Abstract
Utilizing a deep eutectic solvent-like mixture such as choline chloride and lactic acid in a 1:2 molar ratio, clove buds underwent extraction. Furthermore, the determination of the primary components in the clove extracts, namely eugenol, eugenol acetate, and β-caryophyllene, was conducted using the HPLC-DAD method. The total phenolic content (TPC) was also established. Extraction conditions using choline chloride and lactic acid encompassed variables such as extraction temperature (within the range of 40 to 80 °C), water addition (ranging from 5.6 to 40%), and extraction time (ranging from 30 to 90 min). Optimal operational conditions for TPC were pinpointed at 77 °C, 30 min, and a water addition of 40%. The findings showed that clove extracts obtained at 60 °C, 22.8%, and 30 min had the highest amount of eugenol (307.26 ± 8.44 mg/g dry raw material).
1. Introduction
Syzygium aromaticum, commonly referred to as clove, is an esteemed medicinal plant currently undergoing investigation related to its antiviral and antioxidant properties. This botanical specimen is a valuable source of terpenes, mainly bioactive substances such as eugenol, eugenol acetate and β-caryophyllene [1]. Historically, clove has found applications in traditional medicine for addressing respiratory issues, serving as a warming and invigorating agent to alleviate digestion problems while enhancing circulation and metabolism [2,3,4,5,6].
The essential oil content in clove buds ranges from 15 to 20% by weight, with eugenol dominating (70–95%), accompanied by acetyleugenol (up to 20%) and β-caryophyllene (12–17%) in smaller proportions [7]. As proven by science, cloves and their essential oil are biologically beneficial for human health due to their exceptional qualities [4,5,6,8,9,10,11,12,13]. Previous research [13,14,15,16,17,18] has explored the potential of clove extractives in preventing and treating SARS-CoV-2-associated ailments, emphasizing their antiviral, antioxidant, antimicrobial, antifungal, antinociceptive, cytotoxic, anti-inflammatory, and antithrombotic activities [19,20,21,22,23,24,25,26,27,28]. Computational studies [15,16] propose that phytocompounds extracted from cloves, including kaempferol, target the main protease of SARS-CoV-2, which is essential for viral replication, and as such they could be potent drugs against COVID-19 [14]. Clove phytochemicals were tested as possible antiviral agents against the SARS-CoV-2 main protease in another study [26]. A vital technology is the extraction of substances from nature, which can replace materials derived from petrochemicals. Conventional extraction methods possess drawbacks, including the use of toxic solvents, potential thermal degradation, and hydrolysis of constituents. Modern, environmentally friendly extraction techniques provide avenues for process development to enhance efficiency [29,30,31]. A crucial step in obtaining desired product yields is identifying the suitable method and conditions for isolation, and the representation of individual components varies in quantity and quality depending on these conditions. The selection of extraction conditions of particular interest, focusing on replacing organic solvents with green alternatives [32,33,34].
Green extraction techniques with non-toxic solvents have been used by several researchers to study the chemical composition and biological activity of cloves. Supercritical CO2 extraction of clove buds resulted in remarkable yields (13–23.9 wt%) and eugenol content (56.97–87.41%) in studies [35,36,37,38,39,40,41,42]. Eugenol yields (11.5–21.2 wt%) and concentrations (50.3–87.26%) were lower when obtained via conventional extraction methods.
The conventional hydrodistillation method was used by Gonzales-Rivera et al. [43] to analyze clove essential oil. They determined the levels of eugenol (66.9%), β-caryophyllene (24.8%), α-humulene (3.1%), and acetyleugenol (2.7%) in the oil. Oliviera et al. [44] conducted extraction with supercritical CO2 under the experimental temperatures of 40 °C and 50 °C and extraction pressure of 10, 20, and 30 MPa. The results revealed the qualitative chemical composition of the oil, which consisted mainly of eugenol (62.88%), E-caryophyllene (17.13%), eugenol acetate (21.20%), and alfa-humulene (2.62%), with eugenol being the most abundant component in all the oil fractions examined. The results also indicated that eugenol, the major constituent, had a significant role in enhancing phytotoxic activity.
Deep eutectic solvents (DES) encompass a variety of categories based on the nature of the complexing agents involved. Type I comprises quaternary ammonium salts and metal halides, although their use in lignocellulosic biomass processing is limited due to the high melting temperatures of nonhydrated metal halides. Type II includes quaternary ammonium salts and hydrated metal halides, offering better viability for industrial processes. Type III involves a mixture of quaternary ammonium salts and various hydrogen bond donors (HBDs); this type is commonly utilized in biomass processing due to its cost-effectiveness and ease of preparation. Type IV consists of inorganic transition metals and HBDs, although the metal salt typically does not ionize in nonaqueous environments. A relatively novel class, Type V, exclusively comprises non-ionic molecular substances and exhibits hydrophobic characteristics under normal conditions [45,46,47,48,49]. Terminological inconsistencies within the field have led to the adoption of the term “DES-like mixtures” for systems lacking a proven eutectic point, with the aim of maintaining clarity amidst evolving terminology.
Various studies have delved into the structural changes, interactions, and hydrogen bonding within systems involving choline chloride and lactic acid [50,51,52,53,54]. Fourier-transform infrared analysis has confirmed hydrogen bond formation within these systems [50,52,53]. Research by Alcade et al. [51] explored the impact of water on hydrogen bonding and observed minimal disruption within the studied range. 1H NMR analysis has revealed interactions between the OH groups of lactic acid and choline chloride, leading to chemical shifts and the formation of new signals indicative of intermolecular interactions [52,53,54]. Alcalde et al. [51] identified intermolecular interactions facilitated by hydrogen bonding between the chloride ion from choline chloride and lactic acid, with the chloride ion acting as a bridge between choline chloride and multiple lactic acid molecules at a molar ratio greater than 1. In the last 20 years, green solvents have been intensively utilized for the extraction of value-added compounds. Among these, a group of solvents known as deep eutectic solvents (DES), including natural deep eutectic solvents, has gained prominence. Numerous original papers, patents, and conference contributions focus on various aspects of extracting valuable compounds from natural sources each year. Researchers also strive to consolidate these findings in books and review papers [55,56,57].
Deep eutectic solvent-like mixtures (DES-like mixtures) have gained prominence as promising sustainable solvents [58]. These mixtures, derived from natural components, offer unique physicochemical properties aligned with ecological requirements, making them appealing for green extraction processes. Another crucial step in extraction is the setting of extraction conditions. Many factors affect how well the extraction process works, such as the type and quantity of solvents used, the temperature of the solution, the concentration of ions, the extraction time, the properties of the analyte, and the agitation of the mixture. These conditions and their interactions have a significant impact on the quantity and quality of the output of individual compounds [59,60,61,62].
One key component used to prepare these solvents is quaternary ammonium salt (such as choline chloride), which acts as the hydrogen bond acceptor (HBA). Additionally, hydrogen bond donors play a crucial role, and substances like urea, imidazole derivatives, amides, alcohols, and organic carboxylic acids are commonly employed. These solvents achieve promising results in the extraction of various classes of active substances, especially phenols [63,64,65,66,67,68].
In recent years, several articles were published in which these solvents were used for the extraction of these substances, while the combination of choline chloride and lactic acid was also often investigated.
In the work of Soukina et al. [63], the combination of choline chloride and lactic acid achieved the highest yield of polyphenols compared to the other six types of solvents used to extract polyphenols from Mentha pulegium.
However, based on our knowledge, only a few works have been published that used the extraction of DESs and cloves. In the work of Chen et al. [69], DESs were used as pretreatment solvents; they were combined with microwave-assisted hydrodistillation for the extraction of essential oils from the clove buds. They used five DES systems (Choline chloride Fructose 1:2; Choline chloride Lactic acid 1:2; Choline chloride Glucose 1:2; Choline chloride Phenol 1:2; Choline chloride Ethylene glycol 1:2) and the most suitable was evaluated system chloride and lactic acid (1:2). The extraction efficiency of 10 DESs mainly composed of tetrabutylammonium bromide and long-chain alcohols, including choline chloride and lactic acid (2:3), was evaluated for the extraction of terpenes from six spices (cinnamon, cumin, fennel, clove, thyme, and nutmeg); DESs were used as extraction agents for headspace single-drop microextraction [70].
This is an original work because the extraction of selected active substances and the determination of optimal conditions using the DES (deep eutectic solvent) for clove extraction have not yet been explored in published works. Our primary goal was to use choline DES-like mixtures, that is, a selected system of choline chloride and lactic acid for the extraction of substances with antiviral, antioxidant and anti-COVID properties. And it mainly concerns the monitored substances that we analyzed. In this study, we did not deal with the purification of phenolic substances, considering that these extracts can be used as nutritional supplements without subsequent purification, which should have positive effects in the field of prevention in the fight against COVID-19. The study examined the effects of changing the extraction parameters (temperature, water addition, extraction time) on the TPC and identified the major constituents of clove bud extracts, namely eugenol, eugenol acetate, and β-caryophyllene, by applying the HPLC-DAD method.
2. Materials and Methods
2.1. Chemicals
All chemicals, solvents, and reagents utilized in this research were of analytical grade. Choline chloride (ChCl) (≥98.0%), Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl radical, gallic acid, anhydrous sodium carbonate (Na2CO3), and analytical standards, including eugenol (≥98.0%), eugenol acetate (≥98.0%), and β-caryophyllene (≥80.0%), were procured from Sigma-Aldrich (Bratislava, Slovakia). Lactic acid (LacA) (90.0%) solution was obtained from VWR International (Bratislava, Slovakia). Choline chloride was dried in a vacuum oven prior to use to eliminate moisture contamination.
2.2. Plant Materials
Clove buds were acquired from the store Svet plodu (Czech Republic) [71]. These organic cloves originated from Molucca (Indonesia), where they were grown and dried. Before each experimental assay, the raw material underwent grinding in a knife mill and separation through sieves into 0.02–0.04 mesh.
2.3. Preparation of Deep Eutectic Solvents-like Mixtures
The solvent consisted of ChCl and lactic acid in a molar ratio of 1:2. The preparation of the solvent was carried out with constant stirring in a water bath (60 °C; 30 min) and a homogeneous liquid formed as a result [72].
2.4. Design of Experiment
A comprehensive description of the influence of the studied physical parameters on total phenolic content utilized the planned experiment method. A full 23 factorial design was employed, encompassing temperature, water addition, and extraction time as selected physical parameters. After five repetitions of the center point and twenty combinations to measure the standard deviation from the overall mean, the experimental design was complete. The design of the experiment method (DOE) was utilized to represent the mathematical-statistical data of complex formation. The factors that had statistical significance were assessed by analysis of variance (ANOVA). The acquired measurements for each parameter underwent processing based on the principles of experimental design theory using the computer program STATIS, developed by Alexy et al. [73] within MS Excel Visual Basic. The STATIS program output complete regression and statistical analyses, with ANOVA being the employed statistical test, conducted at a 95% probability level. All statistical tests were carried out at a probability level of 95%. For each parameter, the following type of regression Equation (1) was obtained:
where y is an output parameter, x1, x2, and x3 are levels of factors in coded coordinates, b0–b33 are regression coefficients, b0 is a constant, b1, b2 and b3 are the linear coefficients, b12, b13, and b23 are the interaction or crossed coefficients, and b11, b22, and b33 are the quadratic coefficients.
2.5. Extraction Using Deep Eutectic Solvents-like Mixtures
In the work of Chen et al. [70], different DES pretreatments were applied. Choline chloride and lactic acid (1:2) combined with microwave-assisted hydrodistillation for the extraction of essential oils from the clove buds proved to be the most suitable pretreatment. In our research, therefore, the extraction system used choline chloride and lactic acid in a molar ratio of 1:2. The extraction conditions were chosen in a sufficiently wide range and corresponded to the conditions of other works, in which researchers investigated the extraction of polyphenols from other plant sources [73,74].
Under the conditions of the experimental design, the extraction was performed with constant stirring (blender speed 12; Tube Revolver Rotator Thermo Fisher Scientific (Bratislava, Slovakia)) in a closed flask. DOE was used to examine the effect of extraction temperature (t = 40–80 °C), water addition (above 5.6 up to 40%), and extraction time (30–90 min) on total phenolic content. The choline chloride: lactic acid (1:2) mixture had a water content of 5.6%. The water content in the DES-like mixtures was determined by coulometric Karl Fischer titration. The ratio of clove buds to extraction solvent was kept at 1:10 (w/v) throughout the experiments.
2.6. Determination of Total Phenolic Content
The content of total phenolic compounds was determined by the Folin–Ciocalteu (FC) method as described in the study of Jablonský et al. [75].
2.7. HPLC Determination of Eugenol, Eugenol Acetate, β-Caryophyllene
Eugenol, eugenol acetate, and β-caryophyllene were determined by the modified HPLC-DAD method. The analysis was performed using Agilent Technologies HPLC system (series 1100) consisting of a degasser, a binary solvent delivery pump, an autosampler, a column thermostat, and a diode array detector (DAD). Separation of analytes was carried out in reverse-phase mode using a Nucleodur 100-5 C18ec (250 × 4.6 mm i.d., 5 µm particles) column (Macherey-Nagel, Düren, Germany). The mobile phase consisted of acetonitrile (A) and water (B). The gradient program was a 0–10 min linear gradient for (A) component from 40 to 80% then to 100% of (A) over 0.5 min and maintained at 100% of (A) for 10 min. This was followed by a reverse gradient over 0.5 min and maintained at 40/60 (A)/(B) for 4 min. The flow rate was 1.0 mL/min, the injection volume was 20 µL, and the column temperature was maintained at 25 °C. DAD was operated in the wavelength range of 190–400 nm, and the detection wavelengths were set at 210 nm for β-caryophyllene. Eugenol and eugenol acetate were detected at 280 nm. The cloves extracts were diluted 1:10 (v/v) with water before being injected into the HPLC.
Quantification of target analytes was performed using the calibration curve method. The calibration curve was constructed as the dependence of average peak areas of analyte versus concentration of analyte in standard solution. Mixed-analytes calibration solutions at six concentration levels ranging from 100 to 1000 µg/mL for eugenol and eugenol acetate, or from 15.5 to 100 µg/mL for β-caryophyllene, were analyzed in triplicate for the construction of the calibration curve. In Table 1, the values of the retention time, resolution, peak symmetry factor, high equivalence of the theoretical plate, linearity, limit of detection, and limit of quantification are summarized.

Table 1.
Determination of eugenol, eugenol acetate, and β-caryophyllene by HPLC-DAD method: system suitability and validation parameters.
Equations (2) and (3), respectively, were followed to calculate the LODs and LOQs using the standard deviation of the response (Sy) and the slope (S) of the calibration curve at levels around the LOD and LOQ.
3. Results and Discussion
Various factors can exert independent or combined influences on extraction efficiency. Altering extraction parameters can impact the transport and solubilization of biological macromolecules, influence the extraction kinetics, and modify the physicochemical properties of DES-like mixtures [76,77]. This study delved into the individual and combined effects of extraction temperature, water addition, and extraction time on the overall yield of phenolic compounds obtained through the extraction process utilizing ChCl:LacA (1:2). Adjustments to water addition were made to enhance the diffusion of polyphenols. The chosen ranges for these factors were based on existing scientific knowledge derived from a literature search [78]. The experimental design conditions, detailed in Table 2, aimed to comprehensively elucidate the impact of selected physical parameters on the extraction process. A complete 23 factorial design of experiments was implemented, encompassing twenty individual experiments. The measured values of the TPCs are outlined in Table 3.

Table 2.
Conditions of experimental design.

Table 3.
Measured parameter TPC; (mg GAE/g dry raw material) and extraction factors—temperature (factor x1; °C), water addition (factor x2; %), time of extraction (factor x3; min.).
The outcomes revealed a significant impact of adding water when utilizing ChCl:LacA (1:2) on the total amount of extracted phenolic compounds from clove buds, as indicated by the regression coefficient b2 (Table 4). The solubility of compounds correlates with the polarity of DES-like mixtures and can be further enhanced by the addition of water. As shown in Figure 1, TPC exhibits variability based on water addition, ranging from approximately 36 mg GAE/g dry raw material to around 112 mg GAE/g dry raw material. As pointed out by several papers [61,62,79,80,81,82], water addition affects the physicochemical properties of solvents and how they interact with biological structures. The presence of a highly polar solvent like water could potentially impact extraction capabilities, such as viscosity. High viscosity is a hindrance for the analytical use of deep eutectic solvents-like mixtures, as it reduces the mass transfer between the sample and the extraction phase. The viscosity of the reaction medium can be lowered by the addition of water, which leads to a better mass transfer, higher extraction efficiency, and enhanced polyphenol diffusion [62,83].

Table 4.
Results of ANOVA.
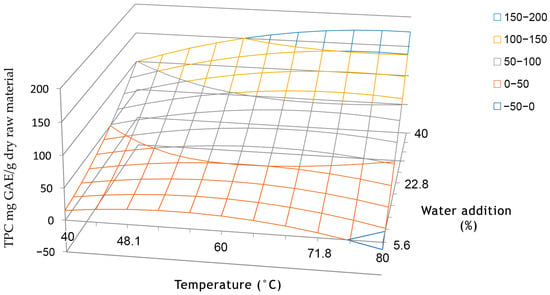
Figure 1.
Response surface of total phenolic content in dependency on factor x1 (temperature, °C) and factor x2 (water addition %) in coded coordinates at constant factor x3, (extraction time 30 min).
The outcomes presented in Table 4 indicate that, within the specified range of values, temperature did not significantly impact the yield of phenolics. Both an elevation in extraction temperature and addition of water led to an increase in TPC, as illustrated by the response surface in Figure 1. The x-axis (extraction temperature) demonstrates a peak in total phenolic content, supported by the negative value of coefficient b11 (refer to Table 4). This mutual interaction between water addition and temperature is further affirmed by the significant interaction coefficient b12 (Table 4). Higher temperatures are known to soften plant tissues, facilitating the extraction of phenolic compounds into the solvent [84]. Additionally, there is a confirmed mutual interaction between water addition and extraction time, as indicated by the interaction coefficient b23 (Table 4). In the realm of deep eutectic solvents (DES), water holds substantial significance [85]. As shown in Figure 2, the viscosity of DES-like mixture decreases when water is added [86,87], improving the mass transfer and extraction efficiency and facilitating polyphenol diffusion [62,83]. The polarity of DESs is also influenced by water content, as observed by Craveiro et al. [76], who found that an increase in water quantity heightened solvent polarity. Besides the viscosity, the density of DESs was also affected by water, as reported by Francisco et al. [87]. They discovered that the density of the DESs they investigated declined with the increase in water content, but this effect was minor.
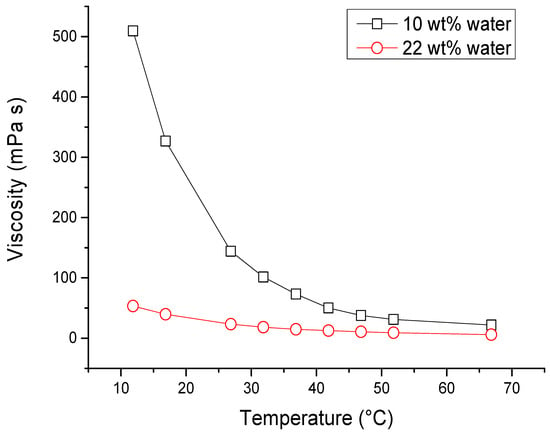
Figure 2.
Dependence of viscosity on temperature and water content in DESs composed of choline chloride and lactic acid (molar ratio 1:2).
The response surface of phenols was influenced by extraction time in the range of 30–90 min, as shown in Figure 3. The maximum value of the response surface was observed at 30 min, demonstrating the significance of extraction time, which was also verified by our regression analysis results (Table 4). The extraction time had a negative correlation with the phenolics yield, which decreased from 30 to 90 min. Phenolic oxidation can occur at longer extraction times unless the solvent system contains reducing agents that can partially prevent these reactions [88].
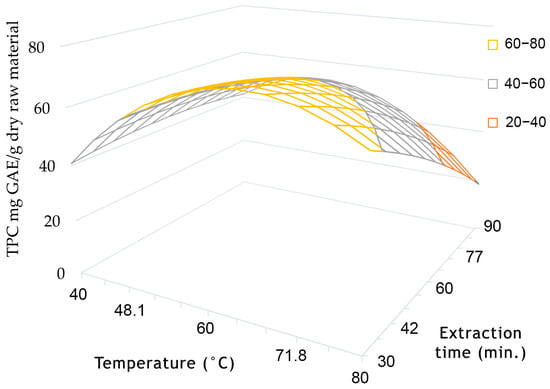
Figure 3.
At constant factor x2 (water addition 22.8%), the total phenolic content in coded coordinates depends on factor x1 (temperature, °C) and factor x3 (extraction time, min.).
The SOLVER subprogram from Microsoft Excel was used to optimize the computations and achieve the highest phenolic content. The optimal conditions for maximum phenolic content were an extraction temperature of 77 °C, time of 30 min, and water addition of 40%. The work also determined the phytogenic bioactive components in the clove extract, which is a mixture of various substances. The main compounds are eugenol, eugenol acetate, and β-caryophyllene. Eugenol and eugenol acetate have excellent antimicrobial activity against fungi and various Gram-negative and Gram-positive bacteria [89,90]. Eugenol has multiple benefits against many life-threatening diseases such as inflammation, oxidative stress, hyperglycemia, nerve disorders, elevated cholesterol, and cancer [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93]. Eugenol has several benefits against various life-threatening diseases such as inflammation, oxidative stress, hyperglycemia, nerve disorders, elevated cholesterol, and cancer [91,92,93]. Eugenol also has antiviral activity against the influenza A virus, as demonstrated by Dai et al. [94]. Eugenol can limit viral infection and replication specifically against herpes simplex viruses, with IC50 values between 16.2 mg/mL and 25.6 mg/mL, as evaluated by the plaque reduction assay. Eugenol has been confirmed to be effective against clinical isolates of herpes simplex virus-1 [95,96]. Musthafa et al. [97] examined eugenol acetate for its antiviral potential against both Gram-negative and Gram-positive pathogens. The results of their work show the potential of eugenol acetate as an alternative candidate to control the pathogenicity of both Gram-negative and Gram-positive organisms. Eugenyl acetate at a concentration of 150 µg/mL significantly inhibited the production of virulence factors, such as pyocyanin and pyoverdin, by Pseudomonas aeruginosa. As shown by several studies, β-caryophyllene, a natural sesquiterpene, affects various organs, such as the liver [98], kidney [99] and brain, in a pharmacological way. β-caryophyllene has different therapeutic benefits, such as functioning as an antioxidant, decreasing inflammation [100], and fighting cancer [101,102]. The HPLC-DAD method was applied to measure eugenol, eugenol acetate, and β-caryophyllene in 20 samples that were extracted using DES-like mixtures. The results of the determination of target analytes by HPLC-DAD method are summarized in Table 5.

Table 5.
HPLC determination of eugenol, eugenol acetate, and β-caryophyllene.
Eugenol was present in the extracts in the highest concentration among the target analytes, according to the HPLC results obtained. The major component of clove extract, eugenol, increased from 168.61 mg/g dry raw material to 307.26 mg/g dry raw material, which is several times lower compared to that of eugenol acetate (11.63–32.70 mg/g dry raw material) and β-caryophyllene (0.57–0.87 mg/g dry raw material). Eugenol was more abundant in the extract obtained at higher temperatures (70 °C) and with less added water (15%).
The chemical composition and content of clove essential oils vary widely according to different studies. One study by Alma et al. [103] analyzed the clove bud essential oil obtained via steam distillation and identified 18 components, with eugenol being the predominant one (87%), followed by eugenol acetate (8.01%) and β-caryophyllene (3.56%). Another study by Razafimamonjison et al. [104] compared the clove bud essential oil from Indonesia, Madagascar, and Zanzibar, which was also extracted via steam distillation for 12 h. They found that eugenol was a major component in all samples, with its content ranging from 77.50% to 79.87%, and β-caryophyllene was the second most abundant, ranging from 4.06% to 6.91%. Chatterjee et al. [105] used a different extraction method, supercritical carbon dioxide extraction, to isolate eugenol from dried clove buds. They optimized the extraction conditions to achieve the highest yield of eugenol (129.86 mg/g dry clove buds), which were 60 °C, 25 MPa, 90 min, and 2 L/min of the CO2 flow rate. Yazdani et al. [38] also used supercritical carbon dioxide extraction and obtained a similar yield of eugenol (86.7%) at 80 °C and 19 MPa. They also compared the eugenol yields from other extraction methods, such as hydrodistillation and microwave-assisted extraction, and found that supercritical extraction resulted in the highest percentage of eugenol (86.70%) compared to hydrodistillation (81.47%) and microwave-assisted extraction (79.08%). The variation in the components and composition of clove essential oils can be attributed to several factors, such as the type and origin of the clove, the environmental conditions, the pretreatment of the plant material, the extraction method, and the extraction parameters.
This study applied the AGREE [106] (analytical environmental performance metric for sample preparation) to assess the environmental performance of the extraction method based on the principles of green chemistry, which aims to reduce the use of hazardous chemicals and energy to improve sustainability and lower the environmental impacts of processes, including extraction methods [107]. The AGREE metric considers several factors that affect the environmental impact of the procedure, such as the sample size, the type of reagents and instruments, the health and safety risks of any reagent, and the waste generation, among others. Figure 4 shows the results for a specific sample type and extraction procedure performed in optimal conditions. The AGREE pictogram shows a mostly yellow-green color rating of the individual aspects. The overall score was 0.63, which indicates a high level of environmental compatibility and low impacts of the proposed extraction procedure. The extraction technique does not use any organic solvents, only DES. The main source of environmental impact is the HPLC method used to analyze the extracts. Reducing the amount of sample used could improve the greenness evaluation of the sample processing.
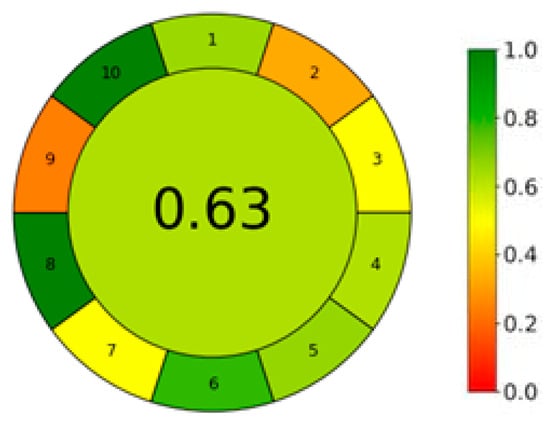
Figure 4.
Evaluation of the greenness of the proposed extraction method using the AGREE (B) assessment metric. Legend: 1. Sample preparation placement, 2. hazardous materials, 3. sustainability, renewability, and reusability of materials, 4. waste, 5. size economy of the sample, 6. sample throughput, 7. integration and automation, 8. energy consumption, 9. post-sample preparation configuration for analysis, 10. operator’s safety.
4. Conclusions
Upon analyzing the trial, our observations infer that extraction conditions significantly influence the total phenolic content, with particular emphasis on water addition and extraction time. Notably, water addition emerged as the most influential factor affecting the total phenolic content, closely followed by the extraction time. The interplay of extraction temperature with the addition of water further underscored the significance of temperature as a contributing factor to total phenolic content (TPC). The culmination of these factors pointed towards an optimal extraction scenario, where the highest TPC was achieved at an extraction temperature of 77 °C, extraction time of 30 min, and a water addition of 40%. The HPLC analysis further confirmed a notable abundance of eugenol in the extracts. Eugenol content exhibited a substantial increase from 168.61 mg/g dry raw material to 307.26 mg/g dry raw material. However, it is noteworthy that this concentration is significantly lower compared to that of eugenol acetate (ranging from 11.63 to 32.70 mg/g dry raw material) and β-caryophyllene (ranging from 0.57 to 0.87 mg/g dry raw material). In summation, this study demonstrates that choline chloride–lactic acid (molar ratio 1:2) is an effective solvent for extracting compounds from clove buds. This research provides useful information for improving the extraction parameters to increase the total phenolic content and the concentrations of specific substances in clove extracts.
Author Contributions
P.S. and V.M. contributed equally to the conceptualization and design of the work; writing—original draft preparation: P.S. and K.H.; data curation: P.S., V.M., K.H. and A.Š.; supervision and critical revision of the manuscript: K.H., I.Š., M.J. and A.H.; project administration: M.J.; funding acquisition: M.J., I.Š. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available from the authors.
Acknowledgments
This work was supported by the Slovak Research and Development Agency under the contracts Nos. APVV-22-0277, APVV-22-0388, and Operational Program Integrated Infrastructure for the project: “Strategic research in the field of SMART monitoring, treatment and preventive protection against coronavirus (SARS-CoV-2)”, Project no. 313011ASS8, co-financed by the European Regional Development Fund. and by the Scientific Grant Agency VEGA of the Slovak Republic (grant number 1/0412/20, 1/0651/23, 1/0332/24), and by the call for doctoral students and young researchers of Slovak University of Technology in Bratislava to start a research career (Grant 23-04-04-B).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; de Oliveira, A.P.; Tilia, C.; de Souza, J.P.; de Sousa, J.P.B.; Bastos, J.K.; et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 149–158. [Google Scholar] [CrossRef]
- Ahmed Khalil, A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Hussain, S.; Rahman, R.; Mushtaq, A.; El Zerey-Belaskri, A. Clove: A review of a precious species with multiple uses. IJCBS Int. J. Chem. Biochem. Sci. 2017, 11, 129–133. [Google Scholar]
- Rani, R.; Jena, M.K. Clove (Syzygium aromaticum): Benefeficial effects on human health: A review. Plant Arch. 2021, 21, 1967–1972. [Google Scholar] [CrossRef]
- Banerjee, S.; Panda, C.K.; Das, S. Clove (Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis 2006, 27, 1645–1654. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abu-Salah, K.M.; Alrokayan, S.A.; Siddiqui, M.J.; Ismail, Z.; Abdul Majid, A.M.S. Syzygium aromaticum extracts as good source of betulinic acid and potential anti-breast cancer. Rev. Bras. Farmacogn. 2012, 22, 335–343. [Google Scholar] [CrossRef]
- Nurdjannah, N.; Bermawie, N. Cloves. In Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 197–215. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Chen, X.; Chen, J.; Li, X.; Qiao, G.; Tian, Y.; Yuan, R.; Su, S.; Liu, X.; et al. Aqueous extract of clove inhibits tumor growth by inducing autophagy through AMPK/ULK pathway. Phytother. Res. 2019, 33, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Schmitz, J.C.; Wei, J.; Cao, S.; Beumer, J.H.; Strychor, S.; Cheng, L.; Liu, M.; Wang, C.; Wu, N.; et al. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol. Res. 2013, 21, 247–259. [Google Scholar] [CrossRef]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann. Microbiol. 2010, 60, 599–604. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Zmantar, T.; Ksouri, R.; Hajlaoui, H.; Mahdouani, K.; Abdelly, C.; Bakhrouf, A. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007, 50, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.d.T.; AlAjmi, M.F.; Hussain, A. Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19. Curr. Pharm. Des. 2020, 27, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Joshi, T.; Sharma, P.; Mathpal, S.; Pundir, H.; Bhatt, V.; Chandra, S. In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4529–4536. [Google Scholar] [CrossRef]
- Pandey, P.; Singhal, D.; Khan, F.; Arif, M. An in silico screening on piper nigrum, syzygium aromaticum and zingiber officinale roscoe derived compounds against SARS-CoV-2: A drug repurposing approach. Biointerface Res. Appl. Chem. 2021, 11, 11122–11134. [Google Scholar] [CrossRef]
- Chaachouay, N.; Douira, A.; Zidane, L. COVID-19, prevention and treatment with herbal medicine in the herbal markets of Salé Prefecture, North-Western Morocco. Eur. J. Integr. Med. 2021, 42, 101285. [Google Scholar] [CrossRef]
- Kanyinda, J.-N. Coronavirus (COVID-19): A Protocol for Prevention And Treatment (Covalyse®). Eur. J. Med. Health Sci. 2020, 2. [Google Scholar] [CrossRef]
- Marwal, A.; Meena, M.; Gaur, R.K. Molecular docking studies of coronavirus proteins with medicinal plant-based phytochemicals. Def. Life Sci. J. 2021, 6, 57–63. [Google Scholar] [CrossRef]
- Paidi, R.K.; Jana, M.; Raha, S.; McKay, M.; Sheinin, M.; Mishra, R.K.; Pahan, K. Eugenol, a Component of Holy Basil (Tulsi) and Common Spice Clove, Inhibits the Interaction Between SARS-CoV-2 Spike S1 and ACE2 to Induce Therapeutic Responses. J. Neuroimmune Pharmacol. 2021, 16, 743–755. [Google Scholar] [CrossRef]
- Aware, D.; Rohane, S. A Role of Herbal Drug as an Immunity Booster during COVID-19 Pandemic. Asian J. Pharm. Res. 2021, 11, 206–211. [Google Scholar] [CrossRef]
- Naik, C.; Pidigam, S. Herbs that heal: A scoping review on COVID-19 pandemic. Ann. Phytomed. Int. J. 2021, 10, 4–12. [Google Scholar] [CrossRef]
- Yunus, G. Herbal compounds from syzygium aromaticum and cassia acutifolia as a shield against SARS-CoV-2 mpro: A molecular docking approach. Biointerface Res. Appl. Chem. 2021, 11, 14853–14865. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; El Hachlafi, N.; Abdallah, E.M.; Jeddi, M.; Assaggaf, H.; Qasem, A.; Alnasser, S.M.; Attar, A.; Naem, M.A.; Lee, L.-H. Exploring the antibacterial mechanisms of chemically characterized essential oils from leaves and buds of Syzygium aromaticum (L.) Merr. et Perry against Staphylococcus aureus and Pseudomonas aeruginosa. Ind. Crops Prod. 2023, 205, 117561. [Google Scholar] [CrossRef]
- Darshan, S.; Doreswamy, R. Patented antiinflammatory plant drug development from traditional medicine. Phytother. Res. 2004, 18, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Chandra Manivannan, A.; Malaisamy, A.K.; Eswaran, M.; Meyyazhagan, A.; Arumugam, V.A.; Rengasamy, K.R.; Balasubramanian, B.; Liu, W.C. Evaluation of Clove Phytochemicals as Potential Antiviral Drug Candidates Targeting SARS-CoV-2 Main Protease: Computational Docking, Molecular Dynamics Simulation, and Pharmacokinetic Profiling. Front. Mol. Biosci. 2022, 604, 918101. [Google Scholar] [CrossRef]
- Strizincova, P.; Jablonsky, M.; Lelovský, M. Bioactive Compounds of Softwood Bark as Potential Agents against Human Diseases Include the SARS-CoV-2 Virus. Biointerface Res. Appl. Chem. 2022, 12, 5860–5869. [Google Scholar] [CrossRef]
- Jablonský, M.; Štekláč, M.; Majová, V.; Gall, M.; Matúška, J.; Pitoňák, M.; Bučinský, L. Molecular docking and machine learning affinity prediction of compounds identified upon softwood bark extraction to the main protease of the SARS-CoV-2 virus. Biophys. Chem. 2022, 288, 106854. [Google Scholar] [CrossRef] [PubMed]
- Easmin, M.S.; Sarker, M.Z.I.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.b.; Uddin, M.S.; Sarker, M.M.R.; Akanda, M.J.H.; Hossain, M.S.; Khalil, H.P.S.A. Bioactive compounds and advanced processing technology: Phaleria macrocarpa (sheff.) Boerl, a review. J. Chem. Technol. Biot. 2015, 90, 981–991. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.v. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Mohammed, G.R. Conventional Extraction Methods Use in Medicinal Plants, their Advantages and Disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ivanovic, J.; Zizovic, I.; Ristic, M.; Stamenic, M.; Skala, D. The analysis of simultaneous clove/oregano and clove/thyme supercritical extraction. J. Supercrit. Fluid. 2011, 55, 983–991. [Google Scholar] [CrossRef]
- Martínez, J.; Rosa, P.T.V.; Meireles, M.A.A. Extraction of Clove and Vetiver Oils with Supercritical Carbon Dioxide: Modeling and Simulation. Open Chem. Eng. J. 2007, 1, 1–7. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Yazdani, F.; Mafi, M.; Farhadi, F.; Tabar-Heidar, K.; Aghapoor, K.; Mohsenzadeh, F.; Darabi, H.R. Supercritical CO2 extraction of essential oil from clove bud: Effect of operation conditions on the selective isolation of eugenol and eugenyl acetate. Z. Naturforsch. B 2005, 60, 1197–1201. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Supercritical extraction of clove bud essential oil: Isolation and mathematical modeling. Chem. Eng. Sci. 1997, 52, 3421–3428. [Google Scholar] [CrossRef]
- Gopalakrishnan, N.; Shanti, P.P.v.; Narayanan, C.S. Composition of clove (Syzygium aromaticum) bud oil extracted using carbon dioxide. J. Sci. Food Agric. 1990, 50, 111–117. [Google Scholar] [CrossRef]
- Della Porta, G.; Taddeo, R.; D’Urso, E.; Reverchon, E. Isolation of clove bud and star anise essential oil by supercritical CO2 extraction. LWT 1998, 31, 454–460. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Petenate, A.J.; Meireles, M.A.A. Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2. J. Supercrit. Fluid. 2014, 93, 56–66. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; da Costa, W.A.; Pereira, D.S.; Botelho, J.R.S.; de Alencar Menezes, T.O.; de Aguiar Andrade, E.H.; da Silva, S.H.M.; da Silva Sousa Filho, A.P.; de Carvalho, R.N. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluid. 2016, 118, 185–193. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Huang, Z.; Guo, Y. Sustainable recovery, and recycling of natural deep eutectic solvent for biomass fractionation via industrial membrane-based technique. Ind. Crops Prod. 2023, 194, 116351. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Wasylka, J.P.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crops Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Yu, Q.; Song, Z.; Zhuang, X.; Liu, L.; Qiu, W.; Shi, J.; Wang, W.; Li, Y.; Wang, Z.; Yuan, Z. Catalytic conversion of herbal residue carbohydrates to furanic derivatives in a deep eutectic solvent accompanied by dissolution and recrystallisation of choline chloride. Cellulose 2019, 26, 8263–8277. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Yagoub, A.E.A.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Al-Risheq, D.I.; Nasser, M.S.; Qiblawey, H.; Hussein, I.A.; Benamor, A. Choline chloride based natural deep eutectic solvent for destabilization and separation of stable colloidal dispersions. Sep. Purif. Technol. 2021, 255, 117737. [Google Scholar] [CrossRef]
- Alcalde, R.; Gutiérrez, A.; Atilhan, M.; Aparicio, S. An experimental and theoretical investigation of the physicochemical properties on choline chloride—lactic acid based natural deep eutectic solvent (NADES). J. Mol. Liq. 2019, 290, 110916. [Google Scholar] [CrossRef]
- Ushchapovskiy, D.; Vorobyova, V.; Vasyliev, G.; Linyucheva, O. Electrodeposition of polyfunctional Ni coatings from deep eutectic solvent based on choline chloride and lactic acid. J. Electrochem. Sci. Eng. 2022, 2, 1025–1039. [Google Scholar] [CrossRef]
- Karimarji, S.; Khorsandi, A.; Azimi, G.; Mardani, Z. Investigation of optical properties of choline chloride-lactic acid deep eutectic solvent under continuous wave laser irradiation regime. Opt. Mater. 2024, 148, 114912. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Basaid, K.; Mir, Y. Deep eutectic solvents as sustainable extraction media for plants and food samples: A review. Sustain. Chem. Pharm. 2023, 31, 100937. [Google Scholar] [CrossRef]
- Zuo, J.; Geng, S.; Kong, Y.; Ma, P.; Fan, Z.; Zhang, Y.; Dong, A. Current progress in natural deep eutectic solvents for the extraction of active components from plants. Crit. Rev. Anal. Chem. 2023, 53, 177–198. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Jablonsky, M.; Šima, J. Is it correct to name DESs deep eutectic solvents? Bioresources 2022, 17, 3880. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH Behavior of Seventeen Deep Eutectic Solvents. Bioresources 2018, 13, 5042–5051. [Google Scholar] [CrossRef]
- Jablonský, M.; Kreps, F.; Ház, A.; Šima, J.; Jablonský, J. Green Solvents, Plant Metabolites, and COVID-19: Challenges and Perspectives. Bioresources 2021, 16, 4667–4670. [Google Scholar] [CrossRef]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Soukaina, K.; Safaa, Z.; Soukaina, H.; Hicham, C.; Bouchra, C. Choline chloride-based deep eutectic solvents (NADES): Potential use as green extraction media for polyphenols from Mentha pulegium, antioxidant activity, and antifungal activity. Microchem. J. 2024, 199, 110174. [Google Scholar] [CrossRef]
- Djaoudene, O.; Bachir-Bey, M.; Schisano, C.; Djebari, S.; Tenore, G.C.; Romano, A. A Sustainable Extraction Approach of Phytochemicals from Date (Phoenix dactylifera L.) Fruit Cultivars Using Ultrasound-Assisted Deep Eutectic Solvent: A Comprehensive Study on Bioactivity and Phenolic Variability. Antioxidants 2024, 13, 181. [Google Scholar] [CrossRef]
- Vo, T.P.; Phan, T.H.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K.; Chung, T.Q.; Nguyen, H.Q.; Chau, P.T.T.; Thinh, L.D.A.; et al. Green extraction of phenolics and terpenoids from passion fruit peels using natural deep eutectic solvents. J. Food Process Eng. 2024, 47, e14503. [Google Scholar] [CrossRef]
- Karpitskiy, D.A.; Bessonova, E.A.; Shishov, A.Y.; Kartsova, L.A. Selective extraction of plant bioactive compounds with deep eutectic solvents: Iris sibirica L. as example. Phytochem. Anal. 2024, 35, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Olfat, A.; Mostaghim, T.; Shahriari, S.; Salehifar, M. Extraction of bioactive compounds of Hypnea flagelliformis by ultrasound-assisted extraction coupled with natural deep eutectic solvent and enzyme inhibitory activity. Algal Res. 2024, 78, 103388. [Google Scholar] [CrossRef]
- Kutlu, N.; Kamiloğlu, A.; Abca, T.E.; Yilmaz, Ö. Ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from persimmon calyx. J. Food Sci. 2024, 89, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, F.; Pang, M.; Jin, X.; Lv, H.; Li, Z.; Lee, M. Microwave-assisted hydrodistillation extraction based on microwave-assisted preparation of deep eutectic solvents coupled with GC-MS for analysis of essential oils from clove buds. Sustain. Chem. Pharm. 2022, 27, 100695. [Google Scholar] [CrossRef]
- Triaux, Z.; Petitjean, H.; Marchioni, E.; Boltoeva, M.; Marcic, C. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal. Bioanal. Chem. 2020, 412, 933–948. [Google Scholar] [CrossRef]
- Svet Plodu Home Page. Available online: https://www.svetplodu.sk/ (accessed on 2 October 2022).
- Jančíková, V.; Jablonský, M. The acidity values of ternary DES-like mixtures based on choline chloride, lactic acid, and dihydric alcohols. J. Mol. Liq. 2023, 389, 122846. [Google Scholar] [CrossRef]
- Alexy, P.; Viselka, M. Základy Plánovania a Vyhodnocovania Experimentov a Programový Modul STATIS pre MS Excel 5.xx až 7.0; Slovenska Technicka Univerzita: Bratislava, Slovakia, 1998; Chapter 13; pp. 132–179. [Google Scholar]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Jablonský, M.; Šima, J.; Strižincová, P.; Hroboňová, K.; Majová, V.; Ház, A. Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid. Green Process. Synth. 2021, 10, 1619–1624. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Leng, K.Y.; Suyin, G. Natural Deep Eutectic Solvent (NADES) as a Greener Alternative for the Extraction of Hydrophilic (Polar) and Lipophilic (Non-Polar) Phytonutrients. Key Eng. Mater. 2019, 797, 20–28. [Google Scholar] [CrossRef]
- San-Myint, W.R.W.; Abu-Bakar, M. Determination of optimal conditions for extraction of alcohol-soluble eugenol containing material from cloves. Pertanika J. Sci. Technol. 1995, 3, 99–106. [Google Scholar]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent advances in hydrophobic deep eutectic solvents for extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Köddermann, T.; Wertz, C.; Heintz, A.; Ludwig, R. The association of water in ionic liquids: A reliable measure of polarity. Angew. Chem. Int. Ed. 2006, 45, 3697–3702. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Bacteria incorporation in deep-eutectic solvents through freeze-drying†. Angew Chem 2009, 122, 2204–2208. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Akshaya, R.; Somasundaram, J.; Anjali, A.K. Eugenol as potential medicine-review. Ann. Rom. Soc. Cell Biol. 2021, 25, 6250–6260. [Google Scholar]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum l. myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Militão, G.C.G.; de Morais, M.C.; de Sousa, D.P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef]
- Dai, J.P.; Zhao, X.F.; Zeng, J.; Wan, Q.Y.; Yang, J.C.; Li, W.Z.; Chen, X.X.; Wang, G.F.; Li, K.S. Drug Screening for Autophagy Inhibitors Based on the Dissociation of Beclin1-Bcl2 Complex Using BiFC Technique and Mechanism of Eugenol on Anti-Influenza A Virus Activity. PLoS ONE 2013, 8, e61026. [Google Scholar] [CrossRef]
- Charan Raja, M.R. Versatile and Synergistic Potential of Eugenol: A Review. Pharm. Anal. Acta 2015, 6, 5. [Google Scholar] [CrossRef]
- Benencia, F.; Courrges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Musthafa, K.S.; Voravuthikunchai, S.P. Anti-virulence potential of eugenyl acetate against pathogenic bacteria of medical importance. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 703–710. [Google Scholar] [CrossRef]
- Hachim, A.K.K.; Shawi, H. Biological Activity of Eugenol Acetate as Antibacterial and Antioxidant Agent, Isolation from Myrtus communis L. Essential Oil. Int. J. Bioeng. Biotechnol. 2016, 1, 6–11. [Google Scholar]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of parkinson’s disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2010, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. β-caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Alma, M.H.; Ertaş, M.; Nitz, S.; Kollmannsberger, H. Chemical composition and content of essential oil from the bud of cultivated Turkish clove (Syzygium aromaticum L.). Bioresources 2007, 2, 265–269. [Google Scholar] [CrossRef]
- Razafimamonjison, G.; Jahiel, M.; Duclos, T.; Ramanoelina, P.; Fawbush, F.; Technique, C.; Tamatave, H.d.; Baillarguet, C.d. Bud, leaf and stem essential oil composition of clove (Syzygium aromaticum L.) from Indonesia, Madagascar and Zanzibar. Agronomiques 2014, 3, 224–233. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, D.; Bhattacharjee, P. Supercritical Carbon Dioxide Extraction of Eugenol from Clove Buds. Food Bioproc. Technol. 2013, 6, 2587–2599. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE–Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Abd Elmonem, H.M.; Al-Harrasi, A.; El Deeb, S. Comparative Evaluation of Reversed Stationary Phase Geometries and Greener Systems on HPLC and UHPLC Using Five Recent Hepatitis-C Antivirals. J. AOAC Int. 2023, 106, 580–587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).