Remote Monitoring the Parameters of Interest in the 18O Isotope Separation Technological Process
Abstract
1. Introduction
- The determination of the structure of the original mathematical model, which describes the behavior of the approached separation cascade, considering that this process is a strong nonlinear fractional-order one.
- The determination of the analytical equations of the parameters associated with the separation cascade is usable for the model parametrization (for example, the separation functions or the steady-state values of the 18O isotope concentration at the top of the two separation columns from the separation cascade structure).
- The proposing of an original method for the simulation of the proposed fractional-order model in the context when the main disturbance which can occur in the process is the variation of the fractional differentiation order (associated with the fractional-order component of the model).
- Defining the structure of the neural networks used as AI techniques for having the possibility to simulate the proposed mathematical model (the neural networks are necessary to implement the nonlinear and the fractional-order components of the proposed mathematical model).
- The implementation of the proposed mathematical model functional diagram in which are highlighted the interconnections between the different components of the model.
2. The modeling of the 18O Isotope Production Process
2.1. Technological Description of the 18O Isotope Separation Cascade
2.2. Experimental Results
2.3. The Proposed Mathematical Model
2.4. The Implementation of the Proposed Model
- they have multiple output signals (each of the two neural networks generates six output signals), an aspect which increases the complexity of the functional dependency between the input signal (the fractional differentiation order) and the output signals;
- in each neural network case, the values of the six output signals present consistent variations from one signal to another one; more exactly, some coefficients have significantly high values and other coefficients have much more small values; in this context, the error obtained after training the two neural networks has to be consistently smaller (of at least 1000 times, for a good accuracy) than the value of the smallest generated coefficient, an aspect which is difficult to be obtained due to the higher values of the other coefficients.
- Analyzing experimental data and displaying them graphically.
- The analytical determination of the steady-state values of the 18O isotope concentrations at the top of the two separation columns (these steady-state values are obtained based on the separations functions, which are nonlinear functions depending on other nonlinear functions (more exactly, on the heights of equivalent theoretical plates)).
- The determination of the system of Equations (8)–(11), which describes the separation cascade behavior in a dynamical regime. As results from (10), the proposed mathematical model is a fractional-order one.
- The application of the proposed iterative procedure used for obtaining the proposed mathematical model’s structure parameters (the set of (T1, T2 and β) parameter values which generate the best fit between the experimental response and the response generated by the proposed mathematical model).
- The determination of the original solution applicable for the simulation of the proposed mathematical model in the context when the main considered parametric disturbance is the variation of the fractional differentiation order (β) of the fractional component of the model.
- The training of the neural networks is used for the implementation of the separation process nonlinearities and its fractional-order component.
- The implementation of the proposed mathematical model functional diagram.
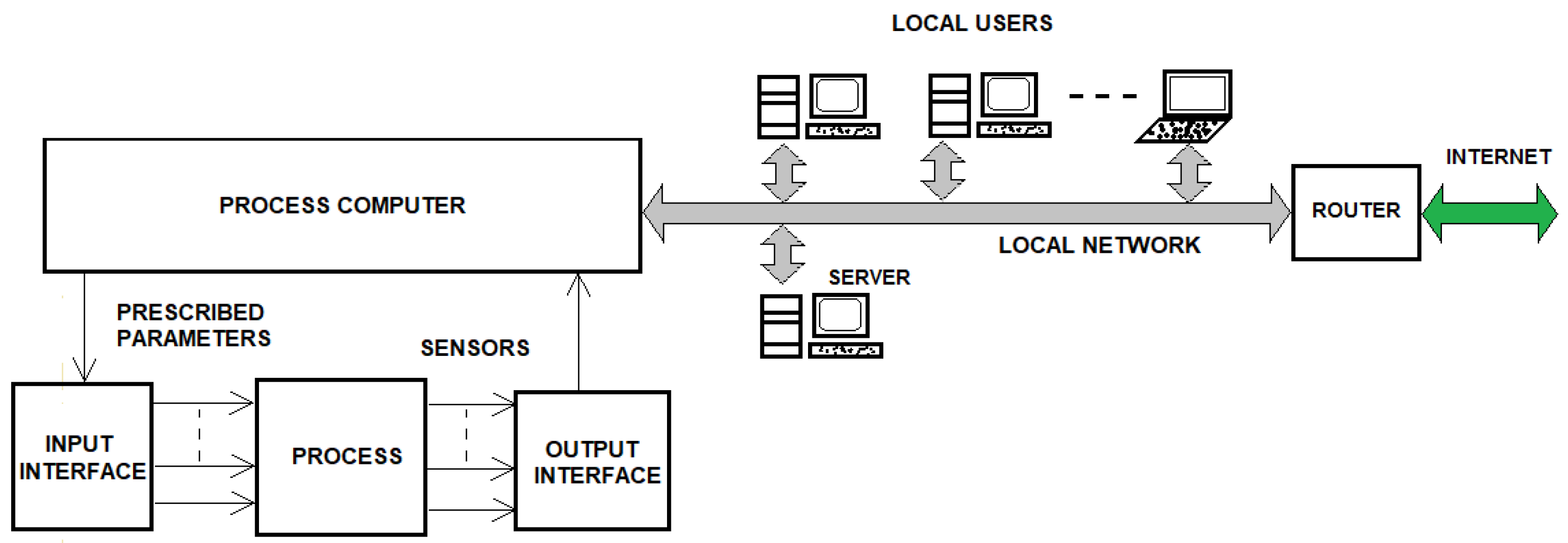
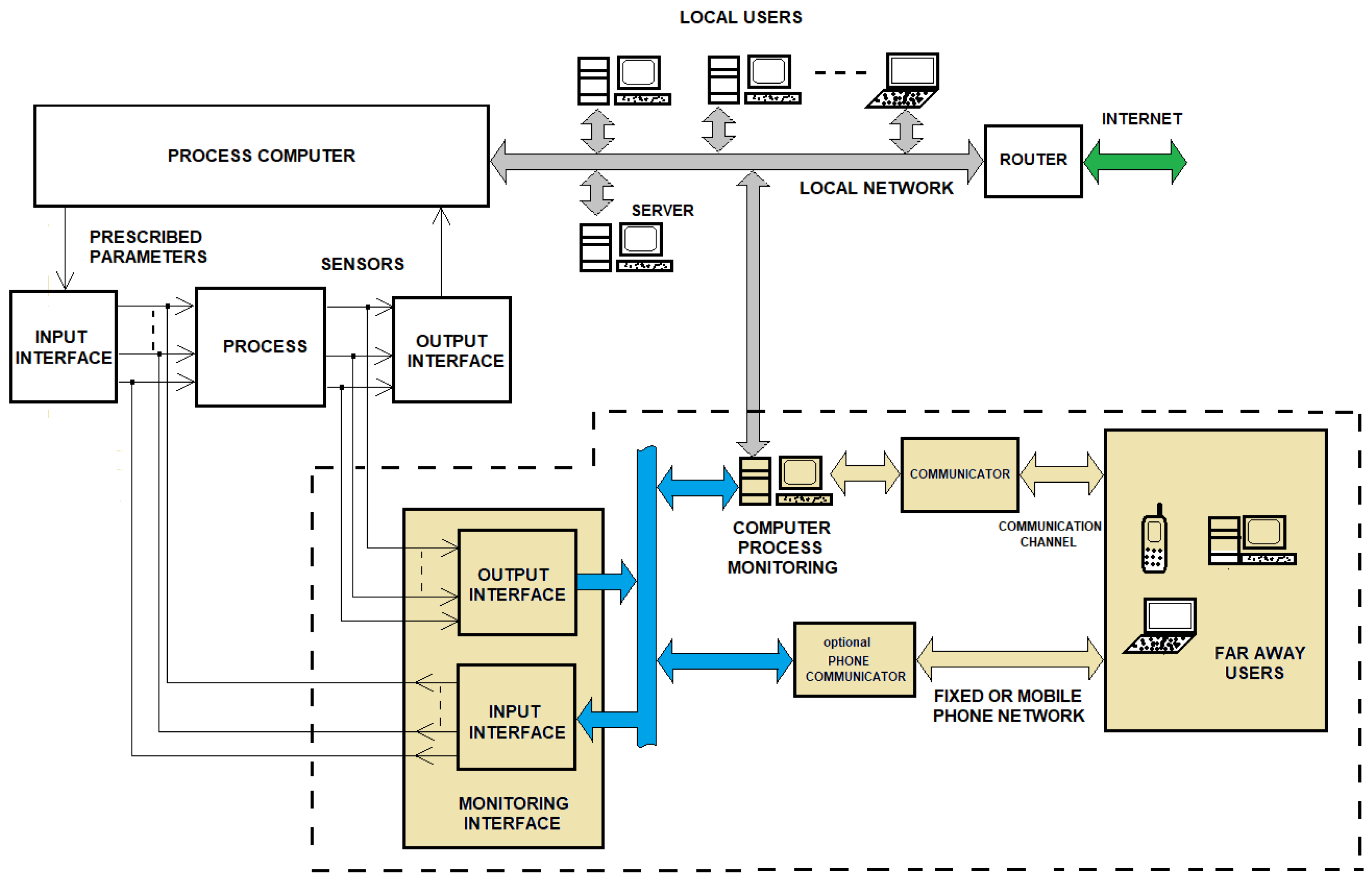
3. Method of Implementing Remote Process Monitoring
3.1. Preliminaries Regarding the Informatic System for a Technological Process Control
3.2. Remote Monitoring Interface of the 18O Isotope Production Process by NO-HNO3 (H2O) Isotopic Exchange Reaction
4. Results
4.1. Interface Board
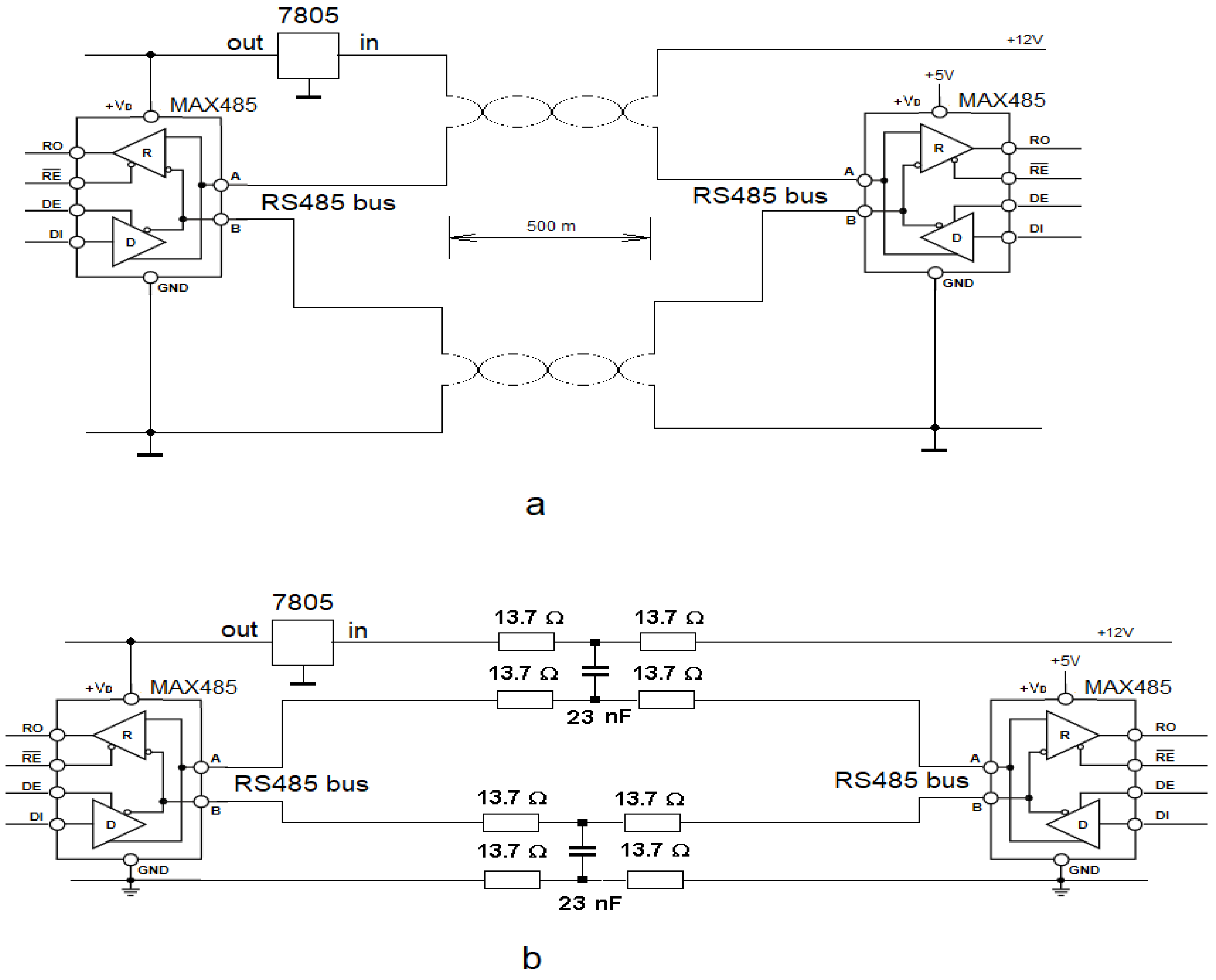
4.2. Simulation of Long Network Buses
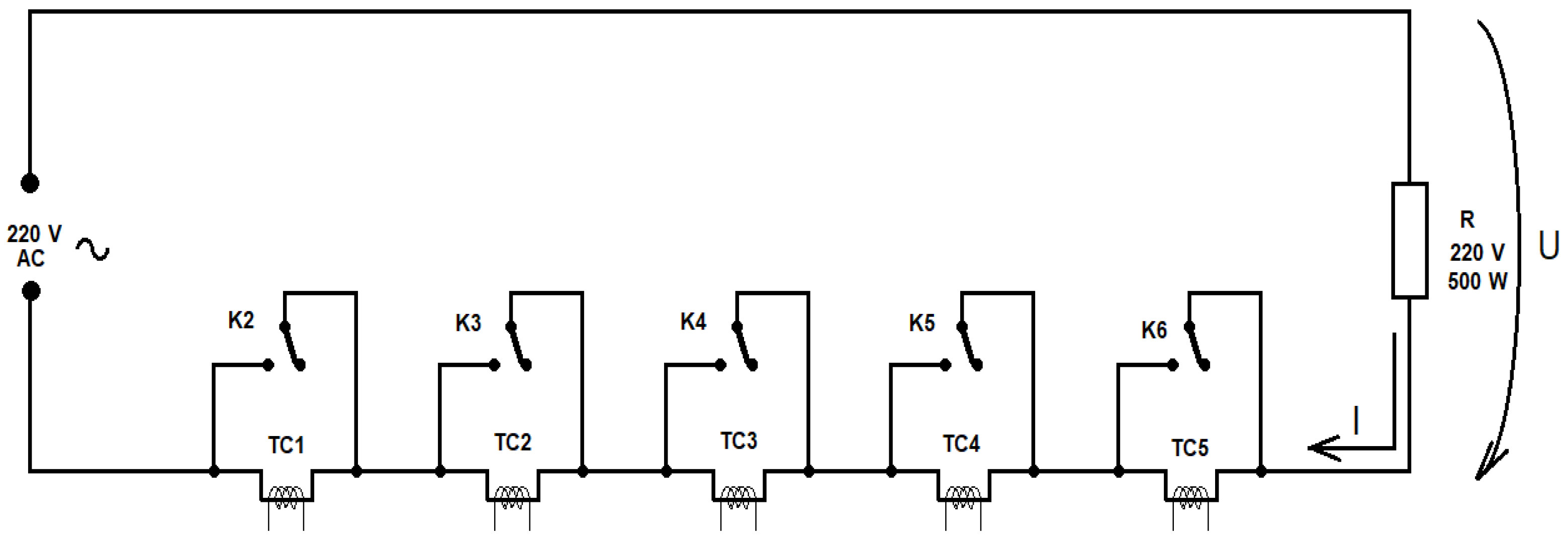
4.3. Issues Related to Remote Powering of Devices
- R—equivalent electrical resistance of the consumer.
- S—voltage stabilizer.
- Rc—equivalent electrical resistance of cables.
- Imax—maximum current absorbed by the consumer.
- -
- increase the supply voltage U > 20 V as the difference U − Uc > 7 V,
- -
- supplying the consumer device locally with a voltage source closer to it so that the voltage drop Uc is lower,
- -
- the use of a step-up switching voltage stabilizer which is not affected by the voltage drop at its input.
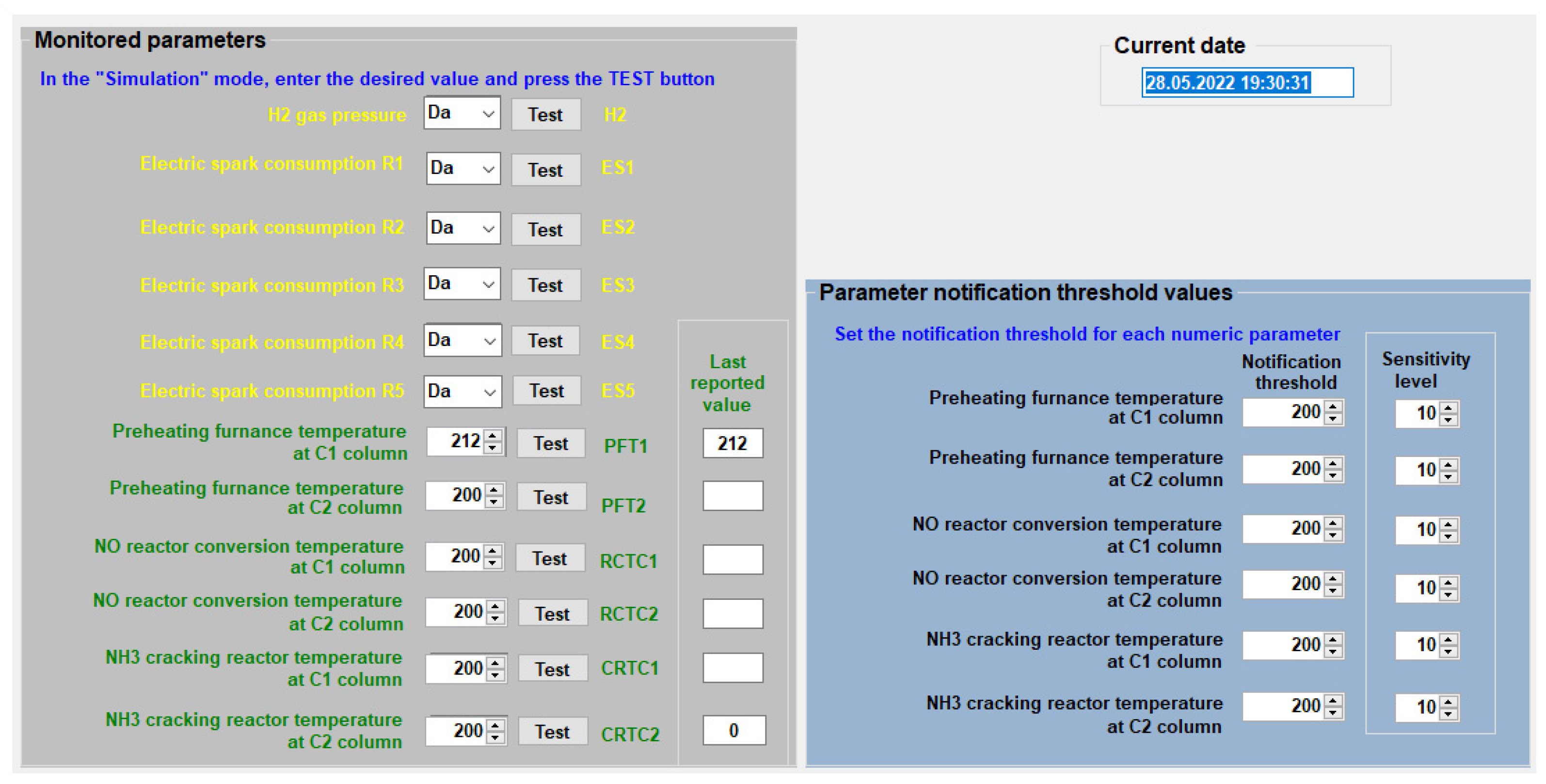
4.4. The Programme for Monitoring the Separation Process of Isotope 18O Isotope Exchange Reaction
- “Normal” working mode, in which the monitoring computer receives the monitored parameter values from microcontroller A via the USB port.
- The “simulation” working mode is only used to test the communication of the program with the users; the computer does not receive any more information from the real process.
- Working mode “off”; monitoring does not work.
- NP—(Parameter Notification) for the user to be notified when the threshold value of the respective parameter is changed or exceeded.
- PIP—(Permission to Query Parameter) so that the user can ask for the value of the parameter at any time, independently of notification.
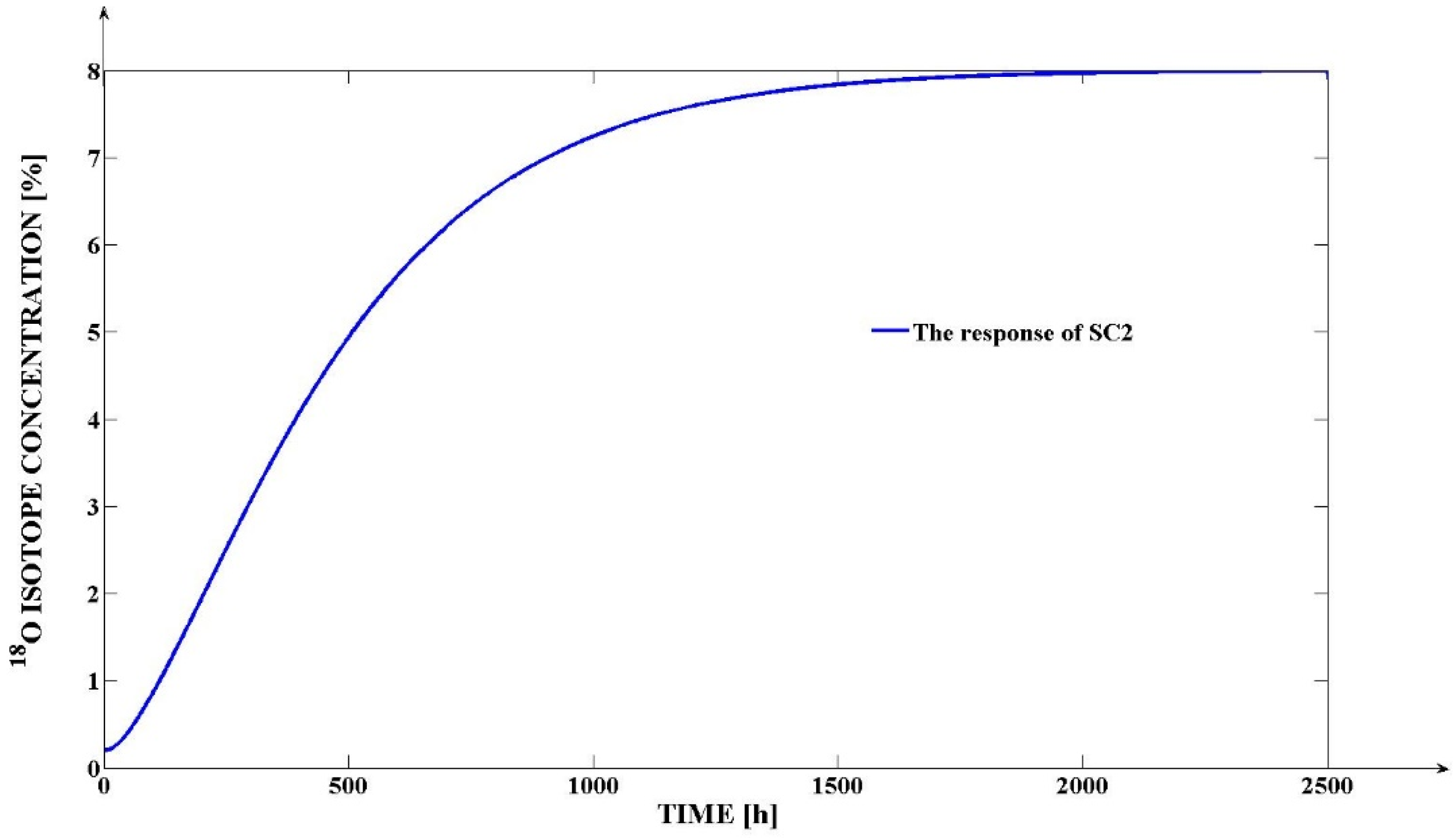
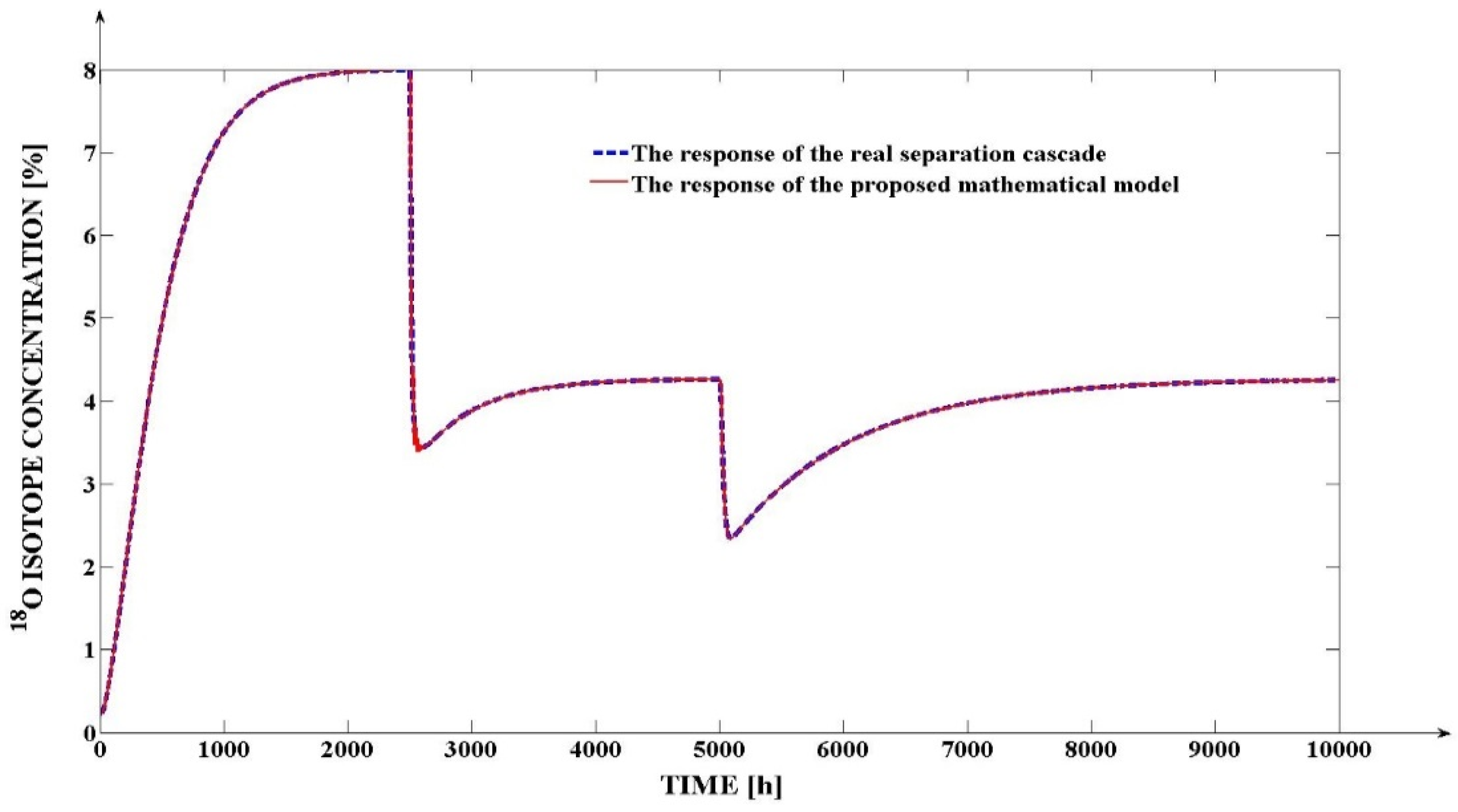
4.5. The Results Obtained through the Simulation of the Proposed Mathematical Model
5. Conclusions and Further Developments
- Expanding the number of remotely monitored parameters, possibly adding some parameters that are not of the isotope production process but of systems that serve the process (e.g., thermal switches, monitoring of their supply voltage value, smoke sensor, gas sensor, etc.).
- Modification of the communication protocol so that the communication interface can detect if the network cable is interrupted or if a network device is not responding (interrupted, disconnected) and communicate this to the monitoring computer.
- Development of a more complex monitoring program with a graphical display (possibly in the LabVIEW environment)
- Development of a client application for users’ mobile devices containing intuitive graphics of the system and monitoring parameters
- Extending the experimental part to the entire installation
- The implementation of an advanced control strategy for the automatic control of all the parameters which occur in the separation cascade operation.
- The implementation, based on the proposed mathematical model, of an optimization system of the separation cascade energy consumption.
- The extension of the proposed mathematical model by considering, also, as a control signal, the temperature in the column (the temperature can be modified through the corresponding control system); the temperature variation would imply the variation of the α elementary separation factor.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Codoban, A.; Abrudean, M.; Silaghi, H.; Dale, S.; Mureşan, V.; Fișcǎ, M.; Ungureşan, M.L.; Cordoş, R. Monitoring System of Production Installation for the Separation of Isotope 18O. In Proceedings of the 2021th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 10–11 June 2021. [Google Scholar]
- Okahashi, N.; Yamada, Y.; Iida, J.; Matsuda, F. Isotope Calculation Gadgets: A Series of Software for Isotope-Tracing Experiments in Garuda Platform. Metabolites 2022, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, D.; Augusto, R.; Liang, J.; Qin, Z.; Liu, J.; Liu, Z. Production Review of Accelerator-Based Medical Isotopes. Molecules 2020, 27, 5294. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Tian, D.; Xu, B.; Ren, J.; Hao, L.; Chen, N.; Li, X. Use of the stable oxygen isotope method to evaluate the difference in water consumption and utilization strategy between alfalfa and maize fields in an arid shallow groundwater area. Agric. Water Manag. J. 2021, 256, 107065. [Google Scholar] [CrossRef]
- Stephens, J.A.; Ducea, M.N.; Killick, D.J.; Ruiz, J. Use of non-traditional heavy stable isotopes in archaeological research. J. Archaeol. Sci. 2021, 127, 105334. [Google Scholar] [CrossRef]
- Vasaru, G. (Ed.) Izotopii Stabili; Editura Tehnica: București, Romania, 1968. [Google Scholar]
- Abrudean, M.; Ungureșan, M.; Pica, E.-M. Preliminaries Concerning the Modeling of the Absorption Process of Nitrogen Oxides in Aqueous Nitric Acid. In Proceedings of the A&Q’98 International Conference of Automation and Quality Control, Cluj-Napoca, Romania, 28–29 May 1998. [Google Scholar]
- Khoroshilov, A.V. Production of stable isotopes of light elements: Past, present and future. J. Phys. Conf. Ser. 2018, 1099, 012002. [Google Scholar] [CrossRef]
- Love, J. Process Automation Handbook—A Guide to Theory And Practice; Springer: London, UK, 2007. [Google Scholar]
- Mureșan, V.; Abrudean, M.; Ungureșan, M.L.; Coloși, T. Modeling and simulation of the isotopic exchange for 18O isotope production. In Proceedings of the 2018 IEEE International Conference on Automation, Quality and Testing, Robotics (AQTR), Cluj-Napoca, Romania, 24–26 May 2018. [Google Scholar]
- Abrudean, M.; Unguresan, M.L.; Silaghi, H.; Muresan, V.; Codoban, A. Experimental Identification of the C-13 Isotope Separation Process by Cryogenic Distillation on a Two-Column Separation Cascade. In Proceedings of the 2019 15th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 13–14 June 2019. [Google Scholar]
- Codoban, A. Monitorizarea de la Distanță a Parametrilor Procesului de Separare a Izotopului 18O. Ph.D. Thesis, University of Oradea, Oradea, Romania, 2021. [Google Scholar]
- Niazi, M.; Protocols for Industrial Remote Monitoring. Control Automation. 3 May 2021. Available online: https://control.com/technical-articles/protocols-for-industrial-remote-monitoring/ (accessed on 14 May 2022).
- Saxena, S.C.; Taylor, T.I. Enrichement of oxygen-18 by the chemical exchange of nitric oxide with nitric acid solution. J. Chem. Phys. 1962, 66, 1480–1487. [Google Scholar] [CrossRef]
- Axente, D.; Abrudean, M.; Baldea, A. Separation of 15N, 18O, 10B, 13C through Isotopic Exchange; Editura Casa Cărţii de Ştiinţă: Cluj-Napoca, Romania, 1995; pp. 249–263. ISBN 973-9654-4-3. [Google Scholar]
- Abrudean, M.; Axente, D.; Bâldea, A. Enrichment of 15N and 18O by chemical Exchange Reaction between Nitrogen Oxides (NO, NO2) and Aqueous Nitric Acid. Isotopenpraxis 1981, 17, 377–382. [Google Scholar] [CrossRef]
- Doyle, J.; Carroll, J. Routing TCP/IP; ciscopress.com: Indianapolis, IN, USA, 2005; Volume I. [Google Scholar]
- Carroll, J.; Doyle, J. Routing TCP/IP: CCIE Professional Development; ciscopress.com: Indianapolis, IN, USA, 2016; Volume II. [Google Scholar]
- Stephens, R. Begin Database Design W/WS; Josey-Bass: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kassi, S.; Karlovets, E.V.; Tashkun, S.A.; Perevalov, V.I.; Campargue, A. Analysis and theoretical modeling of the 18O enriched carbon dioxide spectrum by CRDS near 1.35 μm: (I) 16O 12C 18O, 16O 12C 17O, 12C 16O2 and 13C 16O2. J. Quant. Spectrosc. 2017, 187, 414–425. [Google Scholar] [CrossRef]
- Dulf, E.; Kovacs, L. Fractional order control of the cyber-physical cryogenic isotope separation columns cascade system. In Proceedings of the 2018 IEEE International Conference on Automation, Quality and Testing, Robotics (AQTR), Cluj-Napoca, Romania, 24–26 May 2018. [Google Scholar]
- Mureşan, V.; Abrudean, M.; Ungureşan, M.-L.; Clitan, I.; Coloşi, T. Control of the 18O isotope separation process. In Proceedings of the SACI, Timisoara, Romania, 12–14 May 2016; pp. 283–288. [Google Scholar]
- Monje, C.A.; Chen, Y.; Vinagre, B.M.; Xue, D.; Feliu, V. Fractional-order systems and controls. In Fundamentals and Applications; Springer: New York, NY, USA, 2010; pp. 3–9. [Google Scholar]
- Lin, W.C.; Tsai, C.F.; Zhong, J.R. Deep learning for missing value imputation of continuous data and the effect of data discretization. Knowl. Based Syst. 2022, 239, 108079. [Google Scholar] [CrossRef]
- Chen, L.; Chen, P.; Lin, Z. Artificial Intelligence in Education: A Review. IEEE Access 2020, 8, 75264–75278. [Google Scholar] [CrossRef]
- Haykin, S. Neural Networks and Learning Machines, 3rd ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2009; pp. 818–845. [Google Scholar]
- Kamra, A.; Bhambri, P. Computer Peripherals and Interfaces; Technical Publications Pune: Maharashtra, India, 2008. [Google Scholar]
- System Identification. Available online: https://www.libristo.ro/ro/carte/system-identification_01210326?utm_source=google&utm_medium=surfaces&utm_campaign=shopping%20feed&utm_content=free%20google%20shopping%20clicks%20merchant_ro&gclid=EAIaIQobChMI6e6Ywt3o_QIVoQYGAB3ymgCeEAQYBCABEgI7yfD_BwE (accessed on 14 May 2022).
- Experimental Techiques—Developments, Applications and Tutorials in Experimental Mechanics and Dynamics. Available online: https://www.springer.com/journal/40799?gclid=EAIaIQobChMI4pCO67_p_QIVhdZ3Ch0q-wTuEAAYASAAEgJVDfD_BwE (accessed on 20 May 2022).
- Identification and Classical Control of Linear Multivariable Systems. Available online: https://www.enbook.ro/catalog/product/view/id/2052104?gclid=EAIaIQobChMI6e6Ywt3o_QIVoQYGAB3ymgCeEAQYCSABEgKu1_D_BwE (accessed on 22 July 2022).
- Models and Methods for Parametric Identification. Available online: https://link.springer.com/chapter/10.1007/978-1-4471-3848-8_11 (accessed on 12 September 2022).
- System Identification—Second Edition. Available online: https://www.libristo.ro/ro/carte/system-identification-second-edition_20173614 (accessed on 18 July 2022).
- Using the SDP Identification Method for Electromechanical Systems. Available online: https://reader.elsevier.com/reader/sd/pii/S2405896321012362?token=D86A95AE58908882F60600129D3AA110A55E7645BE23C1CA61D838465FAC13116767A4EAB2E654E62B9E980950183B85&originRegion=eu-west-1&originCreation=20230319193608 (accessed on 20 October 2022).
- Nonlinear System Identification. Available online: https://www.libristo.ro/ro/carte/nonlinear-system-identification_06829824 (accessed on 17 November 2022).
- Karimov, A.I.; Kopets, E.; Nepomuceno, E.G.; Butusov, D. Integrate-and-Differentiate Approach to Nonlinear System Identification. Mathematics 2021, 9, 2999. [Google Scholar] [CrossRef]
- Obeid, S.; Ahmadi, G.; Jha, R. NARMAX Identification Based Closed-Loop Control of Flow Separation over NACA 0015 Airfoil. Fluids 2020, 5, 100. [Google Scholar] [CrossRef]
- Gedon, D.; Wahlström, N.; Schön, T.B.; Ljung, L. Deep state space models for nonlinear system identification. IFAC-PapersOnLine 2021, 54, 481–486. [Google Scholar] [CrossRef]
- Huffaker, R.; Bittelli, M.; Rosa, R. Phase–Space Reconstruction, Chapter 3. In Nonlinear Time Series Analysis with R; Oxford Academic: Oxford, UK, 2017. [Google Scholar]
- Tian, R.; Yang, Y.; van der Helm, F.C.; Dewald, J. A novel approach for modeling neural responses to joint perturbations using the NARMAX method and a hierarchical neural network. Front. Comput. Neurosci. 2018, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Yan, R.; Chen, X. Spline adaptive filter with arctangent-momentum strategy for nonlinear system identification. Signal Process. 2019, 164, 99–109. [Google Scholar] [CrossRef]
- Torres, L.; Besancon, G.; Verde, C.; Georges, D. Parameter identification and synchronization of spatio-temporal chaotic systems with a nonlinear observer. IFAC-PapersOnLine 2012, 45, 267–272. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, M.Z.Q.; Zhang, D. Special Issue on “Advances in Condition Monitoring, Optimization and Control for Complex Industrial Processes”. Processes 2021, 9, 664. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X. An overview on fault diagnosis, prognosis and resilient control for wind turbine systems. Processes 2021, 9, 300. [Google Scholar] [CrossRef]
- Xu, S.; Hashimoto, S.; Jiang, W.; Jiang, Y.; Izaki, K.; Kihara, T.; Ikeda, R. Slow mode-based control method for multi-point temperature control system. Processes 2019, 7, 533. [Google Scholar] [CrossRef]
- Trimmer, W. Understanding and Servicing Alarm Systems; Butterworth-Heinemann: Oxford, UK, 1999. [Google Scholar]
- Blum, J. Exploring Arduino: Tools and Tehniques for Engineering Wizardry; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Margolis, M. Arduino Cookbook; O’Reilly Media: Newton, MA, USA, 2011. [Google Scholar]
- *** Data Transmission Circuits-Line Circuits; Texas Instruments: Dallas, TX, USA, 1996.
- Dong, Y.; Wang, S.; Liu, H. Establishment of Remote Measure and Control System Based on Complex Dynamic Objects. In Proceedings of the 2009 9th International Conference on Electronic Measurement and Instruments, (ICEMI), Beijing, China, 16–19 August 2009. [Google Scholar]
- Codoban, A.; Abrudean, M.; Cordoş, R.; Mureşan, V.; Fișcă, M.; Ungureşan, M.L.; Silaghi, H.; Spoială, V. Preliminaries Regarding the Monitoring of the Parameters of a Separation Cascade for Isotope 18O. In Proceedings of the 2021th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 10–11 June 2021. [Google Scholar]
- Bockmann, C.J.; Klander, L.; Tang, L. Visual Basic Programmer’s Library; Jamsa Pr: Phoenix, AZ, USA ; State University of Pennsylvania: State College, PA, USA, 1998. [Google Scholar]
- Berry, M.V.; Lewis, Z.V. On the Weierstrass-Mandelbrot fractal function. Proc. R. Soc. Lond. A Math. Phys. Sci. 1980, 370, 459–484. [Google Scholar]
- Guariglia, E.; Silvestrov, S. Fractional-Wavelet Analysis of Positive definite Distributions and Wavelets on D’(C). In Engineering Mathematics II; Rancic, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 337–353. [Google Scholar]
- Yang, L.; Su, H.; Zhong, C.; Meng, Z.; Luo, H.; Li, X.; Tang, Y.; Lu, Y. Hyperspectral image classification using wavelet transform-based smooth ordering. Int. J. Wavelets Multiresolut. Inf. Process 2019, 17, 1950050. [Google Scholar] [CrossRef]
- Guariglia, E. Fractional calculus, zeta functions and Shannon entropy. Open Math. 2021, 19, 87–100. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, Y.; Zhou, J. A Framework of Adaptive Multiscale Wavelet Decomposition for Signals on Undirected Graphs. IEEE Trans. Signal Process. 2019, 67, 1696–1711. [Google Scholar]
- Guariglia, E.; Guido, R. Chebyshev wavelet analysis. J. Funct. Spaces 2022, 2022, 5542054. [Google Scholar] [CrossRef]
- Automatic Control Systems. Available online: https://www.fruugo.ro/sisteme-de-control-automat-editia-a-zecea-de-farid-golnaraghibenjamin-kuo/p-48829124-97007997?language=ro&ac=KelkooCSS&gclid=EAIaIQobChMIuK7TneDo_QIVTAWLCh3hgg8lEAQYAyABEgLA2_D_BwE (accessed on 6 June 2022).

















| No. | F1 [L/h] | HETP1 (F1) [cm] | F2 [L/h] | HETP2 (F2) [cm] |
|---|---|---|---|---|
| 1 | 766.9 | 13.1 | 60.1 | 14.9 |
| 2 | 926.3 | 12.1 | 72.6 | 13.9 |
| 3 | 1085.7 | 11.1 | 85.1 | 12.9 |
| 4 | 1245.2 | 10.1 | 97.6 | 11.9 |
| 5 | 1404.6 | 9.1 | 110.1 | 10.9 |
| 6 | 1564.1 | 8.1 | 122.6 | 9.9 |
| 7 | 1642.2 | 7.6 | 128.7 | 9.5 |
| 8 | 1723.5 | 7.1 | 135.1 | 8.9 |
| 9 | 1785.7 | 6.7 | 140 | 8.6 |
| 10 | 1882.9 | 7 | 147.6 | 8.8 |
| 11 | 2042.4 | 7.4 | 160.1 | 9.2 |
| 12 | 2201.8 | 7.8 | 172.6 | 9.6 |
| 13 | 2359.7 | 8.2 | 185 | 10.1 |
| Neural Network | Input Signal/Signals | Output Signal/Signals | Number of Hidden Layers | Size of Hidden Layer/Layers | Size of Output Layer |
|---|---|---|---|---|---|
| NN1 | F1(t) F2(t) | HETP1(F1(t)) HETP2(F2(t)) | 1 | 18 | 2 |
| NN2 | β(t) | (i {0; 1; 2; 3; 4; 5}) | 2 | [23; 10] | 6 |
| NN3 | β(t) | (i {0; 1; 2; 3; 4; 5}) | 2 | [25; 14] | 6 |
| NN4 | FC(t) | F2C(t) | 1 | 14 | 1 |
| NN5 | Zstimp(t) | F2(t) | 1 | 17 | 1 |
| Neural Network | Activation Functions of the Neurons from the Hidden Layer/Layers | Activation Functions of the Neurons from the Output Layer | Maximum Training Epochs | Run Training Epochs | Learning Algorithm |
| NN1 | Bipolar sigmoid | Linear | 50,000 | 43,243 | Levenberg–Marquardt |
| NN2 | Bipolar sigmoid | Linear | 75,000 | 58,234 | Levenberg–Marquardt |
| NN3 | Bipolar sigmoid | Linear | 80,000 | 73,692 | Levenberg–Marquardt |
| NN4 | Bipolar sigmoid | Linear | 30,000 | 18,217 | Levenberg–Marquardt |
| NN5 | Bipolar sigmoid | Linear | 32,000 | 25,875 | Levenberg–Marquardt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codoban, A.; Silaghi, H.; Dale, S.; Muresan, V. Remote Monitoring the Parameters of Interest in the 18O Isotope Separation Technological Process. Processes 2023, 11, 1594. https://doi.org/10.3390/pr11061594
Codoban A, Silaghi H, Dale S, Muresan V. Remote Monitoring the Parameters of Interest in the 18O Isotope Separation Technological Process. Processes. 2023; 11(6):1594. https://doi.org/10.3390/pr11061594
Chicago/Turabian StyleCodoban, Adrian, Helga Silaghi, Sanda Dale, and Vlad Muresan. 2023. "Remote Monitoring the Parameters of Interest in the 18O Isotope Separation Technological Process" Processes 11, no. 6: 1594. https://doi.org/10.3390/pr11061594
APA StyleCodoban, A., Silaghi, H., Dale, S., & Muresan, V. (2023). Remote Monitoring the Parameters of Interest in the 18O Isotope Separation Technological Process. Processes, 11(6), 1594. https://doi.org/10.3390/pr11061594









