Physical Properties and Molecular Interactions Applied to Food Processing and Formulation
Abstract
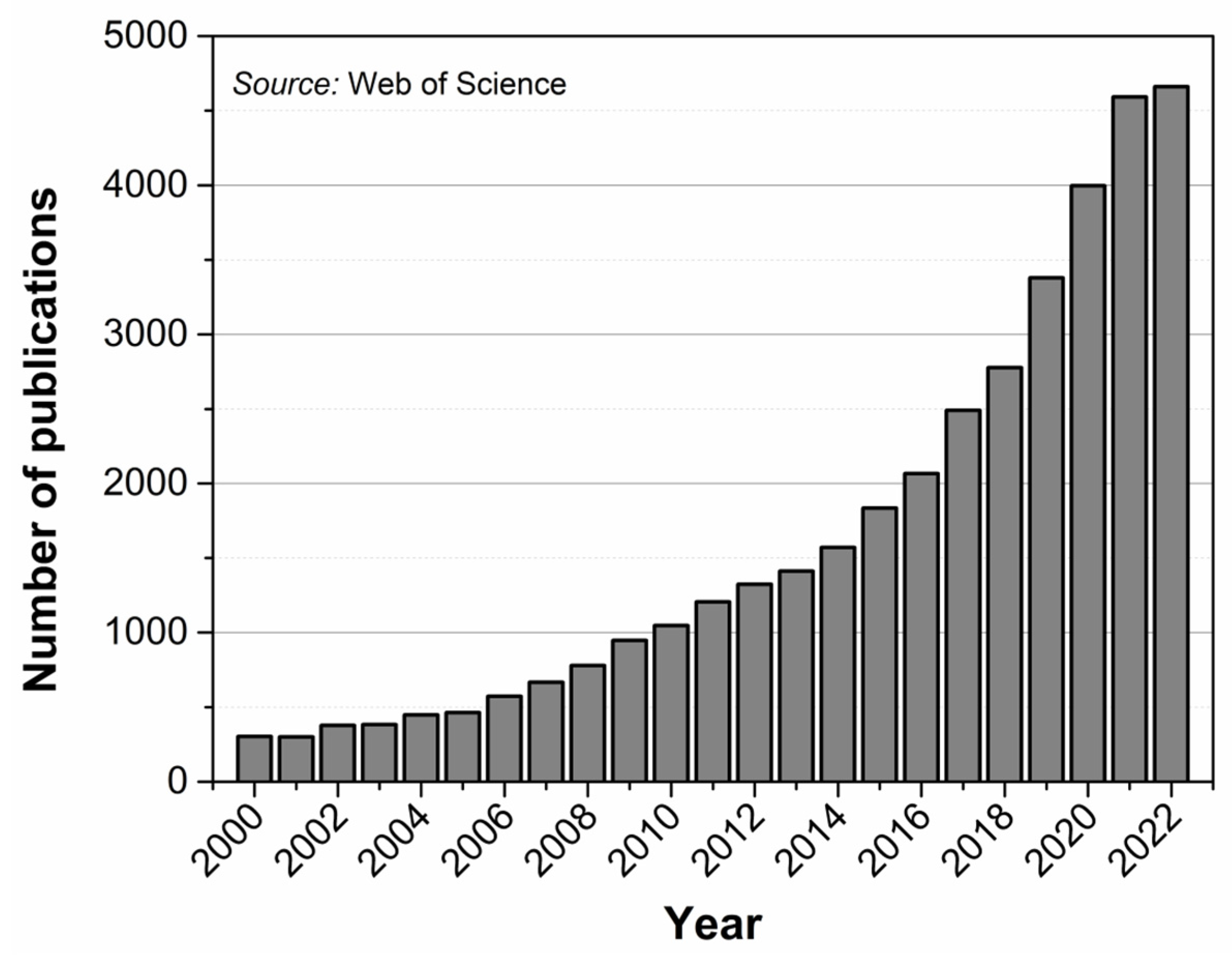
1. Introduction
2. Physical Property Measurements
2.1. Density (ρ)
2.2. Specific Heat (cp)
2.3. Thermal Conductivity (λ)
2.4. Thermal Diffusivity (α)
3. Rheological Behavior of Foods
3.1. Rheology of Solid Foods
3.2. Rheological Classification of Fluid Foods
3.3. Analytical Determination of the Rheological Properties of Fluids
3.4. Rheology of Emulsions
3.5. Food Rheology Applied to Equipment Design
4. Thermodynamic Aspects in Foods
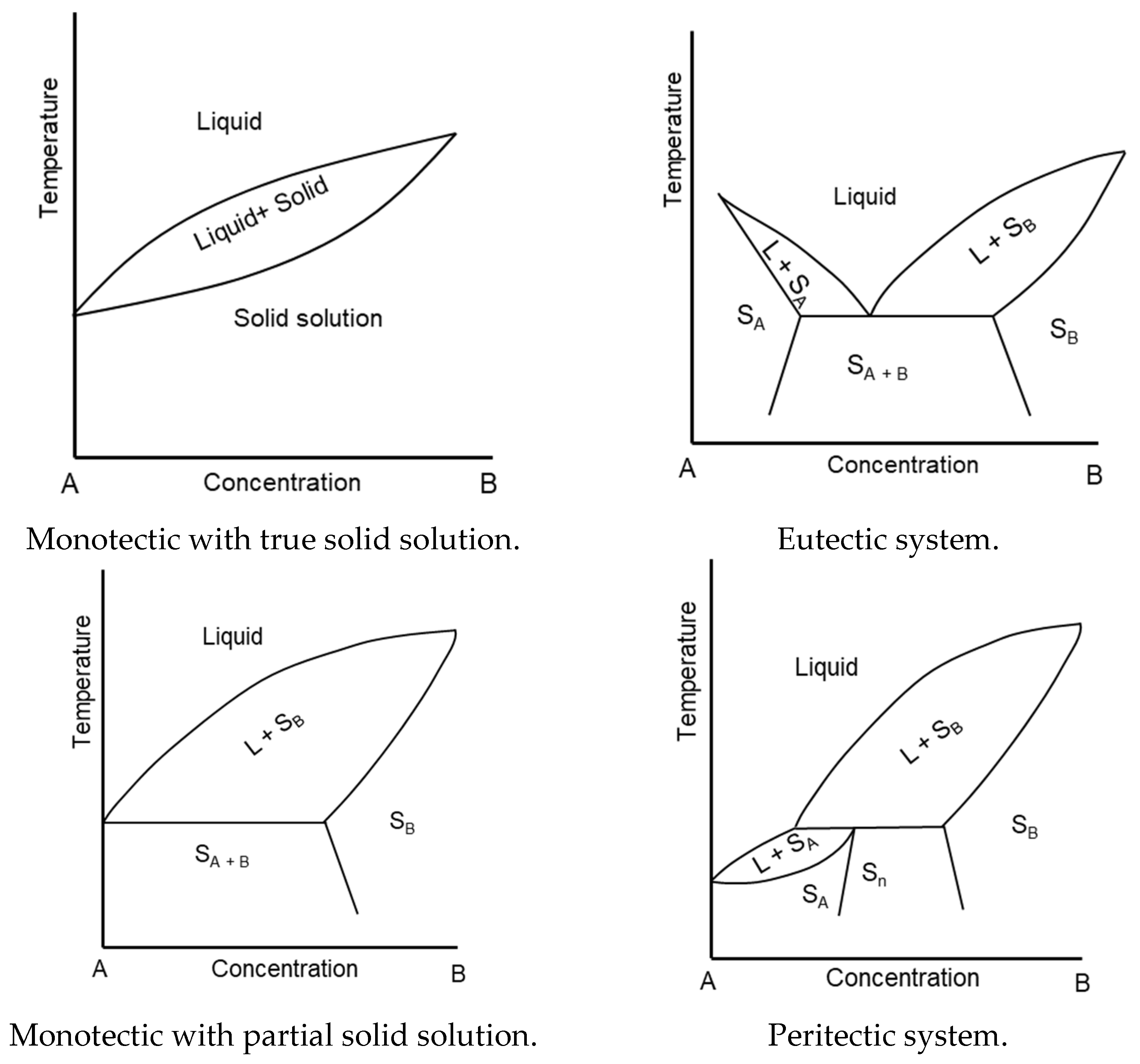
4.1. Systems Containing Triacylglycerols
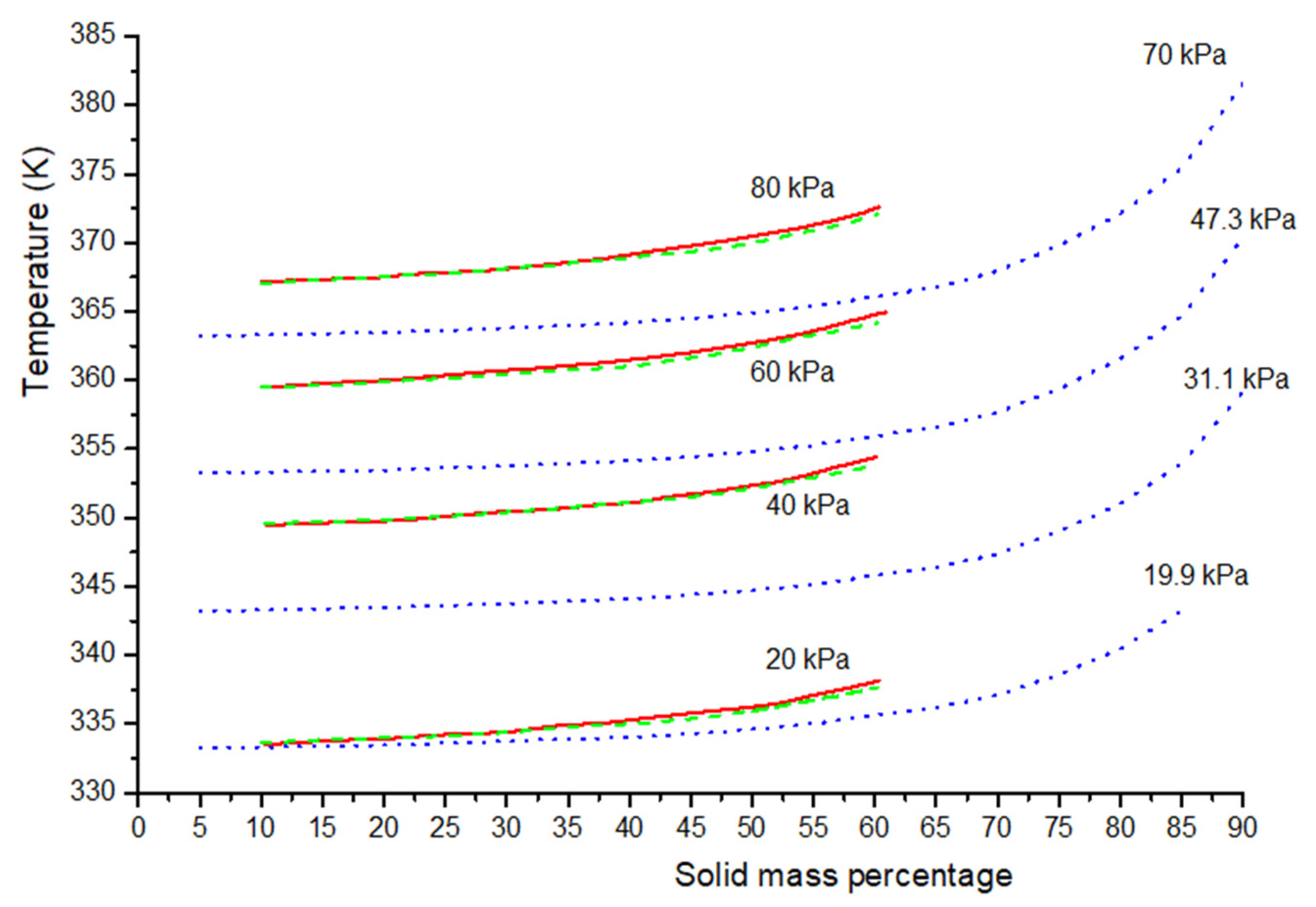
4.2. Sugar-Rich Systems
4.3. Protein and Polysaccharide-Rich Systems
5. Overall Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ρ | Density |
| cp | Specific heat at constant pressure |
| cv | Specific heat at constant volume |
| DSC | Differential scanning calorimetry |
| λ | Thermal conductivity |
| α | Thermal diffusivity |
| τ | Stress–strain |
| η | Apparent fluid viscosity |
| γ | Shear rate |
| k | Coefficient of consistency |
| n | Characteristic flow index |
| τ0 | Yield stress |
| μp | Plastic viscosity |
| μ0 | Viscosity at a reference pressure |
| P | Pressure |
| P0 | Reference pressure |
| α | Adjustment factor known as the viscosity growth coefficient |
| G | Gibbs energy |
| H | Enthalpy |
| T | Temperature |
| S | Entropy |
| γ | Activity coefficient |
| x | Molar fraction of a component |
| R | Universal gas constant |
References
- Carson, J.K. Review of effective thermal conductivity models for foods. Int. J. Refrig. 2006, 29, 958–967. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Carvalho, G.R.; Polachini, T.C.; Augusto, P.E.D.; Telis-Romero, J.; Bon, J. Physical properties of barley grains at hydration and drying conditions of malt production. J. Food Process Eng. 2021, 44, e13644. [Google Scholar] [CrossRef]
- Varnamkhasti, M.G.; Mobli, H.; Jafari, A.; Keyhani, A.R.; Soltanabadi, M.H.; Rafiee, S.; Kheiralipour, K. Some physical properties of rough rice (Oryza sativa L.) grain. J. Cereal Sci. 2008, 47, 496–501. [Google Scholar] [CrossRef]
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials; Gordon and Breach Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- Bellido, G.G.; Scanlon, M.G.; Page, J.H. Measurement of dough specific volume in chemically leavened dough systems. J. Cereal Sci. 2009, 49, 212–218. [Google Scholar] [CrossRef]
- Kelkar, S.; Stella, S.; Boushey, C.; Okos, M. Developing novel 3D measurement techniques and prediction method for food density determination. Procedia Food Sci. 2011, 1, 483–491. [Google Scholar] [CrossRef]
- Palier, J.; Le-Bail, A.; Loisel, C.; Le-Bail, P. Substitution of baking powders in a pound cake by an overpressure mixing process; impact on cake properties. J. Food Eng. 2022, 316, 110824. [Google Scholar] [CrossRef]
- Figura, L.; Teixeira, A.A. Food Physics: Physical Properties—Measurement and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Polachini, T.C.; Betiol LF, L.; Bastos, M.G.; Telis, V.R.N.; Souza, A.C.; Telis-Romero, J. Density, thermal expansion coefficient, and rheological behaviour of meat extract under different temperatures and solids concentrations. Can. J. Chem. Eng. 2016, 94, 988–994. [Google Scholar] [CrossRef]
- Polachini, T.C.; Mulet, A.; Cárcel, J.A.; Telis-Romero, J. Thermophysical properties of dilute acid slurries of cassava bagasse as a function of biomass loading, acid concentration and temperature. Environ. Prog. Sustain. Energy 2020, 40, e13543. [Google Scholar] [CrossRef]
- Gan, T.H.; Pallav, P.; Hutchins, D.A. Non-contact ultrasonic quality measurements of food products. J. Food Eng. 2006, 77, 239–247. [Google Scholar] [CrossRef]
- Calín-Sánchez, A.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochemistry 2019, 57, 73–88. [Google Scholar] [CrossRef]
- Shenoy, P.; Viau, M.; Tammel, K.; Innings, F.; Fitzpatrick, J.; Ahrné, L. Effect of powder densities, particle size and shape on mixture quality of binary food powder mixtures. Powder Technol. 2015, 272, 165–172. [Google Scholar] [CrossRef]
- Apsarina, Y.; Anam, C.; Khasanah, L.U. The impact of blending speed and duration on the characteristics of powdered milk product X in PT. XYZ. AIP Conf. Proc. 2020, 2219, 070001. [Google Scholar]
- Ribeiro, E.F.; Polachini, T.C.; Carvalho, G.R.; Romero, J.T.; Cabral, R.A.F. Thermophysical properties of different olive oils: Evaluating density and rheology through a fluid dynamic approach. Eur. J. Lipid Sci. Technol. 2017, 119, 1600316. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and viscosity of vegetable oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- Aktas, R.N.; Tontul, I. Usability of soapwort and horse chestnut saponin extracts as foaming agents in foam mat drying of pomegranate juice. J. Sci. Food Agric. 2021, 101, 786–793. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Balasubramaniam, V.M.; Sastry, S.K. Determination of In-Situ Thermal Conductivity, Thermal Diffusivity, Volumetric Specific Heat and Isobaric Specific Heat of Selected Foods under Pressure. Int. J. Food Prop. 2012, 15, 169–187. [Google Scholar] [CrossRef]
- Choi, Y.; Okos, M.R. Effects of temperature and composition on the thermal properties of foods. In Food Engineering and Process Aplications; Lemauguer, M., Jelen, M., Eds.; Elsevier Applied Science Publishers: London, UK, 1986; pp. 93–101. [Google Scholar]
- Zhu, X.; Phinney, D.M.; Paluri, S.; Heldman, D.R. Prediction of Liquid Specific Heat Capacity of Food Lipids. J. Food Sci. 2018, 83, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Rizvi, S.S.H. Engineering Properties of Food; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Gupta, M.; Yang, J.; Roy, C. Specific heat and thermal conductivity of softwood bark and softwood char particles. Fuel 2003, 82, 919–927. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, M.; Birwal, P. Differential Scanning Calorimetry (DSC) for the Measurement of Food Thermal Characteristics and Its Relation to Composition and Structure. In Techniques to Measure Food Safety and Quality: Microbial, Chemical, and Sensory; Khan, M.S., Rahman, M.S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 283–328. [Google Scholar]
- Polachini, T.C.; Betiol LF, L.; Bastos, M.G.; Telis, V.R.N.; Telis-Romero, J. Boiling point and specific heat of meat extract. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1392–1402. [Google Scholar] [CrossRef]
- Dash, K.K.; Sharma, M.; Tiwari, A. Heat and mass transfer modeling and quality changes during deep fat frying: A comprehensive review. J. Food Process Eng. 2022, 45, e13999. [Google Scholar] [CrossRef]
- Chen, L.; Irmak, S.; Chaves, B.D.; Subbiah, J. Microbial challenge study and quality evaluation of cumin seeds pasteurized by continuous radio frequency processing. Food Control 2020, 111, 107052. [Google Scholar] [CrossRef]
- Yu, S.S.; Ahn, H.S.; Park, S.H. Heat penetration and quality analysis of retort processed vegetables for home meal replacement foods. Food Sci. Biotechnol. 2023, 32, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.J.N.; Guimarães, B.; Polachini, T.C.; Telis-Romero, J. Thermophysical properties of carbohydrate solutions: Correlation between thermal and transport properties. J. Food Process Eng. 2020, 43, e13483. [Google Scholar] [CrossRef]
- Mohsenin, N.N. Thermal Properties of Foods and Agricultural Materials; Gordon and Breach Science Publishers Ltd.: New York, NY, USA, 1980. [Google Scholar]
- Kausar, A. A review of high performance polymer nanocomposites for packaging applications in electronics and food industries. J. Plast. Film Sheeting 2020, 36, 94–112. [Google Scholar] [CrossRef]
- Horvat, G.; Kotnik, T.; Žvab, K.; Knez, Ž.; Novak, Z.; Kovačič, S. Silica aerogel-filled polymer foams by emulsion-templating: One-pot synthesis, hierarchical architecture and thermal conductivity. Chem. Eng. J. 2022, 450, 138251. [Google Scholar] [CrossRef]
- Carson, J.K. Thermal Conductivity Measurement and Prediction of Particulate Foods. Int. J. Food Prop. 2015, 18, 2840–2849. [Google Scholar] [CrossRef]
- Tavman, I.H.; Tavman, S. Measurement of thermal conductivity of dairy products. J. Food Eng. 1999, 41, 109–114. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Application of Novel Thermal Technology in Foods Processing. Foods 2022, 11, 125. [Google Scholar] [CrossRef]
- Sweat, V.E. Thermal Properties of Foods; Marcel Dekker Inc.: New York, NY, USA, 1986. [Google Scholar]
- Betta, G.; Rinaldi, M.; Barbanti, D.; Massini, R. A quick method for thermal diffusivity estimation: Application to several foods. J. Food Eng. 2009, 91, 34–41. [Google Scholar] [CrossRef]
- Dickerson, R.W. An apparatus for the measurement of thermal diffusivity of foods. Food Technol. 1965, 19, 198–204. [Google Scholar]
- Carbajal-Valdéz, R.; Jiménez-Pérez, J.L.; Gamboa-López, G.; Correa-Pacheco, Z.N.; Hernández-Aguilar, C.; Pérez-González, M.; García-Vidal, U.O.; Netzahual-Lopantzi, A. Determination of the Dependence of Thermal Diffusivity with Moringa Concentration by Thermal Lens as a Sensitive Experimental Technique. Int. J. Thermophys. 2020, 41, 105. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Swapna, M.S.; Devi HV, S.; Sankararaman, S. Nonradiative analysis of adulteration in coconut oil by thermal lens technique. Appl. Phys. B 2019, 125, 113. [Google Scholar] [CrossRef]
- Carciofi, B.A.M.; Faistel, J.; Aragão, G.M.F.; Laurindo, J.B. Determination of thermal diffusivity of mortadella using actual cooking process data. J. Food Eng. 2002, 55, 89–94. [Google Scholar] [CrossRef]
- Mariani, V.C.; Lima, A.G.B.; Coelho, L.S. Apparent thermal diffusivity estimation of the banana during drying using inverse method. J. Food Eng. 2008, 85, 569–579. [Google Scholar] [CrossRef]
- Kostaropoulos, A.E.; Saravacos, G.D. Thermal diffusivity of granular and porous foods at low moisture content. J. Food Eng. 1997, 33, 101–109. [Google Scholar] [CrossRef]
- Silva, W.P.; Medeiros, M.S.; Gomes, J.P.; Silva, C.M.D.P.S. Improvement of methodology for determining local thermal diffusivity and heating time of green coconut pulp during its pasteurization. J. Food Eng. 2020, 285, 110104. [Google Scholar] [CrossRef]
- Nguyen, X.Q.; Le, A.D.; Nguyen, N.P.; Nguyen, H. Thermal Diffusivity, Moisture Diffusivity, and Color Change of Codonopsis javanica with the Support of the Ultrasound for Drying. J. Food Qual. 2019, 2019, 2623404. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Nazarewicz, S.; Góral, D.; Krawczuk, A.; Domin, M. Lyophilized Protein Structures as an Alternative Biodegradable Material for Food Packaging. Sustainability 2019, 11, 7002. [Google Scholar] [CrossRef]
- Samuel, O.D.; Waheed, M.A.; Taheri-Garavand, A.; Verma, T.N.; Dairo, O.U.; Bolaji, B.O.; Afzal, A. Prandtl number of optimum biodiesel from food industrial waste oil and diesel fuel blend for diesel engine. Fuel 2021, 285, 119049. [Google Scholar] [CrossRef]
- Chhabra, R.P.; Richardson, J.F. Non-Newtonian Flow and Applied Rheology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Suhag, R.; Dhiman, A.; Prabhakar, P.K.; Sharma, A.; Singh, A.; Upadhyay, A. Microfluidization of liquid egg yolk: Modelling of rheological characteristics and interpretation of flow behavior under a pipe flow. Innov. Food Sci. Emerg. Technol. 2022, 81, 103119. [Google Scholar] [CrossRef]
- Dobre, I.; Ggeorgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological behavior of different honey types from Romania. Food Res. Int. 2012, 49, 126–132. [Google Scholar] [CrossRef]
- Nambi, V.E.; Thangavel, K.; Rajeswari, K.A.; Manickavasagan, A.; Gita, V. Texture and rheological changes of Indian mango cultivars during ripening. Postharvest Biol. Technol. 2016, 117, 152–160. [Google Scholar] [CrossRef]
- Nikolidaki, E.K.; Mandala, J.; Zogzas, N.P.; Karathanos, V.T. Modeling the rheological properties of currant paste as a function of plasticizers concentration, storage temperature and time and process temperature. Food Res. Int. 2019, 116, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Salunke, P.; Metzger, L.E. Functional characteristics of process cheese product as affected by milk protein concentrate and micellar casein concentrate at different usage levels. Int. Dairy J. 2022, 128, 105324. [Google Scholar] [CrossRef]
- Rocha, R.S.; Silva, R.; Guimarâes, J.T.; Balthazar, C.F.; Pimental, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Esmerino, E.A.; Freitas, M.Q.; Cappato, L.P.; et al. Possibilities for using ohmic heating in Minas Frescal cheese production. Food Res. Int. 2020, 131, 109027. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Sagis, L.M.C.; Bodnár, I.; Boom, R.M.; Goot, A.J.V.D. Non-linear rheology reveals the importance of elasticity in meat and meat analogues. Sci. Rep. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of texture analysis methods for the characterization of cultured meat. Sci. Rep. 2022, 12, 2898. [Google Scholar] [CrossRef] [PubMed]
- Berta, M.; Wiklund, J.; Kotzé, R.; Standing, M. Correlation between in-line measurements of tomato ketchup shear viscosity and extensional viscosity. J. Food Eng. 2016, 173, 8–14. [Google Scholar] [CrossRef]
- Patil, M.H.; Murphy, E.G.; Tanguy, G.; Floch-Fouéré, C.L.; Jeantet, R. Characterization of the superconcentration and granulation steps of a disruptive spray-drying free process for the manufacture of dairy powders. J. Food Eng. 2021, 317, 110865. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Linnemann, A.R.; Heuvel, J.V.D.; Shindano, J.; Smid, E.J. Influence of fermentation temperature on microbial community composition and physicochemical properties of mabisi, a traditionally fermented milk. LWT Food Sci. Technol. 2021, 136, 110350. [Google Scholar] [CrossRef]
- Renoldi, N.; Lucci, P.; Peressini, D. Impact of oleuropein on rheology and breadmaking performance of wheat doughs, and functional features of bread. Int. J. Food Sci. Technol. 2022, 57, 2321–2332. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Schlangen, M.; Kyriakopoulou, K.; Boom, R.M.; Goot, A.J.V.D. Texture methods for evaluating meat and meat analogue structures: A review. Food Control 2021, 127, 108103. [Google Scholar] [CrossRef]
- Morachis-Valdez, A.G.; Santillán-Álvarez, A.; Gómez-Oliván, L.M.; García-Argueta, I.; Islas-Flores, H.; Dublán-Garcia, O. Effects of peppermint extract and chitosan-based edible coating on storage quality of common carp (Cyprinus carpio) fillets. Polymers 2021, 13, 3243. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Xing, J.; Zhu, K. Revealing the effect mechanism of NaCl on the rheological properties of dough of Chinese traditional hand-stretched dried noodles. Food Chem. 2020, 320, 126606. [Google Scholar] [CrossRef]
- Lis, A.; Staniewski, B.; Ziajka, J. A comparison of butter texture measurements with the AP 4/2 penetrometer and TA.XT. Plus texture analyzer. Int. J. Food Prop. 2021, 24, 1744–1757. [Google Scholar] [CrossRef]
- Özdogan, G.; Lin, X.; Sun, D. Rapid and noninvasive sensory analyses of food products by hyperspectral imaging: Recent application developments. Trends Food Sci. Technol. 2021, 111, 151–165. [Google Scholar] [CrossRef]
- Frigaard, I. Simple yield stress fluids. Curr. Opin. Colloid Interface Sci. 2019, 43, 80–93. [Google Scholar] [CrossRef]
- Pang, B.; Wang, S.; Chen, W.; Hassan, M.; Lu, H. Effects of flow behavior index and consistency coefficient on hydrodynamics of power-law fluids and particles in fluidized beds. Powder Technol. 2020, 366, 249–260. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3. [Google Scholar]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M. Effect of high pressure homogenization on mixed juice stability, rheology, physicochemical properties and microorganism reduction. J. Food Sci. Technol. 2020, 57, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ding, F.; Liu, W.; Cheng, Y.; Zhu, J.; Ma, L.; Zhang, Y.; Wang, H. Effect of enzymatic-ultrasonic hydrolyzed chitooligosaccharide on rheology of gelatin incorporated yogurt and 3D printing. Food Hydrocoll. 2022, 132, 107851. [Google Scholar] [CrossRef]
- Shehadeh, A.; Kechagia, D.; Evangelou, A.; Tataridis, P.; Shehadeh, F. Effect of ethanol, glycerol, glucose and tartaric acid on the viscosity of model aqueous solutions and wine samples. Food Chem. 2019, 300, 125191. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Rufo, M.; Paniagua, J.; González-Mohino, A.; Olegário, L.S. New findings of edible oil characterization by ultrasonic parameters. Food Chem. 2022, 374, 131921. [Google Scholar] [CrossRef]
- Hasani, M.; Yazdanpanah, S. The effects of gum cordia on the physicochemical, textural, rheological, microstructural, and sensorial properties of apple jelly. J. Food Qual. 2020, 2020, 8818960. [Google Scholar] [CrossRef]
- Oroian, M. Measurement, prediction and correlation of density, viscosity, surface tension and ultrasonic velocity of different honey types at different temperatures. J. Food Eng. 2013, 119, 167–172. [Google Scholar] [CrossRef]
- Sutariya, S.; Huppertz, T.; Patel, H.A. Influence of milk pre-heating conditions on casein-whey protein interactions and skim milk concentrate viscosity. Int. Dairy J. 2017, 69, 19–22. [Google Scholar] [CrossRef]
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–1995. [Google Scholar] [CrossRef]
- Mohamad, R.; Agus, B.A.P.; Hussain, N. Changes of phytosterols, rheology, antioxidant activity and emulsion stability of salad dressing with cocoa butter during storage. Food Technol. Biotechnol. 2019, 57, 59–67. [Google Scholar] [CrossRef]
- Roman, L.; Cal, E.L.; Gomez, M.; Martinez, M.M. Specific ratio of A-to B-type wheat starch granules improves the quality of gluten-free breads: Optimizing dough viscosity and pickering stabilization. Food Hydrocoll. 2018, 82, 510–518. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Study on the effect of concentration and temperature on rheological properties of whole barley flour suspension by using Mitschka method. J. Texture Stud. 2014, 45, 164–171. [Google Scholar] [CrossRef]
- Crawford, N.C.; Popp, L.B.; Johns, K.E.; Caire, L.M.; Peterson, B.N.; Liberatore, M.W. Shear thickening of corn starch suspensions: Does concentration matter? J. Colloid Interface Sci. 2013, 396, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Noordraven, L.E.C.; Kim, H.; Hoogland, H.; Grauwet, T.; Loey, A.M.V. Potential of chickpea flours with different microstructures as multifunctional ingredient in an instant soup application. Foods 2021, 10, 2622. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.A.; Goh, K.K.T.; Lim, K.; Matia-Merino, L. Rheological characterization of a physically-modified waxy potato starch: Investigation of its shear-thickening mechanism. Food Hydrocoll. 2021, 120, 106908. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements and Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Wilms, P.; Hinrichs, J.; Kohlus, R. Macroscopic rheology of non Brownian suspensions at high shear rates: The infuence of solid volume fraction and non Newtonian behaviour of the liquid phase. Rheol. Acta 2022, 61, 123–138. [Google Scholar] [CrossRef]
- Wallevik, O.H.; Wallevik, J.E. Rheology as a tool in concrete science: The use of rheographs and workability boxes. Cem. Concr. Res. 2011, 41, 1279–1288. [Google Scholar] [CrossRef]
- Zhang, L.; Whang, H.; Wu, A.; Klein, B.; Zhang, X. A constitutive model for thixotropic cemented tailings backfill pastes. J. Non-Newton. Fluid Mech. 2021, 295, 104548. [Google Scholar] [CrossRef]
- Guzmán, E.; Tajuelo, J.; Pastor, J.M.; Rubio, M.A.; Ortega, F.; Rubio, R.G. Shear rheology of fluid interfaces: Closing the gap between macro- and micro-rheology. Curr. Opin. Colloid Interface Sci. 2018, 37, 33–48. [Google Scholar] [CrossRef]
- Rodríguez-González, F.; De Oca, M.A.P.; Ávila-Reyes, S.V.; Camacho-Díaz, B.H.; Alamilla-Beltrán, L.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L. A rheological study of chicory and agave tequilana fructans for use in foods. LWT Food Sci. Technol. 2019, 115, 108137. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Novel Nonanol-Based deep eutectic solvents: Thermophysical properties and their applications in Liquid-Liquid extraction and amino acid detection. J. Mol. Liq. 2021, 336, 116359. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomerated xanthan gum powder used as a food thickener: Effect of sugar binders on physical, microstructural, and rheological properties. Powder Technol. 2020, 362, 301–306. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Adams, G.G.; Zobel, H.; Balance, S.; Wolf, B.; Harding, S.E. Characterisation of the molecular properties of scleroglucan as an alternative rigid rod molecule to xanthan gum for oropharyngeal dysphagia. Food Hydrocoll. 2020, 101, 105446. [Google Scholar] [CrossRef] [PubMed]
- Hazer, B.; Ashby, R.D. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2021, 344, 128644. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Food-grade nanoencapsulation of vitamins. Environ. Chem. Lett. 2019, 17, 991–1002. [Google Scholar] [CrossRef]
- Chaudhary, K.; Mundjal, P.; Singh, K.P. Universal Stokes’s nanomechanical viscometer. Sci. Rep. 2021, 11, 14365. [Google Scholar] [CrossRef]
- Tian, P.; Xia, L.; Zhu, W.; Wang, H.; Jiang, D. Effects of Liquid Viscosity on the Formation and Attenuation of Capillary Waves Induced by AC Electrowetting-on-Dielectric. Langmuir 2023, 39, 265–273. [Google Scholar] [CrossRef]
- Zhai, X.; Lina, D.; Liub, D.; Yanga, X. Emulsions stabilized by nanofibers from bacterial cellulose: New potential food-grade Pickering emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef]
- Tan, C.; Mcclements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Dai, L.; Yang, S.; Wei, Y.; Sun, C.; Mcclements, D.J.; Mao, L.; Gao, Y. Development of stable high internal phase emulsions by pickering stabilization: Utilization of zein-propylene glycol alginate-rhamnolipid complex particles as colloidal emulsifiers. Food Chem. 2019, 275, 246–254. [Google Scholar] [CrossRef]
- Santana, N.S.; Mothé, C.G.; De Souza, M.N.; Mothé, M.G. Thermal and rheological study of artificial and natural powder tabletop sweeteners. Food Res. Int. 2022, 162, 112039. [Google Scholar] [CrossRef]
- Fuhrmann, P.L.; Breunig, S.; Sala, G.; Sagis, L.; Markus, S.M.; Schoolten, E. Rheological behaviour of attractive emulsions differing in droplet-droplet interaction strength. J. Colloid Interface Sci. 2022, 607, 389–400. [Google Scholar] [CrossRef]
- Kongjaroen, A.; Methacanon, P.; Seetapan, N.; Fuongfuchat, A.; Gamonpilas, C.; Nishinari, K. Effects of dispersing media on the shear and extensional rheology of xanthan gum and guar gum-based thickeners used for dysphagia management. Food Hydrocoll. 2022, 132, 107857. [Google Scholar] [CrossRef]
- Timusk, M.; Nigol, I.A.; Vlassov, S.; Oras, S.; Kangur, T.; Linarts, A.; Sutka, A. Low-density PDMS foams by controlled destabilization of thixotropic emulsions. J. Colloid Interface Sci. 2022, 626, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ren, C.; Tian, M.; Yang, X.; Li, J.; Li, B. Emulsion stability and dilatational viscoelasticity of ovalbumin/chitosan complexes at the oil-in-water interface. Food Chem. 2018, 252, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jebalia, I.; Della Valle, G.; Kristiawan, M. Extrusion of pea snack foods and control of biopolymer changes aided by rheology and simulation. Food Bioprod. Process. 2022, 135, 190–204. [Google Scholar] [CrossRef]
- Valencia, G.A.; Moraes, I.C.F.; Hilliou, L.H.G.; Lourenço, R.V.; Sobral, P.J.A. Nanocomposite-forming solutions based on cassava starch and laponite: Viscoelastic and rheological characterization. J. Food Eng. 2015, 166, 174–181. [Google Scholar] [CrossRef]
- Arellano, M.; Flick, D.; Benkhelifa, H.; Alvarez, G. Rheological characterization of sorbet using pipe rheometry during the freezing process. J. Food Eng. 2013, 119, 385–394. [Google Scholar] [CrossRef]
- Lai, S.; Zhang, T.; Wang, Y.; Ouyang, K.; Hu, H.; Hu, X.; Xiong, H.; Zhao, Q. Effects of different extrusion temperatures on physicochemical, rheological and digestion properties of rice flour produced in a pilot-scale extruder. Int. J. Food Sci. Technol. 2022, 57, 6773–6784. [Google Scholar] [CrossRef]
- Amid, B.T.; Mirhosseini, H. Influence of different purification and drying methods on rheological properties and viscoelastic behaviour of durian seed gum. Carbohydr. Polym. 2012, 90, 452–461. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Liu, X.; Ma, Y.; Wang, X. Effects of various oil extraction methods on the gelatinization and retrogradation properties of starches isolated from tigernut (Cyperus esculentus) tubermeals. Int. J. Biol. Macromol. 2020, 156, 144–152. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Ahmed, R.M. A generalized model for apparent viscosity of oil-based muds. J. Pet. Sci. Eng. 2018, 165, 777–785. [Google Scholar] [CrossRef]
- Hothar, M.; SUunden, B.; Wu, Z. Rheological behaviour of IoNanofluids based on [emim][DCA] and [emim][TCM]. J. Mol. Liq. 2022, 348, 118064. [Google Scholar] [CrossRef]
- Lan, B.; Gao, P.-F.; Li, Y.-R.; Yu, J.-J.; Li, P.-C. Numerical Simulation and Theoretical Analysis of Flow Resistance Characteristics in the Honeycomb Ceramic Conduit. Energies 2022, 15, 7330. [Google Scholar] [CrossRef]
- Lin, J.C.-T.; Hsiao, T.-C.; Hsiau, S.-S.; Chen, D.-R.; Chen, Y.-K.; Huang, S.-H.; Chih-Chen, C.; Chang, M.-B. Effects of temperature, dust concentration, and filtration superficial velocity on the loading behavior and dust cakes of ceramic candle filters during hot gas filtration. Sep. Purif. Technol. 2018, 198, 146–154. [Google Scholar] [CrossRef]
- Huilgol, R.J.; Georgiou, G.C. Fluids Mechanics of Viscoplasticity, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Bugarelli, N.A.V.; Biazussi, J.L.; Monte Verde, W.; Perler, C.E.; Castro, M.S.; Bannwart, A.C. A novel criterion based on slip ratio to assess the flow behavior of W/O emulsions within centrifugal pumps. Chem. Eng. Sci. 2022, 247, 117050. [Google Scholar] [CrossRef]
- Li, S.; Kajiwara, S.; Sakai, M. Numerical investigation on the mixing mechanism in a cross-torus paddle mixer using the DEM-CFD method. Powder Technol. 2021, 377, 89–102. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Gómez-Arellano, A.; Jiménez-Islas, H.; Castrejón-González, E.O.; Medina-Torres, L.; Dendooven, L.; Escamilla-Silva, E.M. Rheological behaviour of sesame (Sesamum indicum L.) protein dispersion. Food Bioprod. Process. 2017, 106, 201–208. [Google Scholar] [CrossRef]
- Pereira, C.G. (Ed.) Thermodynamics of Phase Equilibria in Food Engineering; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Co, E.D.; Marangoni, A.G. The Phase Space of Crystallization: Modeling Fat Crystallization Using Thermodynamic and Mass-Transfer Variables. Cryst. Growth Des. 2020, 20, 1628–1637. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Wesdorp, L.H. Structure and Properties of Fat Crystal Networks, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Vehkamäki, H. Classical Nucleation Theory in Multicomponent Systems; Springer: Berlin/Heidelberg, Germany, 2006; p. 176. [Google Scholar]
- Myerson, A.S. Handbook of Industrial Crystallization; Butterworth-Heinemann: Boston, MA, USA, 2002. [Google Scholar]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; Gonçalves, L.A.G. Instrumental Methods for the Evaluation of Interesterified Fats. Food Anal. Methods 2009, 2, 282–302. [Google Scholar] [CrossRef]
- Macridachis-González, J.; Bayés-García, L.; Calvet, T. An Insight into the Solid-State Miscibility of Triacylglycerol Crystals. Molecules 2020, 25, 4562. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, J.L.; Miller, R.L.; Woodley, J.M.; Kiil, S. Thermodynamic Modeling of Multi-phase Solid–Liquid Equilibria in Industrial-Grade Oils and Fats. J. Am. Oil Chem. Soc. 2014, 92, 17–28. [Google Scholar] [CrossRef]
- Timms, R. Phase behavior of fats and their mixtures. Prog. Lipid Res. 1984, 23, 1–38. [Google Scholar] [CrossRef]
- Maximo, G.J.; Costa, M.C.; Coutinho, J.A.P.; Meirelles, A.J.A. Trends and demands in the solid–liquid equilibrium of lipidic mixtures. RSC Adv. 2014, 4, 31840–31850. [Google Scholar] [CrossRef]
- Pereira, E.; Pereira, D.T.V.; Meirelles, A.J.A.; Maximo, G.J. Modeling the solid-liquid equilibrium of binary mixtures of triacylglycerols using UNIFAC and predictive UNIQUAC models. Fluid Phase Equilibria 2022, 554, 113327. [Google Scholar] [CrossRef]
- Marangoni, A.G. Fat Crystal Networks, 2nd ed.; Marcel Dekker Press: New York, NY, USA, 2005. [Google Scholar]
- Hartel, R.W.; Ergun, R.; Vogel, S. Phase/State Transitions of Confectionery Sweeteners: Thermodynamic and Kinetic Aspects. Compr. Rev. Food Sci. Food Saf. 2010, 10, 17–32. [Google Scholar] [CrossRef]
- Rahman, M.S. Food stability determination by macro–micro region concept in the state diagram and by defining a critical temperature. J. Food Eng. 2010, 99, 402–416. [Google Scholar] [CrossRef]
- Roos, Y.H.; Karel, M.; Labuza, T.P.; Levine, H.; Mathlouthi, M.; Reid, D.; Shalaev, E.; Slade, L. Melting and Crystallization of Sugars in High-Solids Systems. J. Agric. Food Chem. 2013, 61, 3167–3178. [Google Scholar] [CrossRef]
- Hurtta, M.; Pitkänen, I.; Knuutinen, J. Melting behaviour of d-sucrose, d-glucose and d-fructose. Carbohydr. Res. 2004, 339, 2267–2273. [Google Scholar] [CrossRef]
- Lee, J.W.; Thomas, L.C.; Schmidt, S.J. Investigation of the Heating Rate Dependency Associated with the Loss of Crystalline Structure in Sucrose, Glucose, and Fructose Using a Thermal Analysis Approach (Part I). J. Agric. Food Chem. 2010, 59, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabrera, M.A.; Rivera-Bautista, C.; Grajales-Lagunes, A.; González-García, R.; Schmidt, S.J. State diagrams for mixtures of low molecular weight carbohydrates. J. Food Eng. 2016, 171, 185–193. [Google Scholar] [CrossRef]
- Sablani, S.S.; Syamaladevi, R.M.; Swanson, B.G. A Review of Methods, Data and Applications of State Diagrams of Food Systems. Food Eng. Rev. 2010, 2, 168–203. [Google Scholar] [CrossRef]
- Peres, A.M.; Macedo, E.A. Thermodynamic properties of sugars in aqueous solutions: Correlation and prediction using a modified UNIQUAC model. Fluid Phase Equilibria 1996, 123, 71–95. [Google Scholar] [CrossRef]
- Simion, A.I.; Grigoraş, C.G.; Lăcrămioara, R.U.S.U.; Dabija, A. Modeling of the thermo-physical properties of aqueous sucrose solutions II. boiling point, specific heat capacity and thermal conductivity. J. Fac. Food Eng. 2011, 10, 49–56. [Google Scholar]
- Maximo, G.J.; Meirelles, A.J.A.; Batista, E.A.C. Boiling point of aqueous d-glucose and d-fructose solutions: Experimental determination and modeling with group-contribution method. Fluid Phase Equilibria 2010, 299, 32–41. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some thermodynamic considerations in food formulation. Food Hydrocoll. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Nicolai, T. Gelation of food protein-protein mixtures. Adv. Colloid Interface Sci. 2019, 270, 147–164. [Google Scholar] [CrossRef]
- Scott, G.; Awika, J.M. Effect of protein–starch interactions on starch retrogradation and implications for food product quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef]
- McClements, D.J. Non-covalent interactions between proteins and polysaccharides. Biotechnol. Adv. 2006, 24, 621–625. [Google Scholar] [CrossRef]
- Magnusson, E.; Nilsson, L. Interactions between hydrophobically modified starch and egg yolk proteins in solution and emulsions. Food Hydrocoll. 2011, 25, 764–772. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and Technofunctional Properties of Protein-Polysaccharide Complexes: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef] [PubMed]
- Amit, K.G.; Prasun, B. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids. In The Complex World of Polysaccharides; InTech: Suffolk County, MA, USA, 2012; pp. 154–196. [Google Scholar]
- Jelesarov, I.; Bosshard, H.R. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J. Mol. Recognit. 1999, 12, 3–18. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Protein -polysaccharide interactions. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 171–198. [Google Scholar]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein-polysaccharide complexes and coacervates. Curr. Opin. Coll. Interf. Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- De Vries, R.; Weinbreck, F.; De Kruif, C.G. Theory of polyelectrolyte adsorption on heterogeneously charged surfaces applied to soluble protein ± polyelectrolyte complexes. J. Chem. Phys. 2003, 118, 4649–4659. [Google Scholar] [CrossRef]
- Schmitt, C.; Aberkane, L.; Sanchez, C. 16-Protein–polysaccharide complexes and coacervates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 420–476. [Google Scholar]
- Grinberg, V.Y.; Tolstoguzov, V.B. Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocoll. 1997, 11, 145–158. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, P. Starch gelatinization, retrogradation, and enzyme susceptibility of retrograded starch: Effect of amylopectin internal molecular structure. Food Chem. 2020, 316, 126036. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Luo, S.; Liu, C.; Liu, W. Effect of food additives on starch retrogradation: A review. Starch Stärke 2015, 67, 69–78. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brennan, C.S. Rheological, pasting and microstructural studies of dairy protein–starch interactions and their application in extrusion-based products: A review. Starch Stärke 2017, 69, 1600273. [Google Scholar] [CrossRef]


| Analysis | Equipment | Properties | Estimated Values | Reference |
|---|---|---|---|---|
| Flow length of ketchup | Bostwick consistometer | Viscosity | 80 Pa·s | [59] |
| Characterization of the flow properties of superconcentrated paste | Ring shear test | Cohesiveness and flowability | Ffc ˂ 1 | [60] |
| Determination of the consistency of fermented milk | Adam’s consistometer | Consistency | 6–9 cm | [61] |
| Bread dough mixing properties | Farinograph | Water absorption | 51–55% | [62] |
| Test to measure meat texture | Warner–Bratzier shear test | Shear force | 23.25–72.59 N | [63] |
| Characterization of gelatine based on gel strength | Bloom gelometer | Gel strength | 250 N | [64] |
| Extensional properties of noodles | Brabender extensograph | Extensibility | 60–120 cm2 | [65] |
| Texture properties of butter | Penetrometer | Texture | 40.67–74.67 °p | [66] |
| Firmness of fruits and vegetables | Magness–Taylor pressure test | Force | 5.53 N | [67] |
| Fluid | Rheological Behavior | Apparent Viscosity (Pa·s) | Temperature (°C) | Reference |
|---|---|---|---|---|
| Juice | pseudoplastic | 0–30 | 25 | [71] |
| Yogurt | pseudoplastic | 100–1000 | 25 | [72] |
| White wine 0% | Newtonian | 0.00353 | 20 | [73] |
| Sunflower oil | Newtonian | 0.0619 | 25 | [74] |
| Jelly | pseudoplastic | 13.222 | 25 | [75] |
| Honey | Newtonian | 05–50 | 25 | [76] |
| Skimmed milk concentrate | pseudoplastic | 0–4 | 55 | [77] |
| Olive oil | Newtonian | 0.074 | 25 | [74] |
| Ketchup | pseudoplastic | 80–166 | 23 | [59] |
| Ice cream | pseudoplastic/thixotropic | 0.1–0.5 | 25 | [78] |
| Salad dressing | pseudoplastic | 2.6 | 30 | [79] |
| Bread dough | pseudoplastic/viscoelastic | 3939 | 25 | [80] |
| Barley flour suspension | pseudoplastic | 0.1–1 | 30 | [81] |
| Corn starch suspension | dilatant | 1–1000 | 25 | [82] |
| Potato starch soups | dilatant | 0.01–0.05 | 60 | [83] |
| Waxy potato starch | rheopectic | 0.2–0.8 | 20 | [84] |
| Process | Shear Rate (s⁻1) | Application | Product | References |
|---|---|---|---|---|
| Extrusion | 1–1000 | Mixture of biopolymers | Pea flour and pea starch | [106] |
| Extrusion | 20–1200 | Mixture of polymers | Extruded corn and wheat | [83] |
| Mixture and agitation | 0.1–100 | Former nanocomposite solutions | Cassava starch and laponite | [107] |
| Tube flow | 4–430 | Pumping | Ice cream | [108] |
| Extrusion | 1–200 | Mixture of polymers | Rice flour | [109] |
| Mixture and agitation | 0.798–150 | Homogenization of liquids | Lily nut drink | [84] |
| Spraying | 10–200 | Drying technique | Durian seed gum | [110] |
| Gelatinization | 0.01–1000 | Folder separation | Tuber meal starch | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polachini, T.C.; Morales, S.A.V.; Filho, L.R.P.; Ribeiro, E.F.; Saraiva, L.S.; Basso, R.C. Physical Properties and Molecular Interactions Applied to Food Processing and Formulation. Processes 2023, 11, 2181. https://doi.org/10.3390/pr11072181
Polachini TC, Morales SAV, Filho LRP, Ribeiro EF, Saraiva LS, Basso RC. Physical Properties and Molecular Interactions Applied to Food Processing and Formulation. Processes. 2023; 11(7):2181. https://doi.org/10.3390/pr11072181
Chicago/Turabian StylePolachini, Tiago Carregari, Sergio Andres Villalba Morales, Luís Roberto Peixoto Filho, Elisa Franco Ribeiro, Larissa Santos Saraiva, and Rodrigo Corrêa Basso. 2023. "Physical Properties and Molecular Interactions Applied to Food Processing and Formulation" Processes 11, no. 7: 2181. https://doi.org/10.3390/pr11072181
APA StylePolachini, T. C., Morales, S. A. V., Filho, L. R. P., Ribeiro, E. F., Saraiva, L. S., & Basso, R. C. (2023). Physical Properties and Molecular Interactions Applied to Food Processing and Formulation. Processes, 11(7), 2181. https://doi.org/10.3390/pr11072181






