSPE/DSPC (saturated). It is −16 °C (DOPE), significantly lower compared to 74 °C/55 °C (DSPE/DSPC). This means that DOPE is liquid at the investigated crystallization temperatures, whereas DSPE and DSPC both may crystallize. A recent study showed that monoglyceride stearate crystallizes at the O/W interface, forming a heterogenous interfacial layer, and also crystallizes inside the droplet [49]. Moreover, it was shown that the deformation of droplets can be the result of surfactants crystallizing at the interface [49,50,51]. Thus, it is reasonable to assume that PLs can also crystallize at the interface and within the droplet and thereby influence the CIN observed in our study. However, the CIN plateau value obtained with DSPC was also lower compared to using β-lg alone, which means that PL crystallinity alone cannot be the reason for this behavior. In summary, this contradicts our assumption that the addition of saturated PLs (DSPC, DSPE) will increase the SFC compared to unsaturated PLs (DOPE) and β-lg alone.
Apart from the general influence of the addition of PLs on droplet crystallization, we expected that the effect would be more pronounced for a greater similarity of the oil phase to the PL lipophilic tail [22,23]. In our case, tripalmitin is more similar to the lipophilic tail of the PLs than trilaurin. When comparing the use of β-lg + DSPE and β-lg + DSPC, no difference in behavior is observed between trilaurin and tripalmitin. For both crystallization temperatures studied, DSPE (saturated) leads to an increase in CIN compared to β-lg. At ϑcryst,2, DSPC (saturated) leads to a decrease in CIN, and at ϑcryst,3, no differences are found. In these cases, the similarity between PL and dispersed phase had no effect on the SFC. Hence, the template effect was not more pronounced. The only small difference can be seen when DOPE (unsaturated) is used. Here, the lowest CIN values are observed when the unsaturated PL is used in tripalmitin dispersions. In the case of trilaurin, DOPE (unsaturated) and DSPC (saturated) always resulted in similar CIN values. Hence, the fact that DOPE might act as a defect hindering nucleation and crystal growth is more pronounced for the more similar dispersed phase, tripalmitin. However, the results do not clearly indicate that the similarity of the oil phase to the LMWE plays an important role in the droplet crystallization for the investigated system.
Furthermore, we expected an influence of the hydrophilic headgroup on the crystallization and, therefore, SFC at similar supercooling. In all cases, the highest SFCs were observed using DSPE (smaller hydrophilic headgroup), compared to DSPC (larger hydrophilic headgroup), although DSPC should be able to better adsorb at the O/W interface. One possible explanation for the behavior could be due to the PL geometry. While PCs, with their large headgroup, have a morphology of a truncated cone, the hydrophobic moiety dominates over the comparably smaller headgroup in PEs [52]. This would lead to the expectation that more PE molecules are able to adsorb at the curved droplet interface and lead to a higher interfacial load. PCs are reported to form more compact interfacial monolayers as compared to PEs due to PC molecules being in a liquid-condensed state, as opposed to PEs being in a liquid-expanded state [53,54,55]. Hence, the differing geometry between DSPC and DSPE leads to differences in the interfacial load and arrangement at the interface. In order for LMWEs to act as a nucleation template for oil droplet crystallization, their lipophilic moiety has to be arranged in such a way that the oil, or in this case TAG molecules, can be incorporated properly to form the crystal lattice [22,23]. It appears that the hydrophilic headgroup of DSPE leads to a favorable arrangement at the interface compared to DSPC. Due to this favorable arrangement, DSPE might act as a nucleation template for the chosen TAGs, and DSPC might not be able to provide this template, even though the lipophilic moiety is the same for both DSPC and DSPE. Furthermore, authors investigating the properties of PLs at O/W interfaces reported that interactions between PCs and water result in PC hydration and, thus, a sharp drop in oil solubility [56]. These hydrated PC molecules then tend to bind with each other, preventing their desorption from the interface into the oil phase. Such behavior has not been reported for PEs yet. In addition, the crystallinity of the PL itself may be the reason for this behavior. DSPC crystallizes at 55 °C, whereas DSPE crystallizes at 74 °C. Theoretically, both PLs should be able to crystallize at the present temperatures. However, bulk crystallization temperatures differ from crystallization temperatures in mixtures. Hence, DSPE might be able to crystallize faster/at lower supercooling under the given conditions and thereby provide a sufficient template for the nucleating oil. In the case of monoacylglycerides (MAGs), crystallized saturated MAGs were reported to be more effective in displacing whey protein from the interface [57,58]. This leads us to the assumption that the crystallized DSPE may have the same effect, and the desorption of β-lg might be higher in the case of DSPE than DSPC. These free patches at the interface can then be covered by DSPE, inducing surface heterogeneous nucleation. Therefore, the solubility of the PL in the oil, in combination with its crystallinity, may also influence the droplet crystallization behavior, leading to higher SFC with DSPE compared to DSPC. Moreover, complex formation between PLs and β-lg was reported to influence their interfacial behavior [33], and the complex formation may differ depending on the PL’s molecular structure. Thereby, the interface may be occupied differently due to the differing interfacial behavior of these complexes, and their solubility in the TAG phase may differ. All these phenomena may, in summary, lead to the observed crystallization behavior of dispersed TAGs. Future studies are needed to clarify the complex underlying processes and prevailing mechanisms.
3.3. Influence of Time for Emulsifier Rearrangement on Dispersed TAG Crystallization
It has been reported that LMWEs are generally able to displace proteins from droplet interfaces [34,35]. Furthermore, our results, presented in Section 3.2, may be the result of insufficient PL quantities at the interface. Hence, we expected an increased CIN when PLs are given more time to adsorb at the droplet interface and displace proteins. The CIN over time of tripalmitin samples for the crystallization temperature ϑcryst,2 = 38 °C is shown in Figure 5 for β-lg (a), β-lg + DSPC (b), β-lg + DOPE (c), and β-lg + DSPE (d). The CIN over time was obtained using a freshly produced emulsion (closed circles) using an emulsion stored at a temperature above ϑmelt,DP for 2 h (open circles). In the case of β-lg, the CIN of the stored sample increases with a higher slope than the fresh sample, but a similar plateau value of ~ 0.48 is reached. This suggests that the nucleation rate was increased, possibly by a change in the interfacial composition. However, this had no effect on the crystallinity the system could reach at this supercooling. Interestingly, the opposite trend was observed when PLs and β-lg were combined as emulsifiers. Here, the CIN of the freshly produced sample is higher than the CIN of the stored one for all cases. This is the opposite of what we expected. The difference in CIN is small for β-lg + DSPC and β-lg + DOPE, whereas it is significantly larger for β-lg + DSPE. One explanation could be that the displacement of protein did not take place as expected. In addition, LMWEs and proteins have been shown to undergo complexation when co-adsorbed at droplet/particle interfaces [33]. The complexes can then have new characteristics differing from those of the individual emulsifiers. This is what might have happened in this case: during the holding period of 2 h, β-lg and the PL molecules may have formed complexes, which hindered the PL from acting as surface heterogeneous nucleation templates as expected. In addition, the complexation of β-lg and the PLs might also change the solubility of the emulsifiers, resulting in a lower oil solubility compared to the single molecules. Therefore, the volume heterogeneous nucleation would also be less pronounced. The opposite behavior of the samples stabilized by β-lg alone provides additional data suggesting that complexation took place and changed the crystallization behavior. However, crystallization is a very stochastic process differing from droplet to droplet, which is why the scattering of crystallinity values can be rather large. Hence, the comparably large standard deviations do not allow for drawing definitive conclusions but rather indicate possible phenomena.
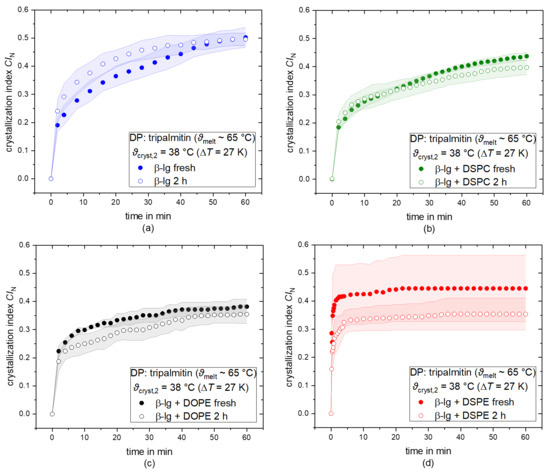
Figure 5.
Number-based crystallization index CIN as a function of time for tripalmitin as dispersed phase (DP) directly after preparation (closed circles) and after a holding time of 2 h above ϑmelt,DP. Droplets are stabilized with β-lg (a, blue), β-lg + DSPC (b, green), β-lg + DOPE (c, black), and β-lg + DSPE (d, red). The CIN is shown for the crystallization temperature ϑcryst,2 = 38 °C.
4. Conclusions
In this study, we investigated the crystallization behavior of trilaurin and tripalmitin droplets emulsified in water, varying the emulsifiers and combinations thereof, as well as the supercooling. We combined the protein β-lg with different PLs as LMWEs, as they are frequently co-formulated to improve the colloidal stability of emulsions and dispersions. Furthermore, LMWEs are able to act as nucleation templates and might thereby help to decrease the required level of supercooling for crystallization. Hence, a suitable choice of protein and LMWE mixtures would allow for achieving specific product properties controlling SFC and take advantage of the stabilization mechanisms of both emulsifiers.
Surprisingly, the addition of PLs did not, in most cases, increase the SFC compared to using β-lg alone, as expected. This leads to the conclusion that droplet crystallization was inhibited in some cases. Our results indicate that the ability of the PL to increase the SFC in the presence of β-lg does not only depend on the lipophilic moiety acting as a nucleating template but also on the size of the hydrophilic moiety. With changing the size of the hydrophilic moiety, differences in oil solubility and packing density at the droplet interface, as well as differences in the interaction with β-lg might occur, which, in turn, can influence droplet crystallization. In addition, complexation between β-lg and PLs might hinder the PL from acting as a surface and volume heterogeneous nucleation template. If the latter is the case, it would be recommendable to start the cooling and crystallization process as soon as possible after the emulsification step. Moreover, if the crystallinity of a system has to be controlled, the interactions of the protein and the PL and their ability to induce droplet nucleation and crystallization must be looked at carefully. An LMWE able to act as a nucleation template does not necessarily provide this ability in combination with a protein.
We acknowledge some limitations in this study. Our study is observational, and our experimental design was based on analytical methods that do not allow us to draw conclusions on the actual composition of the interfacial layer and its changes over time. Future research should attempt to gain a deeper understanding of the underlying mechanisms and try to localize the PLs and their mode of interaction and complex formation with β-lg, as well as the influence of these complexes on the ability of PLs to initiate droplet crystallization.
In summary, we demonstrated in this study that the use of PLs as co-emulsifiers with β-lg can result in a decrease or increase in the dispersed TAG crystallinity compared to systems containing β-lg alone. Whether a decrease or increase in TAG crystallinity is observed highly depends on the nature of the PL. In addition, the duration between emulsification and crystallization can also be an important factor influencing the crystallinity. Our study adds data on TAG droplet crystallization and strongly suggests that there are complex underlying processes that still need much research to clarify and reasonably adjust formulations and process parameters to control the nucleation and crystallization behavior itself.
Author Contributions
Conceptualization, J.R.; methodology, J.R.; formal analysis, J.R., M.S. and L.H; investigation, J.R., M.S. and L.H.; resources, H.P.K.; data curation, J.R., M.S., and L.H.; writing—original draft preparation, J.R.; writing—review and editing, V.G. and H.P.K.; visualization, J.R.; funding acquisition, H.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted in the frame of IGF Project no. 21099 N of the FEI and was supported via AiF within the program for promoting the Industrial Collective Research (IGF) of the Federal Ministry of Economic Affairs and Climate Action (BMWK), based on a resolution of the German Parliament.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
We acknowledge support by the KIT-Publication Fund of the Karlsruhe Institute of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McClements, D.J. Food Emulsions; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780429123894. [Google Scholar]
- Boode, K.; Walstra, P. Partial coalescence in oil-in-water emulsions 1. Nature of the aggregation. Colloids Surf. A Physicochem. Eng. Asp. 1993, 81, 121–137. [Google Scholar] [CrossRef]
- Awad, T.S.; Helgason, T.; Kristbergsson, K.; Decker, E.A.; Weiss, J.; McClements, D.J. Effect of Cooling and Heating Rates on Polymorphic Transformations and Gelation of Tripalmitin Solid Lipid Nanoparticle (SLN) Suspensions. Food Biophys. 2008, 3, 155–162. [Google Scholar] [CrossRef]
- Walstra, P. Overview of Emulsion and Foam Stability. In Food Emulsions and Foams: Interfaces, Interactions and Stability; Dickinson, E., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 1987; pp. 242–257. ISBN 978-1-85573-785-3. [Google Scholar]
- Vanapalli, S.A.; Palanuwech, J.; Coupland, J.N. Stability of emulsions to dispersed phase crystallization: Effect of oil type, dispersed phase volume fraction, and cooling rate. Colloids Surf. A Physicochem. Eng. Asp. 2002, 204, 227–237. [Google Scholar] [CrossRef]
- Töpel, A. Chemie und Physik der Milch; Behr’s Verlag DE: Hamburg, Germany, 2015; ISBN 9783954683604. [Google Scholar]
- Abramov, S.; Ruppik, P.; Schuchmann, H. Crystallization in Emulsions: A Thermo-Optical Method to Determine Single Crystallization Events in Droplet Clusters. Processes 2016, 4, 25. [Google Scholar] [CrossRef]
- Abramov, S.; Berndt, A.; Georgieva, K.; Ruppik, P.; Schuchmann, H.P. Investigation of the influence of mean droplet size and shear rate on crystallization behavior of hexadecane-in-water dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 513–522. [Google Scholar] [CrossRef]
- Abramov, S.; Ahammou, A.; Karbstein, H.P. Influence of external forces during supercooling on dispersion stability during melt emulsification. Chem. Eng. Technol. 2018, 41, 768–775. [Google Scholar] [CrossRef]
- Abramov, S.; Shah, K.; Weißenstein, L.; Karbstein, H. Effect of Alkane Chain Length on Crystallization in Emulsions during Supercooling in Quiescent Systems and under Mechanical Stress. Processes 2018, 6, 6. [Google Scholar] [CrossRef]
- Herhold, A.B.; Ertaş, D.; Levine, A.J.; King, H.E., Jr. Impurity mediated nucleation in hexadecane-in-water emulsions. Phys. Rev. E 1999, 59, 6946. [Google Scholar] [CrossRef]
- Lopez, C.; Lesieur, P.; Keller, G.; Ollivon, M. Thermal and Structural Behavior of Milk Fat. J. Colloid Interface Sci. 2000, 229, 62–71. [Google Scholar] [CrossRef]
- Tippetts, M.; Martini, S. Effect of cooling rate on lipid crystallization in oil-in-water emulsions. Food Res. Int. 2009, 42, 847–855. [Google Scholar] [CrossRef]
- Kaysan, G.; Spiegel, B.; Guthausen, G.; Kind, M. Influence of Shear Flow on the Crystallization of Organic Melt Emulsions—A Rheo-Nuclear Magnetic Resonance Investigation. Chem. Eng. Technol. 2020, 43, 1699–1705. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Cogne, C.; Lafferrere, L.; Salvatori, F.; Ardaud, P.; Zanetti, M.; Puel, F. Effects of surfactants on crystallization of ethylene glycol distearate in oil-in-water emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 93–100. [Google Scholar] [CrossRef]
- Bunjes, H.; Koch, M.H.J.; Westesen, K. Influence of emulsifiers on the crystallization of solid lipid nanoparticles. J. Pharm. Sci. 2003, 92, 1509–1520. [Google Scholar] [CrossRef]
- McClements, D.J.; Dungan, S.R.; German, J.B.; Simoneau, C.; Kinsella, J.E. Droplet Size and Emulsifier Type Affect Crystallization and Melting of Hydrocarbon-in-Water Emulsions. J. Food Sci. 1993, 58, 1148–1151. [Google Scholar] [CrossRef]
- Bunjes, H.; Koch, M.H.J. Saturated phospholipids promote crystallization but slow down polymorphic transitions in triglyceride nanoparticles. J. Control. Release 2005, 107, 229–243. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hartel, R.W. Crystallization in Foods; Food Engineering Series; Aspen Publishers: Frederick, MD, USA, 2001; ISBN 978-0-8342-1634-1. [Google Scholar]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revised. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Palanuwech, J.; Coupland, J.N. Effect of surfactant type on the stability of oil-in-water emulsions to dispersed phase crystallization. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 251–262. [Google Scholar] [CrossRef]
- Awad, T.; Sato, K. Acceleration of crystallisation of palm kernel oil in oil-in-water emulsion by hydrophobic emulsifier additives. Colloids Surf. B Biointerfaces 2002, 25, 45–53. [Google Scholar] [CrossRef]
- Arima, S.; Ueno, S.; Ogawa, A.; Sato, K. Scanning microbeam small-angle X-ray diffraction study of interfacial heterogeneous crystallization of fat crystals in oil-in-water emulsion droplets. Langmuir 2009, 25, 9777–9784. [Google Scholar] [CrossRef]
- Gülseren, İ.; Coupland, J.N. Surface Melting in Alkane Emulsion Droplets as Affected by Surfactant Type. J. Am. Oil Chem. Soc. 2008, 85, 413–419. [Google Scholar] [CrossRef]
- Awad, T.; Sato, K. Effects of hydrophobic emulsifier additives on crystallization behavior of palm mid fraction in oil-in-water emulsion. J. Am. Oil Chem. Soc. 2001, 78, 837–842. [Google Scholar] [CrossRef]
- Awad, T.S. Ultrasonic studies of the crystallization behavior of two palm fats O/W emulsions and its modification. Food Res. Int. 2004, 37, 579–586. [Google Scholar] [CrossRef]
- Katsuragi, T.; Kaneko, N.; Sato, K. Effects of addition of hydrophobic sucrose fatty acid oligoesters on crystallization rates of n-hexadecane in oil-in-water emulsions. Colloids Surf. B Biointerfaces 2001, 20, 229–237. [Google Scholar] [CrossRef]
- McClements, D.; Dickinson, E.; Dungan, S.R.; Kinsella, J.E.; Ma, J.G.; Povey, M.J. Effect of Emulsifier Type on the Crystallization Kinetics of Oil-in-Water Emulsions Containing a Mixture of Solid and Liquid Droplets. J. Colloid Interface Sci. 1993, 160, 293–297. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H.; Koch, M.H.J.; Westesen, K. Effects of surfactants on the crystallization and polymorphism of lipid nanoparticles. In Molecular Organisation on Interfaces; Lagaly, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 7–10. ISBN 978-3-540-43637-9. [Google Scholar]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.A.; van Vliet, T. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef]
- Heiden-Hecht, T.; Drusch, S. Impact of Saturation of Fatty Acids of Phosphatidylcholine and Oil Phase on Properties of β-Lactoglobulin at the Oil/Water Interface. Food Biophys. 2022, 17, 171–180. [Google Scholar] [CrossRef]
- Heiden-Hecht, T.; Taboada, M.L.; Brückner-Gühmann, M.; Karbstein, H.P.; Gaukel, V.; Drusch, S. Towards an improved understanding of spray-dried emulsions: Impact of the emulsifying constituent combination on characteristics and storage stability. Int. Dairy J. 2021, 121, 105134. [Google Scholar] [CrossRef]
- Waninge, R.; Walstra, P.; Bastiaans, J.; Nieuwenhuijse, H.; Nylander, T.; Paulsson, M.; Bergenståhl, B. Competitive adsorption between beta-casein or beta-lactoglobulin and model milk membrane lipids at oil-water interfaces. J. Agric. Food Chem. 2005, 53, 716–724. [Google Scholar] [CrossRef]
- Euston, S.E.; Singh, H.; Munro, P.A.; Dalgleish, D.G. Competitive Adsorption Between Sodium Caseinate and Oil-Soluble and Water-Soluble Surfactants in Oil-in-Water Emulsions. J. Food Sci. 1995, 60, 1124–1131. [Google Scholar] [CrossRef]
- Dickinson, E.; Euston, S.R.; Woskett, C.M. Competitive adsorption of food macromolecules and surfactants at the oil-water interface. In Surfactants and Macromolecules: Self-Assembly at Interfaces and in Bulk; Lindman, B., Rosenholm, J.B., Stenius, P., Eds.; Steinkopff: Darmstadt, Germany, 1990; pp. 65–75. ISBN 978-3-7985-0838-5. [Google Scholar]
- Bylaite, E.; Nylander, T.; Venskutonis, R.; Jönsson, B. Emulsification of caraway essential oil in water by lecithin and beta-lactoglobulin: Emulsion stability and properties of the formed oil-aqueous interface. Colloids Surf. B Biointerfaces 2001, 20, 327–340. [Google Scholar] [CrossRef]
- Fang, Y.; Dalgleish, D.G. Comparison of the effects of three different phosphatidylcholines on casein-stabilized oil-in-water emulsions. J. Am. Oil Chem. Soc. 1996, 73, 437–442. [Google Scholar] [CrossRef]
- Brown, E.M. Interactions of β-Lactoglobulin and α-Lactalbumin with Lipids: A Review. J. Dairy Sci. 1984, 67, 713–722. [Google Scholar] [CrossRef]
- Fang, Y.; Dalgleish, D.G. Interactions between Sodium Caseinate and Dioleoylphosphatidylcholine on Oil–Water Interfaces and in Solution. In Food Colloids; Elsevier: Amsterdam, The Netherlands, 2004; pp. 67–76. ISBN 9781855737839. [Google Scholar]
- Nylander, T.; Arnebrant, T.; Cárdenas, M.; Bos, M.; Wilde, P. Protein/Emulsifier Interactions. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 101–192. ISBN 978-3-030-29185-3. [Google Scholar]
- Dijkstra, A.J. About water degumming and the hydration of non-hydratable phosphatides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600496. [Google Scholar] [CrossRef]
- Bot, F.; Cossuta, D.; O’Mahony, J.A. Inter-relationships between composition, physicochemical properties and functionality of lecithin ingredients. Trends Food Sci. Technol. 2021, 111, 261–270. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Szuhaj, B.F. Effects of lecithins and proteins on the stability of emulsions. Fett Wiss. Technol. 1998, 100, 282–291. [Google Scholar] [CrossRef]
- Reiner, J.; Martin, D.; Ott, F.; Harnisch, L.; Gaukel, V.; Karbstein, H.P. Influence of the Triglyceride Composition, Surfactant Concentration and Time–Temperature Conditions on the Particle Morphology in Dispersions. Colloids Interfaces 2023, 7, 22. [Google Scholar] [CrossRef]
- MacWilliams, S.V.; Sebben, D.A.; Clulow, A.J.; Ferri, J.K.; Gillies, G.; Golding, M.; Boyd, B.J.; Beattie, D.A.; Krasowska, M. The effect of emulsifier type on the secondary crystallisation of monoacylglycerol and triacylglycerols in model dairy emulsions. J. Colloid Interface Sci. 2022, 608, 2839–2848. [Google Scholar] [CrossRef]
- Zhou, X.; Arita-Merino, N.; Meesters, G.; Sala, G.; Sagis, L.M. Emulsifier crystal formation and its role in periodic deformation-relaxation of emulsion droplets upon cooling. J. Food Eng. 2023, 347, 111430. [Google Scholar] [CrossRef]
- Feng, J.; Valkova, Z.; Lin, E.E.; Nourafkan, E.; Wang, T.; Tcholakova, S.; Slavchov, R.; Smoukov, S.K. Minimum surfactant concentration required for inducing self-shaping of oil droplets and competitive adsorption effects. Soft Matter 2022, 18, 6729–6738. [Google Scholar] [CrossRef] [PubMed]
- Reiner, J.; Walter, E.M.; Karbstein, H.P. Assessment of droplet self-shaping and crystallization during temperature fluctuations exceeding the melting temperature of the dispersed phase. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130498. [Google Scholar] [CrossRef]
- Salminen, H.; Helgason, T.; Aulbach, S.; Kristinsson, B.; Kristbergsson, K.; Weiss, J. Influence of co-surfactants on crystallization and stability of solid lipid nanoparticles. J. Colloid Interface Sci. 2014, 426, 256–263. [Google Scholar] [CrossRef]
- Handa, T.; Saito, H.; Miyajima, K. Phospholipid monolayers at the triolein-saline interface: Production of microemulsion particles and conversion of monolayers to bilayers. Biochemistry 1990, 29, 2884–2890. [Google Scholar] [CrossRef] [PubMed]
- Bergenståhl, B.; Alander, J. Lipids and colloidal stability. Current Opinion. Colloid Interface Sci. 1997, 2, 590–595. [Google Scholar]
- Friberg, S.E. Food Emulsions: Emulsion Stability, 3rd ed.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Shchipunov, Y.A.; Kolpakov, A.F. Phospholipids at the oil/water interface: Adsorption and interfacial phenomena in an electric field. Adv. Colloid Interface Sci. 1991, 35, 31–138. [Google Scholar] [CrossRef]
- Barford, N.M.; Krog, N.; Larsen, G.; Buchheim, W. Effects of Emulsifiers on Protein-Fat Interaction in Ice Cream Mix during Ageing I: Quantitative Analyses. Fett Wiss. Technol. 1991, 93, 24–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Goff, H.D. On fat destabilization and composition of the air interface in ice cream containing saturated and unsaturated monoglyceride. Int. Dairy J. 2005, 15, 495–500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).