Conductive MoO3–PEDOT:PSS Composite Layer in MoO3/Au/MoO3–PEDOT:PSS Multilayer Electrode in ITO-Free Organic Solar Cells
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of the Conductive M–PSS Composite
2.3. Device Fabrication
2.4. Characterization
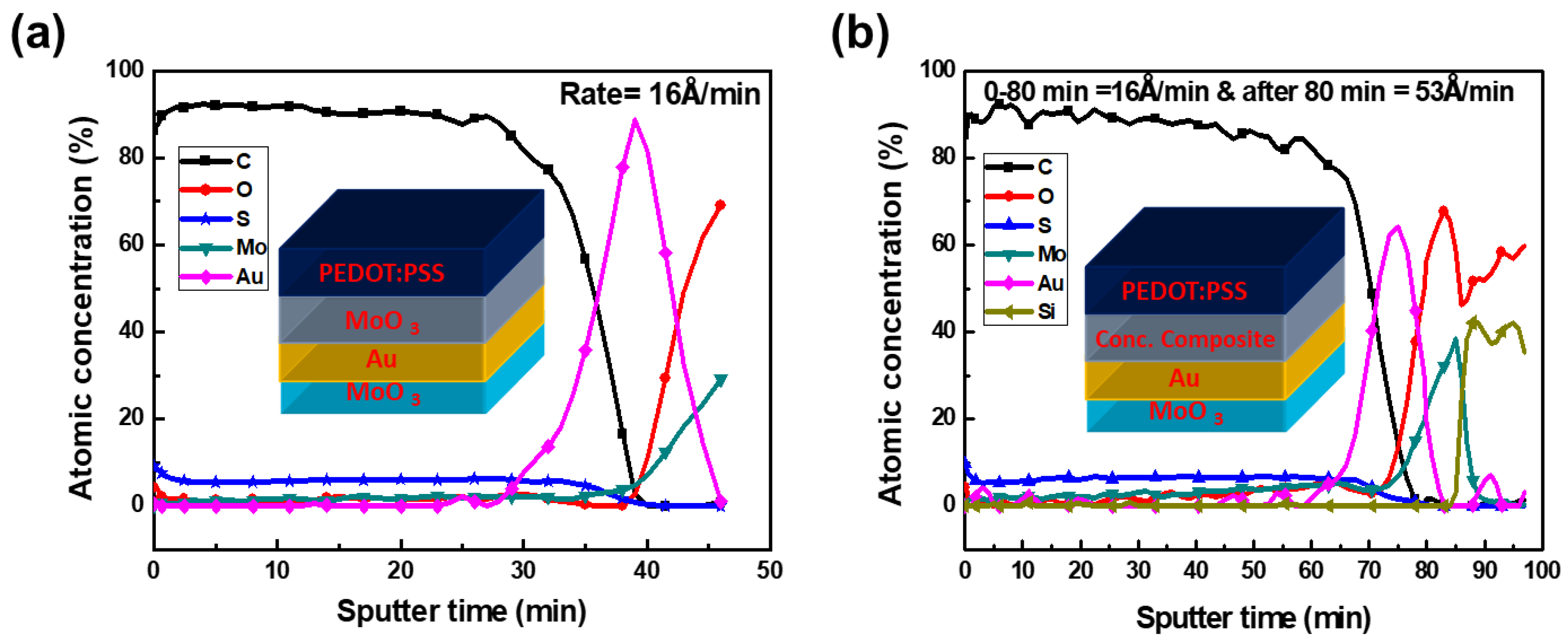
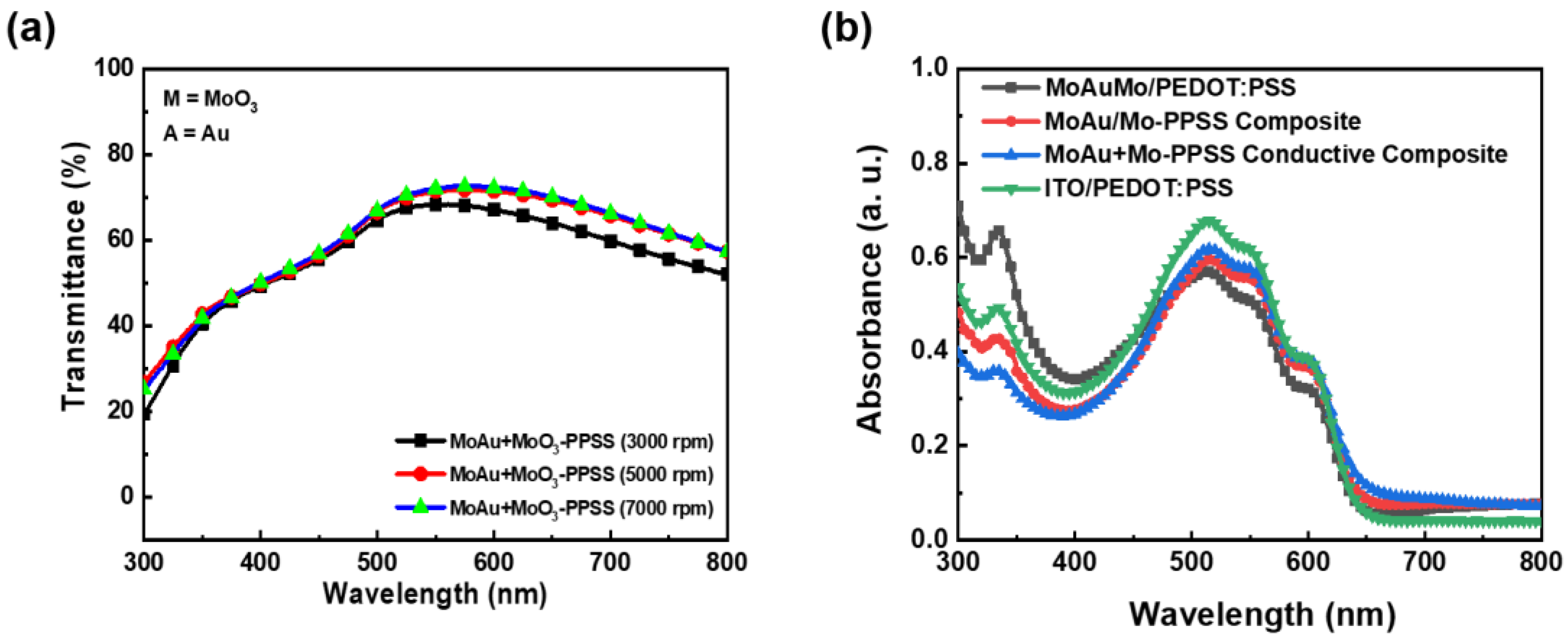
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, W.; Li, J.; Chen, H.; Xue, J. Transparent electrodes for organic optoelectronic devices: A review. J. Photonics Energy 2014, 4, 040990. [Google Scholar] [CrossRef]
- Yao, H.; Hou, J. Recent Advances in Single-Junction Organic Solar Cells. Angew. Chem. 2022, 134, e202209021. [Google Scholar] [CrossRef]
- Lopéz, I.P.; Cattin, L.; Nguyen, D.T.; Morsli, M.; Bernède, J.C. Dielectric/metal/dielectric structures using copper as metal and MoO3 as dielectric for use as transparent electrode. Thin Solid Films 2012, 520, 6419–6423. [Google Scholar] [CrossRef]
- Williams, C.D.; Robles, R.O.; Zhang, M.; Li, S.; Baughman, R.H.; Zakhidov, A.A. Multiwalled carbon nanotube sheets as transparent electrodes in high brightness organic light-emitting diodes. Appl. Phys. Lett. 2008, 93, 183506. [Google Scholar] [CrossRef]
- Krebs, F.C. All solution roll-to-roll processed polymer solar cells free from indium-tin-oxide and vacuum coating steps. Org. Electron. 2009, 10, 761–768. [Google Scholar] [CrossRef]
- Espinosa, N.; Garcia-Valverde, R.; Urbina, A.; Krebs, F.C. A life cycle analysis of polymer solar cell modules prepared using roll-to-roll methods under ambient conditions. Sol. Energy Mater. Sol. Cells 2011, 95, 1293–1302. [Google Scholar] [CrossRef]
- Espinosa, N.; Hösel, M.; Angmo, D.; Krebs, F.C. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 2012, 5, 5117–5132. [Google Scholar] [CrossRef]
- Angmo, D.; Krebs, F.C. Flexible ITO-free polymer solar cells. J. Appl. Polym. Sci. 2013, 129, 1–14. [Google Scholar] [CrossRef]
- Azani, M.R.; Hassanpour, A.; Torres, T. Benefits, problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 2020, 10, 2002536. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef]
- An, T.; Cheng, W. Recent progress in stretchable supercapacitors. J. Mater. Chem. A 2018, 6, 15478–15494. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Kim, Y.J. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahim, A.; Maniruzzaman, M.; Yang, K.; Lee, C.; Nam, H.; Lee, J. ITO-free low-cost organic solar cells with highly conductive poly (3,4 ethylenedioxythiophene): P-toluene sulfonate anodes. Sol. Energy Mater. Sol. Cells 2011, 95, 3573–3578. [Google Scholar] [CrossRef]
- Ali, M.A.; Wu, K.H.; McEwan, J.; Lee, J. Translated structural morphology of conductive polymer nanofilms synthesized by vapor phase polymerization. Synth. Met. 2018, 244, 113–119. [Google Scholar] [CrossRef]
- Yang, K.; Maniruzzaman, M.; Rahman, M.A.; Abdur, R.; Jeong, K.; Nam, H.S.; Lee, J. ITO-free organic solar cell with an PEDOT: PTS/Au/TiO2 grid hybrid electrode as a transparent anode. Curr. Appl. Phys. 2015, 15, S2–S7. [Google Scholar] [CrossRef]
- Song, W.; Fan, X.; Xu, B.; Yan, F.; Cui, H.; Wei, Q.; Ge, Z. All-solution-processed metal-oxide-free flexible organic solar cells with over 10% efficiency. Adv. Mater. 2018, 30, 1800075. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Zheng, Z. Metal-Based Flexible Transparent Electrodes: Challenges and Recent Advances. Adv. Electron. Mater. 2021, 7, 2001121. [Google Scholar] [CrossRef]
- Lee, S.; Jang, J.; Park, T.; Park, Y.M.; Park, J.S.; Kim, Y.K.; Chung, C.H. Electrodeposited silver nanowire transparent conducting electrodes for thin-film solar cells. ACS Appl. Mater. Interfaces 2020, 12, 6169–6175. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, C. The race to replace tin-doped indium oxide: Which material will win? ACS Nano 2010, 4, 11–14. [Google Scholar] [CrossRef]
- Tao, C.; Xie, G.; Liu, C.; Zhang, X.; Dong, W.; Meng, F.; Chen, W. Semitransparent inverted polymer solar cells with MoO3/Ag/MoO3 as transparent electrode. Appl. Phys. Lett. 2009, 95, 206. [Google Scholar] [CrossRef]
- Sang-Hwi, L.; Han-Ki, K. Deposition rate effect on optical and electrical properties of thermally evaporated WO3−x/Ag/WO3−x multilayer electrode for transparent and flexible thin film heaters. Sci. Rep. 2020, 10, 8357. [Google Scholar]
- Xie, H.; Liang, T.; Yin, X.; Liu, J.; Liu, D.; Wang, G.; Que, W. Mechanical Stability Study on PEDOT: PSS-Based ITO-Free Flexible Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 3081–3091. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, Y.M.; Deng, Y.Z.; Kuo, Y.P.; Chen, P.Y.; Tsai, L.; Lin, M.Y. Yb: MoO3/Ag/MoO3 multilayer transparent top cathode for top-emitting green quantum dot light-emitting diodes. Nanomaterials 2020, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Lim, C.H.; Yang, K.; Lee, C.; Nam, H.S.; Lee, J. Indium tin oxide-free PEDOT: PSS/SAM/MoO3/Au/MoO3 multilayer electrodes for organic solar cells. J. Nanosci. Nanotechnol. 2014, 14, 7779–7783. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Wu, K.H.; Lee, J. Solid-state synthesis of conductive polymer PEDOT whiskers. Synth. Met. 2023, 292, 117239. [Google Scholar] [CrossRef]
- Ochiai, S.; Kumar, P.; Santhakumar, K.; Shin, P.K. Examining the effect of additives and thicknesses of hole transport layer for efficient organic solar cell devices. Electron. Mater. Lett. 2013, 9, 399–403. [Google Scholar] [CrossRef]
- Azuma, K.; Sakajiri, K.; Matsumoto, H.; Kang, S.; Watanabe, J.; Tokita, M. Facile fabrication of transparent and conductive nanowire networks by wet chemical etching with an electrospun nanofiber mask template. Mater. Lett. 2014, 115, 187–189. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rahman, M.A.; Jeong, K.; Nam, H.S.; Lee, J. ITO free MoO3/Au/MoO3 structures using Al2O3 as protective barrier between MoO3 and PEDOT: PSS in organic solar cells. Renew. Energ. 2014, 71, 193–199. [Google Scholar] [CrossRef]
- Oh, I.S.; Kim, G.M.; Han, S.H.; Oh, S.Y. PEDOT: PSS-free organic photovoltaic cells using tungsten oxides as buffer layer on anodes. Electron. Mater. Lett. 2013, 9, 375–379. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Voroshazi, E.; Verreet, B.; Buri, A.; Müller, R.; Di Nuzzo, D.; Heremans, P. Influence of cathode oxidation via the hole extraction layer in polymer: Fullerene solar cells. Org. Electron. 2011, 12, 736–744. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Kumar, A.; Asokan, K.; Sachdev, K. High-performance radiation stable ZnO/Ag/ZnO multilayer transparent conductive electrode. Sol. Energy Mater. Sol. Cells 2017, 169, 122–131. [Google Scholar] [CrossRef]
- Zilberberg, K.; Trost, S.; Schmidt, H.; Riedl, T. Solution processed vanadium pentoxide as charge extraction layer for organic solar cells. Adv. Energy Mater. 2011, 1, 377–381. [Google Scholar] [CrossRef]
- Liu, F.; Shao, S.; Guo, X.; Zhao, Y.; Xie, Z. Efficient polymer photovoltaic cells using solution-processed MoO3 as anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 842–845. [Google Scholar] [CrossRef]
- Lee, M.H.; Choi, W.H.; Zhu, F. Solution-processable organic-inorganic hybrid hole injection layer for high efficiency phosphorescent organic light-emitting diodes. Optics Express 2016, 24, A592–A603. [Google Scholar] [CrossRef]
- Lee, M.H.; Chen, L.; Li, N.; Zhu, F. MoO3-induced oxidation doping of PEDOT: PSS for high performance full-solution-processed inverted quantum-dot light emitting diodes. J. Mater. Chem. C 2017, 5, 10555–10561. [Google Scholar] [CrossRef]
- Murase, S.; Yang, Y. Solution processed MoO3 interfacial layer for organic photovoltaics prepared by a facile synthesis method. Adv. Mater. 2012, 24, 2459–2462. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Chen, J.; Kim, H.D.; Gao, P.; Wang, B.; Iriguchi, R.; Ohkita, H. Quadrupolar D–A–D diketopyrrolopyrrole-based small molecule for ternary blend polymer solar cells. Dyes Pigm. Mater. 2018, 158, 213–218. [Google Scholar] [CrossRef]
- Otieno, F.; Kotane, L.; Airo, M.; Erasmus, R.M.; Billing, C.; Wamwangi, D.; Billing, D.G. Comparative investigation of fullerene PC71BM and non-fullerene ITIC-Th acceptors blended with P3HT or PBDB-T donor polymers for PV applications. Front. Energy Res. 2021, 9, 640664. [Google Scholar] [CrossRef]
- Qin, Y.; Uddin, M.A.; Chen, Y.; Jang, B.; Zhao, K.; Zheng, Z.; Yu, R.; Shin, T.J.; Woo, H.Y.; Hou, J. Highly efficient fullerene-free polymer solar cells fabricated with polythiophene derivative. Adv. Mater. 2016, 28, 9416–9422. [Google Scholar] [CrossRef]
- Gélinas, S.; Rao, A.; Kumar, A.; Smith, S.L.; Chin, A.W.; Clark, J.; van der Poll, T.S.; Bazan, G.C.; Friend, R.H. Ultrafast long-range charge separation in organic semiconductor photovoltaic diodes. Science 2014, 3436170, 512–516. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rahman, M.A.; Jeong, K.; Lee, J. MoO3/Au/MoO3–PEDOT: PSS multilayer electrodes for ITO-free organic solar cells. Mater. Sci. Semicond. Process. 2014, 27, 114–120. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Marks, A.; Luscombe, C.K. Molecular design strategies toward improvement of charge injection and ionic conduction in organic mixed ionic-electronic conductors for organic electrochemical transistors. Chem. Rev. 2022, 122, 4325–4355. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Farah, A.A.; Rutledge, S.A.; Schaarschmidt, A.; Lai, R.; Freedman, J.P.; Helmy, A.S. Conductivity enhancement of poly (3,4-ethylenedioxythiophene)-poly (styrenesulfonate) films post-spincasting. J. Appl. Phys. 2012, 112, 113709. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Fehse, K.; Meerheim, R.; Walzer, K.; Leo, K.; Lövenich, W.; Elschner, A. Lifetime of organic light emitting diodes on polymer anodes. Appl. Phys. Lett. 2008, 93, 312. [Google Scholar] [CrossRef]
- Lim, E. Enhanced photovoltaic performance of P3HT: PCBM cells by modification of PEDOT: PSS layer. Mol. Cryst. Liq. Cryst. 2013, 585, 53–59. [Google Scholar] [CrossRef]
- Xie, F.; Choy, W.C.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-temperature solution-processed hydrogen molybdenum and vanadium bronzes for an efficient hole-transport layer in organic electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar] [CrossRef]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.M.; Lu, Z.H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Homes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley DD, C.; Dos Santos, D.A.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 3976715, 121–128. [Google Scholar] [CrossRef]
- Foe, K.; Namkoong, G.; Samson, M.; Younes, E.M.; Nam, I.; Abdel-Fattah, T.M. Stability of high band gap P3HT:PCBM organic solar cells using TiOx interfacial layer. Int. J. Photoenergy 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Charles, J.P.; Abdelkrim, M.; Muoy, Y.H.; Mialhe, P. A practical method of analysis of the current-voltage characteristics of solar cells. Solar Cells 1981, 4, 169–178. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, F.; Liu, Y.; Wei, E.I.; Li, X.; Yang, Y.; Choy, W.C. Efficient hole transport layers with widely tunable work function for deep HOMO level organic solar cells. J. Mater. Chem. A 2015, 3, 23955–23963. [Google Scholar] [CrossRef]





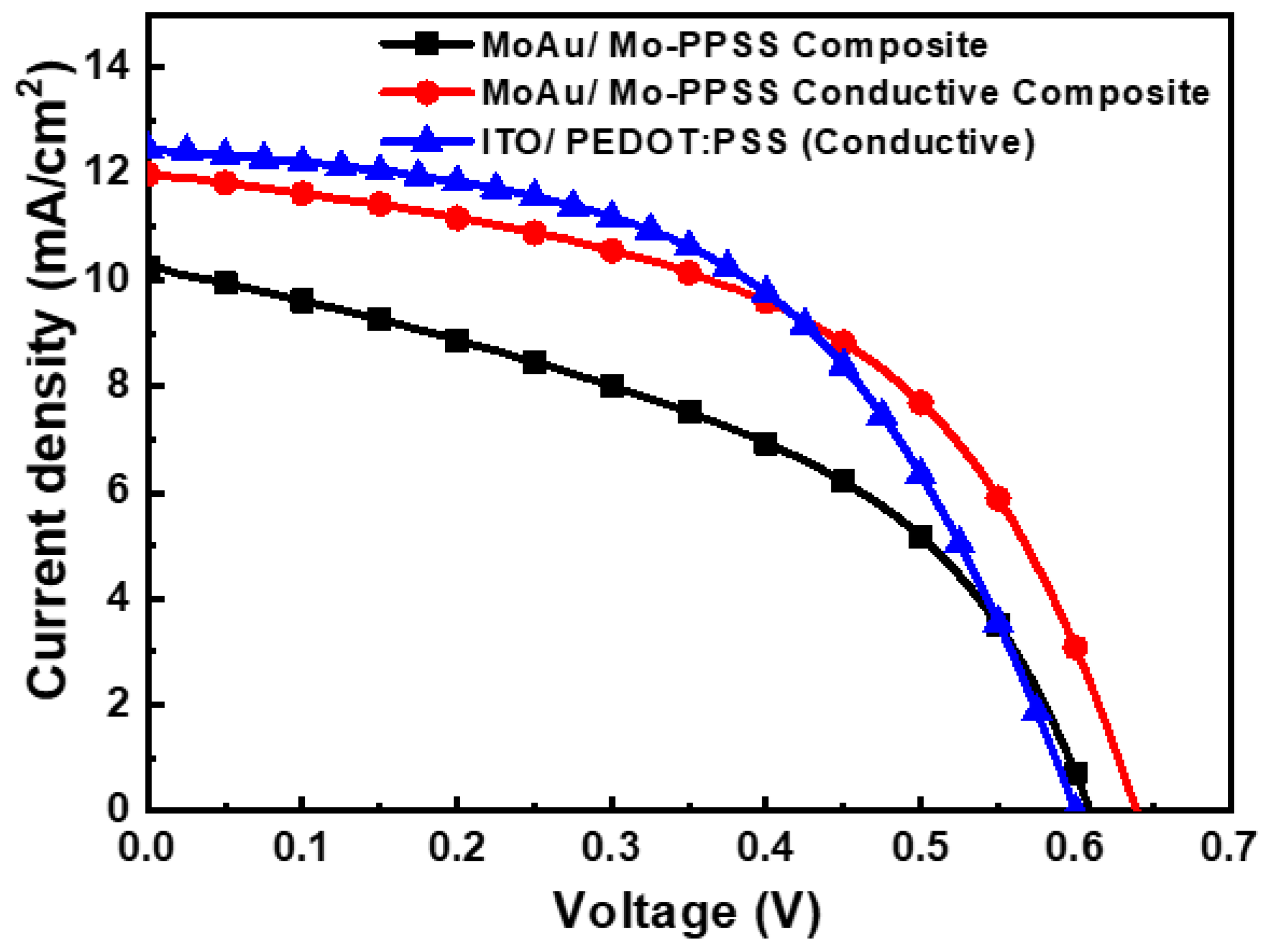
| Jsc mA/cm2 | VOC V | FF % | H % | Rsh Ω cm2 | Rs Ω cm2 | |
|---|---|---|---|---|---|---|
| ITO/PEDOT:PSS | 12.47 ± 0.18 | 0.60 ± 0.01 | 0.52 ± 0.01 | 3.91 ± 0.02 | 576.00 ± 50 | 12.86 ± 0.44 |
| MoAuMo/PEDOT:PSS | 7.75 ± 0.49 | 0.55 ± 0.02 | 0.43 ± 0.02 | 1.84 ± 0.05 | 18.25 ± 95 | 22.21 ± 1.66 |
| MoAu/M-PSS Composite | 10.25 ± 0.39 | 0.61 ± 0.01 | 0.45 ± 0.01 | 2.81 ± 0.10 | 165.57 ± 75 | 13.48 ± 0.84 |
| MoAu/Mo–PPSS Conductive Composite | 11.99 ± 0.19 | 0.64 ± 0.01 | 0.52 ± 0.01 | 3.97 ± 0.03 | 313.00 ± 63 | 11.70 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniruzzaman, M.; Abdur, R.; Kuddus Sheikh, M.A.; Singh, S.; Lee, J. Conductive MoO3–PEDOT:PSS Composite Layer in MoO3/Au/MoO3–PEDOT:PSS Multilayer Electrode in ITO-Free Organic Solar Cells. Processes 2023, 11, 594. https://doi.org/10.3390/pr11020594
Maniruzzaman M, Abdur R, Kuddus Sheikh MA, Singh S, Lee J. Conductive MoO3–PEDOT:PSS Composite Layer in MoO3/Au/MoO3–PEDOT:PSS Multilayer Electrode in ITO-Free Organic Solar Cells. Processes. 2023; 11(2):594. https://doi.org/10.3390/pr11020594
Chicago/Turabian StyleManiruzzaman, Md, Rahim Abdur, Md Abdul Kuddus Sheikh, Son Singh, and Jaegab Lee. 2023. "Conductive MoO3–PEDOT:PSS Composite Layer in MoO3/Au/MoO3–PEDOT:PSS Multilayer Electrode in ITO-Free Organic Solar Cells" Processes 11, no. 2: 594. https://doi.org/10.3390/pr11020594
APA StyleManiruzzaman, M., Abdur, R., Kuddus Sheikh, M. A., Singh, S., & Lee, J. (2023). Conductive MoO3–PEDOT:PSS Composite Layer in MoO3/Au/MoO3–PEDOT:PSS Multilayer Electrode in ITO-Free Organic Solar Cells. Processes, 11(2), 594. https://doi.org/10.3390/pr11020594









