Predicting Final PCDD/F Emissions by Coupling Adsorption Model of Activated Carbon and Relationships of PCDD/Fs and Flue Gas Compositions
Abstract
1. Introduction
2. Materials and Methods
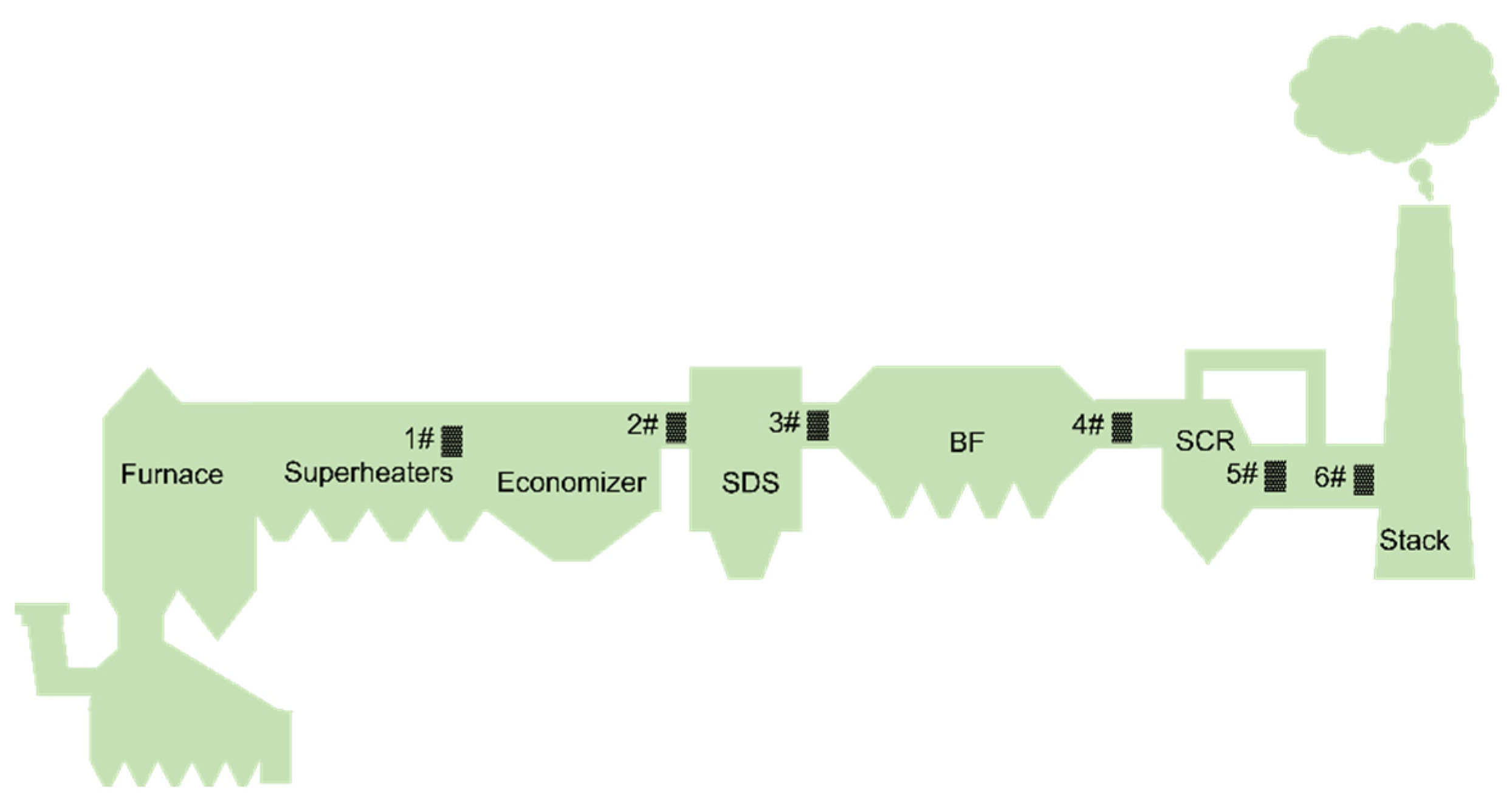
2.1. Sampling
2.2. Instrumental Analysis
3. Results and Discussion
3.1. Correlations of PCDD/Fs and Flue Gas Components
3.2. Modeling PCDD/F Emissions in APCDs
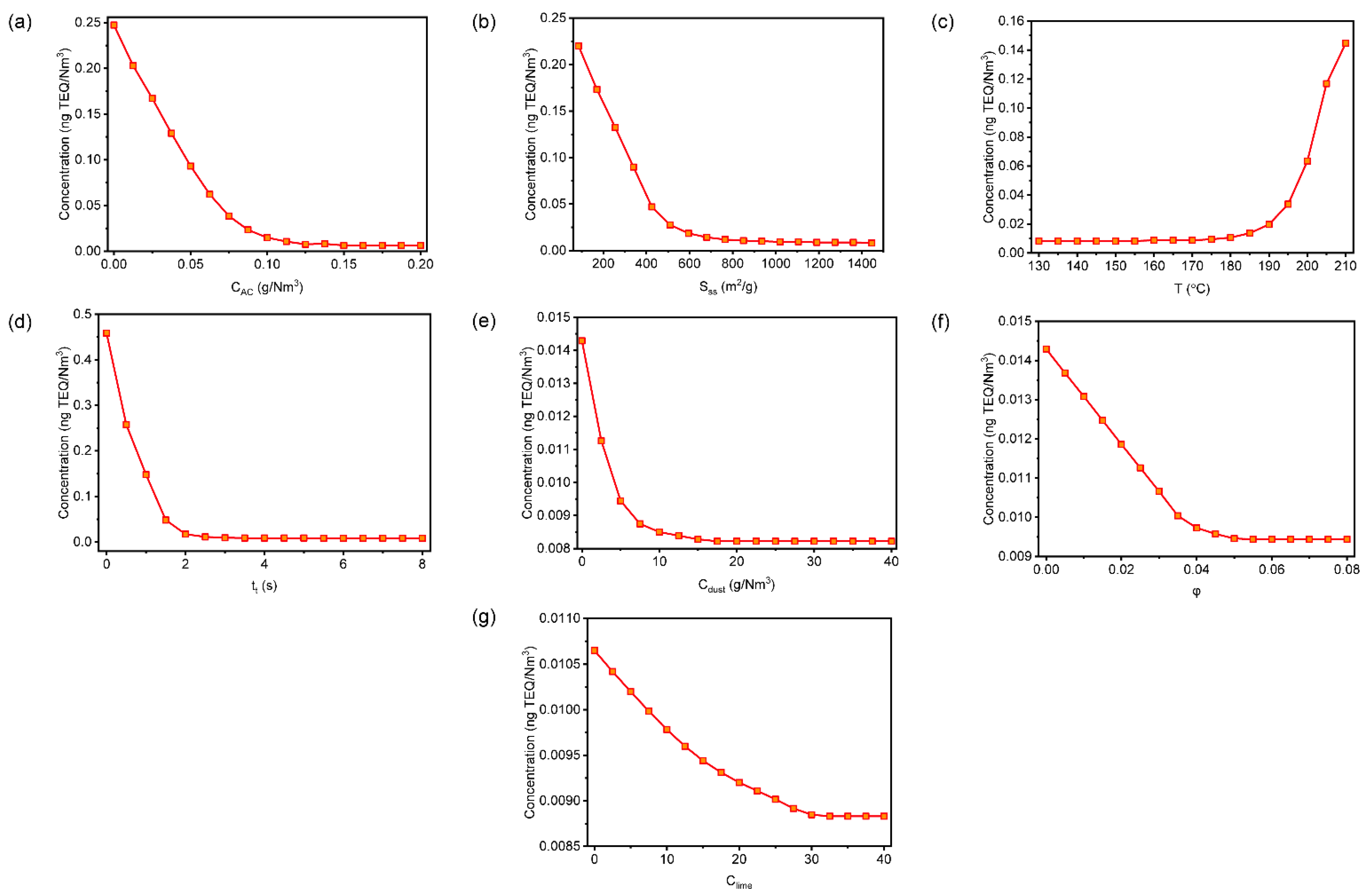
3.3. Effects of Operating Parameters on ∑PCDD/Fs
3.4. Environmental Applications or Suggestions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCDD/F | Polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran |
| MSWI | Municipal solid waste incinerator |
| MSW | Municipal solid waste |
| TEQ | International toxic equivalents |
| APCD | Air pollution control device |
| SDS | Semi-dry scrubber |
| BF | Baghouse filtration |
| SCR | Selective catalytic reduction |
| HRGC/HRMS | High-resolution gas chromatograph/high-resolution mass spectrometer |
| ACI | Activated carbon injection |
| AC | Activated carbon |
| SSA | Specific Surface Area |
| BET | Brunauer–Emmett–Teller |
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Ding, Y.; Zhao, J.; Liu, J.-W.; Zhou, J.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, Ç.; Pan, B.; Li, X.; et al. A review of China’s municipal solid waste (MSW) and comparison with international regions: Management and technologies in treatment and resource utilization. J. Clean. Prod. 2021, 293, 126144. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.-H. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Ssebugere, P.; Sillanpää, M.; Matovu, H.; Mubiru, E. Human and environmental exposure to PCDD/Fs and dioxin-like PCBs in Africa: A review. Chemosphere 2019, 223, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Esposito, V.; Bruno, D.; Maffei, A.; Giua, R.; Capoccia, C.; Nicosia, A.; Ficocelli, S. Long-term emission sampling validation for PCDD/Fs control of compliance to permitted Emission Limit Values at a large sinter plant. Chemosphere 2019, 233, 44–48. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Wang, P.; Su, X.; Fu, J. Polychlorinated biphenyls are still alarming persistent organic pollutants in marine-origin animal feed (fishmeal). Chemosphere 2019, 233, 355–362. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Wang, G.; Zhu, G.; Zhou, X.; Gong, H.; Sun, J.; Wang, L.; Liu, J. Evaluation of atmospheric sources of PCDD/Fs, PCBs and PBDEs around an MSWI plant using active and passive air samplers. Chemosphere 2021, 274, 129685. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- De Titto, E.; Savino, A. Environmental and health risks related to waste incineration. Waste Manag. Res. 2019, 37, 976–986. [Google Scholar] [CrossRef]
- Chen, Z.L.; Tang, M.H.; Lu, S.Y.; Buekens, A.; Ding, J.M.; Qiu, Q.L.; Jianhua, Y. Mechanochemical degradation of PCDD/Fs in fly ash within different milling systems. Chemosphere 2019, 223, 188–195. [Google Scholar]
- Lu, J.-W.; Zhang, S.; Hai, J.; Lei, M. Status and perspectives of municipal solid waste incineration in China: A comparison with developed regions. Waste Manag. 2017, 69, 170–186. [Google Scholar] [CrossRef]
- Pham, M.T.N.; Hoang, A.Q.; Nghiem, X.T.; Tu, B.M.; Dao, T.N.; Vu, D.N. Residue concentrations and profiles of PCDD/Fs in ash samples from multiple thermal industrial processes in Vietnam: Formation, emission levels, and risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 17719–17730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Walsh, P.P. Economic growth, urbanization and energy consumption—A provincial level analysis of China. Energy Econ. 2019, 80, 153–162. [Google Scholar] [CrossRef]
- Gao, D.; Chen, Z.; Zhang, J.; Xu, W.; Wen, D.; Hu, J. Historical production and release inventory of PCDD/Fs in China and projections upon policy options by 2025. Sci. Total Environ. 2023, 876, 162780. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Qiao, J.; Liu, M.; Kołodyńska, D.; Zhang, M.; Dionysiou, D.D.; Ju, Y.; Ma, J.; Chang, M.-B. Detoxification of municipal solid waste incinerator (MSWI) fly ash by single-mode microwave (MW) irradiation: Addition of urea on the degradation of Dioxin and mechanism. J. Hazard. Mater. 2019, 369, 279–289. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, X.; Lu, S.; Li, X.; Yan, J. Suppressing formation pathway of PCDD/Fs by S-N-containing compound in full-scale municipal solid waste incinerators. Chem. Eng. J. 2019, 359, 1391–1399. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yang, Z.; Zhang, Z.; Cai, Z. A field study of polychlorinated dibenzo-p-dioxins and dibenzofurans formation mechanism in a hazardous waste incinerator: Emission reduction strategies. J. Clean. Prod. 2019, 232, 1018–1027. [Google Scholar] [CrossRef]
- Vehlow, J. Air pollution control systems in WtE units: An overview. Waste Manag. 2015, 37, 58–74. [Google Scholar] [CrossRef]
- Zhong, Z.; Jin, B.; Huang, Y.; Zhou, H.; Zhang, M. Experimental study on flue gas purifying of MSW incineration using in-pipe jet adsorption techniques. Waste Manag. 2008, 28, 1923–1932. [Google Scholar] [CrossRef]
- Qiang, N.; He, J.; Miao, H.; Xie, L.; Liu, T.; Cao, Y.; Li, Z. A novel activated carbon injection method to enhance PCDD/Fs removel efficiency in flue gas from a medical waste incinerator in Shanghai, China. Appl. Ecol. Environ. Res. 2018, 16, 2717–2728. [Google Scholar] [CrossRef]
- Tejima, H.; Nakagawa, I.; Shinoda, T.-A.; Maeda, I. PCDDs/PCDFs reduction by good combustion technology and fabric filter with/without activated carbon injection. Chemosphere 1996, 32, 169–175. [Google Scholar] [CrossRef]
- Zhan, M.; Wang, T.; Yang, J.; Ji, L.; Zhou, G.; Chen, T.; Li, X.; Lin, X. The Behaviors and Relationships of PCDD/Fs and Chlorobenzenes in the Whole Process of one Municipal Solid Waste Incinerator. Aerosol Air Qual. Res. 2018, 18, 3134–3146. [Google Scholar] [CrossRef]
- Liu, X.; Ye, M.; Wang, X.; Liu, W.; Zhu, T. Gas-phase and particle-phase PCDD/F congener distributions in the flue gas from an iron ore sintering plant. J. Environ. Sci. 2017, 54, 239–245. [Google Scholar] [CrossRef]
- Chi, K.H.; Chang, M.B. Evaluation of PCDD/F Congener Partition in Vapor/Solid Phases of Waste Incinerator Flue Gases. Environ. Sci. Technol. 2005, 39, 8023–8031. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Hung, C.-Y.; Chen, J.-H.; Chang, C.-T.; Chen, C.-H. Minimum feeding rate of activated carbon to control dioxin emissions from a large-scale municipal solid waste incinerator. J. Hazard. Mater. 2009, 161, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Karademir, A.; Bakoglu, M.; Taspinar, F.; Ayberk, S. Removal of PCDD/Fs from Flue Gas by a Fixed-Bed Activated Carbon Filter in a Hazardous Waste Incinerator. Environ. Sci. Technol. 2004, 38, 1201–1207. [Google Scholar] [CrossRef]
- Chen, M.-W.; Lin, T.-C.; Wang, L.-C.; Chang-Chien, G.-P. The PCDD/F Removal Efficiency of a Medical Waste Incinerator Dual-Bag Filter System. Aerosol Air Qual. Res. 2014, 14, 1223–1231. [Google Scholar] [CrossRef]
- Sam-Cwan, K.; Hwan, J.S.; Il-Rok, J.; Ki-Hun, K.; Myung-Hee, K.; Jae-Hyung, K.; Jun-Heung, Y.; Seung-Jin, K.; Jae-Cheon, Y.; Dong-Hee, J. Removal efficiencies of PCDDs/PCDFs by air pollution control devices in municipal solid waste incinerators. Chemosphere 2001, 43, 773–776. [Google Scholar] [CrossRef]
- Cheruiyot, N.K.; Lee, W.-J.; Yan, P.; Mwangi, J.K.; Wang, L.-C.; Gao, X.; Lin, N.-H.; Chang-Chien, G.-P. An Overview of PCDD/F Inventories and Emission Factors from Stationary and Mobile Sources: What We Know and What is Missing. Aerosol Air Qual. Res. 2016, 16, 2965–2988. [Google Scholar] [CrossRef]
- Abad, E.; Caixach, J.; Rivera, J. Improvements in dioxin abatement strategies at a municipal waste management plant in Barcelona. Chemosphere 2003, 50, 1175–1182. [Google Scholar] [CrossRef]
- Gunes, G.; Saral, A.; Yıldız, Ş.; Kuzu, S.L. Determination of optimum dose of adsorbent for PCDD/F removal in the flue gas of a medical waste incineration plant. Chem. Eng. Res. Des. 2015, 104, 695–702. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J.; Degrève, J. Entrained-Phase Adsorption of PCDD/F from Incinerator Flue Gases. Environ. Sci. Technol. 2003, 37, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ji, Y.; Buekens, A.; Ma, Z.; Jin, Y.; Li, X.; Yan, J. Activated carbon treatment of municipal solid waste incineration flue gas. Waste Manag. Res. J. Sustain. Circ. Econ. 2012, 31, 169–177. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Ni, Y.-W.; Chen, J.-P.; Zhang, Q. Influence of variation in the operating conditions on PCDD/F distribution in a full-scale MSW incinerator. Chemosphere 2008, 70, 721–730. [Google Scholar] [CrossRef]
- Ke, S.; Jianhua, Y.; Xiaodong, L.; Shengyong, L.; Yinglei, W.; Muxing, F. Inhibition of de novo synthesis of PCDD/Fs by SO2 in a model system. Chemosphere 2010, 78, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Mätzing, H.; Baumann, W.; Becker, B.; Jay, K.; Paur, H.-R.; Seifert, H. Adsorption of PCDD/F on MWI fly ash. Chemosphere 2001, 42, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Choi, S.; Oh, J.-E.; Chang, Y.-S. Evaluation of Polychlorinated Dibenzo-p-dioxin/Dibenzofuran (PCDD/F) Emission in Municipal Solid Waste Incinerators. Environ. Sci. Technol. 1999, 33, 2657–2666. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Liu, W.; Fang, M.; Han, Y. Monochlorinated to Octachlorinated Polychlorinated Dibenzo-p-dioxin and Dibenzofuran Emissions in Sintering Fly Ash from Multiple-Field Electrostatic Precipitators. Environ. Sci. Technol. 2018, 52, 1871–1879. [Google Scholar] [CrossRef]
- Weber, R.; Takasuga, T.; Nagai, K.; Shiraishi, H.; Sakurai, T.; Matuda, T.; Hiraoka, M. Dechlorination and destruction of PCDD, PCDF and PCB on selected fly ash from municipal waste incineration. Chemosphere 2002, 46, 1255–1262. [Google Scholar] [CrossRef]
- Burris, S.C.; Li, D.; Riley, J.T. Comparison of Heating Losses and Macro Thermogravimetric Analysis Procedures for Estimating Unburned Carbon in Combustion Residues. Energy Fuels 2005, 19, 1493–1502. [Google Scholar] [CrossRef]
- Oudejans, L.; Touati, A.; Gullett, B.K. Real-Time, On-Line Characterization of Diesel Generator Air Toxic Emissions by Resonance-Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometry. Anal. Chem. 2004, 76, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, J.; Hou, K.; Wang, W.; Qi, Y.; Wang, Y.; Xie, Y.; Hua, L.; Li, H. Online monitoring of trace chlorinated benzenes in flue gas of municipal solid waste incinerator by windowless VUV lamp single photon ionization TOFMS coupled with automatic enrichment system. Talanta 2016, 161, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Gullett, B.K.; Oudejans, L.; Tabor, D.; Touati, A.; Ryan, S. Near-Real-Time Combustion Monitoring for PCDD/PCDF Indicators by GC-REMPI-TOFMS. Environ. Sci. Technol. 2012, 46, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Nakui, H.; Koyama, H.; Takakura, A.; Watanabe, N. Online measurements of low-volatile organic chlorine for dioxin monitoring at municipal waste incinerators. Chemosphere 2011, 85, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Kawamoto, K.; Asada, S.; Fujiyoshi, H.; Miyata, H.; Watanabe, G.; Suzuki, S. Surrogate study for dioxins from municipal waste incinerator in startup condition: Applicability as a dioxin control indicator and an organohalogen emission warning. J. Mater. Cycles Waste Manag. 2010, 12, 254–263. [Google Scholar] [CrossRef]
- Ma, X.; Lv, H.; Yang, L.; Zhang, Z.; Sun, Z.; Wu, H. Removal characteristics of organic pollutants by the adsorbent injection coupled with bag filtering system. J. Hazard. Mater. 2020, 405, 124193. [Google Scholar] [CrossRef]
- Aurell, J.; Marklund, S. Effects of varying combustion conditions on PCDD/F emissions and formation during MSW incineration. Chemosphere 2009, 75, 667–673. [Google Scholar] [CrossRef]


 is the upper limit for 0.1 ng TEQ/Nm3,
is the upper limit for 0.1 ng TEQ/Nm3,  is the lower limit for 0.1 ng TEQ/Nm3,
is the lower limit for 0.1 ng TEQ/Nm3,  is the upper limit for 0.05 ng TEQ/Nm3, and
is the upper limit for 0.05 ng TEQ/Nm3, and  is the lower limit for 0.05 ng TEQ/Nm3. The internationally authoritative measurement of SSA is usually measured by the low-temperature nitrogen adsorption method or static volumetric principle (static volumetric principle; V-Sorb 2800). The Brunauer–Emmett–Teller (BET) theory is commonly used as the testing theory for SSA. In order to facilitate the understanding of more readers of the meaning of the picture more intuitively, in this manuscript, we replaced “SSA” with “BET”.
is the lower limit for 0.05 ng TEQ/Nm3. The internationally authoritative measurement of SSA is usually measured by the low-temperature nitrogen adsorption method or static volumetric principle (static volumetric principle; V-Sorb 2800). The Brunauer–Emmett–Teller (BET) theory is commonly used as the testing theory for SSA. In order to facilitate the understanding of more readers of the meaning of the picture more intuitively, in this manuscript, we replaced “SSA” with “BET”.
 is the upper limit for 0.1 ng TEQ/Nm3,
is the upper limit for 0.1 ng TEQ/Nm3,  is the lower limit for 0.1 ng TEQ/Nm3,
is the lower limit for 0.1 ng TEQ/Nm3,  is the upper limit for 0.05 ng TEQ/Nm3, and
is the upper limit for 0.05 ng TEQ/Nm3, and  is the lower limit for 0.05 ng TEQ/Nm3. The internationally authoritative measurement of SSA is usually measured by the low-temperature nitrogen adsorption method or static volumetric principle (static volumetric principle; V-Sorb 2800). The Brunauer–Emmett–Teller (BET) theory is commonly used as the testing theory for SSA. In order to facilitate the understanding of more readers of the meaning of the picture more intuitively, in this manuscript, we replaced “SSA” with “BET”.
is the lower limit for 0.05 ng TEQ/Nm3. The internationally authoritative measurement of SSA is usually measured by the low-temperature nitrogen adsorption method or static volumetric principle (static volumetric principle; V-Sorb 2800). The Brunauer–Emmett–Teller (BET) theory is commonly used as the testing theory for SSA. In order to facilitate the understanding of more readers of the meaning of the picture more intuitively, in this manuscript, we replaced “SSA” with “BET”.
| Final PCDD/F Eemissions | 0.1 ng TEQ/Nm3 | 0.05 ng TEQ/Nm3 |
|---|---|---|
| CAC | ≥0.037 g/Nm3 | ≥0.050 g/Nm3 |
| Sss | ≥255 m2/g | ≥425 m2/g |
| T | ≤203.4 °C | ≤177.9 °C |
| tt | ≥1.24 s | ≥4.22 s |
| * Cdust | ≥5 g/Nm3 | ≥14 g/Nm3 |
| * φ | ≥0.06 | ≥0.075 |
| * Clime | ≥20 g/Nm3 | ≥35 g/Nm3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Zhu, L.; Huang, D.; Luo, M.; Tang, X.; Liu, W. Predicting Final PCDD/F Emissions by Coupling Adsorption Model of Activated Carbon and Relationships of PCDD/Fs and Flue Gas Compositions. Processes 2023, 11, 1158. https://doi.org/10.3390/pr11041158
Cai J, Zhu L, Huang D, Luo M, Tang X, Liu W. Predicting Final PCDD/F Emissions by Coupling Adsorption Model of Activated Carbon and Relationships of PCDD/Fs and Flue Gas Compositions. Processes. 2023; 11(4):1158. https://doi.org/10.3390/pr11041158
Chicago/Turabian StyleCai, Jianjun, Lingxia Zhu, Da Huang, Ming Luo, Xingying Tang, and Wangqi Liu. 2023. "Predicting Final PCDD/F Emissions by Coupling Adsorption Model of Activated Carbon and Relationships of PCDD/Fs and Flue Gas Compositions" Processes 11, no. 4: 1158. https://doi.org/10.3390/pr11041158
APA StyleCai, J., Zhu, L., Huang, D., Luo, M., Tang, X., & Liu, W. (2023). Predicting Final PCDD/F Emissions by Coupling Adsorption Model of Activated Carbon and Relationships of PCDD/Fs and Flue Gas Compositions. Processes, 11(4), 1158. https://doi.org/10.3390/pr11041158







