Study of the Effect of Eudragit RSPO on the Photostability of Venlafaxine in a Physical Mixture and in a Melt Form
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Physical Mixture and Melt form of VEN and EUD Preparation
2.3. Sample Preparation and Irradiation Conditions
2.4. UHPLC-MS/MS Analysis
2.5. Kinetic Studies
2.6. Scanning Electron Microscopy (SEM) Imaging
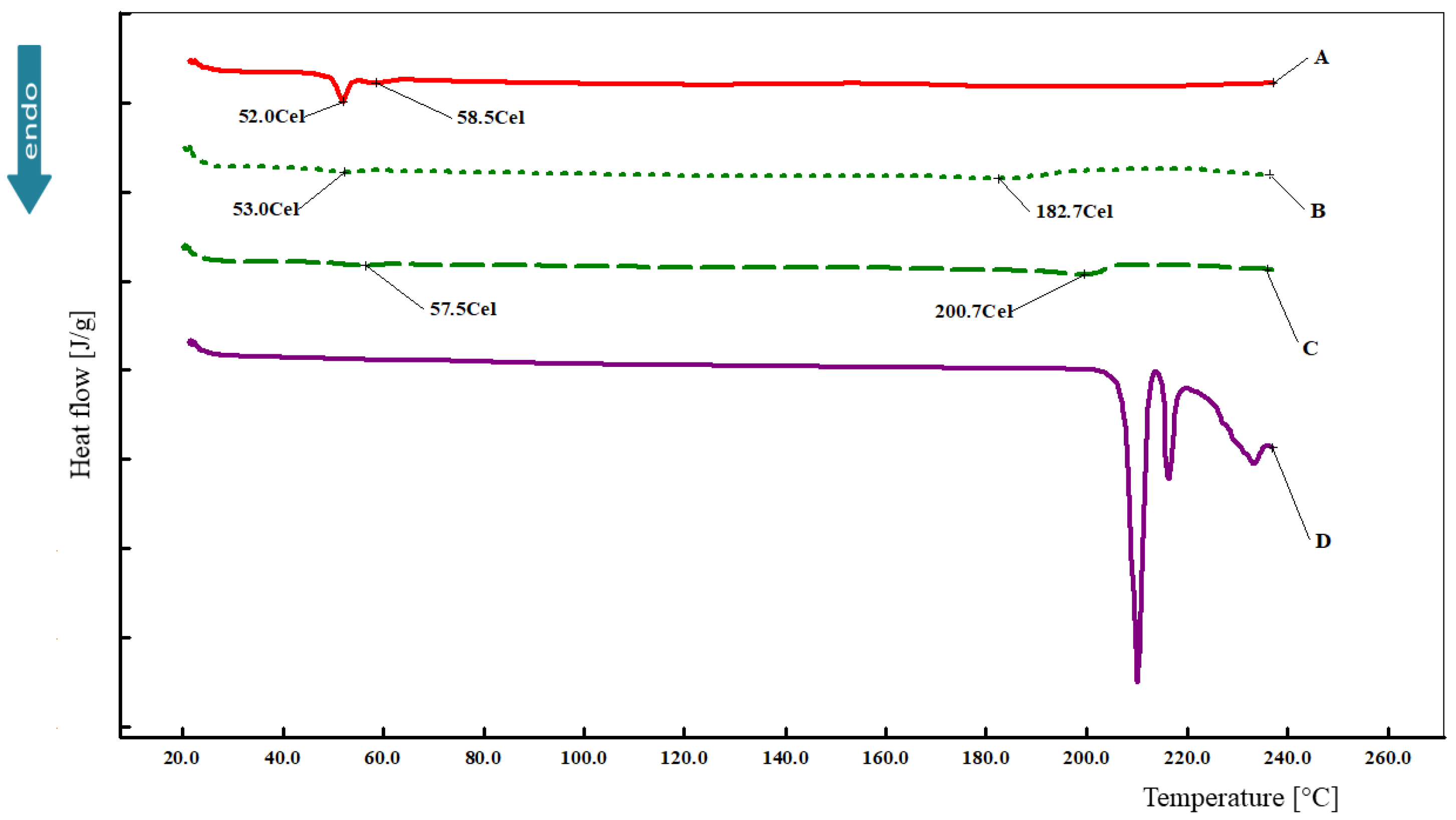
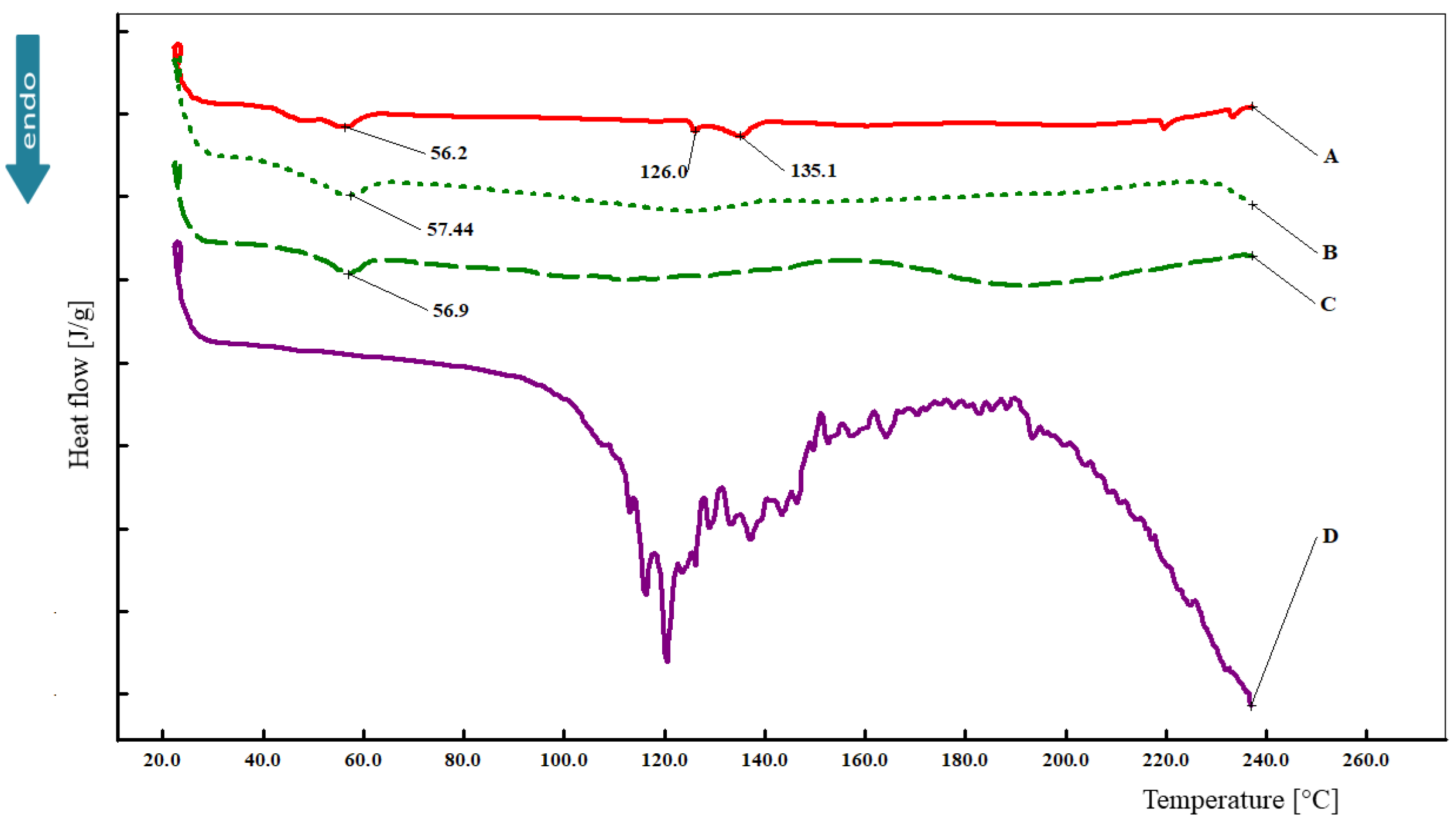
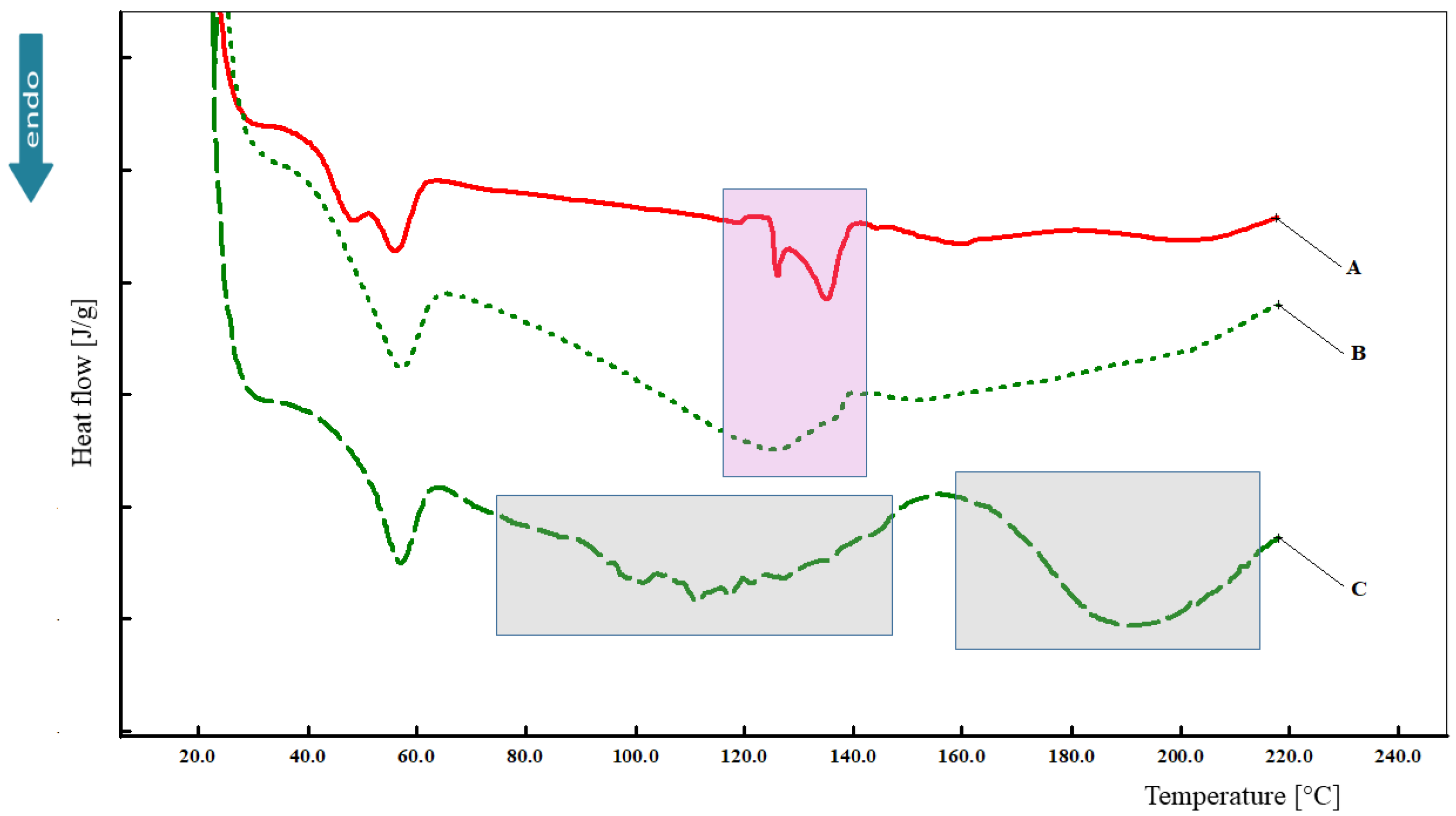
2.7. Differential Scanning Calorimetry
3. Results
3.1. Study of the Photostability of VEN in Substance, Physical Mixture with EUD, and Melt Form
3.2. Identification of Degradation Products
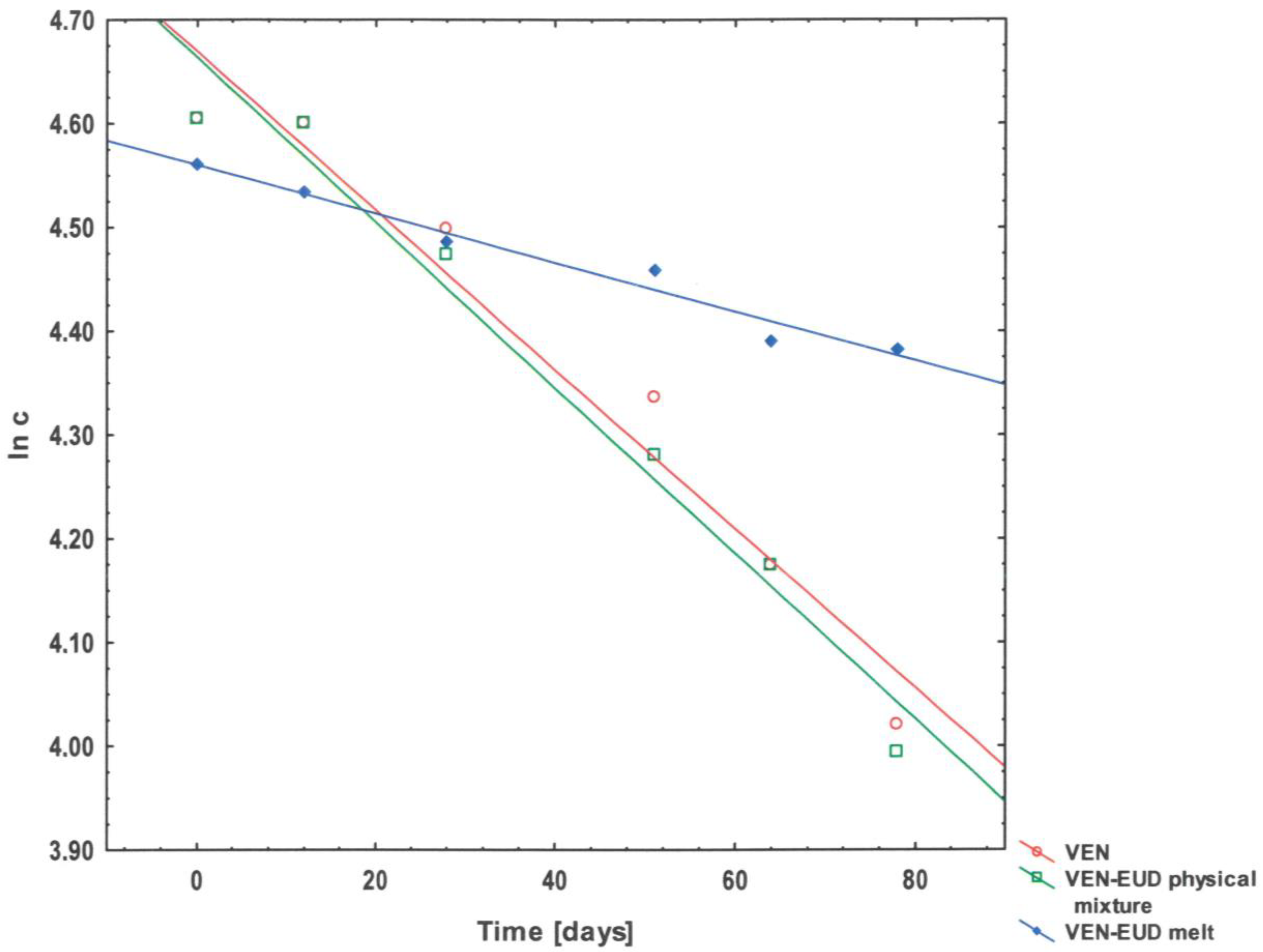
3.3. Kinetic Studies
3.4. Scanning Electron Microscopy Imaging
3.5. Differential Scanning Calorimetry (DSC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ICH. Stability Testing of New Drug Substances and Products, Q1A (R2); ICH: Gunpo-si, Republic of Korea, 2003. [Google Scholar]
- ICH. Stability Testing: Photostability Testing of New Drug Substances and Products, Q1B; ICH: Gunpo-si, Republic of Korea, 2003. [Google Scholar]
- Baertschi, S.W.; Clapham, D.; Foti, C.; Jansen, P.J.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tønnesen, H.H. Implications of In-Use Photostability: Proposed Guidance for Photostability Testing and Labeling to Support the Administration of Photosensitive Pharmaceutical Products, Part 1: Drug Products Administered by Injection. J. Pharm. Sci. 2013, 102, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Foti, C.; Alsante, K. Can Light Absorption and Photostability Data Be Used to Assess the Photosafety Risks in Patients for a New Drug Molecule? J. Photochem. Photobiol. B 2009, 96, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Baertschi, S.W.; Alsante, K.M.; Tønnesen, H.H. A Critical Assessment of the ICH Guideline on Photostability Testing of New Drug Substances and Products (Q1B): Recommendation for Revision. J. Pharm. Sci. 2010, 99, 2934–2940. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Hanne, H. Photostability of Drugs and Drug Formulations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780415303231. [Google Scholar]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and Removal of Transformation Products of PPCPs and Illicit Drugs in Wastewaters: A Review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Olver, J.S.; Burrows, G.D.; Norman, T.R. Third-Generation Antidepressants: Do They Offer Advantages over the SSRIs? CNS Drugs 2001, 15, 941–954. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Serotonin Norepinephrine Reuptake Inhibitors:A Pharmacological Comparison. Innov. Clin. Neurosci. 2014, 11, 37–42. [Google Scholar] [PubMed]
- Marsh, W. Venlafaxine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–5. ISBN 9780080552323. [Google Scholar]
- Yin, L.; Ma, R.; Wang, B.; Yuan, H.; Yu, G. The Degradation and Persistence of Five Pharmaceuticals in an Artificial Climate Incubator during a One Year Period. RSC Adv. 2017, 7, 8280–8287. [Google Scholar] [CrossRef]
- García-Galán, J.J.; Anfruns, A.; Gonzalez-Olmos, R.; Rodríguez-Mozaz, S.; Comas, J. UV/H2O2degradation of the Antidepressants Venlafaxine and O-Desmethylvenlafaxine: Elucidation of Their Transformation Pathway and Environmental Fate. J. Hazard Mater 2016, 311, 70–80. [Google Scholar] [CrossRef]
- Giannakis, S.; Hendaoui, I.; Jovic, M.; Grandjean, D.; De Alencastro, L.F.; Girault, H.; Pulgarin, C. Solar Photo-Fenton and UV/H2O2 Processes against the Antidepressant Venlafaxine in Urban Wastewaters and Human Urine. Intermediates Formation and Biodegradability Assessment. Chem. Eng. J. 2017, 308, 492–504. [Google Scholar] [CrossRef]
- Lambropoulou, D.; Evgenidou, E.; Saliverou, V.; Kosma, C.; Konstantinou, I. Degradation of Venlafaxine Using TiO2/UV Process: Kinetic Studies, RSM Optimization, Identification of Transformation Products and Toxicity Evaluation. J. Hazard Mater 2017, 323, 513–526. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Degradation of Lidocaine, Tramadol, Venlafaxine and the Metabolites O-Desmethyltramadol and O-Desmethylvenlafaxine in Surface Waters. Chemosphere 2013, 90, 1952–1959. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, B.; Ping, S.; Zhang, X.; Ji, Y.; Ren, Y. Ultraviolet Oxidative Degradation of Typical Antidepressants: Pathway, Product Toxicity, and DFT Theoretical Calculation. Chemosphere 2022, 305, 135440. [Google Scholar] [CrossRef]
- Maślanka, A.; Żmudzki, P.; Szlósarczyk, M.; Talik, P.; Hubicka, U. Photodegradation Assessment of Amisulpride, Doxepin, Haloperidol, Risperidone, Venlafaxine, and Zopiclone in Bulk Drug and in the Presence of Excipients. Monatshefte Fur Chem. Chem. Mon. 2020, 151, 483–493. [Google Scholar] [CrossRef]
- Girotti, A.W. Photodynamic Lipid Peroxidation in Biological Systems. Photochem. Photobiol. 1990, 51, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Jamrógiewicz, M.; Jasińska, K.; Sawicki, W. Photolabile Drug Compounds in the Pharmaceutical Dosage Forms—The Range of Problem. Farm Pol. 2012, 68, 858–866. [Google Scholar]
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical Significance of Eudragit: A Review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Raffin, R.P.; Colomé, L.M.; Schapoval, E.E.S.; Pohlmann, A.R.; Guterres, S.S. Increasing Sodium Pantoprazole Photostability by Microencapsulation: Effect of the Polymer and the Preparation Technique. Eur. J. Pharm. Biopharm. 2008, 69, 1014–1018. [Google Scholar] [CrossRef]
- Dhurke, R.; Kushwaha, I.; Desai, B.G. Improvement in Photostability of Pantoprazole Sodium by Microencapsulation. PDA J. Pharm. Sci. Technol. 2013, 67, 43–52. [Google Scholar] [CrossRef]
- Prakash Rao, B.; Rajarajan, S.; Monica, A.; Ramesh, K.; Baby, B.; Nagini, K.S.S.; Vijay Kumar Gupta, N. Spray-Dried Floating Microparticles of Ranitidine HCl by Factorial Design for Oral Delivery. J. Pharm. Sci. Res. 2013, 5, 240–248. [Google Scholar]
- Santos, S.S.; Lorenzoni, A.; Ferreira, L.M.; Mattiazzi, J.; Adams, A.I.H.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Clotrimazole-Loaded Eudragit® RS100 Nanocapsules: Preparation, Characterization and in Vitro Evaluation of Antifungal Activity against Candida Species. Mater. Sci. Eng. C 2013, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; Friedrich, M.L.; dos Santos, J.; Librelotto, D.R.N.; Maurer, L.H.; Emanuelli, T.; da Silva, C.d.B.; Adams, A.I.H. Desonide Nanoencapsulation with Açai Oil as Oil Core: Physicochemical Characterization, Photostability Study and in Vitro Phototoxicity Evaluation. J. Photochem. Photobiol. B 2019, 199, 111606. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Aitipamula, S.; Nangia, A. Thermochemical Analysis of Venlafaxine Hydrochloride Polymorphs 1-5. Cryst. Growth Des. 2005, 5, 2268–2276. [Google Scholar] [CrossRef]
- ICH. Impurities in New Drug Substances—Scientific Guideline, ICH Q3A (R2); ICH: Gunpo-si, Republic of Korea, 2006. [Google Scholar]
- Glass, B.D.; Novák, C.; Brown, M.E. The Thermal and Photostability of Solid Pharmaceuticals: A Review. J. Therm. Anal. Calorim. 2004, 77, 1013–1036. [Google Scholar] [CrossRef]
- Jamrógiewicz, M. Consequences of New Approach of Chemical Stability Tests of Active Pharmaceutical Ingredients (APIs). Front. Pharmacol. 2016, 7, 166291. [Google Scholar] [CrossRef][Green Version]
- Bounartzi, M.; Panagopoulou, A.; Kantiranis, N.; Malamataris, S.; Nikolakakis, I. Effect of Plasticiser Type on the Hot Melt Extrusion of Venlafaxine Hydrochloride. J. Pharm. Pharmacol. 2014, 66, 297–308. [Google Scholar] [CrossRef]
- Pignatello, R.; Ferro, M.; De Guidi, G.; Salemi, G.; Vandelli, M.A.; Guccione, S.; Geppi, M.; Forte, C.; Puglisi, G. Preparation, Characterisation and Photosensitivity Studies of Solid Dispersions of Diflunisal and Eudragit RS100® and RL100®. Int. J. Pharm. 2001, 218, 27–42. [Google Scholar] [CrossRef]
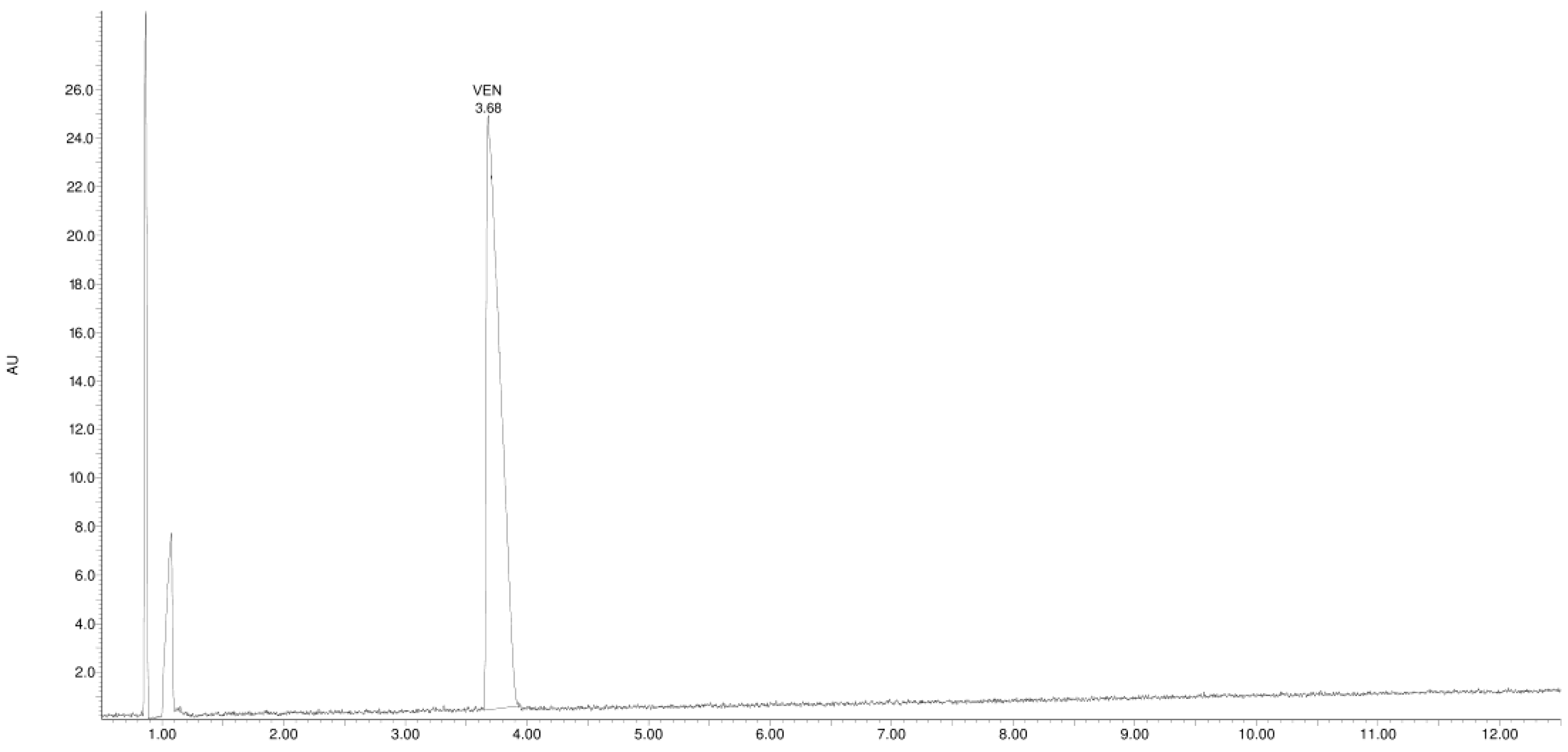
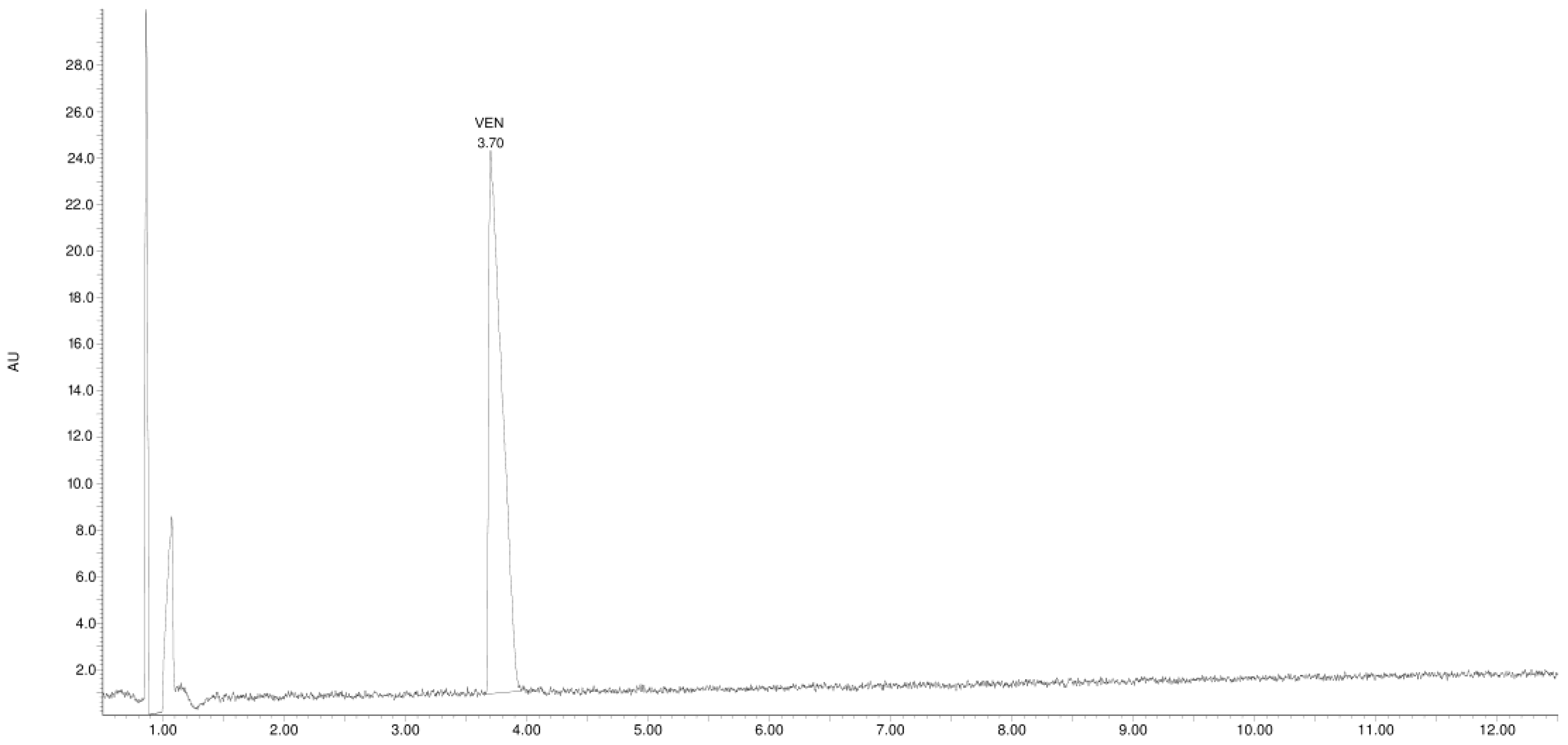
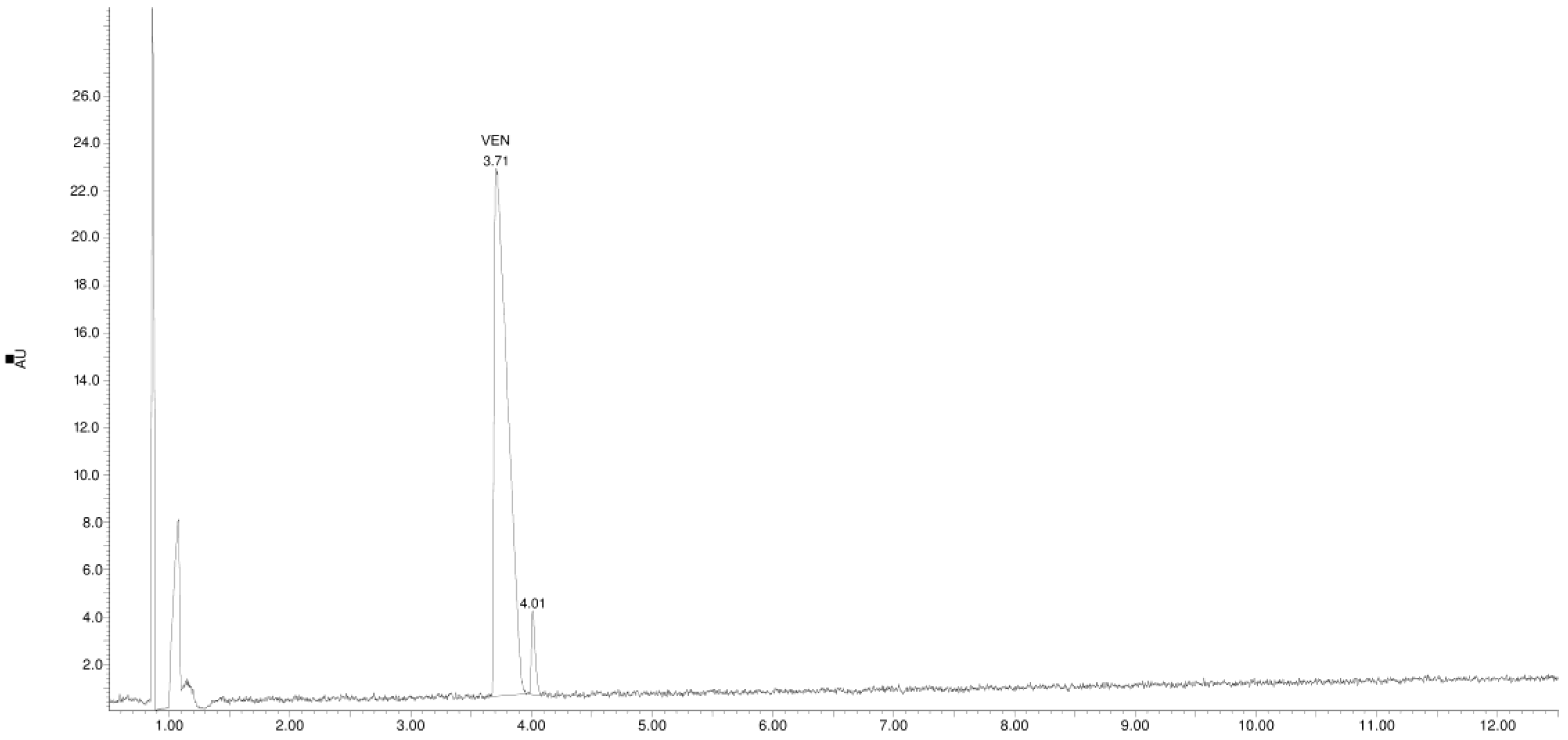
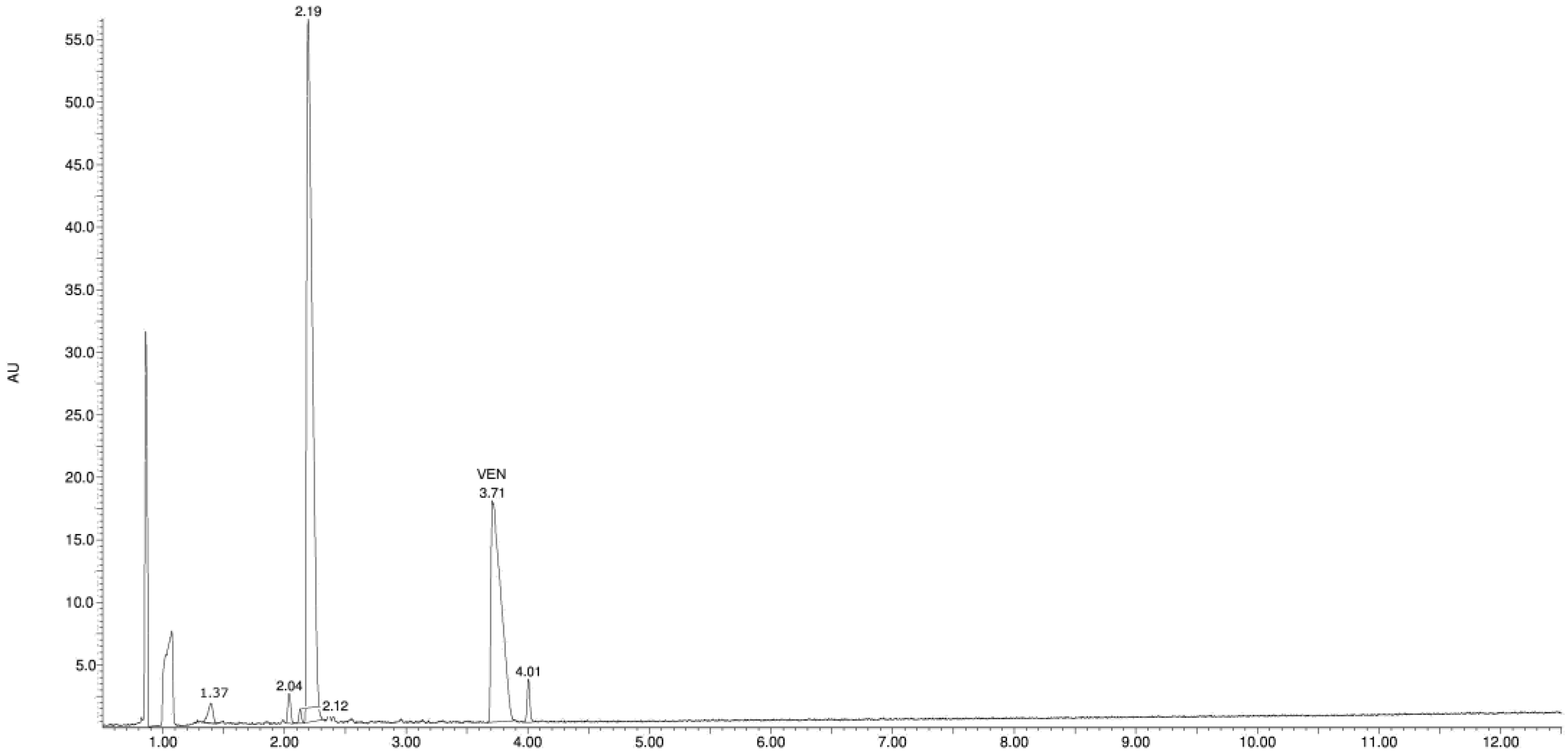
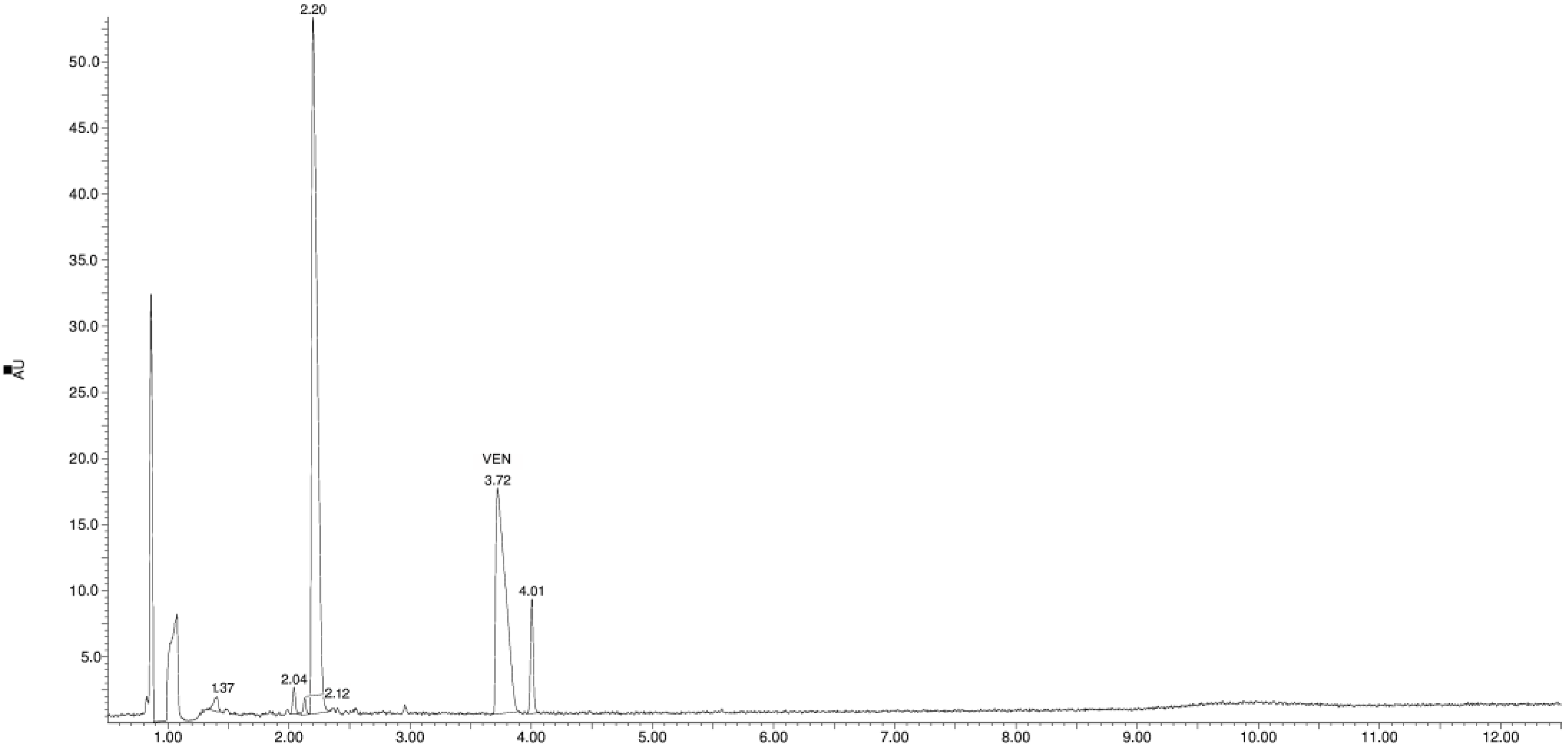
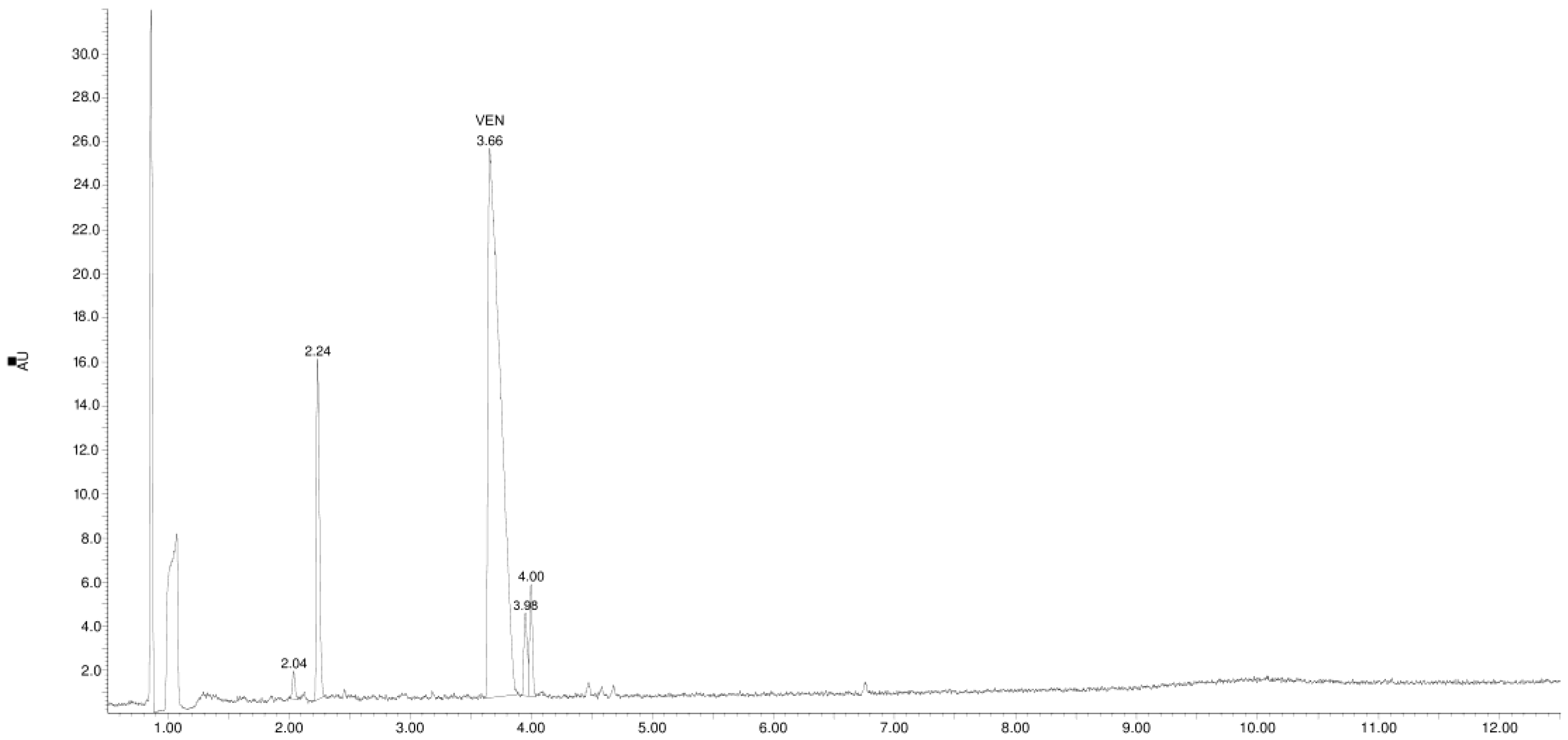
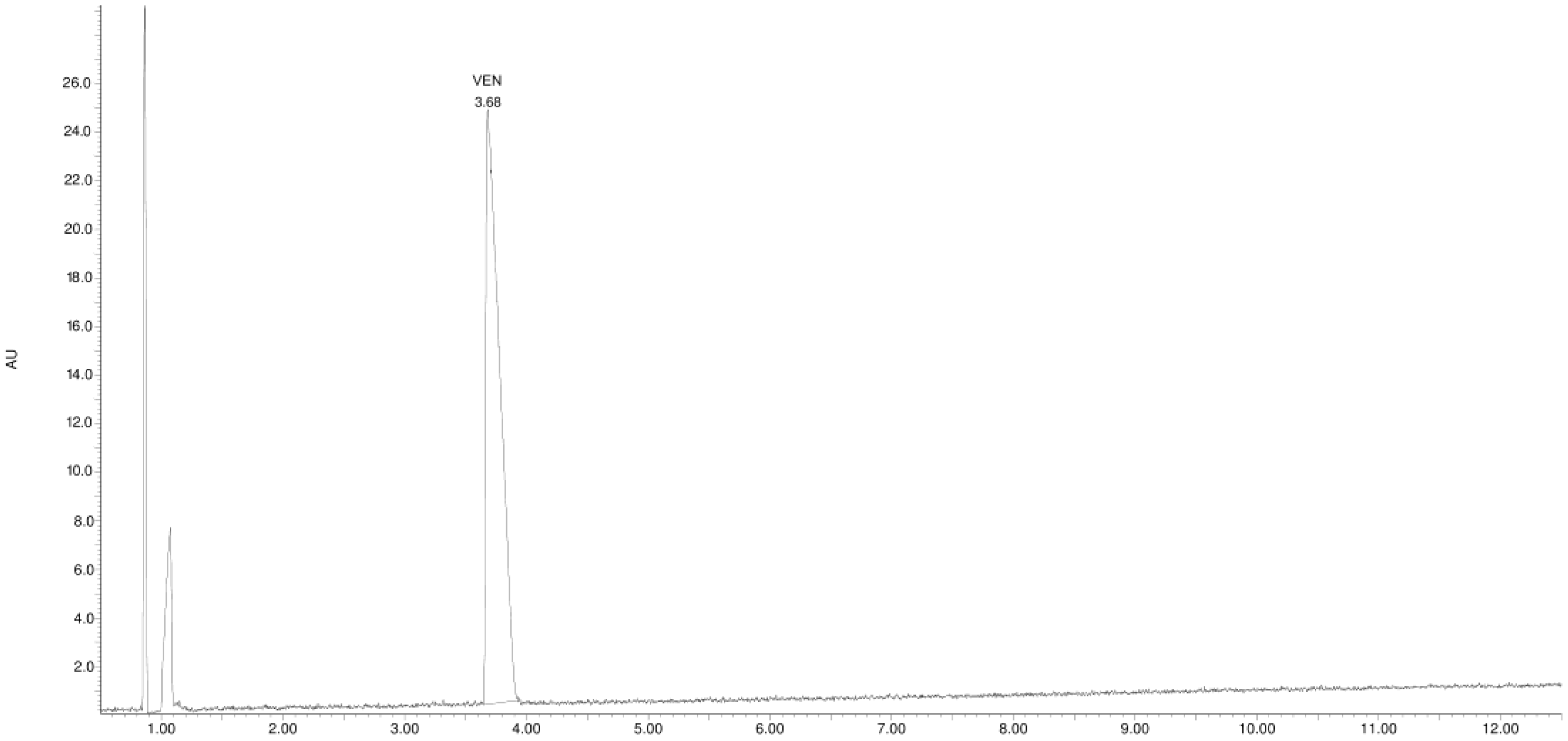
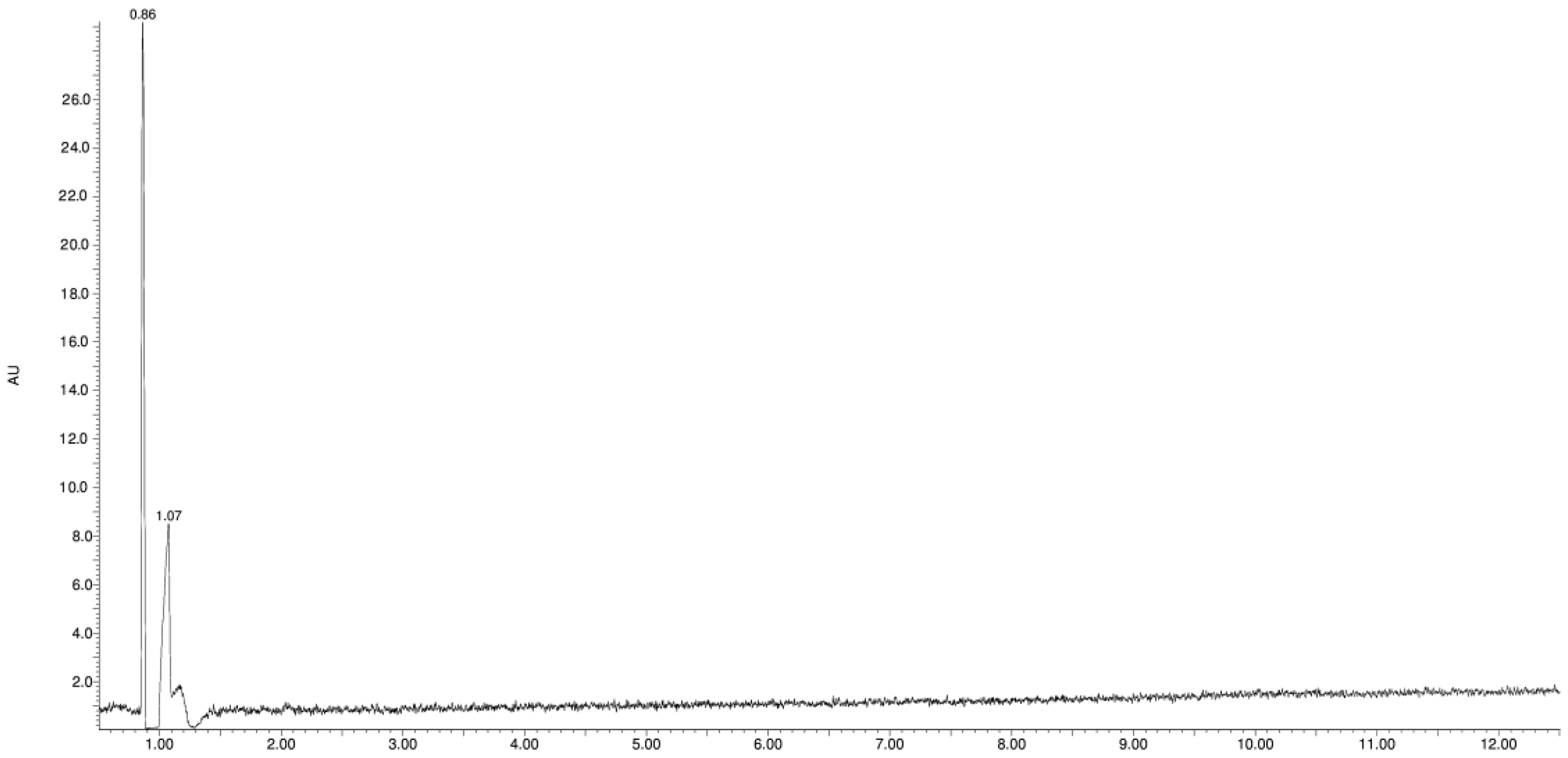


| Product Id | tR | [M + H]+ | Fragmentation Ions | Proposed Structure |
|---|---|---|---|---|
| VP-1 | 1.38 | 180.1 | 107.0, 135.0 | 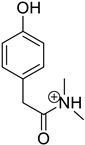 |
| VP-2 | 2.04 | 196.1 | 121.1, 148.1, 162.1, 178.1 |  |
| VP-3 | 2.12 | 180.1 | 121.1, 148.1, 162.1 | 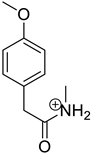 |
| VP-4 | 2.22 | 194.1 | 121.1, 149.1 | 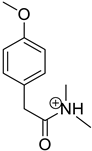 |
| VEN | 3.70 | 278.2 | 121.1, 147.1, 215.1, 260.2 |  |
| VP-5 | 4.00 | 153.1 | 105.1, 135.1 |  |
| Substance Name | k [Day−1] | t0.5 [Days] | t0.1 [Days] | 1 r |
|---|---|---|---|---|
| VEN pure | 7.70 × 10−3 | 90.00 | 13.68 | 0.9779 |
| VEN–EUD physical mixture | 8.00 × 10−3 | 86.60 | 13.16 | 0.9854 |
| VEN–EUD melt | 2.40 × 10−3 | 288.75 | 43.88 | 0.9841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślanka, A.; Szlósarczyk, M.; Talik, P.; Szafraniec-Szczęsny, J.; Woyna-Orlewicz, K.; Żmudzki, P.; Hubicka, U. Study of the Effect of Eudragit RSPO on the Photostability of Venlafaxine in a Physical Mixture and in a Melt Form. Processes 2023, 11, 2479. https://doi.org/10.3390/pr11082479
Maślanka A, Szlósarczyk M, Talik P, Szafraniec-Szczęsny J, Woyna-Orlewicz K, Żmudzki P, Hubicka U. Study of the Effect of Eudragit RSPO on the Photostability of Venlafaxine in a Physical Mixture and in a Melt Form. Processes. 2023; 11(8):2479. https://doi.org/10.3390/pr11082479
Chicago/Turabian StyleMaślanka, Anna, Marek Szlósarczyk, Przemysław Talik, Joanna Szafraniec-Szczęsny, Krzysztof Woyna-Orlewicz, Paweł Żmudzki, and Urszula Hubicka. 2023. "Study of the Effect of Eudragit RSPO on the Photostability of Venlafaxine in a Physical Mixture and in a Melt Form" Processes 11, no. 8: 2479. https://doi.org/10.3390/pr11082479
APA StyleMaślanka, A., Szlósarczyk, M., Talik, P., Szafraniec-Szczęsny, J., Woyna-Orlewicz, K., Żmudzki, P., & Hubicka, U. (2023). Study of the Effect of Eudragit RSPO on the Photostability of Venlafaxine in a Physical Mixture and in a Melt Form. Processes, 11(8), 2479. https://doi.org/10.3390/pr11082479









