Abstract
A large number of aromatic substances can be found in so-called coal tar (containing >10,000 individual compounds), which is a mixture of heavy liquid fractions (dense viscous black liquor, tended to solidification) obtained after the pyrolysis of coal (solid product—coke, gas products, and light liquid products are also produced during the process). Volatile monocyclic aromatic hydrocarbons, which are naturally occurring in coal tar, can be exploited as premium raw materials for the production of graphene by chemical vapor deposition (CVD). Moreover, aromatic chemicals (compounds with benzene rings) can produce graphene at lower temperatures than other small-molecule gas feedstocks (for graphene growth via methane gas, the temperature must be at least 900 °C). The intermediate reaction mechanism involved in the creation of graphene from various temperature ranges of monocyclic aromatic hydrocarbons in benzene ring structures has long been a fascinating enigma. Accordingly, in this paper, we analyze the graphene growth pattern of benzene at different temperatures from 300 to 900 °C. For graphene synthesis in the lower temperature range (300~600 °C), analytical experiments show that benzene rings (almost) do not crack during the gas phase process. Thus, the structure of the benzene ring is directly coupled into graphene in the above temperature range. When benzene is more thoroughly transformed into tiny molecules that are deposited on the surface of copper foil at higher temperatures (700~900 °C), graphene is formed by a complex mixture of carbon sources, including gaseous small molecules (methane and ethane) and benzene. Based on the process above, we provide an alternative solution for the large-scale industrial preparation of graphene, with low energy consumption, via low-temperature synthesis of graphene by the CVD method using the coal tar carbon source at 500 °C, which is the optimal growth temperature of the benzene ring.
1. Introduction
Graphene, a two-dimensional sp2 hybridization carbon nanomaterial with a honeycomb lattice structure [1], has attracted the attention of many scientists due to its powerful optical [2], electrical [3], and mechanical [4] properties. Breakthroughs have been made in graphene preparation methods with graphene-intensive studies [5]. The main graphene preparation methods are mechanical exfoliation [6], chemical exfoliation [7], SiC epitaxial growth [8], redox [9], chemical vapor deposition [10], and the fast Joule heat method [11]. The “top-down” exfoliation method is obviously straightforward and dependable, but it is constrained by the unmanageable number of layers and the small size of the graphene flakes used for practical applications. The high quality, good consistency, and adjustable number of layers offered by the chemical vapor deposition method for producing graphene make it ideal for large-scale industrial manufacturing of the material [12]. Unfortunately, the carbon precursors required for chemical vapor deposition are often relatively expensive small-molecule gases such as methane and acetylene [13,14]. Of course, other hydrocarbons, such as polymers (e.g., PMMA) [15] and liquids (e.g., alcohols and benzene) [15,16], are also used as carbon sources for the CVD growth of graphene. These carbon sources are also not available for large-scale graphene production due to their high preparation costs. Finding affordable and accessible alternative carbon sources has been the subject of current efforts to produce graphene films on a wide scale (industrially) using CVD [17,18]. Even while biomass and food waste have the capacity to generate graphene at a low cost as well [19,20], their unique sources could result in ecological imbalance if employed in significant amounts. Coal, which has been discovered to be inexpensive and plentiful, can also be utilized as a substitute for the carbon precursors needed to produce graphene and is a possible supply of carbon for the sustainable, affordable fabrication of graphene films.
However, due to the complex composition of coal, the different content of alkyl chains and heteroatoms contained in different coals can seriously affect the quality of graphene synthesized from coal [21,22]. The separation of coal into aromatic ring-rich components by certain means plays a role in promoting graphene growth [23]. Coal-based poly-generation technology at Zhejiang University converts coal from a single power generation to the simultaneous production of electricity, gas, and liquid fuels [24]. The key to this technology is the release of volatile fractions such as H2, CO, CO2, and hydrocarbons from coal under a high-temperature inert atmosphere. The solid products are continuously aromatized in their molten states to form semi-coke or coke. The liquid components of the volatile fraction are condensed to form coal tar, in which aromatic hydrocarbon compounds account for more than 85% of the tar (Table 1). The natural volatilization of coal tar produced by using coal poly-generation produces mostly structurally symmetrical monocyclic aromatic hydrocarbons, which is an effective way to separate symmetrical aromatic compounds from coal in a clean and economical manner. Highly symmetric aromatic compounds are the raw material for clean and low-energy consumption-producing graphene, allowing the more efficient synthesis of higher-quality graphene and reducing defects in the graphene lattice. So, the monocyclic aromatic compounds isolated from coal tar are promising for higher-quality CVD synthesis of graphene. This is attributed to the fact that the aromatic component of coal is an effective unit for the synthesis of high-quality graphene, while the heteroatoms in coal as well as aliphatic chain hydrocarbons are responsible for the defects in the graphene lattice [25]. Moreover, aromatic compounds, including benzene, toluene, and polystyrene [15,26,27], are able to synthesize graphene at lower temperatures than small molecule gas carbon sources, such as methane and ethane [12,28]. Therefore, the benzene ring structure in the carbon source must play an important role in graphene synthesis.

Table 1.
Chemical composition of coal tar produced by poly-generation.
It is obvious that the growth mechanism of graphene is influenced by growth conditions (temperature, pressure, time, and the composition of the carbon source in CVD reactors). The mechanism of benzene growth nucleation has received little attention, the basic structure of aromatic carbon source, which is critical to the optimization of graphene synthesis conditions, economics, and the environment using this carbon source including coal tar. It is, therefore, necessary to study the reaction principles of the CVD synthesis process of benzene rings at different temperatures in order to gain insight into the growth mechanism of benzene rings and even graphene films derived from coal tar.
On this basis, the main objective of this paper is to study the synthesis principle of graphene with benzene rings at different temperatures and to select the appropriate temperature for the preparation of high-quality graphene films from coal tar. First, we will predict the benzene ring-derived graphene growth model through gas chromatography (analyze the reaction process at different temperatures) and Raman spectroscopy (analyze the surface state of graphene films). Then with coal tar as raw material, we will prepare the carbon material on the surface of copper foil by the same method under the optimal conditions of temperature. The above-synthesized carbon material of coal tar will be detected as graphene according to the common graphene characterization means, such as the Raman test, scanning electron microscopy, and spherical difference electron microscopy, which proves that coal tar is an effective raw material for the preparation of graphene with low-energy consumption and low costs.
2. Materials and Methods
2.1. Materials
The coal tar used for our experiments was produced from Chinese Shanxi Gemeng coal through pyrolysis in a 1MW double circulating-fluidized-bed (CFB) experimental platform and detected by a laboratory gas chromatography–mass spectrometry system (GCMS, Agilent 7890-5977B) for coal tar components. The composition of the coal tar was analyzed as shown in the following Table 1.
Copper foil (99.8%, thickness 0.025 mm) obtained from Alfa Aesar was used as a substrate for graphene synthesis. For characterization, silicon oxide wafers (oxide layer thickness of 300 nm) were used as substrates to transfer graphene films. Ultrathin pure carbon film with no formvar backing on lacey carbon support film was used for spherical aberration-corrected transmission electron microscopy. Moreover, 1L gas sample bags were purchased for collecting gas components during growth.
2.2. Graphene Synthesis
Graphene was synthesized by a low-pressure chemical vapor deposition method (see experimental equipment in Figure S1): copper foil (2 × 2 cm2) was placed in the center of a quartz tube and heated by a horizontal tube furnace. The carbon precursors, including benzene and coal tar, are contained in a homemade vessel that is isolated from the main system by a valve. During furnace heating, a low-pressure environment of about 50 Pa is maintained in the system and a flow rate of 12 sccm of high-purity hydrogen (99.999%) is introduced to anneal at 1000 °C for 30 min. After annealing, the furnace temperature is cooled to the reaction temperature (300~900 °C). The carbon source valve is opened and the carbon-containing vapor (benzene, coal tar) is introduced into the quartz tube at a certain flow rate, and the flow rate of high-purity hydrogen is reduced to 4 sccm for graphene growth for 30 min. After the growth is completed, the carbon source valve is closed and the equipment is naturally cooled to room temperature under hydrogen purge. Details of graphene low-temperature growth experiments are shown in Figure S2.
2.3. Reaction Gas and Liquids Collection
The released gas is collected in a gas sampling bag attached to the tube furnace exhaust. Prior to pyrolysis, the sampling bags are purged three separate times with hydrogen gas to remove excess air from the sampling bags.
2.4. Characterization
For analysis of the content of the small molecular components of the gas during graphene growth, a gas chromatograph (customized by Agilent Technologies, Ltd., Santa Clara, CA, USA, with a flame ionization detector for detection of low carbon hydrocarbons) was used. The field emission scanning electron microscope (SEM: SU-8010) observes the morphology characteristics of coal tar graphene; a confocal Raman spectrometer with an optical microscope (produced by Horiba Jobin Yvo, type LabRAM HR Evolution) was used to analyze the surface state of benzene rings growing graphene at different temperatures; a spherical aberration-corrected transmission electron microscope (Titan ChemiSTEM, accelerating voltage: 0~200 KV; highest resolution: 0.08 nm) was used to observe the nano-level lattice structure of the coal-tar graphene surface.
3. Results and Discussion
3.1. Mechanism of Graphene Growth by Benzene at Different Temperatures
We are aware that the heteroatoms and aliphatic chains found in coal cause flaws in the graphene lattice, making it difficult to create layers of pure graphene. On the other hand, producing high-grade graphene from structurally symmetric aromatic ring-structured molecules is successful. Liu et al. [29] suggested that petroleum pitch structural units for a large number of aromatic ring structures play an important role in graphene generation: The bitumen will separate the functional groups in the aromatic ring structure by hydrogen etching as well as metal surface catalysis at 400 °C; when the temperature reaches 600 °C, the aromatic rings begin to polymerize, and graphene is produced at 940 °C.
The above explanation, however, is not sufficient to account for the role of the phenyl meta structure in the aromatic ring in graphene growth. No attempt has been made to even explore the reaction mechanism of the benzene ring in graphene growth at different temperatures. Moreover, the basic theoretical aspects of the growth process, particularly the benzene ring in polycrystalline graphene films synthesized on metals at different temperatures, as well as the choice of the fundamental supply units [30] for graphene nucleation in the gas phase reaction process and in the surface reactivity, are still under study despite the fact that the fundamental principles of graphene formation by CVD have been established. Therefore, we used gas chromatography to analyze the gas mixture of the graphene production reactor. The results are shown in Table 2.

Table 2.
Content of gas molecules in the benzene thermal reaction.
Although small molecule gases, such as methane, ethane, propane, and propylene are produced in trace amounts at low temperatures (300~600 °C), at higher temperatures (700~900 °C), methane and ethane contents rise with the temperature, while the contents of other gases stay at the original levels. According to the aforementioned findings, between 3.5 and 11.7% of benzene undergoes a ring-opening reaction [31] that is accelerated by copper foil at higher temperatures between 700 and 900 °C (Figure 1). It is unclear, though, whether benzene rings serve as the only carbon source for the formation of graphene at various temperatures.
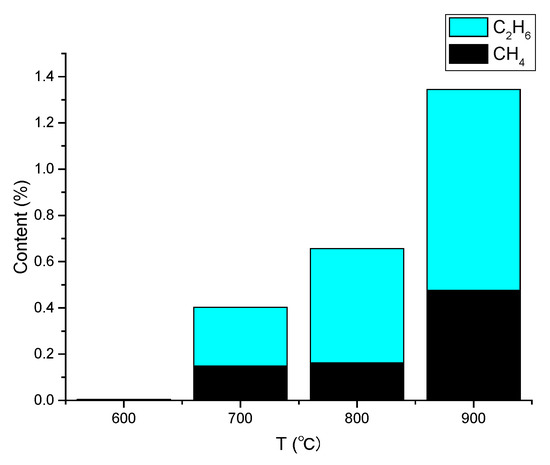
Figure 1.
Analysis of the main gas components of graphene growth environment.
In fact, the benzene ring structure of the macromolecule has been present in the gas phase reaction studies of graphene CVD growth and may play an important role. Lewis et al. [32] simulated the species composition of the gas phase under 100 Pa low-pressure graphene growth conditions and found that in addition to the small molecules of gas containing (C1~C3), larger molecules containing benzene are produced. If we consider the process of graphene synthesis by CVD of benzene as a chemical vapor deposition of pyro carbon [33], A. BECKER et al. [34] found that benzene is able to produce pyro carbon directly without other intermediates in the process of thermal carbon deposition. This finding further suggests that benzene is the most significant intermediate in gas phase reactions of CVD graphene growth and the most active hydrocarbon in the pyrolysis of carbon.
In addition, benzene rings [15,35] as carbon sources have repeatedly been shown to generate graphene via CVD at a low temperature (300~600 °C). Moreover, experiments have shown that in the composition of the gas phase in this interval, almost no breakage of the benzene ring to form small molecules occurred. The small number of small molecules of gas produced by the benzene ring in Table 2 at lower temperatures (300~600 °C), although only at the ppm level under hydrogen atmosphere, causes these small molecules of gas to be difficult to ignore because graphene is a nanomaterial and the number of atoms of carbon required for synthesis is very small. For this reason, we passed ppm levels of alkanes (C1~C3) in the experimental reactor and found that no graphene was produced under low-temperature (300~600 °C) conditions. So, we believe that graphene was synthesized mainly from benzene as the basic unit, and Figure 2 proposes a conjecture of the process of the benzene ring forming graphene on the surface of copper substrates in the reaction temperature interval of 300~600 °C.
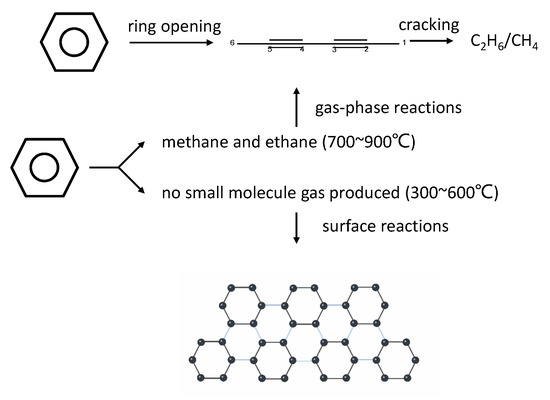
Figure 2.
Mechanism of graphene growth from benzene at different temperatures (300~900 °C).
In the above analysis, together with graphene synthesis by the benzene ring at a low temperature, the benzene ring must dehydrogenate on the surface of copper foil under a hydrogen atmosphere to synthesize graphene in a gas phase reaction (shown in Figure 3). Then, as shown in Figure 4, the benzene dehydrogenated and adsorbed on the surface of the copper foil eventually forms graphene through migration and nucleation. That is, graphene is synthesized by the benzene ring through dehydrogenation and migrating to a certain position as the basic supply unit of graphene to arrange (graphene arrangement synthesis process in video S1).
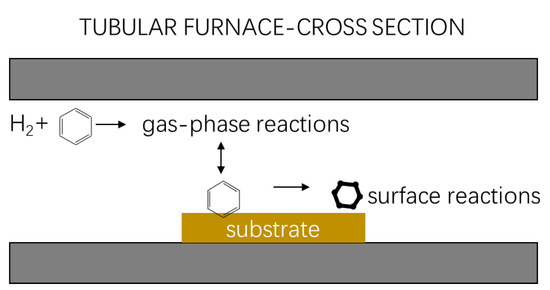
Figure 3.
Gas phase reaction of graphene growth from benzene at low temperatures (300~600 °C).
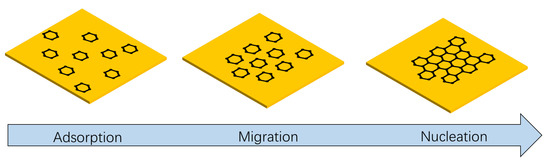
Figure 4.
Surface reaction of graphene growth from benzene at low temperatures (300~600 °C).
More research is needed to determine whether the six-membered benzene ring serves as the basic supply unit of graphene at low temperatures. This is because it is unknown whether the fundamental constituents of graphene islands produced on a specific substrate are mostly carbon monomers or other bigger carbon species. It has been demonstrated that carbon dimers [36] are the main supply units for the formation of graphene islands on metal substrates, and larger forms of carbon supply units, such as trimers, are as stable as dimers on the copper surface, only with higher formation potentials. This suggests that if the substrate surface is enriched with a large number of benzene rings rather than cleaved into other substances at a certain reaction temperature, it also has the possibility that the hexameric carbon after benzene dehydrogenation is directly used as the basic supply unit for graphene growth.
The presence of a significant number of gaseous small molecules in the benzene ring opening reaction causes the graphene growth process to change in the higher temperature range of 700~900 °C, in contrast to the gas phase composition of graphene generated at 300~600 °C. We synthesized graphene on the surface of copper foil using low-pressure chemical deposition at higher reaction temperatures of 700 to 900 °C with benzene as the carbon precursor and hydrogen as the carrier gas. The samples at each temperature were transferred to silicon oxide wafers for Raman analysis. The test results are shown in Figure 5. The intensity of the D-peak of graphene increases significantly when the growth temperature reaches 800 °C in the case of graphene produced via benzene ring deposition at 700 and 900 °C. The intensity of the D peak was much higher than that of the G peak, which is compatible with the formation of ethane [28] at 750 °C. The height of the 2D peak decreased, but its shape did not change as much as that of multilayer graphene with more than three layers. According to previous experimental results, benzene at 800 °C is calculated to have about 5.8% ring-opening cleavage conversion to gaseous carbon sources containing ethane. Therefore, these gaseous small molecules, especially ethane, are more involved in the synthesis of graphene on copper foil and migrate to synthesize graphene as smaller carbon units (e.g., carbon–carbon dimers).
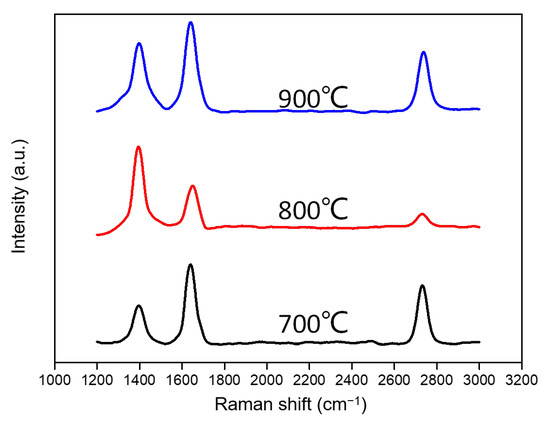
Figure 5.
Raman spectrums of benzene-grown graphene at 700~900 °C.
The above results indicate that the different forms of benzene ring existence at different temperatures are the key to understanding the benzene ring growth of graphene. We believe that at the graphene growth temperature below 600 °C, benzene ring growth is dominated by the direct arrangement of benzene rings due to almost no reaction; the increase of temperature above 700 °C prompts the cleavage of benzene rings, and the small molecule gas products of cleavage also participate in the process of benzene ring growth, which makes the growth process more complicated; when the temperature is greater than 800 °C, with the increase of gas small molecules, gas molecules such as ethane gas participate in the growth process more than other carbon sources.
3.2. Synthesis of Graphene from Coal Tar at 500 °C
The poly-generation coal tar is a favorable starting point for the low-temperature growth of graphene because it contains a lot of aromatic hydrocarbons. However, the temperature of coal tar pitch-derived graphene is always above 900 °C [37]. As a result, more of the aromatic ring structure from coal will be destroyed and more tiny gas molecules will be deposited as graphene. Therefore, we attempted to produce graphene from all the volatile coal tar components containing molecules with phenyl meta-structures at low temperatures.
We first tried to deposit graphene on the surface of copper foil using benzene as the carbon source in order to determine the ideal conditions for the low-temperature development of graphene from coal tar. As shown in Figure 6, the Raman spectrum of graphene grown by benzene in the temperature range of 300~600 °C is shown. In the following, we choose the ideal working conditions for the low-temperature development of benzene using the parameters of each graphene peak from the Raman spectrum. Graphene’s primary Raman peaks are known to be G, D, and 2D, and the peak integration ratio, peak intensity, peak position, and peak shape of these distinguishing peaks can reveal information about the properties of the material [38]. The predominant in-plane vibration induced by sp2-linked carbon atoms produces the G peak (1580 cm−1), which is indicative of the graphitic characteristics of the materials. The D peak (1350 cm−1) depicts the disorder and flaws in the lattice of sp2-hybridized graphene, such as point defects, subdomain borders, etc. The integrated intensity ratio ID/IG of the D and G peaks is widely used to characterize the number of defects in graphitic materials [39]. The D peak’s second-order overtone is the 2D peak (2700 cm−1). Many people use a single, sharp second-order Raman band (2D) as a quick and reliable way to demonstrate the presence of monolayer graphene. The ratio of the integrated intensities of the 2D and G peaks (I2D /IG) and the number of layers of graphene are inversely related by the double resonance mechanism [40].
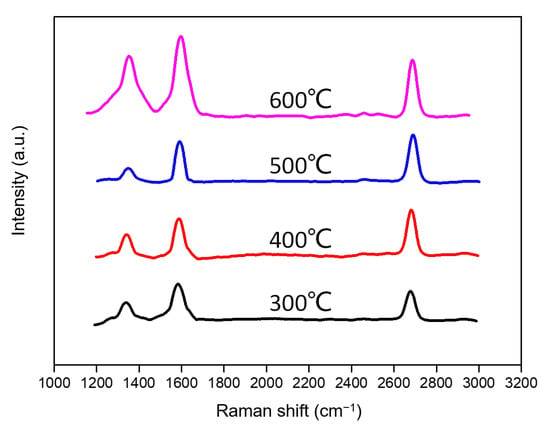
Figure 6.
Raman spectrums of benzene-grown graphene at 300~600 °C.
After comparing peak indicators with temperature in the Raman spectra (Figure 7) of benzene related to graphene at various temperatures (300~600 °C), the layers and defects of graphene can be observed. The D-band and G-band integrated intensity ratio ID/IG = 0.44 at 500 °C is lower than that of the graphene developed at other temperatures, indicating that this temperature has the fewest flaws in graphene. Further evidence that the graphene produced at 500 °C is the thinnest is provided by the integrated intensity ratio of the 2D and G bands at this temperature, which is a minimum of 1.4. According to all available data, 500 °C is the ideal temperature for the low-temperature growth of graphene by benzene under identical operating conditions, both in terms of the number of defects and the thickness of graphene.
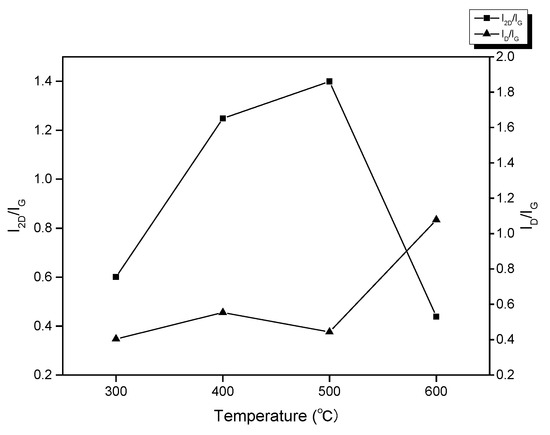
Figure 7.
Property index of graphene by benzene grown at each growth temperature.
In a follow-up to the previous research, 500 °C was selected as the optimal working temperature for the low-temperature synthesis of graphene by benzene. Graphene was then formed by CVD using coal tar as the carbon source at the same temperature. Under the same process flow of growing graphene with benzene as the carbon source, monocyclic aromatic hydrocarbons volatilized from coal tar were successfully deposited on the surface of copper foil at 500 °C to form surface carbon material. Subsequently, we determined the surface carbon material to be graphene by Raman and electron microscopy.
The Raman results of graphene from coal tar obtained are shown in Figure 8a. Among these, the 2D peak structure of graphene made from coal tar is obviously changed in this circumstance. Although the shape and intensity of the 2D peaks of graphene also change significantly compared with those of bulk graphite, the shape of 2D Raman peaks varies with the number of graphene layers [41]. The 2D peak broadens with random orientation between graphene layers relative to monolayer graphene when excited by various Raman lasers, which causes the 2D characteristic peak to broaden. Bulk graphite, for example, is made up of two components, 2D1 and 2D2, which are about 1/4 and 1/2 the height of the G peak, respectively [42]. Likewise, the varied line forms of the 2D peaks of graphene offer an efficient technique to count the number of graphene layers [43]. The number of 4 layers of this graphene can be determined using the peak line form by fitting the 2D peak to the Lorentz peak in Figure 8a. Graphene has a rectangular regular lattice structure [27], as illustrated by scanning electron microscopy (Figure 8b), and an obvious graphene honeycomb carbon arrangement structure can be seen in some areas observed by a spherical aberration-corrected transmission electron microscope. In a word, coal tar can synthesize graphene under 500 °C without destroying the benzene ring structure, providing a cheap carbon source option for graphene growth at low temperatures.
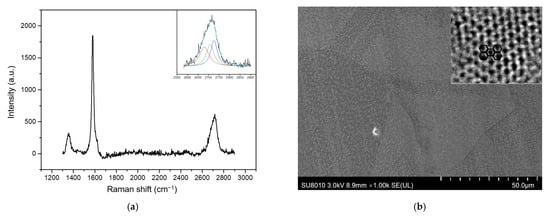
Figure 8.
Characterization of graphene grown from coal tar at 500 °C: (a) Raman spectrum of graphene grown by coal tar at 500 °C (the upper right corner is the result of the fit of the Lorentz splitting of the 2D peak in the spectrum); (b) SEM of graphene grown by coal tar at 500 °C (the upper right corner is Graphene lattice image under spherical aberration microscope).
4. Conclusions
The benzene ring was used as an example of an aromatic ring structure in this paper to examine the mechanism of growing graphene at low temperatures. We found that at low temperatures (300~600 °C), the benzene rings almost do not undergo cleavage in the gas phase reaction, but instead are deposited as complete six-membered ring structures on the surface of copper foil. So, we have good grounds to believe that the basic supply unit for graphene deposition is a carbon-6 phenyl ring. The benzene ring begins to break down into little molecules (primarily CH4 and C2H6) of gas only when the temperature reaches 700 °C. As a natural outcome, the benzene ring structure was destroyed at high temperatures (700~900 °C) before graphene formation began; small molecules of gas produced by the thermal cleavage of benzene rings are also involved in the synthesis of graphene. Furthermore, Raman spectroscopy shows that at 800 °C, ethane becomes the primary carbon source for the formation of graphene on the surface of copper foil. Then we proposed the scenario of using coal tar as a carbon source to achieve clean and low-energy-consumption preparation of graphene and tried to grow the carbon material on the surface of copper foil by the same process of growing graphene with benzene ring at 500 °C (the best graphene synthesis condition of benzene ring). The presence of graphene was also determined using characterization methods, such as Raman and spherical aberration-corrected transmission electron microscope. The above results indicate that the clean and low-energy consumption production of a high-value-added graphene process from coal tar is feasible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11020593/s1, Figure S1: Experimental equipment; Figure S2: Details of graphene low-temperature growth experiments; Video S1: Schematic animation of the low-temperature direct growth of graphene from benzene rings; Figure S3: Gas chromatogram examples of benzene ring chemical vapor deposition on copper foil at different temperatures (300~900 °C); Table S1: The type of coal tar composition compounds from Chinese Shanxi Gemeng coal through pyrolysis in a 1 MW double circulating-fluidized-bed (CFB) experimental platform.
Author Contributions
S.Z., conceptualization, methodology, writing—original draft preparation, visualization, investigation, material synthesis, and characterizations. Z.L. and M.F., results interpretation and writing—reviewing and editing. Q.W. and J.C., supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (2022ZFJH004) and the National Natural Science Foundation of China (no. U1910201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data are included in the text.
Acknowledgments
The authors extend their appreciation to the Fundamental Research Funds for the Central Universities (2022ZFJH004) and the National Natural Science Foundation of China (no. U1910201).
Conflicts of Interest
There are no competing interest that might influence this work.
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.A. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Feng, X. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial graphene on SiC: A review of growth and characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X. Growth of large-area single-and bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Mafra, D.L.; Olmos-Asar, J.A.; Negreiros, F.R. Ambient-pressure CVD of graphene on low-index Ni surfaces using methane: A combined experimental and first-principles study. Phys. Rev. Mater. 2018, 2, 073404. [Google Scholar] [CrossRef]
- Chen, C.S.; Hsieh, C.K. Effects of acetylene flow rate and processing temperature on graphene films grown by thermal chemical vapor deposition. Thin Solid Films 2015, 584, 265–269. [Google Scholar] [CrossRef]
- Li, Z.; Wu, P.; Wang, C. Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef]
- Guermoune, A.; Chari, T.; Popescu, F. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Kumar, E.S.; Sivasankar, V.; Sureshbabu, R. Facile synthesis of few layer graphene from bituminous coal and its application towards electrochemical sensing of caffeine. Adv. Mater. Lett. 2017, 8, 239–245. [Google Scholar] [CrossRef]
- Wang, D.; Vijapur, S.H.; Botte, G.G. Coal char derived few-layer graphene anodes for lithiumion batteries. Photonics 2014, 1, 251–259. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef]
- Ruan, G.; Sun, Z.; Peng, Z. Growth of graphene from food, insects, and waste. ACS Nano 2011, 5, 7601–7607. [Google Scholar] [CrossRef]
- Vijapur, S.H.; Wang, D.; Ingram, D.C. An investigation of growth mechanism of coal derived graphene films. Mater. Today Commun. 2017, 11, 147–155. [Google Scholar] [CrossRef]
- Xu, H.; Lin, Q.; Zhou, T. Facile preparation of graphene nanosheets by pyrolysis of coal-tar pitch with the presence of aluminum. J. Anal. Appl. Pyrolysis 2014, 110, 481–485. [Google Scholar] [CrossRef]
- Wan, X.; Chen, K.; Liu, D. High-quality large-area graphene from dehydrogenated polycyclic aromatic hydrocarbons. Chem. Mater. 2012, 24, 3906–3915. [Google Scholar] [CrossRef]
- Yu, P.; Luo, Z.; Wang, Q. Life cycle assessment of transformation from a sub-critical power plant into a polygeneration plant. Energy Convers. Manag. 2019, 198, 111801. [Google Scholar] [CrossRef]
- Rane, K.; Adams, J.J.; Thode, J.M. Multistep Fractionation of Coal and Application for Graphene Synthesis. ACS Omega 2021, 6, 16573–16583. [Google Scholar] [CrossRef]
- Kang, C.; Jung, D.H.; Lee, J.S. Atmospheric pressure chemical vapor deposition of graphene using a liquid benzene precursor. J. Nanosci. Nanotechnol. 2015, 15, 9098–9103. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R. Low-temperature chemical vapor deposition growth of graphene from toluene on electropolished copper foils. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sun, L. Low-temperature and rapid growth of large single-crystalline graphene with ethane. Small 2018, 14, 1702916. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, Z.; Li, Y. Synthesis of three-dimensional graphene from petroleum asphalt by chemical vapor deposition. Mater. Lett. 2014, 122, 285–288. [Google Scholar] [CrossRef]
- Safron, N.S.; Arnold, M.S. Experimentally determined model of atmospheric pressure CVD of graphene on Cu. J. Mater. Chem. C 2014, 2, 744–755. [Google Scholar] [CrossRef]
- Brooks, C.T.; Peacock, S.J.; Reuben, B.G. Pyrolysis of benzene. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 652–662. [Google Scholar] [CrossRef]
- Lewis, A.M.; Derby, B.; Kinloch, I.A. Influence of gas phase equilibria on the chemical vapor deposition of graphene. ACS Nano 2013, 7, 3104–3117. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Barbera, K. Carbon growth evidences as a result of benzene pyrolysis. Carbon 2013, 59, 296–307. [Google Scholar] [CrossRef]
- Becker, A.; Hüttinger, K.J. Chemistry and kinetics of chemical vapor deposition of pyrocarbon—III pyrocarbon deposition from propylene and benzene in the low temperature regime. Carbon 1998, 36, 201–211. [Google Scholar] [CrossRef]
- Shazni Mohammad Haniff, M.A.; Zainal Ariffin, N.H.; Ooi, P.C. Practical route for the low-temperature growth of large-area bilayer graphene on polycrystalline nickel by cold-wall chemical vapor deposition. ACS Omega 2021, 6, 12143–12154. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Cui, P. Carbon dimers as the dominant feeding species in epitaxial growth and morphological phase transition of graphene on different Cu substrates. Phys. Rev. Lett. 2015, 114, 216102. [Google Scholar] [CrossRef]
- Zhou, Y.; Tong, L.; Wu, L. Review on the research of graphene from coal tar pitch. In Proceedings of the 2022 International Conference on Optoelectronic Information and Functional Materials (OIFM 2022), Chongqing, China, 18–20 March 2022; Volume 12255, pp. 458–462. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Q. The positive influence of boron-doped graphene with pyridine as a probe molecule on SERS: A density functional theory study. J. Mater. Chem. 2012, 22, 15336–15341. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).