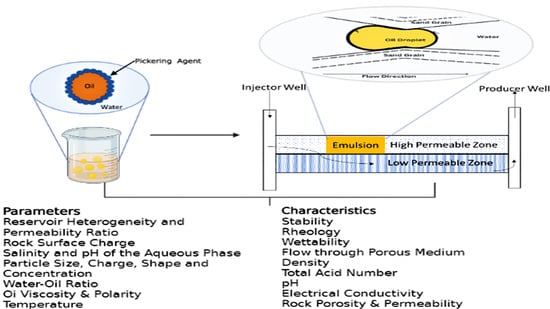
A Perspective on the Prospect of Pickering Emulsion in Reservoir Conformance Control with Insight into the Influential Parameters and Characterization Techniques
Abstract
:1. Introduction
2. Emulsion for Conformance Control
2.1. Type of Emulsions
- i.
- Emulsion type based on Droplet Size:
- ii.
- Type on basis of Dispersion Medium:
- iii.
- Emulsion type on basis of Emulsifier:
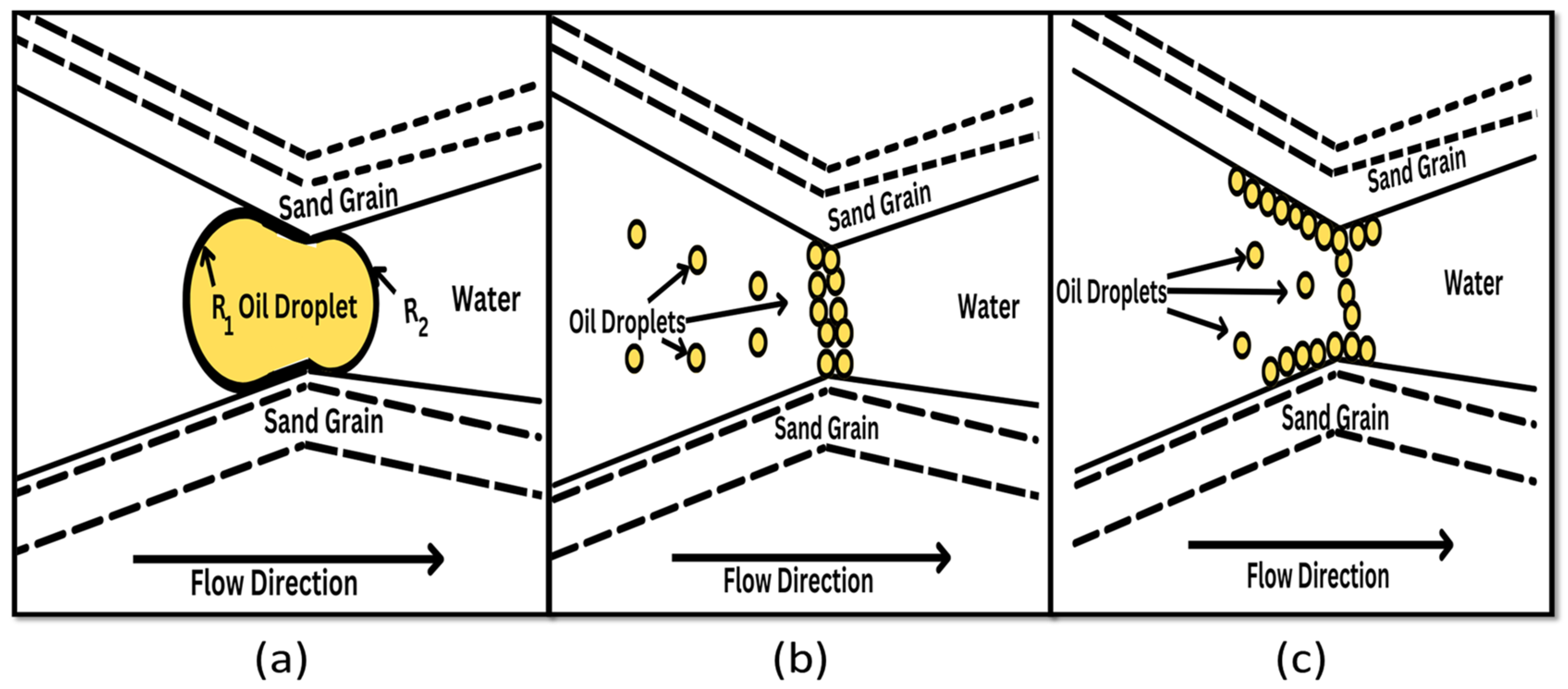
2.2. Mechanism of Plugging in Emulsions
2.3. Drawback of Surfactants and Potential of Pickering Emulsifiers
2.4. Mechanisms of Pickering
3. Influential Parameters Affecting Pickering Emulsion Design and Performance
3.1. Reservoir Heterogeneity and Permeability Ratio
3.2. Rock Surface Charge
3.3. Salinity and pH of the Aqueous Phase
3.4. Particle Characteristics
- i.
- Particle Size:
- ii.
- Particle Charge:
- iii.
- Particle Shape:
- iv.
- Wettability of solid particle:
- v.
- Particle Concentration:
- vi.
- Selection of Pickering Particle:
3.5. Water–Oil Ratio
3.6. Oil Viscosity and Polarity
3.7. Temperature
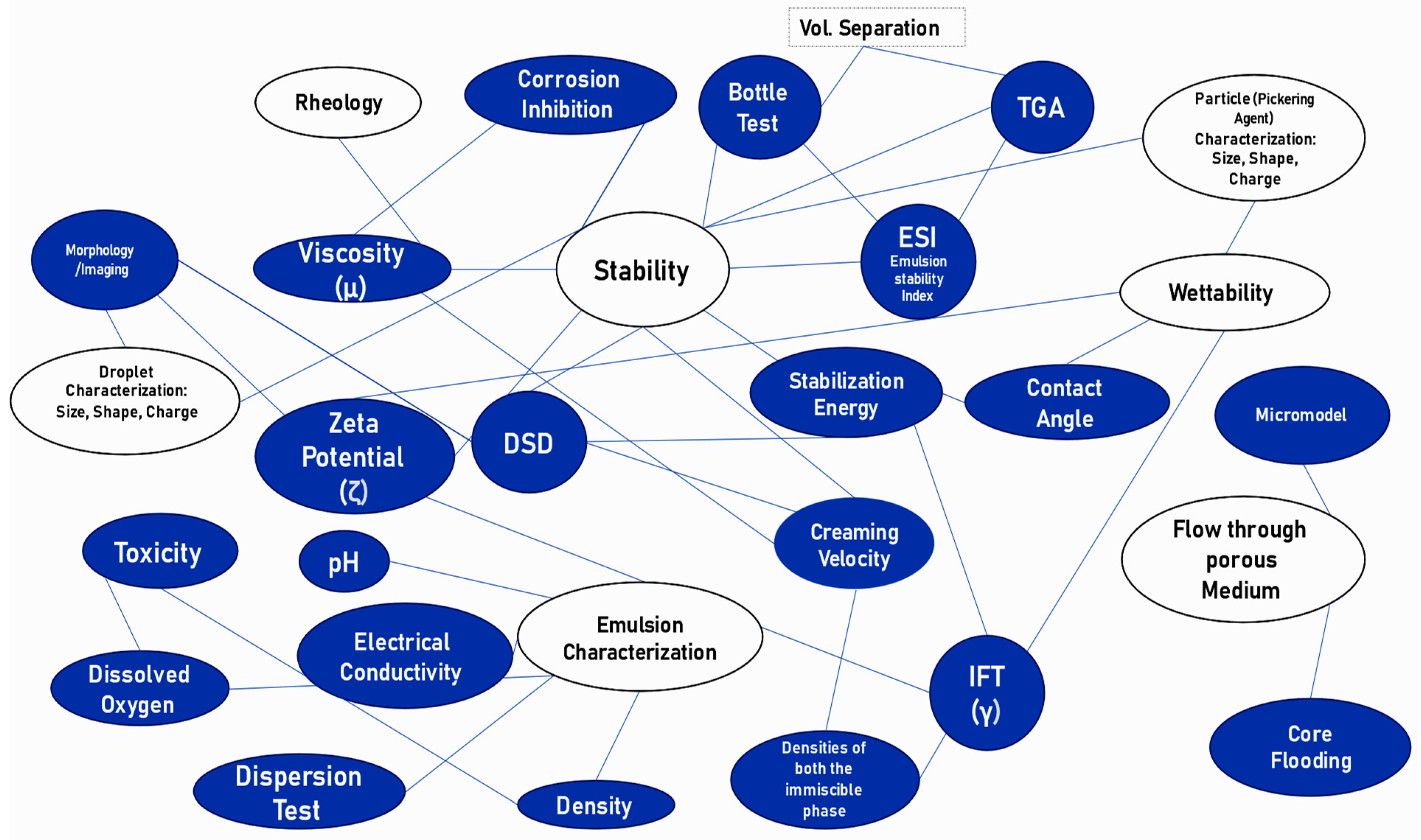
4. Emulsion Characteristics and Measuring Techniques
4.1. Stability
- i.
- Bottle Test:
- ii.
- Emulsion Stability Index (ESI):
- iii.
- Imaging/Microscopy:
- iv.
- Droplet Size Distribution (DSD):
- v.
- Zeta potential:
4.2. Rheology
4.3. Wettability
4.4. Flow through Porous Medium
- Microfluidic Study:
- ii.
- Core Flooding:
4.5. Basic Characterization
- i.
- Density:
- ii.
- Viscosity:
- iii.
- Total acid number:
- iv.
- pH:
- v.
- Electrical Conductivity:
5. In Situ vs. Preformed Emulsion
6. Micro vs. Macroemulsion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA World Energy Outlook. World Energy Outlook. IEA, Paris License: CC BY 4.0. 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 12 May 2023).
- Smith, L.R.; Fast, C.R.; Wagner, O.R. Development and Field Testing of Large Volume Remedial Treatments for Gross Water Channeling. J. Pet. Technol. 1969, 21, 1015–1025. [Google Scholar] [CrossRef]
- Willman, B.T. Method for Control of Water Injection Profiles. U.S. Patent 3,251,414, 17 May 1966. [Google Scholar]
- Grattoni, C.A.; Jing, X.D.; Zimmerman, R.W. Disproportionate permeability reduction when a silicate gel is formed in-situ to control water production. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 25–28 March 2001; p. SPE-69534. [Google Scholar]
- Kabir, A.H. Chemical Water & Gas Shutoff Technology–An Overview. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 8–9 October 2001. SPE-72119-MS. [Google Scholar]
- Borling, D.; Chan, K.; Hughes, T.; Sydnask, R. Pushing out the oil with conformance control. Oilf. Rev. 1994, 6, 44–58. [Google Scholar]
- Zhang, X.; Deng, J.-N.; Yang, K.; Li, Q.; Meng, S.-Y.; Sun, X.-X.; Song, Z.-Z.; Tian, Y.-D.; Zhang, S.-A.; Liu, X.-J.; et al. High-strength and self-degradable sodium alginate/polyacrylamide preformed particle gels for conformance control to enhance oil recovery. Pet. Sci. 2022, 19, 3149–3158. [Google Scholar] [CrossRef]
- Sagbana, P.I.; Abushaikha, A.S. A comprehensive review of the chemical-based conformance control methods in oil reservoirs. J. Pet. Explor. Prod. Technol. 2021, 11, 2233–2257. [Google Scholar] [CrossRef]
- Texter, J. Graphene oxide and graphene flakes as stabilizers and dispersing aids. Curr. Opin. Colloid Interface Sci. 2015, 20, 454–464. [Google Scholar] [CrossRef]
- Gogarty, W.B.; Tosch, W.C. Miscible-Type Waterflooding: Oil Recovery with Micellar Solutions. J. Pet. Technol. 1968, 20, 1407–1414. [Google Scholar] [CrossRef]
- McAuliffe, C.D. Crude oil-in-water emulsions to improve fluid flow in an oil reservoir. J. Pet. Technol. 1973, 25, 721–726. [Google Scholar] [CrossRef]
- McAuliffe, C.D. Oil-in-water emulsions and their flow properties in porous media. J. Pet. Technol. 1973, 25, 727–733. [Google Scholar] [CrossRef]
- Bai, B.; Han, M.; Li, Y.; Wei, M.; Gao, Y.; Coste, J.P. Selective water shutoff technology study and application of W/O emulsions. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
- Davis, J.A., Jr.; Jones, S.C. Displacement mechanisms of micellar solutions. J. Pet. Technol. 1968, 20, 1415–1428. [Google Scholar] [CrossRef]
- Holm, L. Use of soluble oils for oil Recovery. J. Pet. Technol. 1971, 23, 1475–1483. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Chen, W.; Liu, B.; Zhang, X. A comprehensive review of emulsion and its field application for enhanced oil recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef]
- Mandal, A.; Samanta, A.; Bera, A.; Ojha, K. Characterization of Oil−Water Emulsion and Its Use in Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2010, 49, 12756–12761. [Google Scholar] [CrossRef]
- Elraies, K.A.; Tan, I.M. Design and Application of a New Acid-Alkali-Surfactant Flooding Formulation for Malaysian Reservoirs. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Brisbane, Australia, 18–20 October 2010. [Google Scholar] [CrossRef]
- Hoefner, H.S.; Fogler, M.L. Effective Matrix Acidizing in Cabonates using Microemulsions. Chem. Eng. Prog. 1985, 81, 40–44. [Google Scholar]
- Hoefner, M.L.; Michigan, U.; Fogler, H.S.; Michigan, U. Role of Acid Diffusion in Matrix Acidizing of Carbonates. J. Pet. Technol. 1987, 39, 203–208. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Solling, T.; Sultan, A.S.; Saikia, T. Emulsified acid systems for oil well stimulation: A review. J. Pet. Sci. Eng. 2022, 208, 109569. [Google Scholar] [CrossRef]
- Guidry, G.S.; Ruiz, G.A.; Saxon, A. SXE/N2 Matrix Acidizing. In Proceedings of the SPE Middle East Oil Technical Conference and Exhibition, Bahrain, 11–14 March 1989; pp. 1–10, SPE-17951-MS. [Google Scholar] [CrossRef]
- Chang, F.F.; Cairns, A.J.; Sayed, M.A. Acid retardation for deeper stimulation—Revisiting the chemistry and evaluation methods. Can. J. Chem. Eng. 2022, 100, 1298–1308. [Google Scholar] [CrossRef]
- Gardner, T.R.; Dill, W.R.; Ford, W.G.F.; King, K.L. Well Acidizing Compositions and Method. U.S. Patent 5,034,140, 23 July 1991. [Google Scholar]
- Al-Anazi, H.A.; Nasr-El-Din, H.A.; Mohamed, S.K. Stimulation of Tight Carbonate Reservoirs Using Acid-in-Diesel Emulsions: Field Application. In Proceedings of the SPE International Symposium on Formation Damage Control, Lafayette, LA, USA, 18–19 February 1998; pp. 9–17, SPE-39418-MS. [Google Scholar]
- Yousufi, M.M.; Elhaj, M.E.M.; Moniruzzaman, M.; Ayoub, M.A.; Nazri, A.B.M.; Husin, H.B.; Saaid, I.B.M. Synthesis and evaluation of Jatropha oil-based emulsified acids for matrix acidizing of carbonate rocks. J. Pet. Explor. Prod. Technol. 2019, 9, 1119–1133. [Google Scholar] [CrossRef]
- Yousufi, M.M.; Elhaj, M.E.M.; Moniruzzaman, M. Comparative Analysis of Corrosion Inhibition: Between Jatrophacurcas, Palm and Diesel Oil based Emulsified Acids for Acid Stimulation Operations. IOP Conf. Ser. Earth Environ. Sci. 2018, 164, 012006. [Google Scholar] [CrossRef]
- Sayed, M.A.; Nasr-El-Din, H.A.; Nasrabadi, H. Reaction of emulsified acids with dolomite. J. Can. Pet. Technol. 2013, 52, 164–175. [Google Scholar] [CrossRef]
- Sidaoui, Z.; Sultan, A.S. Formulating a Stable Emulsified Acid at High Temperatures: Stability and Rheology Study. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016; pp. 1–17, IPTC-19012-MS. [Google Scholar] [CrossRef]
- Buijse, M.A.; Van Domelen, M.S. Novel Application of Emulsified Acids to Matrix Stimulation of Heterogeneous Formations. In Proceedings of the SPE International Symposium on Formation Damage Control, Lafayette, LA, USA, 18–19 February 1998; pp. 601–611, SPE-39583-MS. [Google Scholar]
- Zakaria, A.S.; Nasr-El-Din, H.A. Application of Novel Polymer Assisted Emulsified Acid System Improves the Efficiency of Carbonate Acidizing. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; p. D021S005R008. [Google Scholar] [CrossRef]
- Xiong, C.; Zhou, F.; Liu, Y.; Yang, X.; Liu, X.; Shi, Y.; Tan, Y.; Zhang, F.; Ji, X.; Qin, S.; et al. Application and Study of Acid Technique Using Novel Selective Emulsified Acid System. In Proceedings of the CPS/SPE International Oil & Gas Conference and Exhibition, Beijing, China, 8–10 June 2010; pp. 4–9, SPE-131216-MS. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, M.; Husein, M.; Bryant, S. Effects of Oil Viscosity on the Plugging Performance of Oil-in-Water Emulsion in Porous Media. Ind. Eng. Chem. Res. 2018, 57, 7301–7309. [Google Scholar] [CrossRef]
- Yu, L.; Dong, M.; Ding, B.; Yuan, Y. Experimental study on the effect of interfacial tension on the conformance control of oil-in-water emulsions in heterogeneous oil sands reservoirs. Chem. Eng. Sci. 2018, 189, 165–178. [Google Scholar] [CrossRef]
- Pal, N.; Alzahid, Y.; AlSofi, A.; Ali, M.; Zhang, X.; Hoteit, H. Technical Evaluation of Conformance Improvement Technology with Anionic Surfactant-Stabilized Microemulsions in Porous Media. SSRN Electron. J. 2022, preprint. [Google Scholar] [CrossRef]
- Gadhave, A.D.; Waghmare, J.T. A Short Review on Microemulsion and Its Application in Extraction of Vegetable Oil. IJRET Int. J. Res. Eng. Technol. 2014, 3, 147–158. Available online: http://ijret.org/Volumes/V03/I09/IJRET_110309022.pdf (accessed on 12 May 2023).
- Al-Mutairi, S.H.; Nasr-El-Din, H.A.; Hill, A.D. Droplet size analysis of emulsified acid. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 9–11 May 2009; pp. 9–11. [Google Scholar] [CrossRef]
- Rezaei, N.; Firoozabadi, A. Macro- and microscale waterflooding performances of crudes which form w/o emulsions upon mixing with brines. Energy Fuels 2014, 28, 2092–2103. [Google Scholar] [CrossRef]
- Tadros, T.F. Fundamental principles of emulsion rheology and their applications. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 39–55. [Google Scholar] [CrossRef]
- Yu, L.; Sang, Q.; Dong, M.; Yuan, Y. Effects of Interfacial Tension and Droplet Size on the Plugging Performance of Oil-in-Water Emulsions in Porous Media. Ind. Eng. Chem. Res. Ind. Eng. Chem. Res. 2017, 56, 9237–9246. [Google Scholar] [CrossRef]
- Soo, H.; Radke, C.J. The Flow Mechanism of Dilute, Stable Emulsions in Porous Media. Ind. Eng. Chem. Fundam. 1984, 23, 342–347. [Google Scholar] [CrossRef]
- Wang, F. Study on Rules of Emulsion Flow in Porous Media; Northwest University: Xi’an, China, 2005. [Google Scholar]
- Al-Yaari, M.; Hussein, I.A.; Al-Sarkhi, A. Pressure drop reduction of stable water-in-oil emulsions using organoclays. Appl. Clay Sci. 2014, 95, 303–309. [Google Scholar] [CrossRef]
- Stavland, A.; Andersen, K.I.; Sandoey, B.; Tjomsland, T.; Mebratu, A.A. How to apply a blocking gel system for bullhead selective water shutoff: From laboratory to field. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Hossain, K.M.Z.; Deeming, L.; Edler, K.J. Recent progress in Pickering emulsions stabilised by bioderived particles. RSC Adv. 2021, 11, 39027–39044. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Presley, C.L.; Militello, M.M.; Barber, C.; Ladd, R.; Laughter, M.; Ferguson, H.; Dewey, J.B.; Pulsipher, K.J.B.; Rundle, C.W.; Dunnick, C.A. The History of Surfactants and Review of Their Allergic and Irritant Properties. Dermatitis 2021, 32, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. Available online: https://www.frontiersin.org/articles/10.3389/fnut.2022.864943 (accessed on 12 May 2023). [CrossRef] [PubMed]
- Sarkar, A.; Zhang, S.; Holmes, M.; Ettelaie, R. Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics. Adv. Colloid Interface Sci. 2019, 263, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ma, Q.; Liao, K.; An, J.; Bai, J.; He, Y. Application of Pickering emulsion in oil drilling and production. Nanotechnol. Rev. 2022, 11, 26–39. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, P.; Colon, T.; Huh, C.; Balhoff, M. A microfluidic investigation of the synergistic effect of nanoparticles and surfactants in macro-emulsion-based enhanced oil recovery. SPE J. 2017, 22, 459–469. [Google Scholar] [CrossRef]
- Kim, I.; Worthen, A.J.; Lotfollahi, M.; Johnston, K.P.; Dicarlo, D.A.; Huh, C. Nanoparticle-stabilized emulsions for improved mobility control for adverse-mobility waterflooding. In Proceedings of the IOR 2017-19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar] [CrossRef]
- Pandey, A.; Telmadarreie, A.; Trifkovic, M.; Bryant, S. Cellulose nanocrystal stabilized emulsions for conformance control and fluid diversion in porous media. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018; pp. 24–26. [Google Scholar] [CrossRef]
- Li, S.; Yu, L.; Lau, H.C.; Stubbs, L.P. Experimental Study of Mobility Control by Clay-Stabilized Pickering Emulsions in High-Salinity Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Saikia, T.; Sultan, A.; Barri, A.A.; Khamidy, N.I.; Shamsan, A.A.; Almohsin, A.; Bataweel, M. Development of pickering emulsified polymeric gel system for conformance control in high temperature reservoirs. J. Pet. Sci. Eng. 2020, 184, 106596. [Google Scholar] [CrossRef]
- Mahboob, A.; Sultan, A.S.; Adewunmi, A.A.; Saikia, T.; Kamal, M.S. Emulsified Silica Gel for Deep Reservoir Water Conformance Control. Energy Fuels 2023, 37, 4331–4340. [Google Scholar] [CrossRef]
- Wang, W.; Dong, X.; Liu, H.; Peng, Y.; Chen, Z.; Li, Y.; Guo, Y. Fly Ash Nanoparticle-Stabilized Emulsions for Improve Mobility Control Application. In Proceedings of the SPE EuropEC-Europe Energy Conference featured at the 83rd EAGE Annual Conference & Exhibition, Madrid, Spain, 6–9 June 2022. [Google Scholar] [CrossRef]
- Binks, B.P.; Desforges, A.; Duff, D.G. Synergistic stabilization of emulsions by a mixture of surface-active nanoparticles and surfactant. Langmuir 2007, 23, 1098–1106. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Pal, N.; Mandal, A. Oil recovery mechanisms of Pickering nanoemulsions stabilized by surfactant-polymer-nanoparticle assemblies: A versatile surface energies’ approach. Fuel 2020, 276, 118138. [Google Scholar] [CrossRef]
- Sadati, E.Y.; Sahraei, E. An experimental investigation on enhancing water flooding performance using oil-in-water emulsions in an Iranian oil reservoir. J. Pet. Explor. Prod. Technol. 2019, 9, 2613–2624. [Google Scholar] [CrossRef]
- Zhou, X.; AlOtaibi, F.M.; Kamal, M.S.; Kokal, S.L. An experimental study on oil recovery performance using in situ supercritical CO2-Emulsion for carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2020. [Google Scholar] [CrossRef]
- Sun, Z.; Pu, W.; Zhao, R.; Pang, S. Study on the mechanism of W/O emulsion flooding to enhance oil recovery for heavy oil reservoir. J. Pet. Sci. Eng. 2022, 209, 109899. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Colloidal Particles at Liquid Interfaces; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Sorbier, Q.M.D.; Aimable, A.; Pagnoux, C. Influence of the electrostatic interactions in a Pickering emulsion polymerization for the synthesis of silica–polystyrene hybrid nanoparticles. J. Colloid Interface Sci. 2015, 448, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Lagaly, G.; Reese, M.; Abend, S. Smectites as colloidal stabilizers of emulsions: I. Preparation and properties of emulsions with smectites and nonionic surfactants. Appl. Clay Sci. 1999, 14, 83–103. [Google Scholar] [CrossRef]
- Teo, S.H.; Chee, C.Y.; Fahmi, M.Z.; Sakti, S.C.W.; Lee, H.V. Review of Functional Aspects of Nanocellulose-Based Pickering Emulsifier for Non-Toxic Application and Its Colloid Stabilization Mechanism. Molecules 2022, 27, 7170. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef]
- Mao, X.; Yang, D.; Xie, L.; Liu, Q.; Tang, T.; Zhang, H.; Zeng, H. Probing the Interactions between Pickering Emulsion Droplets Stabilized with pH-Responsive Nanoparticles. J. Phys. Chem. B 2021, 125, 7320–7331. [Google Scholar] [CrossRef]
- Pal, N.; Alzahid, Y.; AlSofi, A.M.; Ali, M.; Hoteit, H. Review on Microemulsions for Conformance Improvement Technology: Fundamentals, Design Considerations, and Perspectives. Energy Fuels 2023, 37, 858–875. [Google Scholar] [CrossRef]
- Seright, R.; Zhang, G.; Akanni, O.; Wang, D. A Comparison of Polymer Flooding With In-Depth Profile Modification. J. Can. Pet. Technol. 2012, 51, 393–402. [Google Scholar] [CrossRef]
- Izgec, O.; Shook, G.M. Design Considerations of Waterflood Conformance Control with Temperature-Triggered Low Viscosity Sub-Micron Polymer. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 21–23 March 2012; p. SPE-153898-MS. [Google Scholar] [CrossRef]
- Shambhu, V.B.; Bhattacharya, T.K.; Nayak, L.K.; Das, S. Studies on Characterization of Raw Jatropha Oil and its Biodiesels with Relevance of Diesel. Int. J. Emerg. Technol. Adv. Eng. 2013, 9001, 48–54. [Google Scholar]
- Lu, W.; Liu, Q.; Wang, Z.D. Gelling Behaviour of Natural Ester Transformer Liquid under Thermal Ageing. In Proceedings of the 2012 International Conference on High Voltage Engineering and Application, Shanghai, China, 17–20 September 2012. [Google Scholar] [CrossRef]
- Hanamertani, A.S.; Pilus, R.M.; Idris, A.K.; Irawan, S.; Tan, I.M. Ionic liquids as a potential additive for reducing surfactant adsorption onto crushed Berea sandstone. J. Pet. Sci. Eng. 2018, 162, 480–490. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S.E. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhaanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef]
- Nan, F.; Wu, J.; Qi, F.; Fan, Q.; Ma, G.; Ngai, T. Preparation of uniform-sized colloidosomes based on chitosan-coated alginate particles and its application for oral insulin delivery. J. Mater. Chem. B 2014, 2, 7403–7409. [Google Scholar] [CrossRef]
- Ge, S.; Xiong, L.; Li, M.; Liu, J.; Yang, J.; Chang, R.; Liang, C.; Sun, Q. Characterizations of Pickering emulsions stabilized by starch nanoparticles: Influence of starch variety and particle size. Food Chem. 2017, 234, 339–347. [Google Scholar] [CrossRef]
- Velikov, K.P.; Velev, O.D. Stabilization of thin films, foams, emulsions and bifluid gels with surface-active solid particles. Colloid Stability and Application in Pharmacy; Wiley Online Library: Hoboken, NJ, USA, 2007; p. 277À306. [Google Scholar]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Leal-Calderon, F.; Faure, C. O/W Pickering emulsions stabilized by cocoa powder: Role of the emulsification process and of composition parameters. Food Res. Int. 2018, 116, 755–766. [Google Scholar] [CrossRef]
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering Emulsions for Sustainable Cosmetic Applications. Polymers 2020, 12, 2385. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Effects of oil type and aqueous phase composition on oil–water mixtures containing particles of intermediate hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Wong, S.F.; Law, M.C.; Samyudia, Y.; Dol, S.S. A review on the effects of emulsions on flow behaviours and common factors affecting the stability of emulsions. J. Appl. Sci. 2015, 15, 167–172. [Google Scholar] [CrossRef]
- Zhang, T.; Davidson, A.; Bryant, S.L.; Huh, C. Nanoparticle-stabilized emulsions for applications in enhanced oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Volume 2, pp. 1009–1026. [Google Scholar] [CrossRef]
- Jurinak, J.J.; Summers, L.E. Oilfield applications of colloidal silica gel. SPE Prod. Eng. 1991, 6, 406–412. [Google Scholar] [CrossRef]
- Zhao, X.J.; Wang, C.J. A study on microscopic profiling/oil displacing mechanisms in weak gel flooding. Oilf. Chem. 2004, 21, 56–60. [Google Scholar]
- Thomas, S.; Ali, S.M.F. Flow of emulsions in porous media, and potential for enhanced oil recovery. J. Pet. Sci. Eng. 1989, 3, 121–136. [Google Scholar] [CrossRef]
- French, T.R.; Broz, J.S.; Lorenz, P.B.; Bertus, K.M. Use of emulsions for mobility control during steamflooding. In Proceedings of the SPE California Regional Meeting, Oakland, CA, USA, 2–4 April 1986; pp. 43–54. [Google Scholar] [CrossRef]
- Chesters, A.K. Modelling of coalescence processes in fluid-liquid dispersions. A review of current understanding. Chem. Eng. Res. Des. 1991, 69, 227–259. [Google Scholar]
- AfzaliTabar, M.; Alaei, M.; Khojasteh, R.R.; Motiee, F.; Rashidi, A.M. Preference of multi-walled carbon nanotube (MWCNT) to single-walled carbon nanotube (SWCNT) and activated carbon for preparing silica nanohybrid pickering emulsion for chemical enhanced oil recovery (C-EOR). J. Solid State Chem. 2017, 245, 164–173. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Sellier, E.; Leal-Calderon, F.; Ravaine, V.; Schmitt, V. Origin and control of adhesion between emulsion drops stabilized by thermally sensitive soft colloidal particles. Langmuir 2012, 28, 3744–3755. [Google Scholar] [CrossRef]
- Tsuji, S.; Kawaguchi, H. Thermosensitive pickering emulsion stabilized by poly(N-isopropylacrylamide)-carrying particles. Langmuir 2008, 24, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1131-20; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ISO 11358-1:2022; Plastics—Thermogravimetry (TG) of Polymers—Part 1: General Principles. ISO: Geneva, Switzerland, 2022; p. 13. Available online: https://www.iso.org/standard/79999.html (accessed on 12 May 2023).
- ISO/TR 13097:2013; Guidelines for the Characterization of Dispersion Stability. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/52802.html (accessed on 12 May 2023).
- 2.2.25. Absorption Spectrophotometry, Ul-traviolet and Visible, EUROPEAN PHARMACOPOEIA 6.0, 6th ed.; EUROPEAN PHARMACOPOEIA: Strasbourg, France; Available online: https://pasg.nhs.uk/downloads.php?did=268 (accessed on 12 May 2023).
- ISO 20998-1:2006; Measurement and Characterization of Particles by Acoustic Methods—Part 1: Concepts and Procedures in Ultrasonic Attenuation Spectroscopy. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/39869.html (accessed on 12 May 2023).
- ISO 13320:2020; Particle size analysis—Laser diffraction methods. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/69111.html (accessed on 12 May 2023).
- ISO 13099-2:2012; Colloidal systems—Methods for zeta-potential determination—Part 2: Optical methods. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/52832.html (accessed on 12 May 2023).
- Llopis, P.M.; Senft, R.A.; Ross-Elliott, T.J.; Stephansky, R.; Keeley, D.P.; Koshar, P.; Marqués, G.; Gao, Y.-S.; Carlson, B.R.; Pengo, T.; et al. Best practices and tools for reporting reproducible fluorescence microscopy methods. Nat. Methods 2021, 18, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- ISO 19749:2021; Nanotechnologies—Measurements of particle size and shape distributions by scanning electron microscopy. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/66235.html (accessed on 12 May 2023).
- ISO 21363:2020; Nanotechnologies—Measurements of particle size and shape distributions by transmission electron microscopy. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/70762.html (accessed on 12 May 2023).
- EUROPEAN PHARMACOPOEIA. 2.2.10. Viscosity—Rotating Viscometer Method. 2008, pp. 28–30. Available online: https://www.drugfuture.com/Pharmacopoeia/EP7/DATA/20210E.PDF (accessed on 12 May 2023).
- ASTM D7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D971-20; Standard Test Method for Interfacial Tension of Insulating Liquids Against Water by the Ring Method. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ISO 304:1985; Surface active agents—Determination of surface tension by drawing up liquid films. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/4238.html (accessed on 12 May 2023).
- ASTM D4520-18; Standard Practice for Determining Water Injectivity Through the Use of On-Site Floods. ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- American Petroleum Institute. “Recommended Practices for Core Analysis RP-40, 2nd ed.; American Petroleum Institute: Washington, DC, USA, 1998; p. 236. [Google Scholar]
- ASTM D4052-22; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D664-18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM D1293-18; Standard Test Methods for pH of Water. ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- DIN 55659-2:2012-08; Coating materials—Determination of the pH value—Part 2: pH electrodes with ISFET technology. Deutsches Institut für Normung: Berlin, Germany, 2012. [CrossRef]
- ASTM D2624-22; Standard Test Methods for Electrical Conductivity of Aviation and Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D4404-18; Standard Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry. ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D4525-13e2; Standard Test Method for Permeability of Rocks by Flowing Air. ASTM: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/d4525-13e02.html (accessed on 12 May 2023).
- Güzey, D.; McClements, D.J. Influence of Environmental Stresses on O/W Emulsions Stabilized by β-Lactoglobulin–Pectin and β-Lactoglobulin–Pectin–Chitosan Membranes Produced by the Electrostatic Layer-by-Layer Deposition Technique. Food Biophys. 2006, 1, 30–40. [Google Scholar] [CrossRef]
- Hu, Y.T.; Ting, Y.; Hu, J.Y.; Hsieh, S.C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–267. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. 2-Impact of processing on the chemistry and functionality of food proteins. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 27–45. [Google Scholar] [CrossRef]
- Chang, P.S. Determination of emulsion stability index in W/O emulsion. Anal. Sci. Technol. 1994, 7, 233–236. [Google Scholar]
- Russ, J.C. Image Analysis of Food Microstructure; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bunker, K.L.; McAllister, D.; Allison, K.; Wagner, K.; Rickabaugh, K.; Levine, A.; Strohmeier, B.; Lee, R. TEM and FESEM: The right combination for enhanced particle characterization. Microsc. Microanal. 2008, 14 (Suppl. S2), 580–581. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Jain, S.; Winuprasith, T. Properties and stability of pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact of oil type and emulsifier concentration. Songklanakarin J. Sci. Technol. 2020, 42, 468–476. [Google Scholar] [CrossRef]
- Chu, B. Laser light Scattering: Basic Principles and Practice; Courier Corporation: North Chelmsford, Chelmsford, MA, USA, 2007. [Google Scholar]
- HORIBA Scientific. A Guidebook to Particle Size Analysis. Distribution. 2016. Available online: https://www.horiba.com/fileadmin/uploads/Scientific/Documents/PSA/PSA_Guidebook.pdf (accessed on 20 August 2017).
- Wriedt, T. Mie Theory: A Review BT—The Mie Theory: Basics and Applications; Hergert, W., Wriedt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–71. [Google Scholar] [CrossRef]
- Malvern Ltd. Zeta Potential: An Introduction in 30 Minutes. Zetasizer Nano Serles Tech. Note. MRK654-01 2011. Available online: https://www.research.colostate.edu/wp-content/uploads/2018/11/ZetaPotential-Introduction-in-30min-Malvern.pdf (accessed on 12 May 2023).
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 227–258. [Google Scholar] [CrossRef]
- Laurén, S. What Is a Sessile Drop Method? Biolin Scientific. Available online: https://www.biolinscientific.com/blog/what-is-a-sessile-drop-method (accessed on 12 May 2023).
- Laurén, S. 7 Ways to Measure Contact Angle. Biolin Scientific. 2021. Available online: https://www.biolinscientific.com/blog/7-ways-to-measure-contact-angle (accessed on 12 May 2023).
- What is Contact Angle? Brighton Science. Available online: https://www.brighton-science.com/what-is-contact-angle (accessed on 12 May 2023).
- Hu, H.H.; Joseph, D.D. Evolution of a liquid drop in a spinning drop tensiometer. J. Colloid Interface Sci. 1994, 162, 331–339. [Google Scholar] [CrossRef]
- Interfacial Tension Measurement. Biolin Scientific. Available online: https://www.biolinscientific.com/measurements/interfacial-tension#interfacial-tension-measurement-with-optical-tensiometer (accessed on 12 May 2023).
- Singh, R.; Sivaguru, M.; Fried, G.A.; Fouke, B.W.; Sanford, R.A.; Carrera, M.; Werth, C.J. Real rock-microfluidic flow cell: A test bed for real-time in situ analysis of flow, transport, and reaction in a subsurface reactive transport environment. J. Contam. Hydrol. 2017, 204, 28–39. [Google Scholar] [CrossRef]
- Alzahid, Y.A.; Mostaghimi, P.; Gerami, A.; Singh, A.; Privat, K.; Amirian, T.; Armstrong, R.T. Functionalisation of Polydimethylsiloxane (PDMS)- Microfluidic Devices coated with Rock Minerals. Sci. Rep. 2018, 8, 15518. [Google Scholar] [CrossRef] [PubMed]
- Salhin, A.; Ali, M.; Abdurrhman, A.M. Determination of Free Fatty Acids in Palm Oil Samples by Non-Aqueous Flow Injection Using Colorimetric Reagent. Chem. Mater. Eng. 2013, 1, 96–103. [Google Scholar] [CrossRef]
- Mitsubishi Chemical Analytech, Determination of Acid Number of Palm Oil. 2014. Available online: https://www.mccat.co.jp/archives/003/201703/044_GT200-OF030E.pdf (accessed on 12 May 2023).
- Wei, Z.; Li, M.; Lin, M.; Luo, T.; Yao, C. Electrical Conductivity and Stability of O/W Emulsions. Acta Pet. Sin. Pet. Process. Sect. 2008, 24, 592. Available online: http://www.syxbsyjg.com/EN/abstract/abstract477.shtml (accessed on 12 May 2023).
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Amaral, C.N.R.; Oliveira, P.F.; Roman, I.O.; Mansur, C.R.E. Preformed particle gels with potential applicability for conformance control of oil reservoirs. J. Appl. Polym. Sci. 2020, 137, 48554. [Google Scholar] [CrossRef]
- Gidley, J.L.; Schechter, R.S.; Williams, B.B. Acidizing Fundamentals; Society of Petroleum Engineers of AIME: New York, NY, USA; Dallas, TX, USA, 1979. [Google Scholar]
- Shafiq, M.U.; Mahmud, H.B. Sandstone matrix acidizing knowledge and future development. J. Pet. Explor. Prod. Technol. 2017, 7, 1205–1216. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Solares, J.R.; Al-Mutairi, S.H.; Mahoney, M.D. Field Application of Emulsified Acid-Based System to Stimulate Deep, Sour Gas Reservoirs in Saudi Arabia. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001; pp. 1–16, SPE-71693-MS. [Google Scholar] [CrossRef]
- Kasza, P.; Dziadkiewicz, M.; Czupski, M. From Laboratory Research to Successful Practice: A Case Study of Carbonate Formation Emulsified Acid Treatments. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2006; pp. 1–7, SPE-98261-MS. [Google Scholar] [CrossRef]
- Eghbal, M.D.; Behzad, S. Hydrophobic silica nanoparticle-stabilized invert emulsion as drilling fluid for deep drilling. Pet. Sci. 2017, 14, 105–115. [Google Scholar] [CrossRef]
- Shah, S.N.; Ph, D.; Shanker, N.H.; Ogugbue, C.C. Future Challenges of Drilling Fluids and Their Rheological Measurements. Am. Assoc. Drill. Eng. 2010, 10, 1–16. [Google Scholar]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Karunaratne, D.N.; Pamunuwa, G.; Ranatunga, U. Introductory Chapter: Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 1. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Wan, F.; Sun, Y. Carbon quantum dots-stabilized Pickering emulsion to prepare NIR light-responsive PLGA drug delivery system. Mater. Today Commun. 2020, 23, 100951. [Google Scholar] [CrossRef]
- Sakthivel, S.; Adebayo, A.; Kanj, M.Y. Experimental Evaluation of Carbon Dots Stabilized Foam for Enhanced Oil Recovery. Energy Fuels 2019, 33, 9629–9643. [Google Scholar] [CrossRef]
- Saafie, N.; Sambudi, N.S.; Wirzal, M.D.H.; Sufian, S. Effect of Hydrothermal Conditions on Kenaf-Based Carbon Quantum Dots Properties and Photocatalytic Degradation. Separations 2023, 10, 137. [Google Scholar] [CrossRef]


| S. No. | Author | Emulsion Type | Composition | Droplet Size | Formation | Outcome |
|---|---|---|---|---|---|---|
| 1 | Kim et al., 2017 [52] | O/W (Macroemulsion) | Oil Phase: Decane Aqueous Phase: Brine (8% NaCl and 2% CaCl2) Emulsifier: Particle: Hydrophilic Silica (5 nm), Concentration = 0.01 wt% Surfactant: 4 types (Cationic, Nonionic, Zwitterionic, Anionic) | 41–47 microns | Ottawa Sands | → Surfactants which produced most stable emulsions are in the following order: Cationic > Nonionic > Zwitter ionic > Anionic → Emulsion possessed shear thinning behavior. Hybrid emulsions presented higher viscosity than those made solely with surfactants or particles. → Slight amount of NP aggregation due to high brine salinity prevents coalescence as particles form a network. → The ideal pH for sole NP emulsion formation is between 6 and 8; higher and rapid coalescence starts at lesser pH particles get neutralized due to large ionic volume in medium. → Nonionic surfactants are overall feasible, especially against surfactant loss than others. |
| 2 | Xu et al., 2017 [51] | O/W In situ Macroemulsion | Oil Phase: Decane Aqueous Phase: Deionized Water Emulsifier: Particle: Hydrophilic Silica (5 nm) 20 wt% diluted to 2 wt% Surfactant: Tween 40 (Nonionic Surfactant) (0.05 wt%) | 190–210 microns | Bentheimer Sandstone replicated on glass | → A microfluidic study was conducted in which the oil droplets moved towards the high permeable zones courtesy of NP adhered to O/W, forming a network and compressing in the high permeable zone while the aqueous phase sidelined into low permeable regions. This provided better blockage of high permeable zones and sweep efficiency of displacing fluid. Particle + surfactant makes a denser fluid than surfactant based. |
| 3 | Pandey et al., 2018 [53] | O/W (Macroemulsion) Preformed 50:50 ratio | Oil Phase: Dodecane Aqueous Phase: Water Emulsifier: Particle: CNC (250 nm, rod-shaped, treated with 12 M HCl | 7 microns | Sandpack | → HCl-treated CNCs at 20 mgCNC/mLo concentration were found to be most stable and had smallest droplet size; thus, continuous phase viscosity increased as a result creaming rate was slowed down. This is because of the excess particles, along with significant oil viscosity that formed a strong droplet network which provided effective blockage and robust stability. → Oil could enter emulsion-saturated zones at a pressure gradient 5× lower pressure gradient for water. → Compared with surfactant and polymer-based emulsions, which failed to block water flow, CNC-based Pickering emulsion performed effectively. |
| 4 | Chen et al., 2018 [33] | O/W Emulsion Ratio: 10:90 | Oil Phase: Crude Oil (diluted with Kerosene for required Viscosity) Oil Viscosity for Core Flood was kept at 47.4 cp. Aqueous Phase Deionized Water + NaOH Surfactant: Dioctyl Sodium Sulfosuccinate | 3–4 microns (avg 3.6 microns) | Sandpack | The following aspects were observed via core flooding: → Pressure drop increases (3.8 times) as oil viscosity increases. → Increase in oil viscosity increases emulsion viscosity, but emulsion viscosity reduces as injection rate rises. → Increase in oil viscosity leads to rise in frictional resistance, but too much increment in oil viscosity can cause increase in slipping behavior and change in path, which does not improve plugging efficiency. |
| 5 | Sadati et al., 2019 [61] | O/W Emulsion Optimum ratio: 20:80 | Oil Phase: Intermediate Iranian Field Crude Oil Aqueous Phase: Synthesized Sea Water Emulsifier: Surfactant: Sodium dodecyl sulfate (HLB = 40) | ___ | Sandpack | → Micelle solution of Sodium dodecyl sulfate and gasoil was injected to create in situ emulsion with water phase percentages (60–90% with 10% increments) into cores. → As observed from core flooding, emulsion injection resulted in incremental oil recovery up to 20%. → Emulsion with 80% water cut was technically and economically most feasible. |
| 6 | X. Zhou et al., 2020 [62] | In situ Emulsion EmulsionPolymer + Surfactant + CO2 CO2: Water Polymer Gel Ratio = 40:60 | CO2 Phase: CO2 Aqueous Phase: Water Emulsifier: Surfactant: Polysaccharide linear polymer and a foaming agent | ___ | Carbonate | → Two physical models were tested out: (1) Isolation of low permeable zone while opening the high permeable one. (2) CO2 flooding followed by injection of a base gel into the cores while keeping both permeable zones open. → Model was more technically and economically feasible. Upon injection of 0.2 PV, it produced 46% incremental oi, while model 1 recovered merely 5% upon utilization of 0.4 PV of slug injection. |
| 7 | S. Li et al., 2020 [54] | O/W (Macroemulsion) | Oil Phase: Decane (5 vol.%) Aqueous Phase: Brine (10 wt% NaCl with 0.5 wt% clay particles in it) Emulsifier: Particle: Sodium Montmorillonite, (Clay particle dia. = 300 nm) | 2.2–3 microns | Quartz Sand | → Good long-term coalescence stability but poor creaming stability. → The clay-stabilized colloid system overall provided a 16% (67% to 84%) incremental oil recovery and mobilized all trapped oil in high saline conditions. |
| 8 | Saikia et al., 2020 [55] | W/O Emulsified In situ Polymeric Gel | Oil Phase: Diesel Oil Aqueous Phase: Field Mixing Water Emulsifier: Pickering Agent: Organoclay (Cloisite 20A) Polymers: (1) Polyacrylamide (3 wt.%) (2) Polyethyleneimine (1 wt.%) | 1.485 microns | Berea Sandstone and Carbonate | → Uniform penetration in both cases with unrestricted oil flow contrary to water flow. → After gel-based plugging, injections of Diesel oil followed by KCl flooding were conducted to check plugging. → The gel could withstand a differential pressure as high as 2000 psi. → In sandstone, Diesel Oil was only produced equal to the amount injected. → In carbonate, KCl brine found a channel to bypass plugged zones and got produced. |
| 9 | Pal et al., 2022 [35] | Microemulsion (ME), W1(O/W)-W3(O/ME/W)-W2(W/O) | Oil phase: Decane Aqueous phase: Deionized Water + Brine Emulsifier: Surfactant: SDS (sodium dodecyl sulfate, Anionic) Co-surfactant: Isopropanol | 0.034 microns (Without salt) 0.138 microns (10,000 ppm) to 0.211 microns (80,000 ppm) | Sandstone | → Microemulsion reduced 98% water cut by 30%, but with time (halfway through 0.84 PV of 1.15 PV), viscosity reduced as a result blocking efficiency decreased, and water cut increased to 53%. Showed slight inefficiency of the synthesized microemulsion. |
| 10 | Sun et al., 2022 [63] | W/O (Macroemulsion) | Oil Phase: Heavy Crude Oil of Xinjiang Oil Field Aqueous Phase: Formation Water varied from 20–90% at different salinities Emulsifier: Natural surfactants present in crude oil (Asphaltenes, resins, naphthenic acids) | 0.2–4 microns with change from 20 to 90% in water content. | ___ | → Emulsion non-Newtonian behavior (pseudoplastic/shear thinning). → Emulsion stability and DSD uniformity worsened with increase in water content. → Emulsion viscosity initially increased with water content from 20 to 40% but started decreasing after 40% to 90%. → Salinity had little impact on the stability of the emulsion due to naturally occurring surfactants (polar components) present in the heavy crude oil (used as the oil phase). → High viscosity allows for piston-like displacement due to high mobility control provided by high viscosity of the emulsion. |
| 11 | W. Wang et al., 2022 [57] | O/W (in situ micelle) | Oil phase: Kerosene Aqueous Phase: Brine (Salinity = 4263 mg/L), 0.5 wt% Emulsifier: Fly ash: Spherical and irregular, 150 nm (after milling) Surfactants: 0.2 wt% (Cationic, Anionic, and Nonionic) | ___ | Double-layered heterogeneous Sandpack Core | → Stability was checked using a microscope and rheometer. The emulsion made from fly ash with cationic or nonionic were highly stable in comparison to surfactant and particle-based emulsions and worked effectively. → The cationic + fly ash emulsion improved oil recovery by 8.5% in intraformation heterogeneous cores. It was also used for sequestering the flyvash to the rock layers. |
| 12 | Mahboob et al., 2023 [56] | W/O Emulsified In situ Polymeric Gel O/W = 70:30 | Oil Phase: Diesel Oil Aqueous Phase: Synthetic Sea Water and Formation Water Emulsifier: Pickering Agent: Organoclay (Cloisite 20 A) Polymer: Silica (28%) + NaCl (10%) | 41.15 Microns | ___ | → 10% NaCl and 28% Silica produce optimum gel. → Nonemulsified gel had a decrease in gelation time upon increase in salinity and temperature (100 °C), and upon increasing silica concentration, gel strength increased → Whereas emulsified gel had longer gelation time, although had good resistance at high temperatures and salinity. However, gel strength reduced with increase in silica concentration. → Bulk mixing produces direct emulsion (O/W), and drop-by-drop method generates inverse emulsion (W/O), → Optimized emulsified colloidal silica gel is made using drop-by-drop method. |
| Publications | Pickering Agent |
|---|---|
| [51,52] | Hydrophilic silica (5 nm) |
| [53] | CNC (250 nm, rod-shaped, treated with 12 M HCl) |
| [54] | Sodium montmorillonite, (clay particle dia. = 300 nm) |
| [55,56] | Organoclay, <10 μm |
| [57] | Fly ash, spherical and irregular, 150 nm (after milling) |
| Characteristics | Techniques | Methods | Standards/Practices | |
|---|---|---|---|---|
| Stability | Visual Observation | Bottle Test | --- | |
| Thermal Analysis/Mass Loss | TGA (Thermalgravimetric Analysis) | [99,100] | ||
| Turbidity/Absorbance | Turbidity-based (Multiple Light Scattering) | [101] | ||
| Spectrometry/Absorbance | UV-Vis Spectrophotometry | [102] | ||
| Droplet Size Analysis | Ultrasonic Spectrometry (Acoustic-based) | [103] | ||
| Light Scattering (Static/Dynamic) | [104] | |||
| Surface Charge of Droplet | Zeta Potential | Electroacoustic-based | [105] | |
| Microelectrophoresis | [105] | |||
| Imaging | Fluorescence Microscope | Best practices and tools for reporting reproducible fluorescence microscopy methods by Paula Montero Llopis [106] | ||
| Cross Polarized Microscope | --- | |||
| Scanning Electron Microscope | [107] | |||
| Field Emission Electron Microscope | ||||
| Transmission Electron Microscope | [108] | |||
| Shear Thinning | Rheology | Rotational Rheometer | [109] | |
| Wettability | Contact Angle/ Interfacial Tension | Sessile Drop Method | [110] | |
| Pendant Drop Method | ||||
| Spinning Drop Method (Modified Pendant Drop Method) | ||||
| Du Nouy Ring Method | [111] | |||
| Wilhelmy Plate Method | [112] | |||
| Flow through Porous Medium | Microfluidic Study | --- | --- | |
| Core Flooding | --- | [113,114] | ||
| Density | Oscillating U-tube Method | [115] | ||
| Total Acid Number | Titration Method | [116] | ||
| pH | Colorimetric Method | --- | ||
| Metal/Glass-electrode Method | [117] | |||
| Semiconductor Sensor Method | [118] | |||
| Electrical Conductivity | Potentiometric method | [119] | ||
| Rock Porosity | Mercury Intrusion Porosimetry | [120] | ||
| Gas Pycnometry | --- | |||
| Rock Permeability | Steady State Method | [121] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousufi, M.M.; Dzulkarnain, I.b.; Elhaj, M.E.M.; Ahmed, S. A Perspective on the Prospect of Pickering Emulsion in Reservoir Conformance Control with Insight into the Influential Parameters and Characterization Techniques. Processes 2023, 11, 2672. https://doi.org/10.3390/pr11092672
Yousufi MM, Dzulkarnain Ib, Elhaj MEM, Ahmed S. A Perspective on the Prospect of Pickering Emulsion in Reservoir Conformance Control with Insight into the Influential Parameters and Characterization Techniques. Processes. 2023; 11(9):2672. https://doi.org/10.3390/pr11092672
Chicago/Turabian StyleYousufi, Muhammad Mohsin, Iskandar bin Dzulkarnain, Mysara Eissa Mohyaldinn Elhaj, and Shehzad Ahmed. 2023. "A Perspective on the Prospect of Pickering Emulsion in Reservoir Conformance Control with Insight into the Influential Parameters and Characterization Techniques" Processes 11, no. 9: 2672. https://doi.org/10.3390/pr11092672
APA StyleYousufi, M. M., Dzulkarnain, I. b., Elhaj, M. E. M., & Ahmed, S. (2023). A Perspective on the Prospect of Pickering Emulsion in Reservoir Conformance Control with Insight into the Influential Parameters and Characterization Techniques. Processes, 11(9), 2672. https://doi.org/10.3390/pr11092672