Folic Acid and Its Role in Oral Health: A Narrative Review
Abstract
1. Introduction
2. The Role of FA in Pregnancy
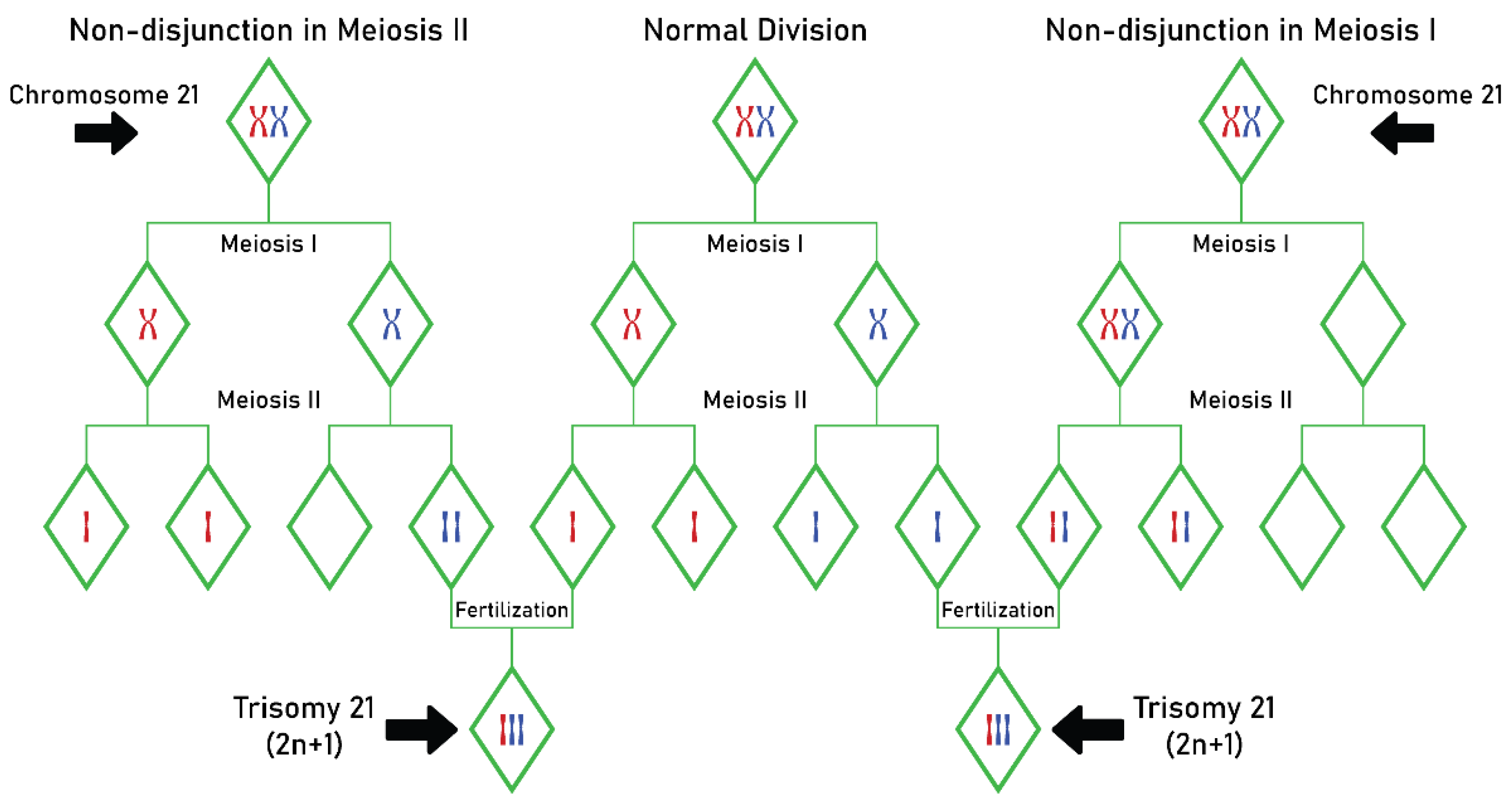
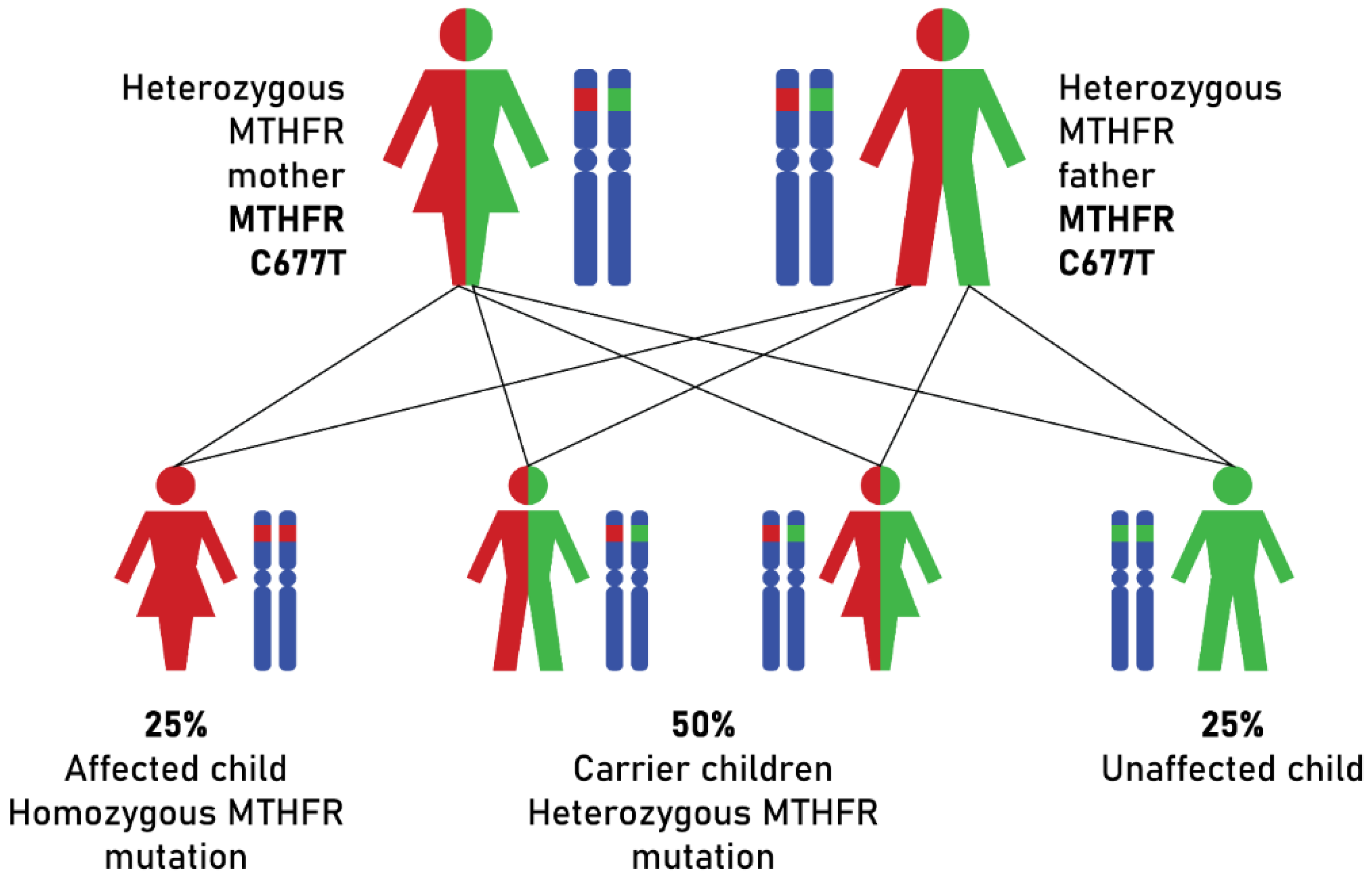
Relationships between MTHFR C677T Gene Mutation and Maternal Risk of Down Syndrome
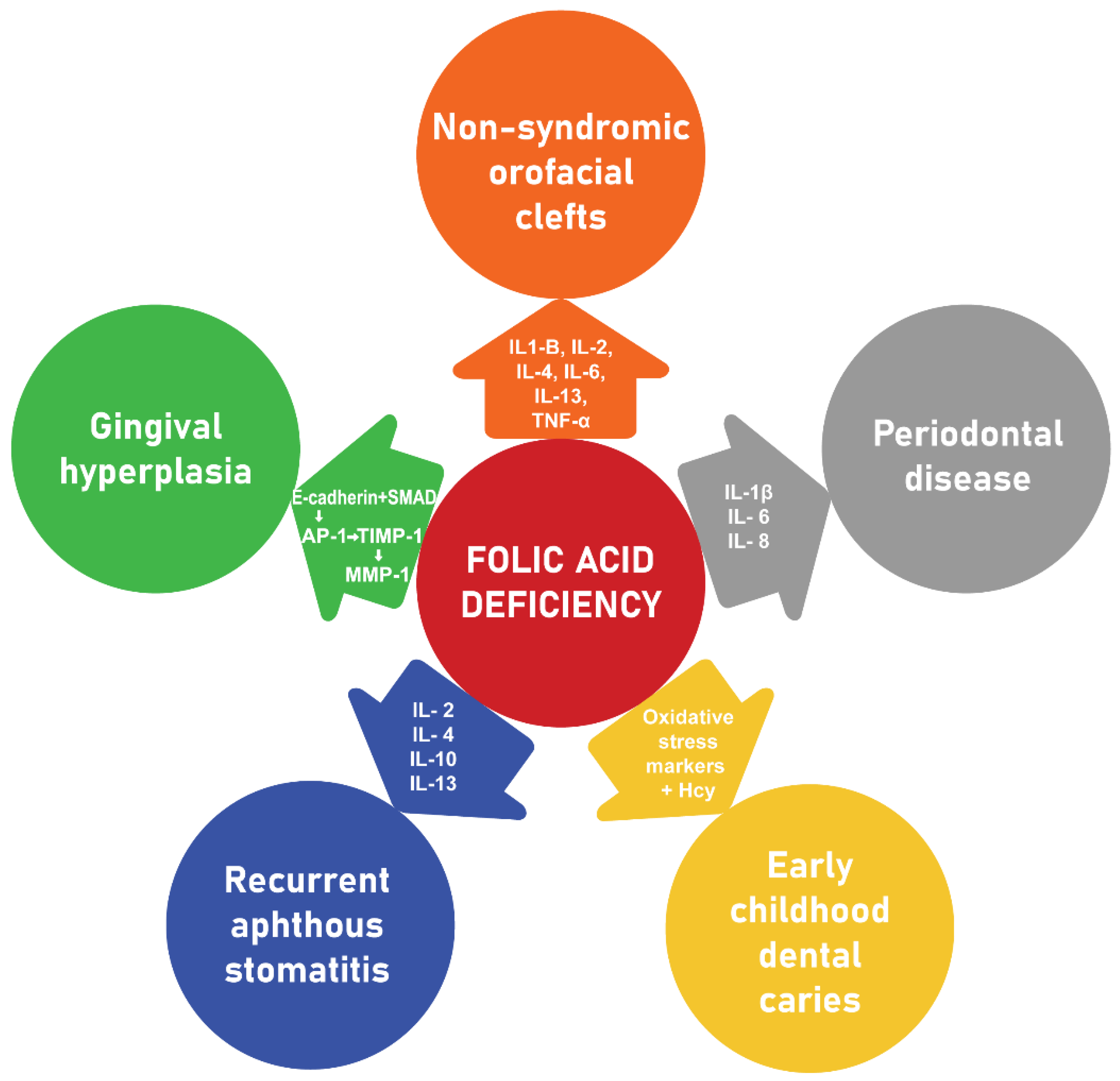
3. FA and Its Role in Oral Health
3.1. Relationships between FA and Non-Syndromic OFC
CKs and Their Role in Orofacial Clefting
3.2. Relationships between FA and Periodontal Disease
The Role of CKs in Periodontal Disease
3.3. Relationships between FA and Early Childhood Dental Caries
The Linkage between Oxidative Stress Markers and Hcy in FA Deficiency and Their Role in Early Childhood Dental Caries
3.4. Relationships between FA and Recurrent Aphthous Stomatitis
3.5. Relationships between FA and Gingival Hyperplasia
4. Suggestions for Future Research
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Romero, A.C.; Hernández, E.G.O.; Cerón, T.; Chávez, A.Á. The Exogenous Antioxidants. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Watanabe, H.; Miyake, T. Folic and Folate Acid. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Bajic, Z.; Sobot, T.; Skrbic, R.; Stojiljkovic, M.P.; Ponorac, N.; Matavulj, A.; Djuric, D.M. Homocysteine, Vitamins B6 and Folic Acid in Experimental Models of Myocardial Infarction and Heart Failure—How Strong Is That Link? Biomolecules 2022, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Duan, H.; Zou, Y.; Qiu, R.; Wang, C. Quantification of Total Folate, Folate Species and Polyglutamyl Folate Distribution in Winged Beans (Psophocarus tetragonolobus (L) DC) from Different Cultivars and Growth Stages by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Nutr. Sci. Vitaminol. 2017, 63, 69–80. [Google Scholar] [PubMed]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Shohag, M.J.; Wei, Y.Y.; Yu, N.; Zhang, J.; Wang, K.; Patring, J.; He, Z.L.; Yang, X.E. Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. J. Agric. Food Chem. 2011, 59, 12520–12526. [Google Scholar]
- Delchier, N.; Ringling, C.; Maingonnat, J.F.; Rychlik, M.; Renard, C.M. Mechanisms of folate losses during processing: Diffusion vs. heat degradation. Food Chem. 2014, 157, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Bekaert, S.; Storozhenko, S.; Mehrshahi, P.; Bennett, M.J.; Lambert, W.; Gregory, J.F., 3rd; Schubert, K.; Hugenholtz, J.; Van Der Straeten, D.; Hanson, A.D. Folate biofortification in food plants. Trends Plant. Sci. 2008, 13, 28–35. [Google Scholar] [CrossRef]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert. Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Affonfere, M.; Chadare, F.J.; Fassinou, F.T.K.; Linnemann, A.R.; Duodu, K.G. In-vitro Digestibility Methods and Factors Affecting Minerals Bioavailability: A Review. Food Rev. Int. 2021, 37, 1014–1042. [Google Scholar] [CrossRef]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Hannon-Fletcher, M.P.; Armstrong, N.C.; Scott, J.M.; Pentieva, K.; Bradbury, I.; Ward, M.; Strain, J.J.; Dunn, A.A.; Molloy, A.M.; Kerr, M.A.; et al. Determining bioavailability of food folates in a controlled intervention study. Am. J. Clin. Nutr. 2004, 80, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Ohrvik, V.E.; Witthoft, C.M. Human Folate Bioavailability. Nutrients 2011, 3, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, P.; McNulty, H.; Mastroiacovo, P.; McDowell, I.F.; Melse-Boonstra, A.; Finglas, P.M.; Gregory, J.F., 3rd; UK Food Standards Agency. Folate bioavailability: UK Food Standards Agency workshop report. Br. J. Nutr. 2003, 90, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Winkels, R.M.; Brouwer, I.A.; Siebelink, E.; Katan, M.B.; Verhoef, P. Bioavailability of food folates is 80% of that of folic acid. Am. J. Clin. Nutr. 2007, 85, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Imbard, A.; Benoist, J.F.; Blom, H.J. Neural tube defects, folic acid and methylation. Int. J. Environ. Res. Public. Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef]
- Khan, K.M.; Jialal, I. Folic Acid Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sharma, J.; Krupenko, S.A. Folate pathways mediating the effects of ethanol in tumorigenesis. Chem. Biol. Interact. 2020, 324, 109091. [Google Scholar] [CrossRef]
- Tanaka, K.; Ao, M.; Kuwabara, A. Insufficiency of B vitamins with its possible clinical implications. J. Clin. Biochem. Nutr. 2020, 67, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Moosavian, S.P.; Nazarian, B.; Afrisham, R.; Kelishadi, M.R.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Inflammatory Markers: A Grade-Assessed Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2327. [Google Scholar] [CrossRef]
- Gutierrez Gossweiler, A.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. Monogr. Oral Sci. 2020, 28, 59–67. [Google Scholar]
- Bencze, M.A.; Imre, M.; Albu, C.C.; Albu, S.D.; Albu, D.F.; Tancu, A.M.C. Early non-invasive diagnosis of fetal anencephaly. Eur. J. Biomed. Pharm. Sci. 2021, 8, 171–175. [Google Scholar]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Padmanabhan, N.; Jia, D.; Geary-Joo, C.; Wu, X.; Ferguson-Smith, A.C.; Fung, E.; Bieda, M.C.; Snyder, F.F.; Gravel, R.A.; Cross, J.C.; et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 2013, 155, 81–93. [Google Scholar] [CrossRef]
- Jalambadani, Z.; Delavari Heravi, M.; Noori Sistani, M. Folic acid consumption based on the theory of planned behaviour in pregnant women. J. Obstet. Gynaecol. 2020, 40, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.M.; Khalifeh, H. Perinatal mental health: A review of progress and challenges. World Psychiatry 2020, 19, 313–327. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.R.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Yadav, U.; Kumar, P.; Yadav, S.K.; Mishra, O.P. Maternal methylenetetrahydrofolate reductase C677T polymorphism and down syndrome risk: A meta-analysis from 34 studies. PLoS ONE 2014, 9, e108552. [Google Scholar] [CrossRef]
- Kokotas, H.; Grigoriadou, M.; Mikkelsen, M.; Giannoulia-Karantana, A.; Petersen, M.B. Investigating the impact of the Down syndrome related common MTHFR 677C>T polymorphism in the Danish population. Dis. Markers 2009, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pavarino, E.C.; Zampieri, B.L.; Biselli, J.M.; Bertollo, E.M.G. Abnormal Folate Metabolism and Maternal Risk for Down Syndrome. In Genetics and Etiology of Down Syndrome; Dey, S., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Albu, D.F.; Onofriescu, M.; Nada, E.S.; Ion, G.; Milicescu, S.; Albu, S.D.; Albu, C.C. The importance of customized biometric correlations in the prevention of growth and development disorders-a determining factor in the social integration of children and adolescents with mental disabilities. Rev. Cercet. Interv. Soc. 2021, 72, 324–337. [Google Scholar] [CrossRef]
- Allen, E.G.; Freeman, S.B.; Druschel, C.; Hobbs, C.A.; O’Leary, L.A.; Romitti, P.A.; Royle, M.H.; Torfs, C.P.; Sherman, S.L. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: A report from the Atlanta and National Down Syndrome Projects. Hum. Genet. 2009, 125, 41–52. [Google Scholar] [CrossRef]
- Atli, E.I. What Causes Down Syndrome? In Down Syndrome and other Chromosome Abnormalities; Dey, S.K., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Albu, C.C.; Vasilache, A.; Stanciu, I.A.; Suciu, I.; Teodorescu, E.; Dragomirescu, A.O.; Albu, S.D.; Nada, E.S.; Albu, D.F.; Ionescu, E. De novo apparently balanced translocation with a novel abnormal phenotype: Review and case presentation. Rom. J. Leg. Med. 2021, 29, 196–204. [Google Scholar] [CrossRef]
- Albu, C.; Cilievici, S.E.; Albu, D.; Albu, S.; Patrascu, A.; Goganau, A.M. Impact of Material Serum Screening in Early Prenatal Diagnosis and Management of Congenital Anomalies. Rev. Chim. 2019, 70, 1534–1538. [Google Scholar] [CrossRef]
- Albu, C.C.; Albu, D.; Albu, S.; Patrascu, A.; Musat, A.; Goganau, A.M. Early Prenatal Diagnosis of an Extremely Rare Association of Down Syndrome and Transposition of the Great Vessels. Rev. Chim. 2019, 70, 2574–2578. [Google Scholar] [CrossRef]
- Ghosh, S.; Dey, S.K. Risk Factors for Down Syndrome Birth: Understanding the Causes from Genetics and Epidemiology. In Down Syndrome; Dey, S.K., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Kolgeci, S.; Kolgeci, J.; Azemi, M.; Shala-Beqiraj, R.; Gashi, Z.; Sopjani, M. Cytogenetic study in children with down syndrome among kosova Albanian population between 2000 and 2010. Mater. Sociomed. 2013, 25, 131–135. [Google Scholar] [CrossRef]
- Simmons, C.J.; Mosley, B.S.; Fulton-Bond, C.A.; Hobbs, C.A. Birth defects in Arkansas: Is folic acid fortification making a difference? Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 559–564. [Google Scholar] [CrossRef]
- Scala, I.; Granese, B.; Sellitto, M.; Salomè, S.; Sammartino, A.; Pepe, A.; Mastroiacovo, P.; Sebastio, G.; Andria, G. Analysis of seven maternal polymorphisms of genes involved in homocysteine/folate metabolism and risk of Down syndrome offspring. Genet. Med. 2006, 8, 409–416. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huo, J.; Sun, J.; Huang, J.; Piao, W.; Yin, J. Meta-analysis on relationship between the Chinese maternal MTHFR gene polymorphism(C677T) and neural tube defects in offspring. Wei Sheng Yan Jiu 2018, 47, 312–317. [Google Scholar]
- Graydon, J.S.; Claudio, K.; Baker, S.; Kocherla, M.; Ferreira, M.; Roche-Lima, A.; Rodríguez-Maldonado, J.; Duconge, J.; Ruaño, G. Ethnogeographic prevalence and implications of the 677C>T and 1298A>C MTHFR polymorphisms in US primary care populations. Biomark. Med. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, H.H.; El-Hefnawy, K.A.; Elalawi, S.M. C677T MTHFR Gene Polymorphism is Contributing Factor in Development of Renal Impairment in Young Hypertensive Patients. Indian J. Clin. Biochem. 2021, 36, 213–220. [Google Scholar] [CrossRef]
- Wu, X.; Yang, K.; Tang, X.; Sa, Y.; Zhou, R.; Liu, J.; Luo, Y.; Tang, W. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: A meta-analysis. J. Assist. Reprod. Genet. 2015, 32, 797–805. [Google Scholar] [CrossRef]
- Al-Gazali, L.I.; Padmanabhan, R.; Melnyk, S.; Yi, P.; Pogribny, I.P.; Pogribna, M.; Bakir, M.; Hamid, Z.A.; Abdulrazzaq, Y.; Dawodu, A.; et al. Abnormal folate metabolism and genetic polymorphism of the folate pathway in a child with Down syndrome and neural tube defect. Am. J. Med. Genet. 2001, 103, 128–132. [Google Scholar] [CrossRef]
- Caudill, M.A. Folate bioavailability: Implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 2010, 91, 1455S–1460S. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, S.T.; Cogger, E.A.; Caudill, M.A. Heterogeneity in the prevalence of methylenetetrahydrofolate reductase gene polymorphisms in women of different ethnic groups. J. Am. Diet. Assoc. 2003, 103, 200–207. [Google Scholar] [CrossRef]
- Acosta-Elias, J.; Espinosa-Tanguma, R. The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front. Pharmacol. 2020, 11, 1062. [Google Scholar] [CrossRef]
- Ashfield-Watt, P.A.; Pullin, C.H.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; Lewis, M.J.; Powers, H.J.; McDowell, I.F. Methylenetetrahydrofolate reductase 677C-->T genotype modulates homocysteine responses to a folate-rich diet or a low-dose folic acid supplement: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, E.P.; Gregory, J.F., 3rd. Reassessing folic acid consumption patterns in the United States (1999 2004): Potential effect on neural tube defects and overexposure to folate. Am. J. Clin. Nutr. 2007, 86, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Cuckle, H.S. Primary prevention of Down’s syndrome. Int. J. Med. Sci. 2005, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, W.; Yang, Y.; Ji, T.; Xu, J. More severe toxicity of genetic polymorphisms on MTHFR activity in osteosarcoma patients treated with high-dose methotrexate. Oncotarget 2017, 9, 11465–11476. [Google Scholar] [CrossRef]
- Dutta, S.; Das, A.B.; Mukhopadhyay, K. Risk of Down syndrome conferred by MTHFR C677T polymorphism: Ethnic variations. Indian J. Hum. Genet. 2007, 13, 76–77. [Google Scholar]
- Irani, M.; Amirian, M.; Sadeghi, R.; Lez, J.L.; Latifnejad Roudsari, R. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol. J. 2017, 14, 4069–4078. [Google Scholar]
- Silva, C.; Keating, E.; Pinto, E. The impact of folic acid supplementation on gestational and long term health: Critical temporal windows, benefits and risks. Porto Biomed. J. 2017, 2, 315–332. [Google Scholar] [CrossRef]
- Wehby, G.L.; Murray, J.C. Folic acid and orofacial clefts: A review of the evidence. Oral Dis. 2010, 16, 11–19. [Google Scholar] [CrossRef]
- Dhamo, B.; Jaddoe, V.; Steegers, E.; Wolvius, E.B.; Ongkosuwito, E.M. The association of maternal folic acid supplementation and prenatal folate and vitamin B12 concentrations with child dental development. Community Dent. Oral Epidemiol. 2021, 49, 445–453. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Lie, R.T.; Solvoll, K.; Taylor, J.; McConnaughey, D.R.; Abyholm, F.; Vindenes, H.; Vollset, S.E.; Drevon, C.A. Folic acid supplements and risk of facial clefts: National population based case-control study. BMJ 2007, 334, 464. [Google Scholar] [CrossRef]
- Millacura, N.; Pardo, R.; Cifuentes, L.; Suazo, J. Effects of folic acid fortification on orofacial clefts prevalence: A meta-analysis. Public Health Nutr. 2017, 20, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Calzolari, E.; Ciulli, L.; Cordier, S.; Gualandi, F.; Pierini, A.; Mossey, P. Environment and genetics in the etiology of cleft lip and cleft palate with reference to the role of folic acid. Epidemiol. Prev. 2000, 24, 21–27. [Google Scholar]
- Komiyama, Y.; Koshiji, C.; Yoshida, W.; Natsume, N.; Kawamata, H. 5,10-Methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms in patients with nonsyndromic cleft lip and palate. Biomed. Rep. 2020, 13, 57. [Google Scholar] [CrossRef]
- Kirke, P.N.; Mills, J.L.; Molloy, A.M.; Brody, L.C.; O’Leary, V.B.; Daly, L.; Murray, S.; Conley, M.; Mayne, P.D.; Smith, O.; et al. Impact of the MTHFR C677T polymorphism on risk of neural tube defects: Case-control study. BMJ 2004, 328, 1535–1536. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, R.S.; Fatahi-Meibodi, N.; Meibodi, B.; Javaheri, A.; Abbasi, H.; Hadadan, A.; Bahrami, R.; Mirjalili, S.R.; Karimi-Zarchi, M.; Neamatzadeh, H. Association of Fetal MTHFR C677T Polymorphism with Susceptibility to Neural Tube Defects: A Systematic Review and Update Meta-Analysis. Fetal Pediatr. Pathol. 2022, 41, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Jeon, Y.J.; Choi, W.I.; Choi, Y.S.; Kim, S.Y.; Chong, S.Y.; Oh, D.; Kim, N.K. The 677C>T mutation of the MTHFR gene increases the risk of venous thromboembolism in Koreans and a meta-analysis from Asian population. Clin. Appl. Thromb. Hemost. 2013, 19, 309–314. [Google Scholar] [CrossRef]
- Pan, X.; Wang, P.; Yin, X.; Liu, X.; Li, D.; Li, X.; Wang, Y.; Li, H.; Yu, Z. Association between Maternal MTHFR Polymorphisms and Nonsyndromic Cleft Lip with or without Cleft Palate in Offspring, A Meta-Analysis Based on 15 Case-Control Studies. Int. J. Fertil. Steril. 2015, 8, 463–480. [Google Scholar]
- Abbasi, I.; Abbasi, F.; Wang, L.; Abd El Hack, M.E.; Swelum, A.A.; Hao, R.; Yao, J.; Cao, Y. Folate promotes S-adenosyl methionine reactions and the microbial methylation cycle and boosts ruminants production and reproduction. AMB Express 2018, 8, 65. [Google Scholar] [CrossRef]
- Seelan, R.S.; Mukhopadhyay, P.; Philipose, J.; Greene, R.M.; Pisano, M.M. Gestational folate deficiency alters embryonic gene expression and cell function. Differentiation 2021, 117, 1–15. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Ferreira, V.L.; Borba, H.H.; de F. Bonetti, A.; Leonart, L.P.; Pontarolo, R. Cytokines and Interferons: Types and Functions. In Autoantibodies and Cytokines; Khan, W.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Ramani, T.; Auletta, C.S.; Weinstock, D.; Mounho-Zamora, B.; Ryan, P.C.; Salcedo, T.W.; Bannish, G. Cytokines: The Good, the Bad, and the Deadly. Int. J. Toxicol. 2015, 34, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Zídek, Z.; Anzenbacher, P.; Kmonícková, E. Current status and challenges of cytokine pharmacology. Br. J. Pharmacol. 2009, 157, 342–361. [Google Scholar] [CrossRef]
- Pilmane, M.; Sidhoma, E.; Akota, I.; Kazoka, D. Characterization of Cytokines and Proliferation Marker Ki67 in Cleft Affected Lip Tissue. Medicina 2019, 55, 518. [Google Scholar] [CrossRef]
- Pilmane, M.; Jain, N.; Jain, S.; Akota, I.; Kroiča, J. Quantification of Cytokines in Lip Tissue from Infants Affected by Congenital Cleft Lip and Palate. Children 2021, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Samblas, M.; Martínez, J.A.; Milagro, F. Folic Acid Improves the Inflammatory Response in LPS-Activated THP-1 Macrophages. Mediat. Inflamm. 2018, 2018, 1312626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Li, X.; Geng, J. Preliminary analysis of immunoregulatory mechanism of hyperhomocysteinemia-induced brain injury in Wistar-Kyoto rats. Exp. Ther. Med. 2021, 21, 483. [Google Scholar] [CrossRef]
- Gildestad, T.; Bjørge, T.; Vollset, S.E.; Klungsøyr, K.; Nilsen, R.M.; Haaland, Ø.A.; Øyen, N. Folic acid supplements and risk for oral clefts in the newborn: A population-based study. Br. J. Nutr. 2015, 114, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yi, L.; Deng, C.; Zhao, Z.; Ran, L.; Ren, Z.; Zhao, S.; Zhou, T.; Zhang, G.; Liu, H.; et al. Maternal periconceptional folic acid supplementation reduced risks of non-syndromic oral clefts in offspring. Sci. Rep. 2021, 11, 12316. [Google Scholar] [CrossRef]
- Bortolus, R.; Blom, F.; Filippini, F.; van Poppel, M.N.; Leoncini, E.; de Smit, D.J.; Benetollo, P.P.; Cornel, M.C.; de Walle, H.E.; Mastroiacovo, P.; et al. Prevention of congenital malformations and other adverse pregnancy outcomes with 4.0 mg of folic acid: Community-based randomized clinical trial in Italy and the Netherlands. BMC Pregnancy Childbirth 2014, 14, 166. [Google Scholar] [CrossRef]
- Melnick, M. Cleft Lip and Palate: From Origin to Treatment. Am. J. Hum. Genet. 2003, 72, 503. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Puranik, A.S.; Shintre, P.; Joshi, K.; Patwardhan, B.; Lamba, J.; Niewold, T.B.; Chopra, A. Folate metabolic pathway single nucleotide polymorphisms: A predictive pharmacogenetic marker of methotrexate response in Indian (Asian) patients with rheumatoid arthritis. Pharmacogenomics 2015, 16, 2019–2034. [Google Scholar] [CrossRef]
- Umesh, S.G.; Ramachandran, L.; Karthikeyan, J.; Mani, A. Genetics and Periodontal Disease: An Explicit Insight. In Periodontology—Fundamentals and Clinical Features; Surlin, P., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- Alpan, A.L.; Karakan, N.C. Folate in Dentistry. In B Group Vitamins—Current Uses and Perspectives; J. LeBlanc, G., de Giori, G., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- George, J.P.; Shobha, R.; Lazarus, F.J. Folic acid: A positive influence on periodontal tissues during health and disease. Int. J. Health. Allied. Sci. 2013, 2, 145–152. [Google Scholar] [CrossRef]
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef]
- Surlin, P.; Rauten, A.M.; Popescu, M.R.; Constantin Daguci, C.; Bogdan, M. Periodontal Changes and Oral Health. In Emerging Trends in Oral Health Sciences and Dentistry; Virdi, M.S., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Sumona, B.; Sheetal, S.; Anil, M.; Suvarna, P. Comparative evaluation of serum folic acid levels in smokers and non-smokers with chronic periodontitis. Bangladesh J. Medical. Sci. 2011, 10, 83–90. [Google Scholar] [CrossRef]
- Whitehead, N.; Reyner, F.; Lindenbaum, J. Megaloblastic changes in the cervical epithelium: Association with oral contraceptive therapy and reversal with folic acid. JAMA. 1973, 226, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.I.; Fink, R.A.; Schneider, L.C.; Frank, O.; Baker, H. The effect of folic acid on gingival health. J. Periodontol. 1976, 47, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Morita, M.; Akhter, R.; Akino, K.; Honda, O. Relationship between folic acid intake and gingival health in non-smoking adults in Japan. Oral Dis. 2010, 16, 96–101. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Canakci, V.; Yildirim, A.; Canakci, C.F.; Eltas, A.; Cicek, Y.; Canakci, H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J. Periodontol. 2007, 78, 1602–1611. [Google Scholar] [CrossRef]
- Goutoudi, P.; Diza, E.; Arvanitidou, M. Effect of periodontal therapy on crevicular fluid interleukin-6 and interleukin-8 levels in chronic periodontitis. Int. J. Dent. 2012, 2012, 362905. [Google Scholar] [CrossRef]
- McGee, J.M.; Tucci, M.A.; Edmundson, T.P.; Serio, C.L.; Johnson, R.B. The relationship between concentrations of proinflammatory cytokines within gingiva and the adjacent sulcular depth. J. Periodontol. 1998, 69, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.M.; Grbíc, J.T.; Lamster, I.B. Interleukin-8 and beta-glucuronidase in gingival crevicular fluid. J. Clin. Periodontol. 1977, 24, 146–152. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Steiner, L.; Mayer, W.; De Luca, C.; Korkina, L.G. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dent. J. 2020, 8, 10. [Google Scholar] [CrossRef]
- Wang, R.P.; Huang, J.; Chan, K.W.Y.; Leung, W.K.; Goto, T.; Ho, Y.S.; Chang, R.C. IL-1β and TNF-α play an important role in modulating the risk of periodontitis and Alzheimer’s disease. J. Neuroinflammation 2023, 20, 71. [Google Scholar] [CrossRef]
- Akpınar, A.; Karakan, N.C.; Alpan, A.L.; Dogan, S.; Goze, F.; Poyraz, O. Comparative effects of riboflavin, nicotinamide and folic acid on alveolar bone loss: A morphometric and histopathologic study in rats. Srp. Arh. Celok. Lek. 2016, 144, 273–279. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Policy on early childhood caries (ECC): Classifications, consequences, and preventive strategies. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2020; pp. 79–81. [Google Scholar]
- Alazmah, A. Early Childhood Caries: A Review. J. Contemp. Dent. Pract. 2017, 18, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Yadav, K.; Prakash, S. Dental Caries: A Microbiological Approach. J. Clin. Infect. Dis. Pract. 2017, 2, 118. [Google Scholar] [CrossRef]
- Skutnik-Radziszewska, A.; Zalewska, A. Salivary Redox Biomarkers in the Course of Caries and Periodontal Disease. Appl. Sci. 2020, 10, 6240. [Google Scholar] [CrossRef]
- Șaramet, V.; Meleșcanu-Imre, M.; Țâncu, A.M.C.; Albu, C.C.; Ripszky-Totan, A.; Pantea, M. Molecular Interactions between Saliva and Dental Composites Resins: A Way Forward. Materials 2021, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Shih, T.S.; Huang, H.R.; Huang, S.C.; Lee, L.H.; Huang, Y.C. Plasma homocysteine is associated with increased oxidative stress and antioxidant enzyme activity in welders. Sci. World J. 2013, 2013, 370487. [Google Scholar] [CrossRef] [PubMed]
- Fakhrzadeh, H.; Ghotbi, S.; Pourebrahim, R.; Nouri, M.; Heshmat, R.; Bandarian, F.; Shafaee, A.; Larijani, B. Total plasma homocysteine, folate, and vitamin B12 status in healthy Iranian adults: The Tehran homocysteine survey (2003–2004)/a cross—sectional population based study. BMC Public Health 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef]
- Mahjoub, S.; Ghasempour, M.; Gharage, A.; Bijani, A.; Masrourroudsari, J. Comparison of total antioxidant capacity in saliva of children with severe early childhood caries and caries-free children. Caries Res. 2014, 48, 271–275. [Google Scholar] [CrossRef]
- Jurge, S.; Kuffer, R.; Scully, C.; Porter, S. Number VI Recurrent aphthous stomatitis. Oral Dis. 2006, 12, 1–21. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Noor, S.; Menzel, A.; Gasmi, A. Oral Aphthous: Pathophysiology, Clinical Aspects and Medical Treatment. Arch. Razi. Inst. 2021, 76, 1155–1163. [Google Scholar] [PubMed]
- Scully, C.; Gorsky, M.; Lozada-Nur, F. The diagnosis and management of recurrent aphthous stomatitis. J. Am. Dent. Assoc. 2003, 134, 200–207. [Google Scholar] [CrossRef]
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Rahmani, S. Diagnostic Features of Common Oral Ulcerative Lesions: An Updated Decision Tree. Int. J. Dent. 2016, 2016, 7278925. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Porter, S. Oral mucosal disease: Recurrent aphthous stomatitis. Br. J. Oral Maxillofac. Surg. 2008, 46, 198–206. [Google Scholar] [CrossRef]
- Bao, Z.X.; Shi, J.; Yang, X.W.; Liu, L.X. Hematinic deficiencies in patients with recurrent aphthous stomatitis: Variations by gender and age. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e161–e167. [Google Scholar] [CrossRef]
- Plewa, M.C.; Chatterjee, K. Aphthous Stomatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Piskin, S.; Sayan, C.; Durukan, N.; Senol, M. Serum iron, ferritin, folic acid, and vitamin B12 levels in recurrent aphthous stomatitis. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 66–67. [Google Scholar] [CrossRef]
- Barnadas, M.A.; Remacha, A.; Condomines, J.; de Moragas, J.M. Hematologic deficiencies in patients with recurrent oral aphthae. Med. Clin. 1997, 109, 85–87. [Google Scholar]
- Lokossou, A.G.; Dechavanne, C.; Bouraïma, A.; Courtin, D.; Le Port, A.; Ladékpo, R.; Noukpo, J.; Bonou, D.; Ahouangninou, C.; Sabbagh, A.; et al. Association of IL-4 and IL-10 maternal haplotypes with immune responses to P. falciparum in mothers and newborns. BMC Infect. Dis. 2013, 13, 215. [Google Scholar] [CrossRef]
- Ślebioda, Z.; Szponar, E.; Kowalska, A. Recurrent aphthous stomatitis: Genetic aspects of etiology. Postepy Dermatol. Alergol. 2013, 2, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, R.; Thubashini, M.; Sundharam, B.S. Detection of salivary interleukin-2 in recurrent aphthous stomatitis. J. Oral Maxillofac. Pathol. 2014, 18, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Pekiner, F.N.; Aytugar, E.; Demirel, G.Y.; Borahan, M.O. Interleukin-2, interleukin-6 and T regulatory cells in peripheral blood of patients with Behçet’s disease and recurrent aphthous ulcerations. J. Oral Pathol. Med. 2012, 41, 73–79. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Borilova Linhartova, P.; Janos, J.; Slezakova, S.; Bartova, J.; Petanova, J.; Kuklinek, P.; Fassmann, A.; Dusek, L.; Izakovicova Holla, L. Recurrent aphthous stomatitis and gene variability in selected interleukins: A case-control study. Eur. J. Oral Sci. 2018, 26, 485–492. [Google Scholar] [CrossRef]
- Brown, R.S.; Arany, P.R. Mechanism of drug-induced gingival overgrowth revisited: A unifying hypothesis. Oral Dis. 2015, 21, e51–e61. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Gulati, S.; Kabra, M.; Sahu, J.K.; Kalra, V. Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children. Neurology 2011, 76, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.N.; Chawla, H.S.; Goyal, A.; Gauba, K.; Singhi, P. Folic acid and phenytoin induced gingival overgrowth—Is there a preventive effect. J. Indian. Soc. Pedod. Prev. Dent. 2004, 22, 82–91. [Google Scholar] [PubMed]
- Garg, U.; Sandritter, T.L.; Gaedigk, A. Pediatric therapeutic drug monitoring, toxicology and pharmacogenomics. In Biochemical and Molecular Basis of Pediatric Disease, 5th ed.; Dietzen, D., Bennett, M., Wong, E., Haymond, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 25; pp. 849–908. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, C.-C.; Bencze, M.-A.; Dragomirescu, A.-O.; Suciu, I.; Tănase, M.; Albu, Ş.-D.; Russu, E.-A.; Ionescu, E. Folic Acid and Its Role in Oral Health: A Narrative Review. Processes 2023, 11, 1994. https://doi.org/10.3390/pr11071994
Albu C-C, Bencze M-A, Dragomirescu A-O, Suciu I, Tănase M, Albu Ş-D, Russu E-A, Ionescu E. Folic Acid and Its Role in Oral Health: A Narrative Review. Processes. 2023; 11(7):1994. https://doi.org/10.3390/pr11071994
Chicago/Turabian StyleAlbu, Cristina-Crenguța, Maria-Angelica Bencze, Anca-Oana Dragomirescu, Ioana Suciu, Mihaela Tănase, Ştefan-Dimitrie Albu, Emily-Alice Russu, and Ecaterina Ionescu. 2023. "Folic Acid and Its Role in Oral Health: A Narrative Review" Processes 11, no. 7: 1994. https://doi.org/10.3390/pr11071994
APA StyleAlbu, C.-C., Bencze, M.-A., Dragomirescu, A.-O., Suciu, I., Tănase, M., Albu, Ş.-D., Russu, E.-A., & Ionescu, E. (2023). Folic Acid and Its Role in Oral Health: A Narrative Review. Processes, 11(7), 1994. https://doi.org/10.3390/pr11071994





