Study on Oxy-Methane Flame Stability in a Cylindrical Porous Medium Burner
Abstract
:1. Introduction
2. Research Method
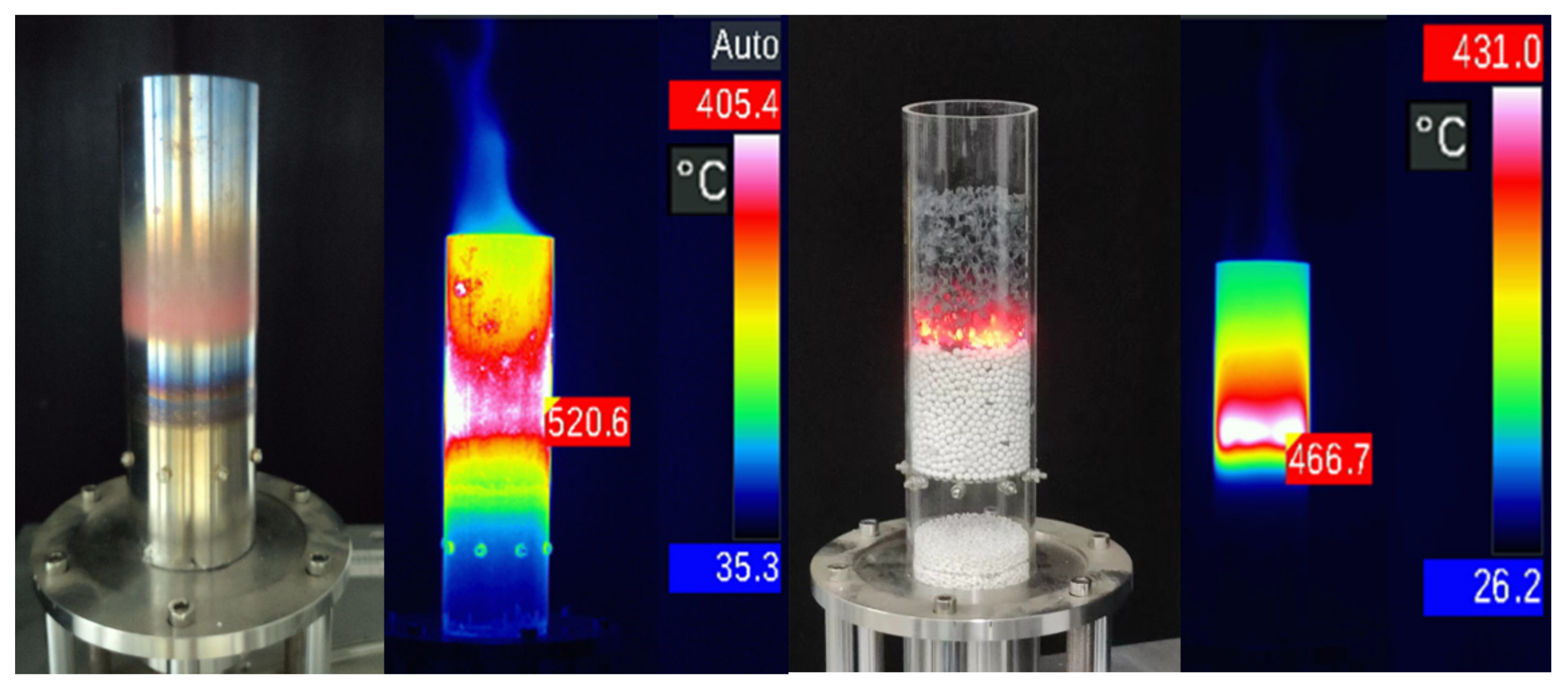
2.1. Experimental Setup
2.2. Mathematical Model
2.2.1. Governing Equations
2.2.2. Boundary Conditions
3. Results and Discussion
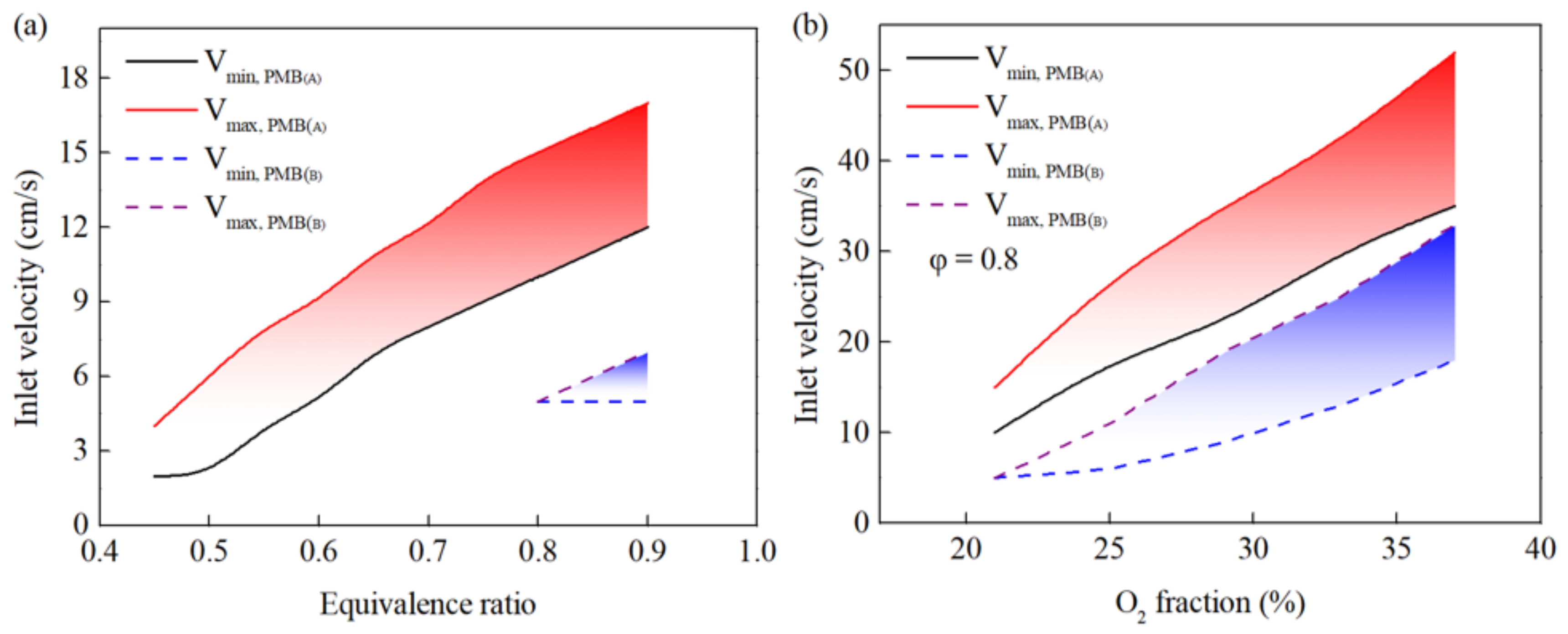
3.1. Combustion Behavior of PMB(A)
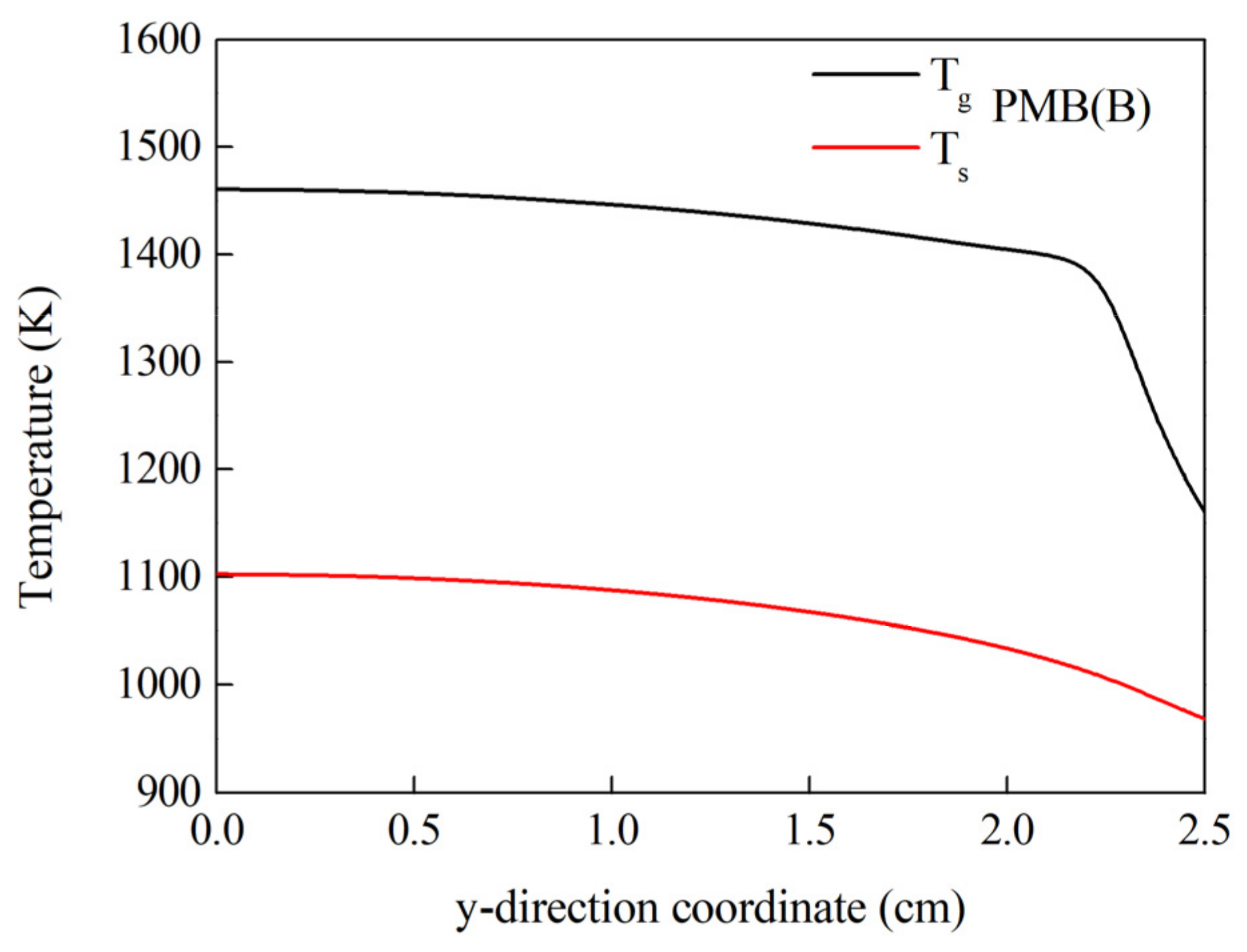
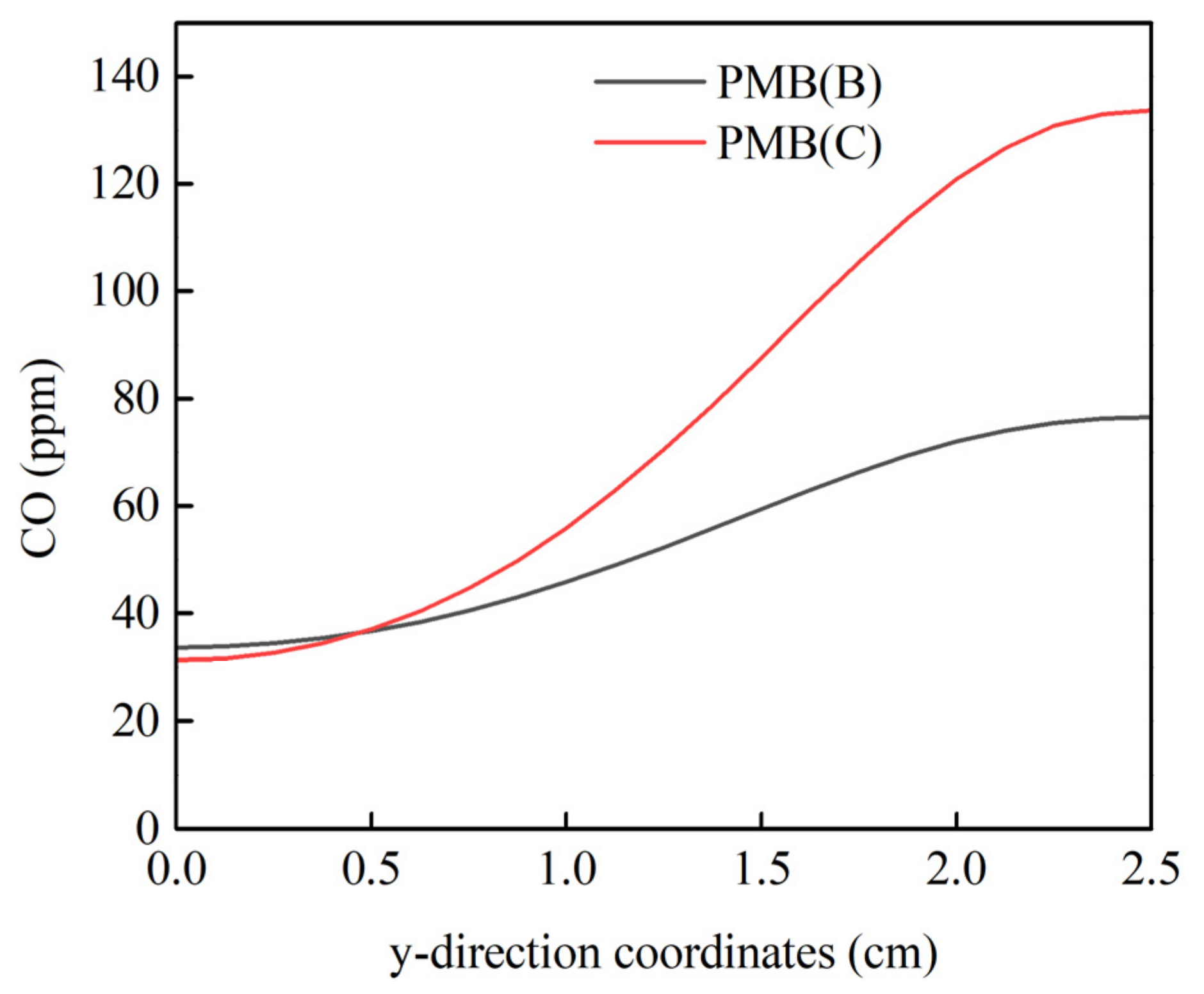
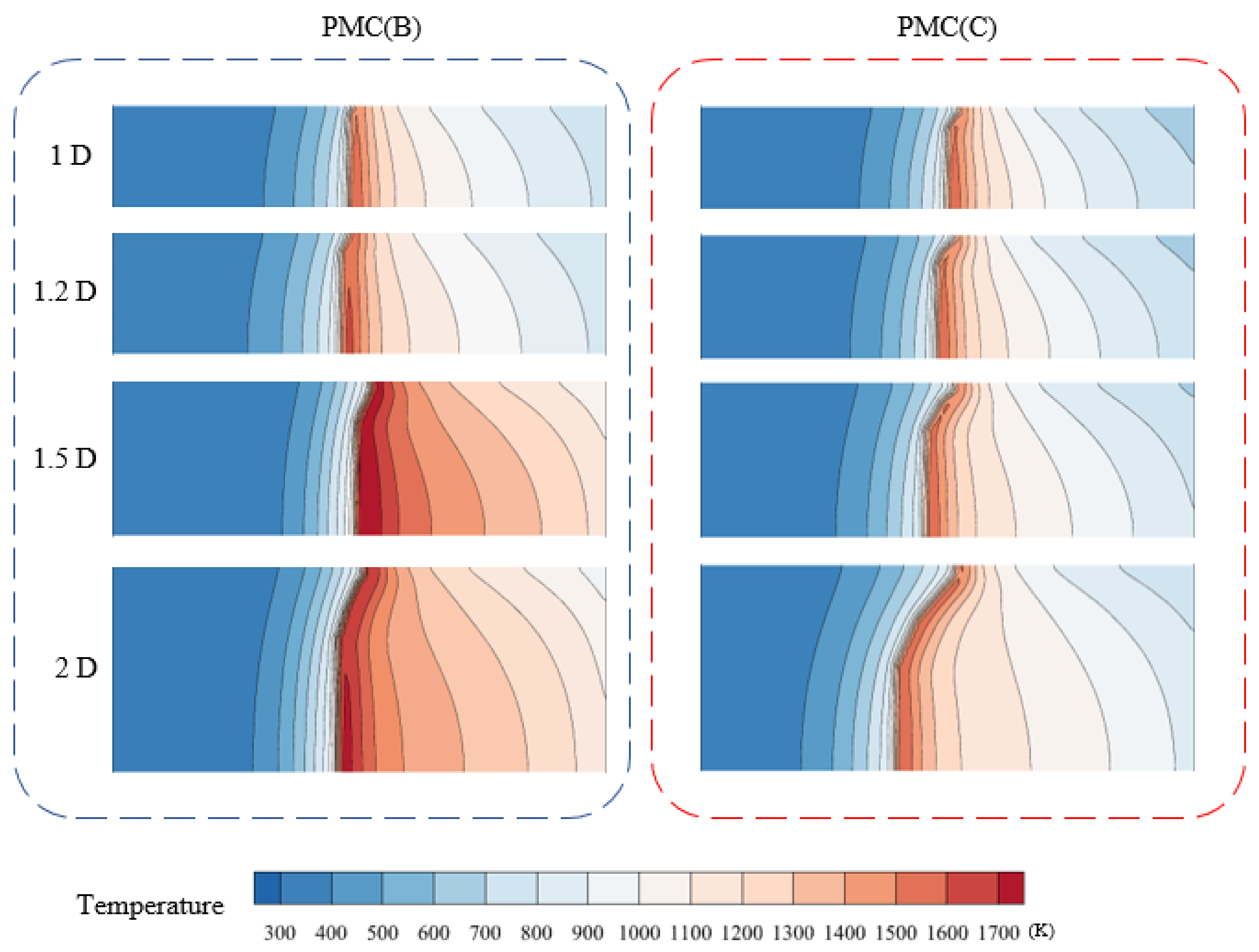
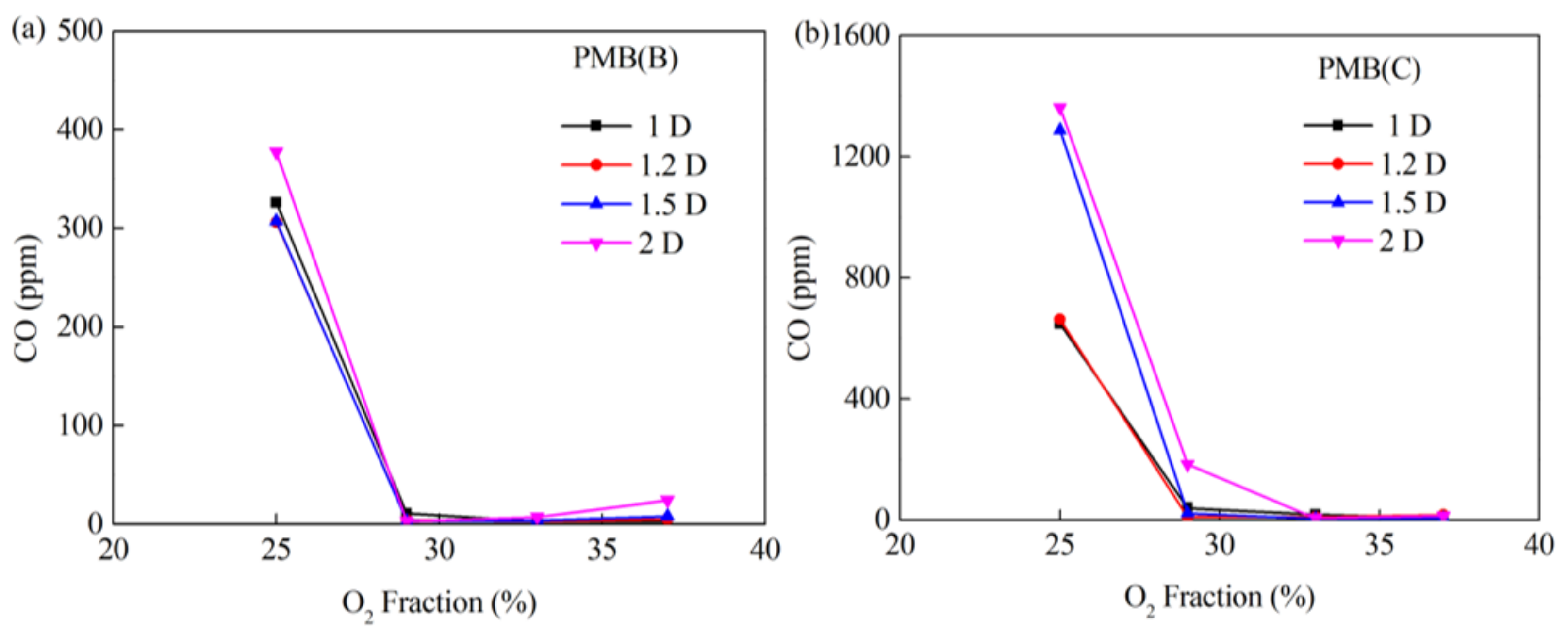
3.2. Combustion Behavior of PMB(B) and PMB(C)
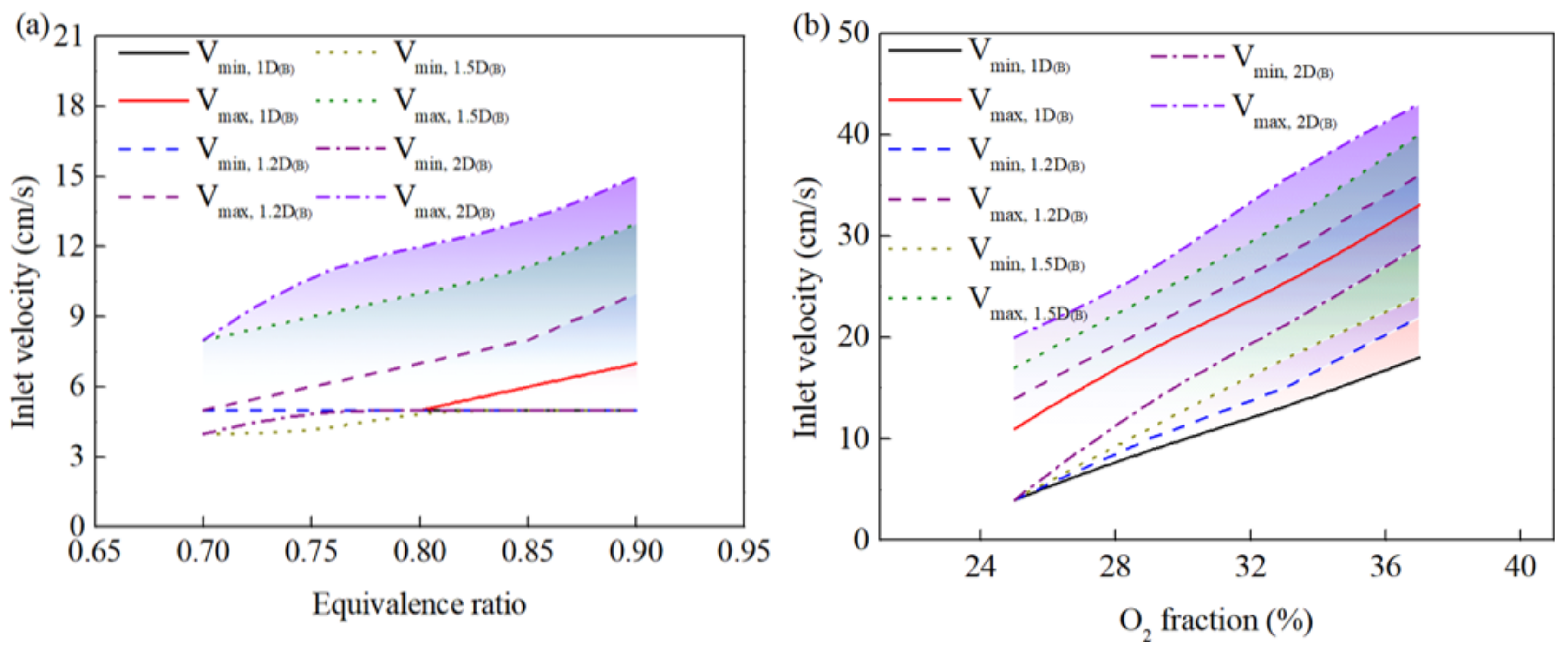
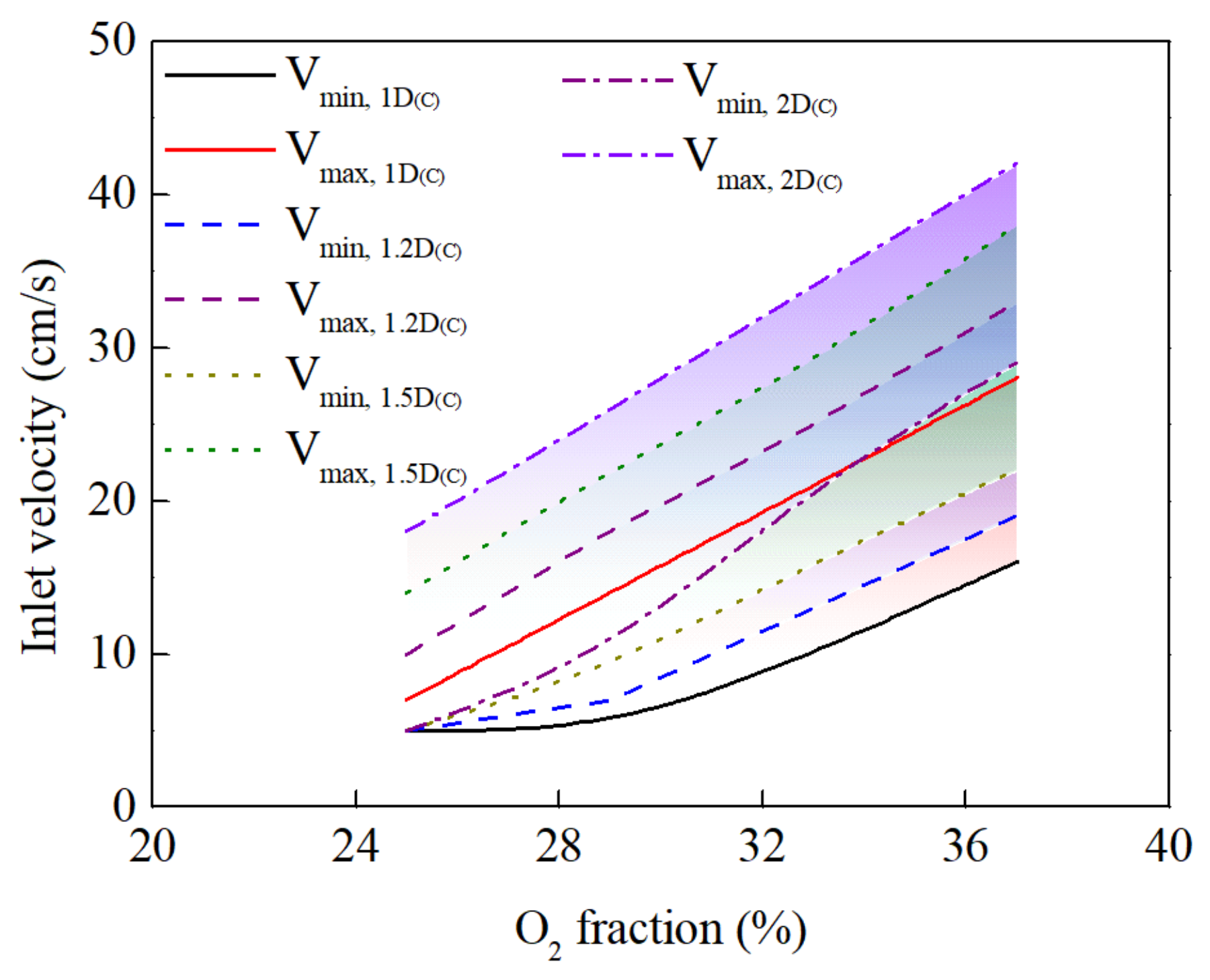
3.3. Effect of Burner Diameter on Combustion Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mostafa, M.M.H.; Liu, R.; Wang, Z.; Chen, H.; Yang, J.; Manickam, S. Study on the Effect of Standardization on Energy Demand and Economic Growth. E3S Web Conf. 2021, 261, 01018. [Google Scholar] [CrossRef]
- Lei, R.; Feng, S.; Lauvaux, T. Country-scale trends in air pollution and fossil fuel CO2 emissions during 2001–2018: Confronting the roles of national policies and economic growth. Environ. Res. Lett. 2021, 16, 014006. [Google Scholar] [CrossRef]
- Wu, F.; Argyle, M.D.; Dellenback, P.A.; Fan, M.H. Progress in O2 separation for oxy-fuel combustion—A promising way for cost-effective CO2 capture: A review. Prog. Energy Combust. Sci. 2018, 67, 188–205. [Google Scholar] [CrossRef]
- Zheng, L. Oxy-Fuel Combustion for Power Generation and Carbon Dioxide (CO2) Capture; Woodhead Publishing: Sawston, UK, 2011; pp. 1–13. [Google Scholar]
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; la O’, G.J.; Shao-Horn, Y.; Mancini, N.D.; Mitsos, A. A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2011, 35, 741–764. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, L.; Zhu, X.; Zheng, C. Experiment investigation of coal MILD-Oxy combustion integrated with flue gas recirculation at a 0.3 MW th furnace. Fuel Process. Technol. 2017, 162, 126–134. [Google Scholar] [CrossRef]
- Yin, C. Prediction of air-fuel and oxy-fuel combustion through a generic gas radiation property model. Appl. Energy 2017, 189, 449–459. [Google Scholar] [CrossRef]
- Peng, Q.; Jiaqiang, E.; Chen, J.; Zuo, W.; Zhao, X.; Zhang, Z. Investigation on the effects of wall thickness and porous media on the thermal performance of a non-premixed hydrogen fueled cylindrical micro combustor. Energy Convers. Manag. 2018, 155, 276–286. [Google Scholar] [CrossRef]
- Mathis, W.M.; Ellzey, J.L. Flame stabilization, operating range, and emissions for a methane/air porous burner. Combust. Sci. Technol. 2003, 175, 825–839. [Google Scholar] [CrossRef]
- Sanmiguel, J.E.; Mehta, S.A.; Moore, R.G. An Experimental Study of Controlled Gas-Phase Combustion in Porous Media for Enhanced Recovery of Oil and Gas. J. Energy Resour. Technol. 2003, 125, 64–71. [Google Scholar] [CrossRef]
- Keramiotis, C.; Founti, M.A. An experimental investigation of stability and operation of a biogas fueled porous burner. Fuel 2013, 103, 278–284. [Google Scholar] [CrossRef]
- Akbari, M.H.; Riahi, P.; Roohi, R. Lean flammability limits for stable performance with a porous burner. Appl. Energy 2009, 86, 2635–2643. [Google Scholar] [CrossRef]
- Zangeneh, V.; Alipoor, A.; Lund, H.; Kaiser, M.J. Stability study of hydrogen-air flame in a conical porous burner. Energy 2021, 215, 119140. [Google Scholar] [CrossRef]
- Fruzza, F.; Lamioni, R.; Tognotti, L.; Galletti, C. Flashback of H2-enriched premixed flames in perforated burners: Numerical prediction of critical velocity. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Gao, H.B.; Qu, Z.G.; Feng, X.B.; Tao, W.Q. Combustion of methane/air mixtures in a two-layer porous burner: A comparison of alumina foams, beads, and honeycombs. Exp. Therm Fluid Sci. 2014, 52, 215–220. [Google Scholar] [CrossRef]
- Gao, H.B.; Qu, Z.G.; Feng, X.B.; Tao, W.Q. Methane/air premixed combustion in a two-layer porous burner with different foam materials. Fuel 2014, 115, 154–161. [Google Scholar] [CrossRef]
- Shi, J.; Mao, M.; Li, H.; Liu, Y.; Liu, Y.; Deng, Y. Influence of Chemical Kinetics on Predictions of Performance of Syngas Production From Fuel-Rich Combustion of CO2/CH4 Mixture in a Two-Layer Burner. Front. Chem. 2019, 7, 902. [Google Scholar] [CrossRef]
- Arrieta, C.E.; García, A.M.; Amell, A.A. Experimental study of the combustion of natural gas and high-hydrogen content syngases in a radiant porous media burner. Int. J. Hydrogen Energy 2017, 42, 12669–12680. [Google Scholar] [CrossRef]
- Coutinho, J.E.A.; de Lemos, M.J.S. Laminar flow with combustion in inert porous media. Int. Commun. Heat Mass Transf. 2012, 39, 896–903. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Hashemi, S.A. Flame stability analysis of the premixed methane-air combustion in a two-layer porous media burner by numerical simulation. Fuel 2017, 202, 56–65. [Google Scholar] [CrossRef]
- Pan, J.F.; Yu, H.; Ren, H.M.; Zhang, Y.; Lu, Q.B.; Quaye, E.K. Study on combustion characteristics of premixed methane/oxygen in meso-combustors with different cross-sections. Chem. Eng. Process. -Process Intensif. 2020, 154, 108025. [Google Scholar] [CrossRef]
- Ahmed, P. Characteristics of air and oxy-fuel combustion in micro-channels. In Proceedings of the HEFAT2012 9th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, St. Julian’s, Malta, 16–18 July 2012. [Google Scholar]
- Habib, R.; Yadollahi, B.; Saeed, A.; Doranehgard, M.H.; Karimi, N. On the Response of Ultralean Combustion of CH4/H2 Blends in a Porous Burner to Fluctuations in Fuel Flow-an Experimental Investigation. Energy Fuels 2021, 35, 8909–8921. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Hayden, A. Premixed gas combustion stabilized in fiber felt and its application to a novel radiant burner. Fuel 2006, 85, 1094–1100. [Google Scholar] [CrossRef]
- Chou, S.K.; Yang, W.M.; Li, J.; Li, Z.W. Porous media combustion for micro thermophotovoltaic system applications. Appl. Energy 2010, 87, 2862–2867. [Google Scholar] [CrossRef]
- Gentillon, P.; Southcott, J.; Chan, Q.N.; Taylor, R.A. Stable flame limits for optimal radiant performance of porous media reactors for thermophotovoltaic applications using packed beds of alumina. Appl. Energy 2018, 229, 736–744. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; Jiaqiang, E.; Xu, H.; Li, Z.; Yu, W.; Tu, Y.; Wu, Y. Experimental investigation on premixed hydrogen/air combustion in varied size combustors inserted with porous medium for thermophotovoltaic system applications. Energy Convers. Manag. 2019, 200, 112086. [Google Scholar] [CrossRef]
- Xie, M.Z.; Shi, J.R.; Deng, Y.B.; Liu, H.; Zhou, L.; Xu, Y.N. Experimental and numerical investigation on performance of a porous medium burner with reciprocating flow. Fuel 2009, 88, 206–213. [Google Scholar] [CrossRef]
- Liao, M.; Jia, S.; Wang, Q.; Chan, T.L.; Li, Y.; Xu, X.; He, Z. Numerical Simulation of Methane Combustion in Two-Layer Porous Media Under Oxy-Fuel Condition. Flow Turbul. Combust. 2022, 110, 649–670. [Google Scholar] [CrossRef]
- Zheng, C.; Cheng, L.; Saveliev, A.; Luo, Z.; Cen, K. Gas and solid phase temperature measurements of porous media combustion. Proc. Combust. Inst. 2011, 33, 3301–3308. [Google Scholar] [CrossRef]
- Banerjee, A.; Paul, D. Developments and applications of porous medium combustion: A recent review. Energy 2021, 221, 119868. [Google Scholar] [CrossRef]
- Yakovlev, I.; Zambalov, S. Three-dimensional pore-scale numerical simulation of methane-air combustion in inert porous media under the conditions of upstream and downstream combustion wave propagation through the media. Combust. Flame 2019, 209, 74–98. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Sobhani, S.; Ihme, M. Pore-resolved simulations of porous media combustion with conjugate heat transfer. Proc. Combust. Inst. 2021, 38, 2127–2134. [Google Scholar] [CrossRef]
- Stagni, A.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Skeletal mechanism reduction through species-targeted sensitivity analysis. Combust. Flame 2016, 163, 382–393. [Google Scholar] [CrossRef]
- Macdonald, I.F.; El-Sayed, M.S.; Mow, K.; Dullien, F.A.L. Flow through Porous Media-the Ergun Equation Revisited. Ind. Eng. Chem. Fundam 1979, 18, 199–208. [Google Scholar] [CrossRef]
- Achenbach, E. Heat flow characterstics of packed beds. Exp. Therm. Fluid Ence 1995, 10, 17–27. [Google Scholar] [CrossRef]
- Youngis, B.L.; Viskanta, R. Experimental determination of the volumetric heat transfer coefficient between stream of air and ceramic foam. Int. J. Heat Mass Transf. 1993, 36, 1425–1434. [Google Scholar] [CrossRef]
- Dobrego, K.V.; Gnesdilov, N.N.; Lee, S.H.; Choi, H.K. Overall chemical kinetics model for partial oxidation of methane in inert porous media. Chem. Eng. J. 2008, 144, 79–87. [Google Scholar] [CrossRef]
- Hsu, P.F.; Howell, J.R. Measurements of thermal conductivity and optical properties of porous partially stabilized zirconia. Exp. Heat Transf. 1993, 5, 293–313. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. OpenSMOKE++: An object-oriented framework for the numerical modeling of reactive systems with detailed kinetic mechanisms. Comput. Phys. Commun. 2015, 192, 237–264. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, S.; Qiu, T.; Liu, C.; Zhang, S. Bi-Fo time scaling method in the numerical simulation of transient conjugate heat transfer. Propuls. Power Res. 2021, 10, 15. [Google Scholar] [CrossRef]
- Maffulli, R.; Marinescu, G.; Li, H. On the Validity of Scaling Transient Conjugate Heat Transfer Characteristics. J. Eng. Gas Turbines Power 2020, 142, 031021. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Zhao, D.; Rashid, M.T.; Wang, N. Effects of porous media on partially premixed combustion and heat transfer in meso-scale burners fuelled with ethanol. Energy 2021, 224, 120191. [Google Scholar] [CrossRef]
- Wang, G.; Tang, P.; Li, Y.; Xu, J.; Durst, F. Flame front stability of low calorific fuel gas combustion with preheated air in a porous burner. Energy 2018, 170, 1279–1288. [Google Scholar] [CrossRef]







| Property | Unit | Preheating Zone | Combustion Zone |
|---|---|---|---|
| Density | kg/m3 | 3900 | 3200 |
| Heat capacity | J/(kg·K) | 910 | 825 |
| Porosity | - | 0.4 | 0.8 |
| Extinction coefficient | 1/m | 800 | 234.1 |
| Thermal conductivity | W/(m·K) | 0.28 | 0.71 |
| Pore diameter | mm | 3 | 2.56 |
| Equipment | Model | Measurement Range | Error or Uncertainty |
|---|---|---|---|
| Mass flow controller | D07-9E D07-7B D07-7B | 0~50 SLM for CO2 0~10 SLM for O2 0~5 SLM for CH4 | 2% F.S. |
| Gas analyzer | Testo350 pro | 0~10,000 ppm for CO | ±2 ppm (0~39.9); 5% measured value (40~500 ppm) |
| Thermocouple | S-type | 273~1400 K | ±2.5 K |
| Thermocouple | K-type | 233~650 K | ±2.5 K |
| Thermal imaging camera | FLUKE TiX660 | 233~1473 K | ±1.5 K or 1.5% |
| Thermocouple input model | NI 9214 | 233~1700 K | ±4.5 K |
| φ | O2 Fraction (%) | Vmin (cm/s) | Vmax (cm/s) | Numerical | Experimental | Numerical | Experimental | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg,vmin (K) | Tg,vmax (K) | Tg,vmin (K) | Tg,vmax (K) | COvmin (ppm) | COvmax (ppm) | COvmin (ppm) | COvmax (ppm) | ||||
| 0.45 | 21 | 2 | 4 | 1242 | 1366 | — | — | 2.6 | 0.9 | — | — |
| 0.5 | 21 | 2 | 6 | 1292 | 1433 | — | — | 1.4 | 0.7 | — | — |
| 0.55 | 21 | 4 | 8 | 1405 | 1533 | — | — | 0.5 | 1.2 | — | — |
| 0.6 | 21 | 5 | 9 | 1460 | 1574 | 1323 | 1381 | 0.5 | 1.9 | 34 | 4 |
| 0.65 | 21 | 7 | 11 | 1558 | 1619 | 1380 | 1473 | 1.0 | 4.3 | 16 | 2 |
| 0.7 | 21 | 8 | 12 | 1584 | 1684 | 1427 | 1488 | 1.7 | 9.8 | 8 | 3 |
| 0.75 | 21 | 9 | 14 | 1645 | 1727 | 1410 | 1522 | 3.8 | 22.9 | 2 | 5 |
| 0.8 | 21 | 10 | 15 | 1670 | 1781 | 1540 | 1610 | 7.5 | 53.0 | 5 | 34 |
| 0.6 | 17 | 2 | 4 | 1259 | 1501 | — | — | 2.1 | 8.7 | — | — |
| 0.6 | 25 | 9 | 14 | 1618 | 1698 | — | — | 1.3 | 7.1 | — | — |
| 0.6 | 29 | 13 | 18 | 1759 | 1806 | — | — | 5.7 | 19.0 | — | — |
| 0.6 | 33 | 17 | 24 | 1903 | 1930 | — | — | 21.1 | 68.9 | — | — |
| 0.6 | 37 | 21 | 29 | 2008 | 2062 | — | — | 55.0 | 197.0 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; He, Z.; Liang, X.; Chan, T.L.; Li, Y.; Xu, X. Study on Oxy-Methane Flame Stability in a Cylindrical Porous Medium Burner. Processes 2023, 11, 2182. https://doi.org/10.3390/pr11072182
Liao M, He Z, Liang X, Chan TL, Li Y, Xu X. Study on Oxy-Methane Flame Stability in a Cylindrical Porous Medium Burner. Processes. 2023; 11(7):2182. https://doi.org/10.3390/pr11072182
Chicago/Turabian StyleLiao, Mingjian, Zhu He, Xiong Liang, Tat Leung Chan, Yawei Li, and Xuecheng Xu. 2023. "Study on Oxy-Methane Flame Stability in a Cylindrical Porous Medium Burner" Processes 11, no. 7: 2182. https://doi.org/10.3390/pr11072182
APA StyleLiao, M., He, Z., Liang, X., Chan, T. L., Li, Y., & Xu, X. (2023). Study on Oxy-Methane Flame Stability in a Cylindrical Porous Medium Burner. Processes, 11(7), 2182. https://doi.org/10.3390/pr11072182







