Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
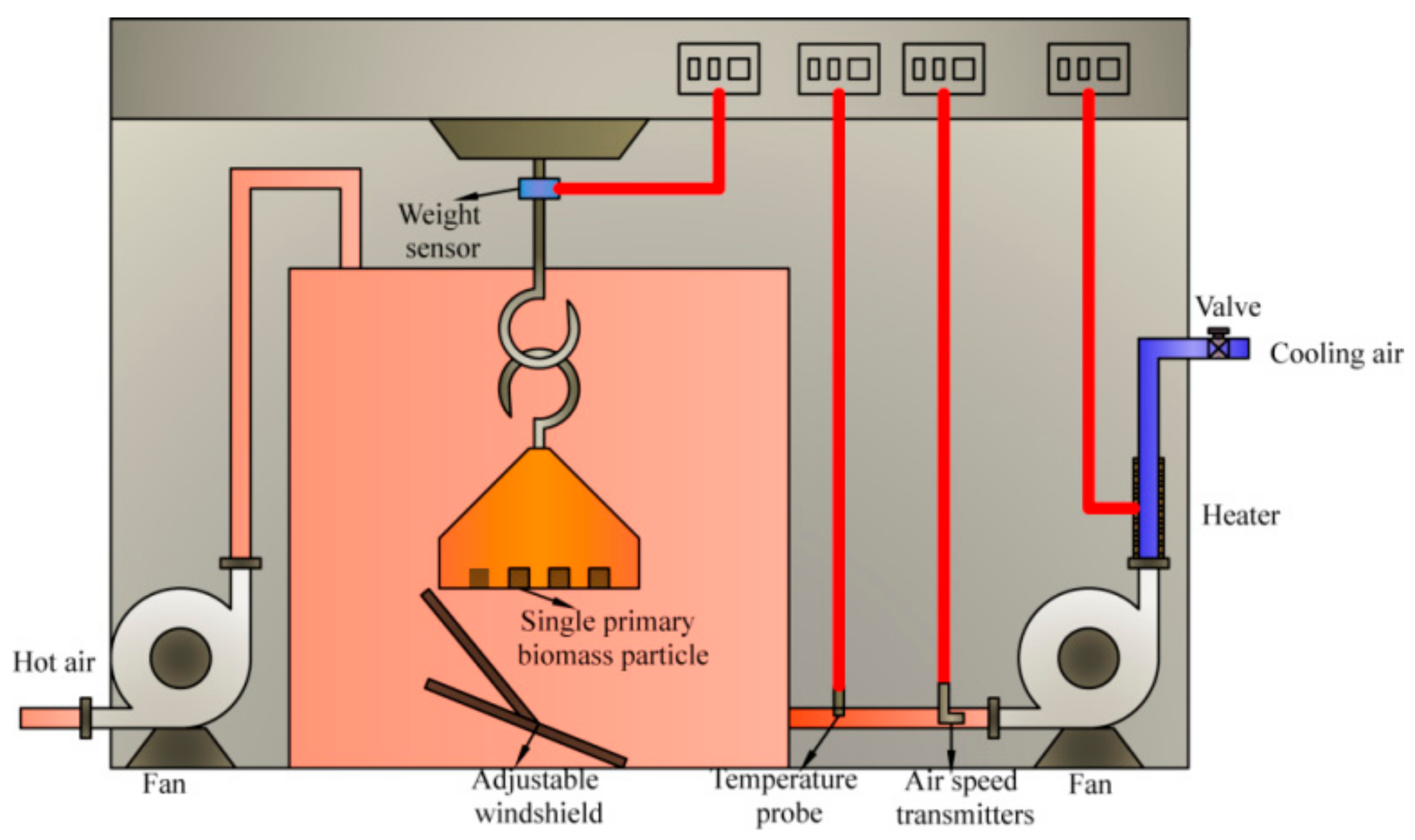
2.2. Experimental Apparatus and Methods
2.3. Mathematical Modeling of Drying Curves
3. Results and Discussion
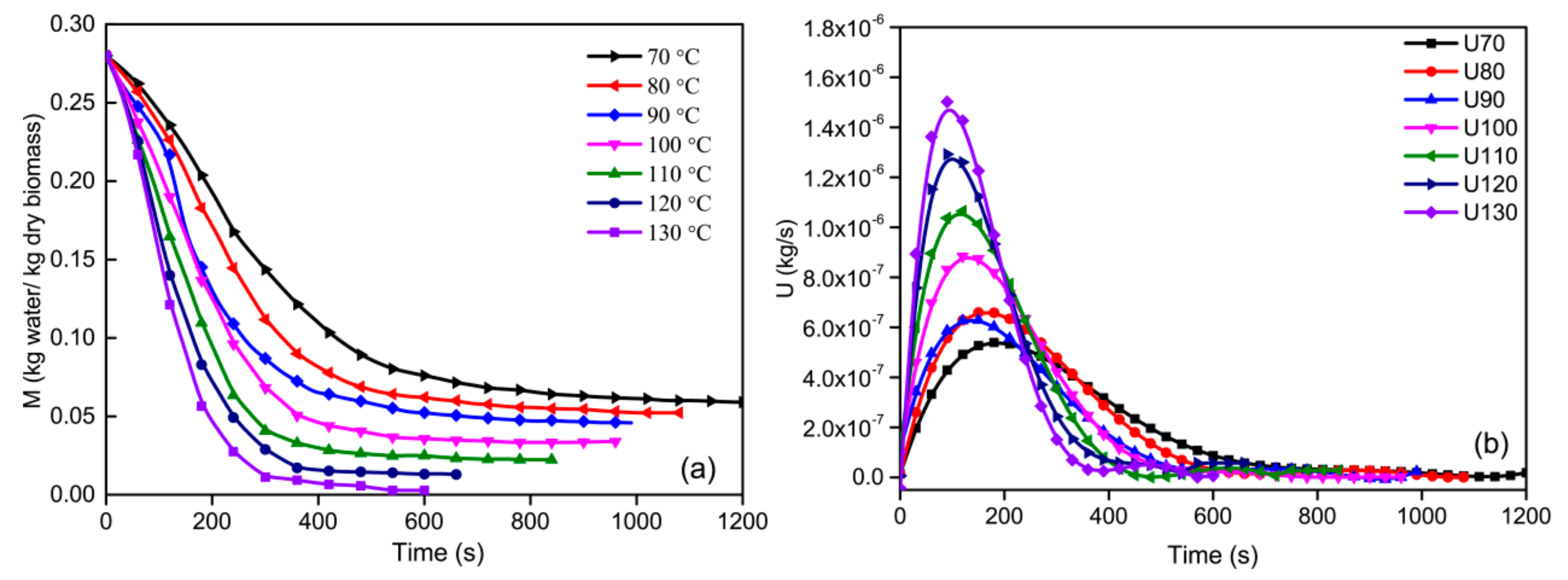
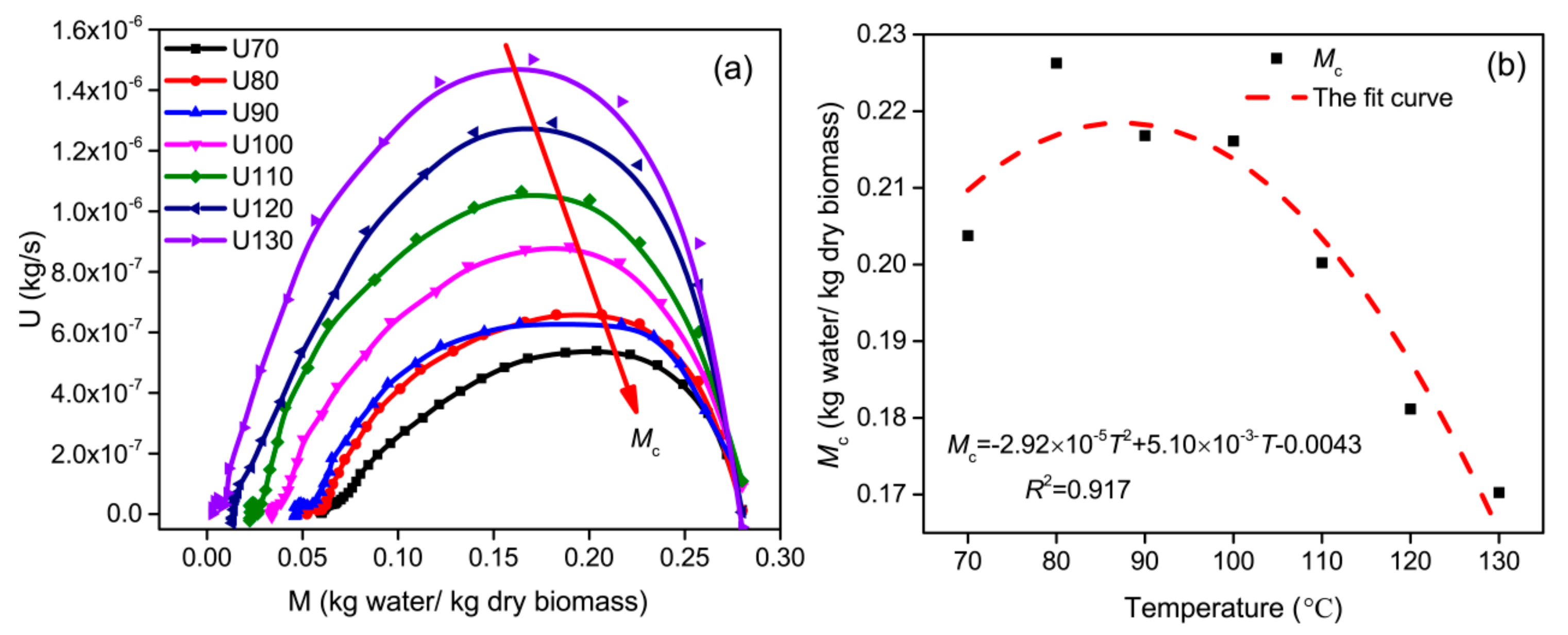
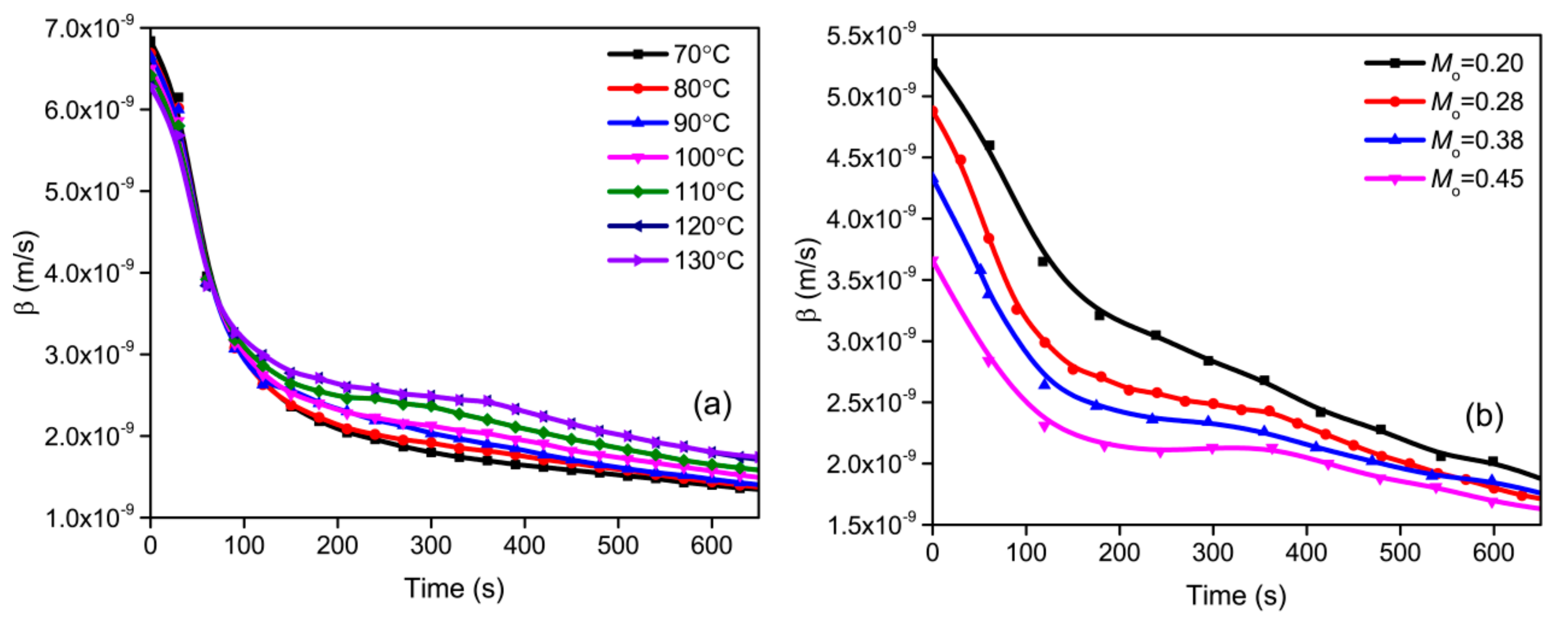
3.1. Effect of Drying Temperature
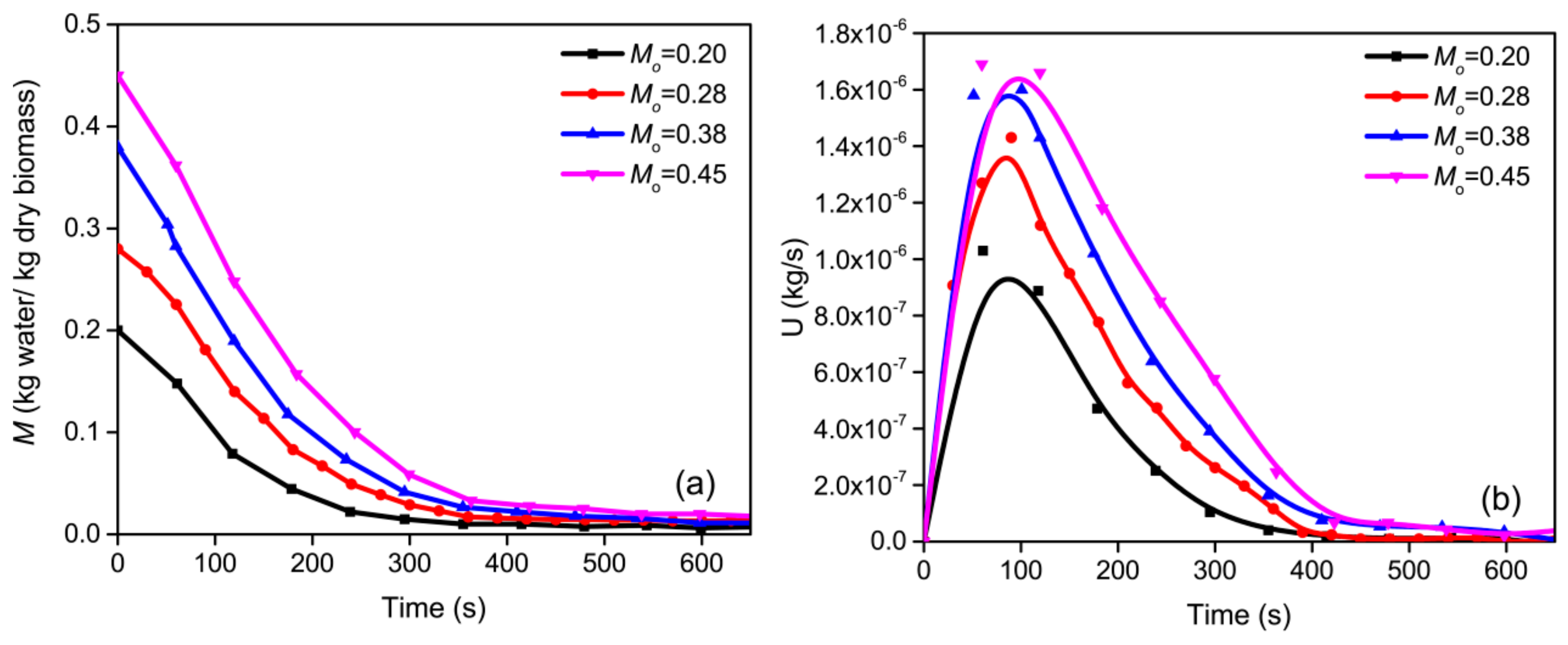
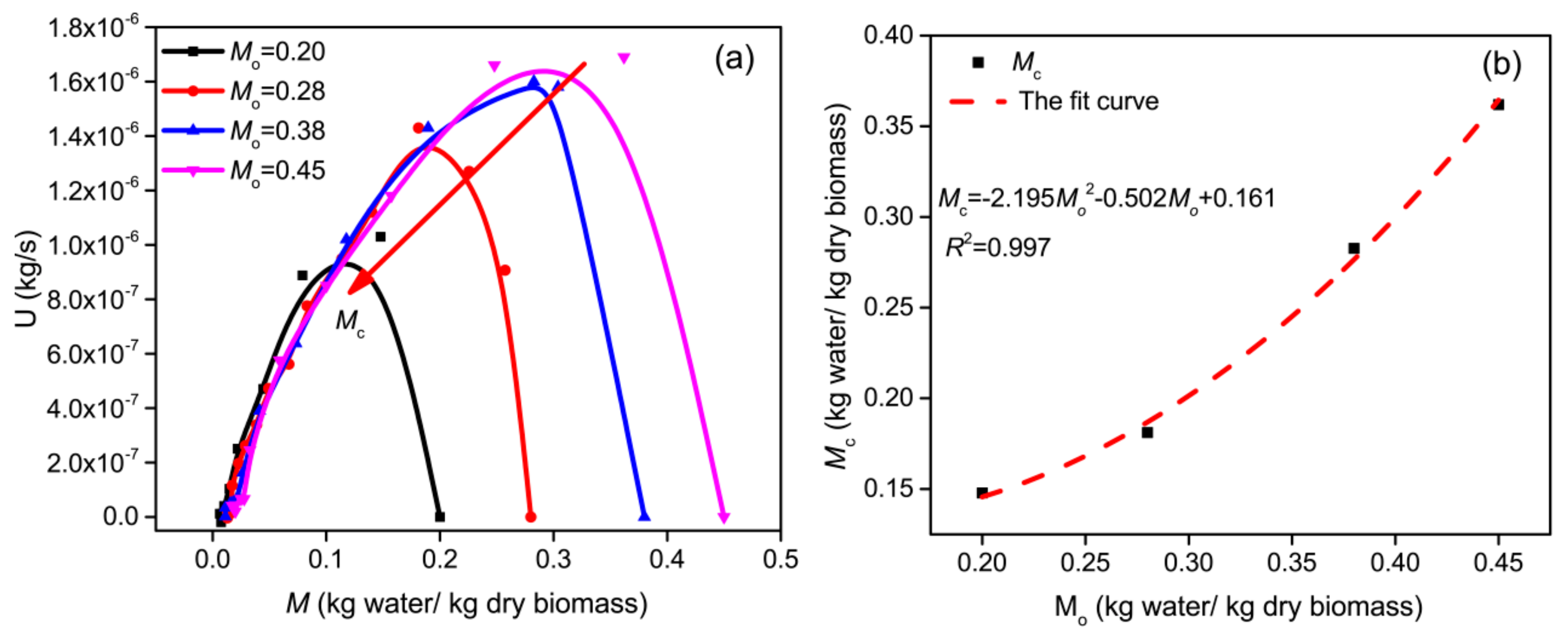
3.2. Effect of Initial Moisture Content
3.3. Characteristics of Drying Curves
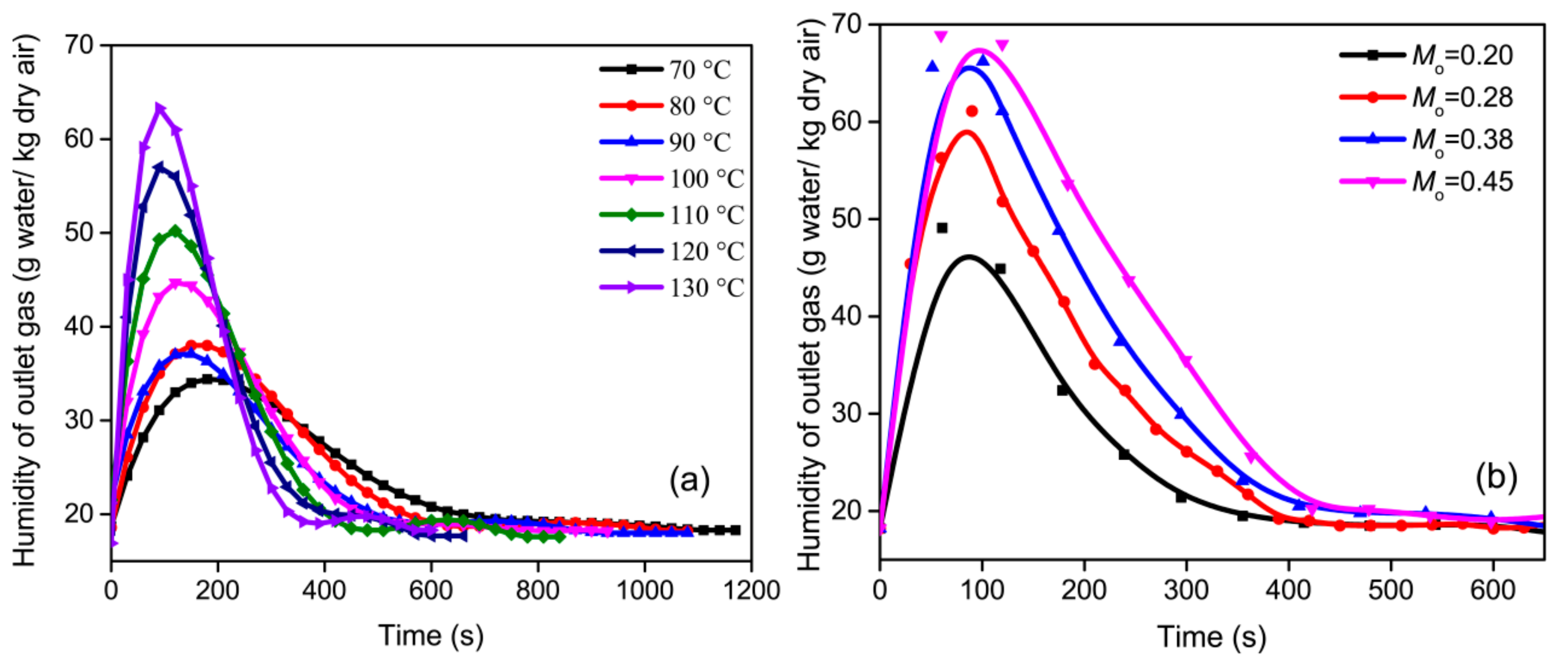
3.3.1. Humidity of Outlet Air
3.3.2. Critical Moisture Content
3.3.3. Effective Diffusivity
3.3.4. Coefficient of Mass Transfer
3.3.5. Normalized Drying Curves
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, J.; Zeng, R.; Zheng, W.; Wang, S.; Han, J.; Li, K.; Luo, M.; Tang, X. Synergistic effects of co-gasification of municipal solid waste and biomass in fixed-bed gasifier. Process Saf. Environ. Prot. 2021, 148, 1–12. [Google Scholar] [CrossRef]
- Yana, S.; Nizar, M.; Mulyati, D. Biomass waste as a renewable energy in developing bio-based economies in Indonesia: A review. Renew. Sustain. Energy Rev. 2022, 160, 112268. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, B.; Zhang, W.; Wang, X.; Mo, Q. Investigations into the drying kinetics of biomass in a fluidized bed dryer using electrostatic sensing and digital imaging techniques. Fuel 2022, 308, 122000. [Google Scholar] [CrossRef]
- Womac, A.R.; Igathinathane, C.; Sokhansanj, S.; Pordesimo, L.O. Biomass moisture relations of an agricultural field residue: Corn stover. Trans. Asae 2005, 48, 2073–2083. [Google Scholar] [CrossRef]
- Rezaei, H.; Lim, C.J.; Sokhansanj, S. A computational approach to determine the residence time distribution of biomass particles in rotary drum dryers. Chem. Eng. Sci. 2022, 247, 116932. [Google Scholar] [CrossRef]
- Holmberg, H.; Isaksson, J.; Lahdelma, R. Minimization of total drying costs for a continuous packed-bed biomass dryer operating at an integrated chemical pulp and paper mill. Biomass Bioenergy 2014, 71, 431–442. [Google Scholar] [CrossRef]
- Song, H.; Starfelt, F.; Daianova, L.; Yan, J.Y. Influence of drying process on the biomass-based polygeneration system of bioethanol, power and heat. Appl. Energy 2012, 90, 32–37. [Google Scholar] [CrossRef]
- Van den Broek, R.; Faaij, A.; van Wijk, A. Biomass combustion for power generation. Biomass Bioenergy 1996, 11, 271–281. [Google Scholar] [CrossRef]
- Pang, S.; Mujumdar, A.S. Drying of Woody Biomass for Bioenergy: Drying Technologies and Optimization for an Integrated Bioenergy Plant. Dry. Technol. 2010, 28, 690–701. [Google Scholar] [CrossRef]
- Granström, K.; Javeed, A. Javeed, Emissions from sawdust in packed moving bed dryers and subsequent pellet production. Dry. Technol. 2016, 34, 258–266. [Google Scholar] [CrossRef]
- Brammer, J.G.; Bridgwater, A.V. Bridgwater, the influence of feedstock drying on the performance and economics of a biomass gasifier-engine CHP system. Biomass Bioenergy 2002, 22, 271–281. [Google Scholar] [CrossRef]
- Suherman; Peglow, M.; Tsotsas, E. On the Applicability of Normalization for Drying Kinetics. Dry. Technol. 2007, 26, 90–96. [Google Scholar] [CrossRef]
- Suherman, T.K.; Praba, A.M. Derivation of Single Particle Drying Kinetics of Tapioca Flour. Adv. J. Food Sci. Technol. 2013, 5, 565–570. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. In-depth investigation on the pyrolysis kinetics of raw biomass. Part I: Kinetic analysis for the drying and devolatilization stages. Bioresour. Technol. 2013, 131, 40–46. [Google Scholar] [CrossRef]
- Burgschweiger, J.; Groenewold, H.; Hirschmann, C.; Tsotsas, E. From hygroscopic single particle to batch fluidized bed drying kinetics. Can. J. Chem. Eng. 1999, 77, 333–341. [Google Scholar] [CrossRef]
- Karim, M.A.; Hawlader, M.N.A. Drying characteristics of banana: Theoretical modelling and experimental validation. J. Food Eng. 2005, 70, 35–45. [Google Scholar] [CrossRef]
- Groenewold, C.; Moser, C.; Groenewold, H.; Tsotsas, E. Determination of single-particle drying kinetics in an acoustic levitator. Chem. Eng. J. 2002, 86, 217–222. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Defendi, R.O.; Nicolin, D.J.; Paraíso, P.R.; Jorge, L.M.D.M. Assessment of the initial moisture content on soybean drying kinetics and transport properties. Dry. Technol. 2015, 34, 360–371. [Google Scholar] [CrossRef]
- Johann, G.; da Silva, E.A.; Pereira, N.C. Modelling and optimisation of grape seed drying: Equivalence between the lumped and distributed parameter models. Biosyst. Eng. 2018, 176, 26–35. [Google Scholar] [CrossRef]
- Vargas-González, S.; Núñez-Gómez, K.S.; López-Sánchez, E.; Tejero-Andrade, J.M.; Ruiz-López, I.I.; García-Alvarado, M.A. Thermodynamic and mathematical analysis of modified Luikov’s equations for simultaneous heat and mass transfer. Int. Commun. Heat Mass Transf. 2021, 120, 105003. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Wang, Y.F.; El-Kolaly, W.; Gao, M.; Sun, W.; Li, M. Effects of drying variables on the characteristic of the hot air drying for gastrodia elata: Experiments and multi-variable model. Energy 2021, 222, 119982. [Google Scholar] [CrossRef]
- Perazzini, H.; Perazzini, M.T.B.; Freire, F.B.; Freire, F.B.; Freire, J.T. Modeling and cost analysis of drying of citrus residues as biomass in rotary dryer for bioenergy. Renew. Energy 2021, 175, 167–178. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Uribe, E.; Perez, M.; Tabilo-Munizaga, G.; Vergara, J.; Garcia-Segovia, P.; Lara, E.; Di Scala, K. Effect of high hydrostatic pressure pretreatment on drying kinetics, antioxidant activity, firmness and microstructure of Aloe vera (Aloe barbadensis Miller) gel. Lwt-Food Sci. Technol. 2011, 44, 384–391. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zheng, Y.; Zhu, X.F. Determination of effective moisture diffusivity and drying kinetics for poplar sawdust by thermogravimetric analysis under isothermal condition. Bioresour. Technol. 2012, 107, 451–455. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhang, Y.; Zhu, X.F. Drying Kinetics of Rice Straw under Isothermal and Nonisothermal Conditions: A Comparative Study by Thermogravimetric Analysis. Energy Fuels 2012, 26, 4189–4194. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Evrendilek, F.; Zhang, X.; Buyukada, M. TG-FTIR and Py-GC/MS analyses of pyrolysis behaviors and products of cattle manure in CO2 and N2 atmospheres: Kinetic, thermodynamic, and machine-learning models. Energ. Convers. Manage. 2019, 195, 346–359. [Google Scholar] [CrossRef]
- Cai, J.M.; Liu, R.H. New distributed activation energy model: Numerical solution and application to pyrolysis kinetics of some types of biomass. Bioresour. Technol. 2008, 99, 2795–2799. [Google Scholar] [CrossRef]
- Vanmeel, D.A. Adiabatic convection batch drying with recirculation of air. Chem. Eng. Sci. 1958, 9, 36–44. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Kuzu, A., Gokasan, M., Bogosyan, S., Eds.; Wseas Transactions on Systems and Control (Greece): Athena, Greece, 1975; Volume 8, pp. 625–626. ISSN 1991-8763. [Google Scholar]
- Kucuk, H.; Midilli, A.; Kilic, A.; Dincer, I. A review on thin-layer drying-curve equations. Dry. Technol. 2014, 32, 757–773. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Díaz, L.P.; Lopez, L.; Rodriguez, K.; Di Scala, K. Effective moisture diffusivity determination and mathematical modelling of the drying curves of the olive-waste cake. Bioresour. Technol. 2010, 101, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, İ. Air-drying characteristics of tomatoes. J. Food Eng. 2007, 78, 1291–1297. [Google Scholar] [CrossRef]
- Doymaz, İ. Thin-layer drying characteristics of sweet potato slices and mathematical modelling. Heat Mass Transf. 2010, 47, 277–285. [Google Scholar] [CrossRef]
- Zhang, K.; You, C. Experimental and Numerical Investigation of Convective Drying of Single Coarse Lignite Particles. Energy Fuels 2010, 24, 6428–6436. [Google Scholar] [CrossRef]


| Proximate Analysis (wt %,DB) | Ultimate Analysis (wt %,DB) | HHV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | V | FC | A | C | H | O | N | S | MJ/kg, DB |
| 8.39 | 84.31 | 6.80 | 0.50 | 46.40 | 6.25 | 47.33 | 0.08 | 0.04 | 18.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Zhu, L.; Wei, Q.; Huang, D.; Luo, M.; Tang, X. Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion. Processes 2023, 11, 984. https://doi.org/10.3390/pr11040984
Cai J, Zhu L, Wei Q, Huang D, Luo M, Tang X. Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion. Processes. 2023; 11(4):984. https://doi.org/10.3390/pr11040984
Chicago/Turabian StyleCai, Jianjun, Lingxia Zhu, Qiuxia Wei, Da Huang, Ming Luo, and Xingying Tang. 2023. "Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion" Processes 11, no. 4: 984. https://doi.org/10.3390/pr11040984
APA StyleCai, J., Zhu, L., Wei, Q., Huang, D., Luo, M., & Tang, X. (2023). Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion. Processes, 11(4), 984. https://doi.org/10.3390/pr11040984






