Abstract
This research aimed to assess the anti-inflammatory and antioxidant potential of methanol extract of Wilckia maritima, a plant belonging to the family Brassicaceae, which is enriched with natural antioxidants. Qualitative phytochemical studies showed the presence of numerous compounds including glycosides, phenols, triterpenoids, and GC-MS studies revealed the presence of 35 bioactive components, including n-hexadecanoic acid (26.96%), 9,12,15 octadecatrienoic acid (cis) (25.52%), 3,5 di-hydroxy-6-methyl 2,3-di-hydro-4-pyran 4-one (14.35%), and 3-tertiary butyl-4-hydroxy-anisol (11.68%) as major components, which are thought to be responsible for anti-inflammatory and antioxidant potentials of methanol extract of W. maritima., flavonoids, steroids, tannins, and saponins. The antioxidant potential of the extract was determined by performing various assays, including DPPH free radical scavenging, ferrous reducing, and hydrogen peroxide assays, which showed significant percentage inhibition (83.55 ± 0.89, 79.40 ± 1.17, and 81.26 ± 0.36%, respectively) as compared to ascorbic acid (standard). The extract also exhibited significant anti-inflammatory activity with percentage inhibition 65.66 ± 0.42% compared to standard ibuprofen, which showed 73.20 ± 0.21% inhibition. In vivo analysis further confirmed this anti-inflammatory potential of the extract, showing a 75.55 ± 0.11% reduction in edema at 300 mg/kg as compared with standard diclofenac sodium 70.27 ± 0.012%. Moreover, in silico investigations revealed that the phytocompounds in W. maritima exhibited excellent antioxidant and anti-inflammatory characteristics, which could provide novel biological molecules for target receptors. Overall, our findings suggest that W. maritima can be utilized as a potential resource of natural compounds with antioxidants and anti-inflammatory potential, with promising therapeutic effect in relieving various ailments related to inflammatory response.
Keywords:
phenols; Wilckia maritima; GC-MS; antioxidant; radicals; anti-inflammatory; hexadecanoic acid 1. Introduction
Human diseases arise due to the invasion of pathogens and toxins into the body and the disturbance of immune regulations, which initiate a series of different inflammatory responses in a living system. The release of proteases from mast cell starts a chain of redox reactions by producing reactive oxygen species (ROS), which play a useful role against cellular signal transduction. It can be dangerous when cells become unregulated and uncontrolled progression of these pro-inflammatory responses are associated with complications such as inflammation, diabetes, arthritis, arteriosclerosis, hypertension and Alzheimer’s disease [1]. Synthetic antioxidants used for monitoring unwanted redox action are expensive and produce undesirable side effects. NSAIDs including coxibs and aspirin are associated with high risk of cardiovascular and gastro-intestinal complications upon prolonged use [2,3]. Plant-based medicines are thought to be a potent solution to these challenges. To overcome these challenges, natural antioxidants have attracted attention over time as they are economical, have fewer side effects compared with their synthetic counterparts and are available in a wide range of plants [4]. The characterized phytochemical constituents responsible for anti-oxidant and anti-inflammatory effects are flavonoids (anthocyanidins and flavones), polyphenols and terpenes [5,6].
Free radicals such as hydroxyl, lipid peroxide and superoxide free radicals are unstable and very reactive molecules that immediately combine with other chemical species to obtain stability and start a cascade of chain reactions that are harmful to living cells [7,8]. Oxidative stress arises due to an imbalance between the antioxidant agents and the free radicals in living cells and causes cell death by damaging vital cellular components such as membrane lipids, proteins and DNA [9]. Medicinal plants are potential sources of new drug development. The WHO reported that approximately 80% of developing areas still use medicinal plants as a primary source of antioxidant agents [10,11].
GC-MS is an extensively used analytical technique for the assessment of components in test samples due to its great sensitivity, specificity, resolution and high-throughput potential. It has gained more consideration due to its high degree of reproducibility and accuracy [12]. It is used for the separation of thermally stable and volatile substitutes in test sample and fragments of GC-MS analyte identified on the base of mass. In the GC-MS evaluation, chemical ionization and electron impact techniques are used for the characterization of components according to ratio of mass of particle to electrostatic charge on particle represented as m/z (mass to charge ratio) [13].
In this study, we investigated the ornamental plant Wilckia maritima from the Brassicaceae family for its phytochemical composition and potential therapeutic benefits. There are six species of genus Wilckia native to Africa, Europe and the Mediterranean region; however, only two species including Wilckia maritima are cultivated in Asia, in Pakistan. Traditionally, the species of this genus were used as mineral sources and possess antioxidant and antibacterial effects [14]. Wilckia maritima, commonly known as Virginia stock, and is characterized by its beautiful heart-shaped petalled flowers. The height of the plant is in the 6–12 inches range. Specifically, we analyzed the methanol extract of the plant for its anti-oxidant and anti-inflammatory properties, and characterized its phytochemical composition using GC-MS analysis. The methanol extract of Wilckia maritima contains a diverse array of phytochemicals with potential therapeutic benefits, including anti-inflammatory compounds. Additionally, the extract demonstrated strong anti-oxidant and anti-inflammatory activities, indicating its potential use in the treatment of diseases involving inflammation. The GC-MS analysis allowed for the quantitative analysis of specific phytochemicals present in the extract, providing important insights into the composition of the plant and its potential therapeutic properties. The molecular docking was also used for the anti-oxidant and anti-inflammatory activities with the target proteins and interactions.
2. Experimental
2.1. Plant Collection and Preparation of Extract
The Wilckia maritima was collected in April 2021 from Fazal Nursery Multan, Pakistan, and identified as such. Prof. Dr. Altaf Dasti of the Botany Department at Bahauddin Zakariya University in Multan, Pakistan, identified the specimen with the voucher number WM-964-2021.The Wilckia maritima (whole plant) was shade-dried for 27 days, grinded to a fine powder and accurately weighed. A total of 700 g of powder was macerated in 2.5 L methanol for three successive days [15]. Using a rotary evaporator Buchi R-400 Switzerland, the excess solvent was evaporated in order to obtain the W. maritima whole plant methanol extract, which was then placed in an airtight container for future use.
2.2. Phytochemical Investigation
Preliminary phytochemicals tests for qualitative analysis were performed to determine phytoconstituents such as flavonoids, glycosides, triterpenoids, steroids, phenols, tannins, alkaloids and saponins in W. maritima by adopting standard procedures [16].
2.3. GC-MS Technique
The study conducted an analysis of the bioactive compounds present in the methanolic extract of W. maritima using Agilent 7000 A triple quad technologies with GC-model 7890-A, which had an HP-5 MS-column (0.25 µm thickness of film ×250 µ diameter ×30 m length). The characterization of the compounds was carried out using high-energy electrons (70 eV) in an electron ionization system. The carrier gas used was 99.995% pure helium gas, with a flow rate of 1 mL/min.
The analysis was performed by starting at a temperature of 50 °C, which was gradually increased up to 200 °C for 30 min, and then finally raised up to 300 °C for 10 min at a rate of 10 °C.
Temperatures of the transfer line and injection port were set at 260 °C and 250 °C, respectively. A 2.5 µL sample solution (diluted with 1% methanol) was introduced into split mode at a ratio of 10:1. The retention indices of bioactive components in the methanolic extract of W. maritima were recalculated using n-alkanes.
2.4. Methodology for Molecular Docking
2.4.1. Protein Targets’ Identification and Selection
Crystal structures of urate oxidase and lipoxygenase protein were obtained from the RCSB protein data bank using the PDB IDs 1R4U for urate oxidase and 3O8Y for lipoxygenase, respectively, to carry out the virtual screening procedure [17,18].
2.4.2. Software Required
For the purpose of molecular docking, we made use of MGLtools, the binary files for Autodock4 and Autogrid4 [19], and the Discovery Studio Visualizer that was provided by the BIOVIA [20] for 2D structures chemdraw ultra [21], and for 3D structure and minimization chemdraw 3D pro [22].
2.4.3. Target Protein Preparation
To enable the use of the autodock suite, the target protein was downloaded from RCSB and processed. We removed all heteroatoms from the protein molecule in the discovery studio visualizer of BIOVIA, along with co-crystal ligand and solvent molecules. A clean protein structure was obtained; then, using autodock tools, it was made to be optimized for docking by providing each atom with the proper polar hydrogen and Kollman charges and storing the file as a pdbqt [23].
2.4.4. Preparation of Ligand and Molecular Docking
The structures were created in chemdraw ultra using the IUPAC names of the compounds, and the energy minimization was finished in chem 3D pro. The compound structures were saved in SDF files. These structures were then converted into a pdbqt file using the openbabel GUI software, which autodock can read. After that, the structure-based virtual screening was carried out using Autodock4. The box dimensions used to create the grid parameter file were the same as those used to create the co-crystal ligand. Using a bespoke force field dubbed Autodock4Zn and a Lamarckian genetic process, docking parameter files were created (LGA). We employed a population size of 300 and a total of 50 postures to ensure the best level of accuracy [24]. After preparation, the ligand library was separately docked into each protein’s active site [19].
2.4.5. Visualization
For this, we employed the ligand–protein interaction analyzer BIOVIA discovery studio visualizer. The pdbqt and autodock output files were fed into BIOVIA’s Discovery Studio in order to generate all 2D and 3D conformations. The bonding and non-bonding interactions between the ligand and active pocket were found.
2.4.6. Validation
The docking method was validated using the RMSD value and by re-docking the co-crystal ligand into the protein’s active pocket. Pose acceptance requires docking and experimental ligand RMSD values of less than 2.0 [25].
2.5. Antioxidant Activity
2.5.1. DPPH Free Radical Scavenging Potential
Antioxidant potential was investigated using diphenyl-1-picrylhydrazyl (DPPH) radical scavenging potential as explained by Awah et al., 2010 [26] with some modification. Briefly, different concentrations of extract solution 200–1000 ppm were prepared. A 1 mL sample solution of different concentrations and a 2 mL DPPH solution (0.004% w/v) were mixed together. The mixture was vortexed and sited in a dark place for 30 min, and absorbance was measured at 517 nm considering ascorbic acid (standard). All experiments were performed in triplicate and the results are reported as mean ± SD (n = 3) using the following equation:
A0 = absorbance of DPPH (0.004% w/v), whereas A = absorbance of control with extract solution.
2.5.2. H2O2 Assay
The hydrogen peroxide assay was investigated according to the method presented by Ruch et al., 1989 [27] with some modifications. Briefly, the H2O2 solution was prepared in phosphate buffer maintained at pH 7.4. Extract solutions of different concentrations 200–1000 ppm were prepared and mixed with 1 mL of H2O2 solution. Absorbance was measured at 230 nm and compared with ascorbic acid. The experiment was performed in triplicate and the result is expressed as mean ± SD (n = 3) using the following equation.
A0 = absorbance of H2O2 solution, whereas A = absorbance of control having extract solution.
2.5.3. Ferrous Reducing Activity
The ferrous reducing power of extract was investigated according to the method presented by Mariga et al., 2012 [28]. Different extract concentrations 200–1000 ppm were mixed with 2.5 mL 0.2 mM buffer of 6.6 pH and 2.5 mL of 1% [K3Fe(CN)6]. The mixture was vortexed and placed for incubation at 50 °C for 25 min. After incubation, 2.5 mL trichloroactetic acid was added to mixture and centrifuged at 1000 RPM for 15 min. Then, 2.5 mL of deionized water was added to supernatant (2.5 mL), followed by the addition of 0.5 mL of ferric chloride solution; the absorbance was measured at 700 nm using ascorbic acid as the standard.
2.6. Anti-Inflammatory Activity
To determine the anti-inflammatory potential of the methanolic extract of W. maritima, the luminol-enhanced chemiluminescence assay was used as presented by Jabeen et al., 2015 [29] with some modifications. The standard drug used in the study was Ibuprofen (50 µg/mL).
In the assay, 25 µL of blood was diluted with HBSS++ (Hanks Balanced Salt Solution, which includes magnesium chloride and calcium chloride), and 25 µL of three different concentrations of the methanolic extract (10, 25, and 50 µg/mL) were mixed together and incubated. A control well was also included with only the HBSS++ solution. The test was carried out in 96-well plates that were previously incubated for 20 min at 37 °C in the thermostat chamber of a luminometer. To each well, 25 µL of serum opsonized zymosan (SOZ), 25 µL of an intracellular reactive oxygen species detecting probe, and luminol were added. The level of reactive oxygen species (ROS) was recorded using a luminometer and expressed in relative light units (RLU).
2.7. Anti-Inflammatory Activity (In Vivo)
The in vivo anti-inflammatory effect of the hydro-methanol extract of W. maritima was determined using carrageenan-induced paw edema in Wister albino rats. A plethysmometer was used to calculate the reduction in inflammation. To initiate the inflammation, a solution of carrageenan (0.11 mL in physiological saline) was injected into the hind paw of Wister albino rats (either gender) weighing between 105 and 160 g. After 30 min of the carrageenan injection, the hydro-methanolic extract was orally administered at concentrations of 200 mg/kg and 300 mg/kg. The paw volume was monitored using a plethysmograph at 0 min and then at intervals of 30, 60, 120, 180, and 240 min, respectively. Diclofenac sodium (5 mg/kg) was used as a standard for comparison [30].
2.8. Statistical Analysis
The data was evaluated using graph prism 8.0.1 software and the final results were reported as mean with standard deviation (mean ± S.D.). Analysis of variance (ANOVA) with t-test was applied, in which p < 0.05 at 95% confidence interval was considered significant.
3. Results and Discussion
The extractive value was found to be 8.9% from 700 g powder drug macerated with methanol. Preliminary phytochemical analysis exhibited the presence of important phytochemicals such as tannins, phenolics, saponins, flavonoids, fatty acids, glycosides, triterpenoids, and steroids that exert significant effects in curing various illness [31]. The extract is a rich source of polyphenols and saponins followed by flavonoids, triterpenoids and tannins. The results are presented in Table 1.

Table 1.
Phytochemical investigation of W. maritima.
3.1. GC-MS Technique
The composition of methanol extract of W. maritima was determined using the GC-MS technique electron ionization positive mode (+EI Scan). GC–MS is an analytical technique for identification of broad range molecules. A major strength of GC–MS with its standard electron ionization (EI) ion sources is its ability to provide easy sample identification with names and structures at the isomer level through the use of 70 eV EI libraries. However, GC–MS suffers from a limitation, in that only a relatively small range of volatile, thermally stable compounds are amenable for analysis. Methanol extract of W. maritima revealed the presence of 35 compounds belonging to phenols, carboxylic acids, esters, alcohols, flavonoids and steroids. The chromatogram is shown in Figure 1 and details of compounds, including retention time and percentage area, are presented in Table 2.
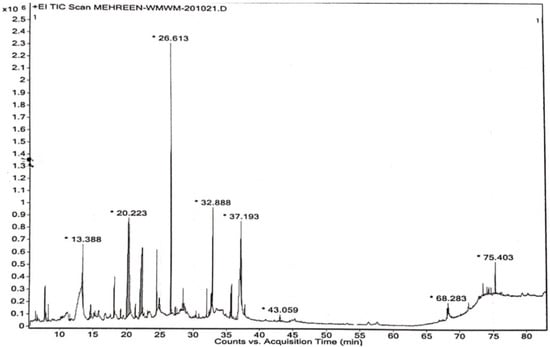
Figure 1.
GC-MS chromatogram of methanol extract of W. maritima. * Representing the retention time of most abundant compound.

Table 2.
List of compounds characterized using gas chromatography mass spectrometry.
GC-MS studies characterized 35 phytochemicals in the crude methanol extract. Out of these, nine compounds are responsible for remarkable antioxidant and anti-inflammatory activities. n-Hexadecanoic acid (26.96%) possesses antiandrogenic, antioxidant, hemolytic, 5-alpha- reductase inhibitory, hypocholesterolemic, nematicides, pesticide activities and is also utilized as a flavoring agent [34]. 9,12,15-Octadecatrienoic acid,(Z,Z,Z) exhibits antiarthritic, antiandrogenic, hepatoprotective, anti-inflammatory, antieczemic, 5-alpha reductase, hypocholesterolemic, antiacne, and antihistaminic effects [34,35]. The compound 3,5-diihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (14.35%) belongs to flavonoids, possessing anticancer, anti-inflammatory and antioxidant activities [36]. 3-ter-butyl-4-hydroxyanisol is phenolic and possesses anti-cancer, anti-inflammatory, and antioxidant potentials. 2-methoxy-4-vinylphenol, also known as vinyl guaiacol (7.81%), and 4-((1E)3-Hyrdoxy-1-propenyl)-2-methoxyphenol (5.33%) are also phenolic in nature and exhibit analgesic, anti-inflammatory, anti-microbial and antioxidant potentials [37,38]. β-sitosterol (5.86%) is an important phytochemical responsible for various activities including, anti-cancer, antioxidant, anti-inflammatory, anti-microbial, anti-nociceptive, anti-fertility, anti-diabetic and immunomodulatory effects. Vitamin E (1.71%) is used for its anti-inflammatory, anti-spasmodic, anti-leukemic, anti-microbial, analgesic anti-tumor, hepatoprotective and antioxidant characteristics. In addition to these, vitamin E is also used in various skin problems and issues related to the growth and development of hairs [39]. Hexadecanoic acid methyl ester possesses antioxidant, anti-microbial, nematicides, pesticide and insecticide hypocholesterolemic and anti-androgenic effects. Not only are 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester and 4-hydroxy-3-methoxy benzoic acid, also known as vanillic acid, used as flavoring agents, but these are also responsible for important pharmacological actions such as antioxidant, anti-bacterial, anti-fungal, anti-hypertensive, anti-depressant, anti-cancer, anti-nociceptive, hepatoprotective and wound-healing activities [40]. Butyrolactone possesses anti-cancer, antioxidants, anti-inflammatory, neuroprotective, hypoglycemic, and immunosuppressive effects. The fragmentation patterns of bioactive compounds responsible for antioxidant and anti-inflammatory effects are listed in Figure 2.
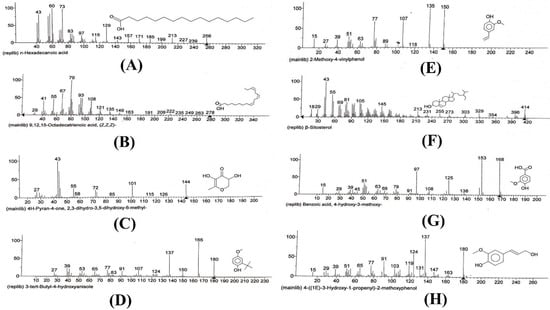
Figure 2.
Fragmentation patterns of compounds with anti-inflammatory and antioxidant effects. (A) Fragmentation pattern of n-hexadecanoic acid. (B) Fragmentation pattern of 9,12,15 octadecatrienoic acid (Z,Z,Z)-. (C) Fragmentation pattern of 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one. (D), Fragmentation pattern of 3-tert-butyl-4-hydroxyanisole. (E) Fragmentation pattern of 2-methoxy-4-vinylphenol. (F) Fragmentation pattern of β-sitosterol. (G) Fragmentation pattern of benzoic acid, 4-hydroxy-3-methoxy. (H) Fragmentation pattern of 4-((1E)-3-hydroxy-1-propenyl-2-methoxyphenol.
3.2. Molecular Docking
Molecular docking of compounds 3-benzoyl-2—tert-butyl-4-(1hydroxyethyl)-4-[2-(methylsulfanyl)ethyl]-1,3-oxazolidin-5-one (A), Pyrimidin-2,4-dione, 1,2,3,4-tetrahydro-5-methyl-1-[[2-hydroxymethyl-3-dimethylamino]tetrahydrofur-5-yl (B) Iberin nitrile (C), Sulfamic acid, 1,7-heptanediyl ester (D), (d)-(+)-(2R,3R)-2,3-Dibenzoyltartaric acid (E), [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester (F) and methyl 13-eicosenoate (G) was carried out, and Table 3, as well as Figure 3 and Figure 4, describes the outcomes.

Table 3.
Predicted binding energies via molecular docking.
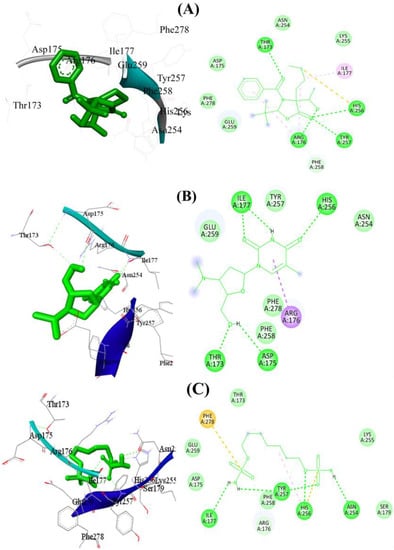
Figure 3.
The 2D and 3D binding mode of compounds (A–C) using target protein 1R4U. Green dashes denote hydrogen bonding, whereas red dashes denote hydrophobic interactions. Detailed Figure for all investigated ligands is shown in Figure S1.
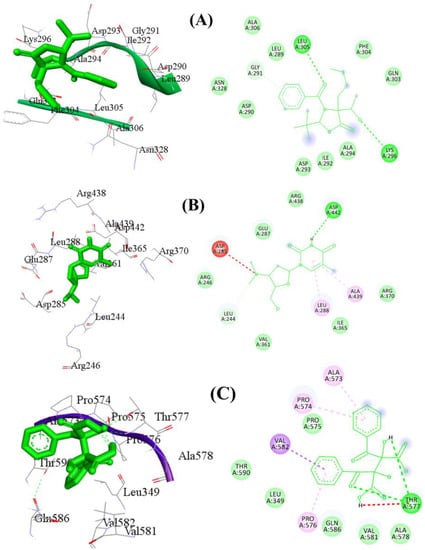
Figure 4.
The 2D and 3D binding mode of compound (A–C) using target protein 3O8Y. Green dashes denote hydrogen bonding, whereas red dashes denote hydrophobic interactions. Detailed Figure for all investigated ligands is shown in Figure S2.
Seven different compounds were screened to determine their ability to bind to the antioxidant protein 1R4U. Among them, compounds A and B showed promising results in terms of their hydrophobic, hydrophilic, and hydrogen bond interactions with the protein. Compound A had the strongest hydrophobic and hydrophilic interactions with the protein’s active site residues. Specifically, Asp175, Thr173, His256, Asn254, Glu259, Ile177, and Arg176 were found to be important amino acid residues involved in these interactions. Additionally, three hydrogen bonds contributed to the stability of the protein–ligand complex, with bond lengths of 2.97 Å between Arg176, 2.99 Å between His256, and 3.14 Å between Thr173. The docking score for compound A was determined to be −6.88 kcal/mol. Similarly, compound B showed stronger molecular interactions with the protein’s active site residues, including Arg176, His256, Asp175, Thr173, Ile177, Glu259, Phe258, Phe278, and Asn254. In particular, Phe258 and Glu259 were connected through hydrophobic interactions. Furthermore, five significant hydrogen bonds contributed to the conformational stability of the protein–ligand complex, with bond lengths of 2.2 Å involving His256, 2.29 Å involving Thr173 and Asp175, 2.79 Å involving Asp175, and 3.34 Å involving Ile177. Van der Waals interactions played a crucial role in stabilizing the complex as well. The docking score for compound B was −6.18 kcal/mol. Compound D was another hit in the virtual screening, displaying robust molecular interactions with amino acid residues in the active site of the antioxidant protein 1R4U. It was the second-best candidate medication identified from the screening process. Amino acid residues including His256, Asn254, Tyr257, Ile177, Arg176, Asp175, Glu259, Phe278, Thr173, Lys255, Phe258, and Ser179 were found to participate in bonding and nonbonding interactions with compound D. Four significant hydrogen bonds with short bond lengths were identified as contributing to the complex’s stability. The oxygen atom of compound D formed an electronegative hydrogen bond with the His256 residue of the target protein. Additionally, Asn254 was involved in a second hydrogen bond with a bond length of 3.16 Å, while the third and fourth hydrogen bonds were with Ile177 and Tyr257, with bond lengths of 2.99 Å and 3.21 Å, respectively.
Figure 3 illustrates the potential 2D and 3D binding mechanisms of compounds A, B, and D. The entire docking data, including docking scores and binding interactions for the antioxidant protein with all the ligands, can be found in Figure S1 and Table S1 of the supplementary material. Overall, the results suggest that compounds A, B and D have the potential to bind effectively to the antioxidant protein 1R4U through a combination of hydrophobic, hydrophilic, and hydrogen bond interactions. These findings provide useful insights for the development of new antioxidant drugs that can target this protein. However, further experimental studies are needed to validate the efficacy of these compounds as potential drug candidates.
This study utilized molecular virtual screening to identify potential drug candidates for anti-inflammatory therapy targeting protein 3O8Y. Seven compounds were initially screened, and the results indicated that compounds A, B, and E had the strongest binding interactions with the target protein. Compound A displayed the most promising hydrophobic and hydrophilic interactions, with important chemical interactions involving several amino acid residues, including Leu305, Lys296, and Asp293. Two hydrogen bonds were identified, contributing to the stability of the protein–ligand combination. Similarly, compound B showed strong molecular interactions with several amino acid residues, such as Asp442, Arg438, and Leu288. It also had two significant hydrogen bonds, contributing to the conformational stability of the complex. Compound E also exhibited strong interactions with amino acid residues in the active site, with two significant hydrogen bonds contributing to the complex’s stability. The docking scores of compounds A, B, and E were −6.21 kcal/mol, −6.41 kcal/mol, and −5.93 kcal/mol, respectively. The entire docking data, including docking scores and binding interactions for the antioxidant protein with all the ligands, are provided in Figure 4 and Figure S2 and Table S1 of the Supplementary Materials. The study’s findings indicate that compounds A, B, and E have potential as anti-inflammatory drugs, with strong molecular interactions with the target protein. These compounds can be further investigated for their efficacy and safety in preclinical and clinical studies.
3.3. Antioxidant Activity
Table 4 shows the antioxidant potential of methanol extract of W. maritima at different concentrations (200 µg/mL to 1000 µg/mL) using three different assays: DPPH free radical scavenging effect, hydrogen peroxide assay, and ferrous reducing power. As the concentration of the extract increased, the antioxidant activity also increased. Ascorbic acid was used as a standard for comparison. The percentage inhibitions for each assay are presented in the table. At a concentration of 1000 µg/mL, the methanol extract of W. maritima showed a significant antioxidant potential with a percentage inhibition of 83.55 ± 0.89% for DPPH assay, 79.40 ± 1.17% for H2O2 assay, and 81.26 ± 0.36% for reducing power assay. IC 50 values for DPPH, H2O2 and ferrous reducing assay were calculated as 0.36 ± 0.05, 0.37 ± 1.3 and 0.38 ± 0.56 mg/mL, respectively. The results suggest that W. maritima is a rich source of phenolic and flavonoid compounds, which are natural antioxidants found in plants and offer resistance against oxidative stress by inhibiting free radicals and lipid peroxidations [41]. The results of the study are presented in Figure 5. Overall, the table provides important information about the antioxidant potential of W. maritima and the potential health benefits associated with the consumption of this plant.

Table 4.
Antioxidant potential of methanol extract of W. maritima.
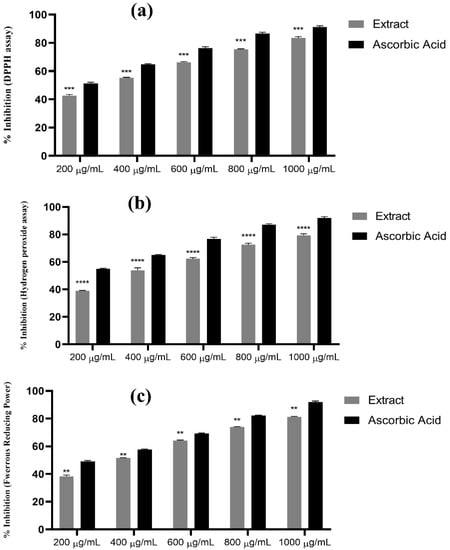
Figure 5.
Antioxidant potential of methanol extract of W. maritima. (a) Percentage inhibition using DPPH assay, results were significant with p =< 0.0001 at CI 95% n = 3. (b) Percentage inhibition using H2O2 assay, p =< 0.0001 at 95% CI. n = 3. (c) Percentage inhibition using ferrous reducing assay, p =< 0.002 at 95% CI n = 3. “**” indicating p =< 0.002, “***” indicating p =< 0.001 and “****” indicating p =< 0.0001.
3.4. Anti-Inflammatory Effect
Table 5 presents the results of the impact of W. maritima hydro-methanol extract on the reduction in paw edema produced by carrageenan. The paw volume of rats was measured at different time intervals (0, 30, 60, 120, 180, and 240 min) after the administration of carrageenan. The rats were then treated with either 200 mg/kg or 300 mg/kg of the extract or diclofenac (5 mg/kg) as a standard for comparison. The control group received no treatment. The results showed that the paw volume of rats in the control group increased significantly over time, while the rats treated with the extract or diclofenac exhibited a decrease in paw volume. The percentage inhibition of edema in paw volume was calculated and found to be 71.47% and 75.55% for the extracts at 200 mg/kg and 300 mg/kg, respectively. IC50 value was calculated as 0.19± 0.01 at a dose of 300 mg/kg. Diclofenac showed a percentage inhibition of 70.27% with IC50 value 3.5 ± 0.08 mg/kg. These results suggest that W. maritima extract has a significant anti-inflammatory effect and could potentially be used as a natural treatment for inflammation-related conditions. The results are shown in Figure 6 and Figure 7.

Table 5.
Impact of W. maritima hydro-methanol extract on the reduction in paw edema produced by carrageenan.
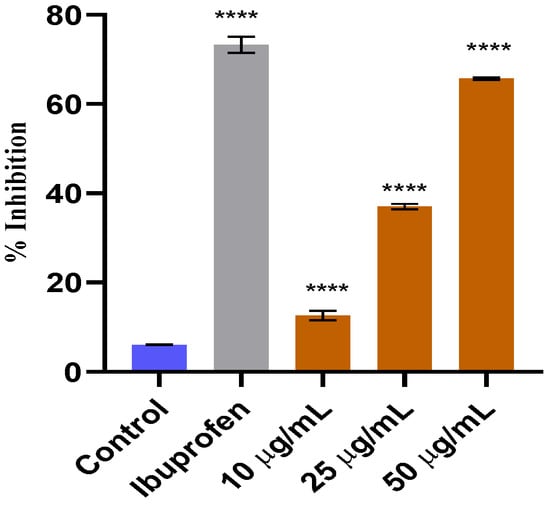
Figure 6.
In vitro anti-inflammatory effect of methanol extract of W. maritima. **** indicating that the data is significant.
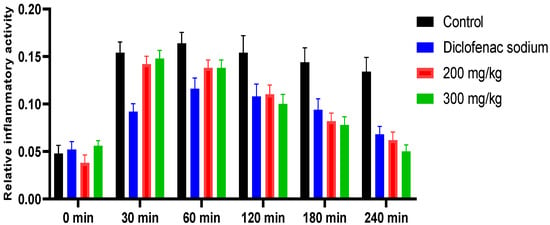
Figure 7.
Graphical representation of in vivo anti-inflammatory effect of hydro-methanol extract of W. maritima.
4. Conclusions
In conclusion, the methanol extract of W. maritima contains 35 phytochemicals, 8 of which have potent antioxidant properties that can prevent the entrapment of free radicals associated with various diseases, including inflammation. The in vitro and in vivo anti-inflammatory studies confirm that W. maritima can serve as a valuable source of effective anti-inflammatory agents. Furthermore, the in silico analyses indicate that W. maritima’s phytocompounds possess significant antioxidant and anti-inflammatory potential and can serve as a source of novel bioactive molecules for related target receptors. These findings can save researchers both time and money by providing fresh leads. Additional experimental research is suggested for future prospects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11051497/s1.
Author Contributions
The author contributions for this study are as follows: M.J. and M.U. conceptualized the study, performed the experiments, and wrote the manuscript. M.S.K. assisted with plant collection, biological activities, and manuscript corrections. M.H. refined and improved the manuscript. F.S. supervised the study, performed in silico work, and contributed to manuscript refinement and improvement. A.M.S., H.-A.N. and M.B.: reviewing and editing, investigations, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Researchers Supporting Project number (RSP-2023R437), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The mentioned authors of the work consented to the publication of the provided data, including all figures and supporting materials.
Informed Consent Statement
We certify that the information in this submission has not been previously published and is not currently under review by any other journal. In addition, all authors have agreed to submit their paper for possible publication in this journal.
Data Availability Statement
All data are already present in manuscript and supplementary materials.
Acknowledgments
The authors with to express their gratitude to Natural Product Chemistry Laboratory, Department of Pharmaceutical Chemistry, Bahauddin Zakariya University, South Punjab, Multan, Pakistan, for providing all research facilities and instrumentation regarding this work. The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia, for funding this work through project number (RSP-2023R437).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2016, 778, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Borquaye, L.S.; Laryea, M.K.; Gasu, E.N.; Boateng, M.A.; Baffour, P.K.; Kyeremateng, A.; Doh, G. Anti-inflammatory and antioxidant activities of extracts of Reissantia indica, Cissus cornifolia and Grosseria vignei. Cognet Biol. 2020, 6, 1785755. [Google Scholar] [CrossRef]
- Rivera, J.O.; Loya, A.M.; Ceballos, R. Use of herbal medicines and implications for conventional drug therapy medical sciences. Altern. Integr. Med. 2013, 2, 1–6. [Google Scholar]
- Oguntibeju, O.O. Medicinal plants with antiinflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focusgroup study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, S. Perspective on Plant Products as Antimicrobials Agents: A Review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Piechocka, J.; Wieczorek, M.; Głowacki, R. Gas Chromatography–Mass Spectrometry Based Approach for the Determination of Methionine-Related Sulfur-Containing Compounds in Human Saliva. Int. J. Mol. Sci. 2020, 21, 9252. [Google Scholar] [CrossRef]
- Ashish, C.; Manish, K.G.; Priyanka, C. GC-MS Technique and its Analytical Applications in Science and Technology. J. Anal. Bioanal. Tech. 2014, 5, 1–5. [Google Scholar]
- Hamayun, K.A.J.S.; Mehwish, J.; Rabia, S.; Zahid, K.; Aftab, A.; Sher, Z.S. Imran, nutritional composition and antioxidant activities od selected edible plants. J. Food Biochem. 2015, 40, 61–70. [Google Scholar]
- Patel, S.B.; Attar, U.A.; Sakate, D.M.; Ghane, S.G. Efficient extraction of cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: Optimization using response surface methodology, extraction methods and study of some important bioactivities. Sci. Rep. 2010, 10, 2109. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M.; Saud, I. Physicochemical and phytochemical standardization of berries of Myrtus communis Linn. J. Pharm. Bioallied Sci. 2012, 4, 322–326. [Google Scholar]
- Available online: https://www.rcsb.org/3O8Y (accessed on 1 November 2022).
- URATE OXIDASE FROM ASPERGILLUS FLAVUS COMPLEXED WITH ITS INHIBITOR OXONIC ACID. Available online: https//www.rcsb.org/1R4U (accessed on 1 November 2022).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Accelrys Software Inc. Discovery Studio Visualizer, 2; Accelrys Software Inc.: San Diego, CA, USA, 2005. [Google Scholar]
- CambridgeSoft. ChemDraw Ultra 12.0 0 (Copyright) 1986 to 2009; CambridgeSoft Corp.: Cambridge, MA, USA, 2009. [Google Scholar]
- CambridgeSoft. Chem 3D Pro 12.0 (Copyright) 1986 to 2009; CambridgeSoft Corp.: Cambridge, MA, USA, 2009. [Google Scholar]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Zentgraf, M.; Steuber, H.; Koch, C.; La Motta, C.; Sartini, S.; Sotriffer, C.A.; Klebe, G. How reliable are current docking approaches for structure-based drug design? Lessons from aldose reductase. Angew. Chem. Int. Ed. 2007, 46, 3575–3578. [Google Scholar] [CrossRef]
- Yusuf, D.; Davis, A.M.; Kleywegt, G.J.; Schmitt, S. An alternative method for the evaluation of docking performance: RSR vs. RMSD J. Chem. Inf. Model 2008, 48, 1411–1422. [Google Scholar] [CrossRef]
- Awah, F.M.; Uzoegwu, P.N.; Oyugi, J.O.; Rutherford, J.; Ifeonu, P.; Yao, X.J.; Fowke, K.R.; Eze, M.O. Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chem. 2010, 119, 1409–1416. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Meriga, B.; Mopuri, R.; MuraliKrishna, T. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac. J. Trop. Biomed. 2012, 5, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.A.F.; Uzair, M.; Chaudhary, B.A.; Jillani, U.; Zafar, Z. Phytochemical screening and biological potential of Phyla nodiflora (Verbenaceae) and Pterospermum acerifolium (sterculiaceae). J. Pharm. Biol. 2015, 5, 230–234. [Google Scholar]
- Hanif, M.; Ameer, N.; Mahmood, M.K.; Shehzad, A.; Azeem, M.; Rana, H.L.; Usman, M. Improved anti-inflammatory effect of curcumin by designing self emulsifying drug delivery system. Drug Dev. Ind. Pharm. 2022, 47, 1432–1438. [Google Scholar] [CrossRef]
- Koche, D.; Shirsat, R.; Kawale MA HE, S.H. An overerview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 1, 0976–2124. [Google Scholar]
- Harborne, A.J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysi; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; pp. 54–84. [Google Scholar]
- Khandelwal, K. Practical Pharmacognosy; Pragati Books Pvt. Ltd.: Nagpur, India, 1997; Volume 218. [Google Scholar]
- Jananie, R.K.; Priya, V.; Vijayalakshmi, K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. N. Y. Sci. J. 2011, 4, 16–20. [Google Scholar]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS studies of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Shalini, K.; Ilango, K.J.P.J. Preliminary phytochemical studies, GC-MS analysis and In-vitro antioxidant activity of selected medicinal plants and its polyherbal formulation. Pharmacogn. J. 2021, 13, 648–659. [Google Scholar] [CrossRef]
- Al-Marzoqi, A.H.; Hadi, M.Y.; Hameed, I.H. Determination of metabolities products by Cassia angustifolia and evaluate antimicrobial activity. J. Pharmacogn. Phytother. 2016, 19, 151–157. [Google Scholar]
- Vadivel, E.; Gopalakrishnan, S. GC-MS analysis of some bioactive constituents of Mussaenda frondosa Linn. Int. J. Pharm. Biol. Sci. 2014, 2, 312–320. [Google Scholar]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity and determination of bioactive components from leaves of Aegle marmelos. Biomed. Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef]
- Ingole, A.S.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U.; et al. A review of pharmacological activities of vanillic acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).