Non-Thermal Plasma as a Biomass Pretreatment in Biorefining Processes
Abstract
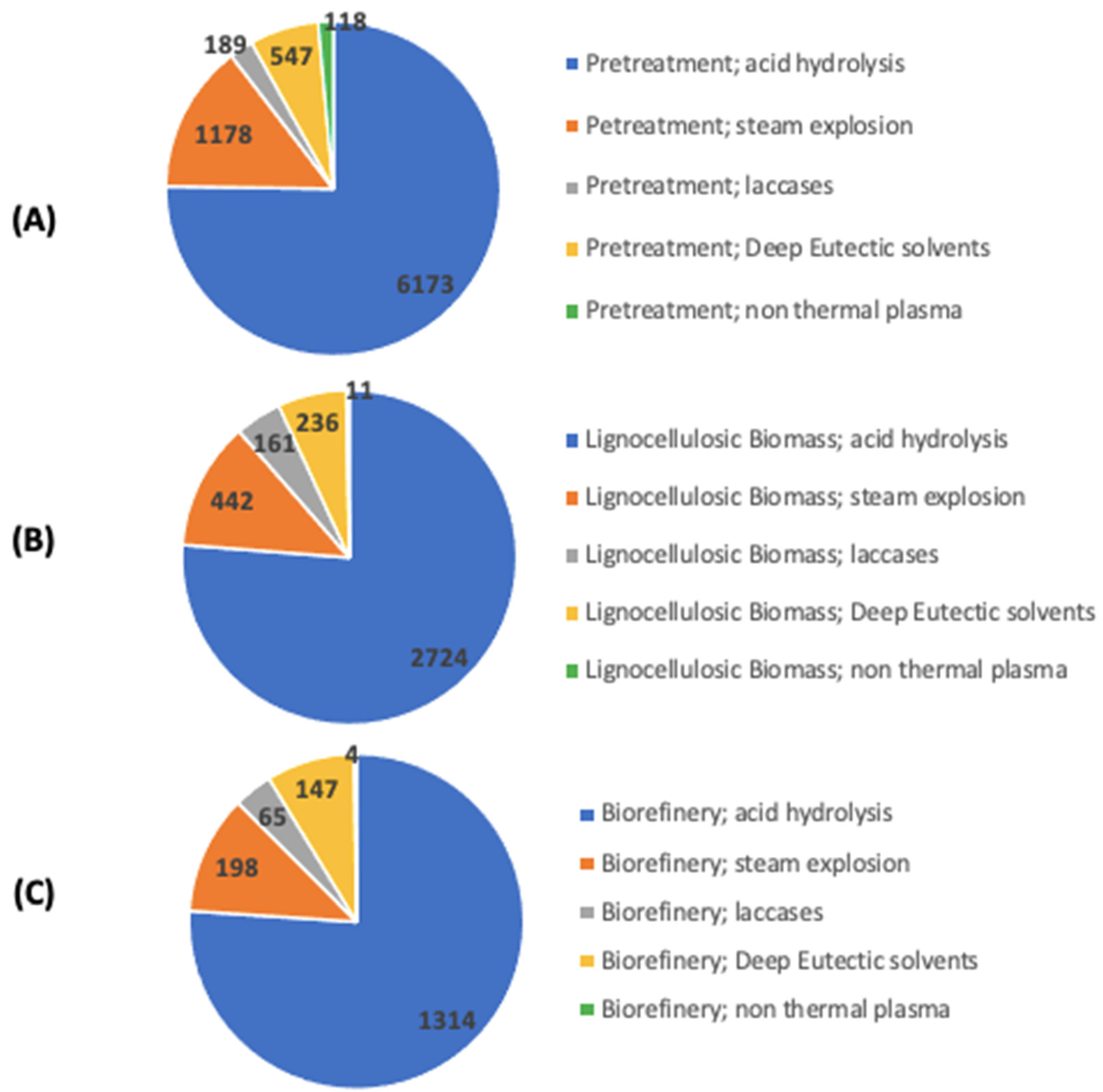
1. Introduction
2. Feedstocks for Biorefining Processes
3. Feedstock Pretreatments
3.1. Conventional Pretreatments
- Chemical (organosolvents, acid/alkaline solutions, ionic solvents, etc.);
- Physicochemical (steam explosion, ammonia fiber explosion, CO2 explosion, etc.);
- Physical (extrusion, milling);
- Biological (enzymes or micro-organisms).
3.2. Non-Conventional Pretreatments
- Le Chatelier’s principle: if the pressure changes, the volume also changes;
- The isostatic principle: pressure is transmitted throughout the biomass [39].
4. Plasma Technology
4.1. Chemical Effects of Non-Thermal Plasma
4.2. Configuration of the Non-Thermal Plasma Reactor
- Their geometrical configuration is simple;
- They can be easily used for industrial applications;
- The plasma conditions are stable and reproducible;
- They can work at atmospheric pressure.
4.3. Application of Non-Thermal Plasma in Biorefineries
4.4. Chemistry of Non-Thermal Plasma Pretreatment
5. Conclusions
6. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Department of Economic and Social Affairs. World Population Prospects 2019: Highlights; United Nations Department for Economic and Social Affairs: New York, NY, USA, 2019; Volume 11, p. 125.
- Mathimani, T.; Mallick, N. A comprehensive review on harvesting of microalgae for biodiesel–key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Kannah, Y.; Bhatia, S.K.; Kumar, A.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar]
- Singh, T.A.; Sharma, M.; Sharma, M.; Sharma, G.D.; Passari, A.K.; Bhasin, S. Valorization of agro-industrial residues for production of commercial biorefinery products. Fuel 2022, 322, 124284. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Zhou, Y.; Stuart-Williams, H.; Farquhar, G.D.; Hocart, C.H. The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 2010, 71, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Gao, Y.; Uner, N.B.; Thimsen, E.; Foston, M.B. Accessing unconventional biofuels via reactions far from local equilibrium. Fuel 2018, 226, 472–478. [Google Scholar] [CrossRef]
- Sabat, K.C. Physics and Chemistry of Solid State Direct Reduction of Iron Ore by Hydrogen Plasma. Phys. Chem. Solid State 2021, 22, 292–300. [Google Scholar] [CrossRef]
- Pereira, G.N.; Cesca, K.; Vieira Cubas, A.L.; de Oliveira, D. Use of non-thermal plasma in lignocellulosic materials: A smart alternative. Trends Food Sci. Technol. 2021, 109, 365–373. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Zhang, J.; Yan, Z. Improved enzymatic hydrolysis of lignocellulosic biomass through pretreatment with plasma electrolysis. Bioresour. Technol. 2014, 171, 469–471. [Google Scholar] [CrossRef]
- Lusi, A.; Hu, H.; Bai, X. Producing high yield of levoglucosan by pyrolyzing nonthermal plasma-pretreated cellulose. Green Chem. 2020, 22, 2036–2048. [Google Scholar]
- Souza-Corrêa, J.; Oliveira, C.; Nascimento, V.; Wolf, L.; Gómez, E.; Rocha, G.; Amorim, J. Atmospheric pressure plasma pretreatment of sugarcane bagasse: The influence of biomass particle size in the ozonation process. Appl. Biochem. Biotechnol. 2014, 172, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.; Ennaert, T.; Vanhulsel, A.; Sels, B. Unconventional pretreatment of lignocellulose with low-temperature plasma. ChemSusChem 2017, 10, 14–31. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, M.; Yang, P.; Chen, M.; Wang, S.; Hua, H.; Chen, W.; Zhou, X. Atmospheric low-temperature plasma-induced changes in the structure of the lignin macromolecule: An experimental and theoretical investigation. J. Agric. Food Chem. 2019, 68, 451–460. [Google Scholar] [CrossRef]
- Shao, S.; Ye, Z.; Sun, J.; Liu, C.; Yan, J.; Liu, T.; Li, X.; Zhang, H.; Xiao, R. A review on the application of non-thermal plasma (NTP) in the conversion of biomass: Catalyst preparation, thermal utilization and catalyst regeneration. Fuel 2022, 330, 125420. [Google Scholar] [CrossRef]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Willke, T.; Skiadas, I.; Van Ree, R.; de Jong, E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Biorefin. 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Cherubini, F.; Ulgiati, S. Crop residues as raw materials for biorefinery systems–A LCA case study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Smeets, E.M.; Lewandowski, I.M.; Faaij, A.P. The economical and environmental performance of miscanthus and switchgrass production and supply chains in a European setting. Renew. Sustain. Energy Rev. 2009, 13, 1230–1245. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962. [Google Scholar] [CrossRef]
- Cardona, C.; Quintero, J.; Paz, I. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Yadav, S.K. Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Chang, V.S.; Burr, B.; Holtzapple, M.T. Lime pretreatment of switchgrass. In Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 1997; pp. 3–19. [Google Scholar]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Alberts, G.; Ayuso, M.; Bauen, A.; Boshell, F.; Chudziak, C.; Gebauer, J.P.; German, L.; Kaltschmitt, M.; Nattrass, L.; Ripken, R. Innovation Outlook: Advanced Liquid Biofuels; International Renewable Energy Agency (IRENA): New York, NY, USA, 2016.
- Mathew, A.K.; Chaney, K.; Crook, M.; Humphries, A.C. Dilute acid pre-treatment of oilseed rape straw for bioethanol production. Renew. Energy 2011, 36, 2424–2432. [Google Scholar] [CrossRef]
- Rai, R.; Bibra, M.; Chadha, B.; Sani, R.K. Enhanced hydrolysis of lignocellulosic biomass with doping of a highly thermostable recombinant laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Gelosia, M.; Giannoni, T.; Temporim, R.B.L.; Nicolini, A.; Cotana, F.; Bertini, A. Acid-catalyzed steam explosion for high enzymatic saccharification and low inhibitor release from lignocellulosic cardoon stalks. Biochem. Eng. J. 2021, 174, 108121. [Google Scholar] [CrossRef]
- Gu, B.-J.; Wang, J.; Wolcott, M.P.; Ganjyal, G.M. Increased sugar yield from pre-milled Douglas-fir forest residuals with lower energy consumption by using planetary ball milling. Bioresour. Technol. 2018, 251, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.K.; Chaney, K.; Crook, M.; Humphries, A.C. Alkaline pre-treatment of oilseed rape straw for bioethanol production: Evaluation of glucose yield and pre-treatment energy consumption. Bioresour. Technol. 2011, 102, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Liyakathali, N.A.M.; Muley, P.D.; Aita, G.; Boldor, D. Effect of frequency and reaction time in focused ultrasonic pretreatment of energy cane bagasse for bioethanol production. Bioresour. Technol. 2016, 200, 262–271. [Google Scholar] [CrossRef]
- Gogate, P.R. Hydrodynamic cavitation for food and water processing. Food Bioprocess Technol. 2011, 4, 996–1011. [Google Scholar] [CrossRef]
- Madison, M.J.; Coward-Kelly, G.; Liang, C.; Karim, M.N.; Falls, M.; Holtzapple, M.T. Mechanical pretreatment of biomass–Part I: Acoustic and hydrodynamic cavitation. Biomass Bioenergy 2017, 98, 135–141. [Google Scholar] [CrossRef]
- Castañón-Rodríguez, J.; Torrestiana-Sánchez, B.; Montero-Lagunes, M.; Portilla-Arias, J.; de León, J.R.; Aguilar-Uscanga, M. Using high pressure processing (HPP) to pretreat sugarcane bagasse. Carbohydr. Polym. 2013, 98, 1018–1024. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Tayyab, M.; Noman, A.; Islam, W.; Waheed, S.; Arafat, Y.; Ali, F.; Zaynab, M.; Lin, S.; Zhang, H.; Lin, W. Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: A review. Appl. Ecol. Environ. Res 2018, 16, 225–249. [Google Scholar] [CrossRef]
- Miranda, F.; Rabelo, S.; Pradella, J.; Carli, C.D.; Petraconi, G.; Maciel, H.; Pessoa, R.; Vieira, L. Plasma in-liquid using non-contact electrodes: A method of pretreatment to enhance the enzymatic hydrolysis of biomass. Waste Biomass Valorization 2020, 11, 4921–4931. [Google Scholar] [CrossRef]
- Gabhane, J.; William, S.; Vaidya, A.N.; Anand, D.; Wate, S. Pretreatment of garden biomass by alkali-assisted ultrasonication: Effects on enzymatic hydrolysis and ultrastructural changes. J. Environ. Health Sci. Eng. 2014, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; Kamoei, D.V.; Ahmed, M.A.; da Silva, S.S.; Han, J.-I.; Dos Santos, J.C. A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason. Sonochem. 2018, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Lee, J.T.; Bai, H.-W.; Kim, U.-J.; Bae, H.-J.; Wi, S.G.; Cho, J.-Y. Enhanced enzymatic hydrolysis of poplar bark by combined use of gamma ray and dilute acid for bioethanol production. Radiat. Phys. Chem. 2012, 81, 1003–1007. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V.; Palma, V. Non-thermal plasma coupled with catalyst for the degradation of water pollutants: A review. Catalysts 2020, 10, 1438. [Google Scholar] [CrossRef]
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A review on non-thermal plasma treatment of water contaminated with antibiotics. J. Hazard. Mater. 2021, 417, 125481. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Prakash, G.V.; Ahammad, S.Z.; Satyananda, K.; Sreekrishnan, T. Development of low power non-thermal plasma jet and optimization of operational parameters for treating dyes and emerging contaminants. Plasma Sci. Technol. 2022, 24, 105501. [Google Scholar] [CrossRef]
- Zhao, Q.; Bu, D.; Li, Z.; Zhang, X.; Di, L. Cold Plasma Preparation of Pd/Graphene Catalyst for Reduction of p-Nitrophenol. Nanomaterials 2021, 11, 1341. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Application of Non-Thermal Plasma on Biofilm: A Review. Appl. Sci. 2019, 9, 3548. [Google Scholar] [CrossRef]
- Meichsner, J.; Schmidt, M.; Schneider, R.; Wagner, H.-E. Nonthermal Plasma Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bouregba, N.; Benmimoun, Y.; Meddah, B.; Tilmatine, A.; Ouldmoumna, A. Ozonation of wastewater in Algeria by dielectric barrier discharge. Desalin. Water Treat. 2016, 57, 1824–1835. [Google Scholar] [CrossRef]
- Piferi, C.; Barni, R.; Roman, H.E.; Riccardi, C. Current filaments in asymmetric surface dielectric barrier discharge. Appl. Sci. 2021, 11, 2079. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Delikonstantis, E.; Stefanidis, G.; Vlachos, D. Plasma technology for lignocellulosic biomass conversion toward an electrified biorefinery. Green Chem. 2022, 24, 2680–2721. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Shaghaleh, H.; Xu, X.; Liu, H.; Wang, S.; Hamoud, Y.A.; Dong, F.; Luo, J. The effect of atmospheric pressure plasma pretreatment with various gases on the structural characteristics and chemical composition of wheat straw and applications to enzymatic hydrolysis. Energy 2019, 176, 195–210. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Kádár, Z.; Thomsen, A.B.; Bindslev, H.; Leipold, F. Plasma-assisted pretreatment of wheat straw for ethanol production. Appl. Biochem. Biotechnol. 2011, 165, 1010–1023. [Google Scholar] [CrossRef]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic feedstock: A review of a sustainable platform for cleaner production of nature’s plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Hayashi, J.i.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Kou, L.; Zhang, X.; Tan, T. Bioethanol production from cellulose obtained from the catalytic hydro-deoxygenation (lignin-first refined to aviation fuel) of apple wood. Fuel 2019, 250, 245–253. [Google Scholar] [CrossRef]
- García-Cubero, M.T.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 2009, 100, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, C.; Lu, P.; Behan, P.; Bourke, P.; Cullen, P. Humic acid and trihalomethane breakdown with potential by-product formations for atmospheric air plasma water treatment. J. Ind. Eng. Chem. 2018, 59, 350–361. [Google Scholar] [CrossRef]
- Schoemaker, H.; Leisola, M. Degradation of lignin by Phanerochaete chrysosporium. J. Biotechnol. 1990, 13, 101–109. [Google Scholar] [CrossRef]
- Crema, A.P.S.; Borges, L.D.P.; Micke, G.A.; Debacher, N.A. Degradation of indigo carmine in water induced by non-thermal plasma, ozone and hydrogen peroxide: A comparative study and by-product identification. Chemosphere 2020, 244, 125502. [Google Scholar] [CrossRef]
- Su, Z.; Kim, H.-H.; Tsutsui, M.; Takashima, K.; Mizuno, A. OH radical generation by atmospheric pressure plasma and its quantitative analysis by monitoring CO oxidation. In Proceedings of the Conference Record of the 1999 IEEE Industry Applications Conference—Thirty-Forth IAS Annual Meeting, Phoenix, AZ, USA, 3–7 October 1999; pp. 1473–1477. [Google Scholar]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour. Technol. 2017, 239, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Lu, P.; Cullen, P.; Bourke, P.; Jaiswal, A.K. Improving enzymatic hydrolysis of brewer spent grain with nonthermal plasma. Bioresour. Technol. 2019, 282, 520–524. [Google Scholar] [CrossRef]
- Wright, A.; Bandulasena, H.; Ibenegbu, C.; Leak, D.; Holmes, T.; Zimmerman, W.; Shaw, A.; Iza, F. Dielectric barrier discharge plasma microbubble reactor for pretreatment of lignocellulosic biomass. AIChE J. 2018, 64, 3803–3816. [Google Scholar] [CrossRef]


| Pretreatment | Energy | Average Time (min) | Wastes | Production of Fermentation Inhibitors | Results | Limitations to Improvement | TRL | Reference |
|---|---|---|---|---|---|---|---|---|
| Organosolv treatment | 0.02–0.04 kWh/mol of xylan | 10–90 | Residual solvents | Yes | 55% of xylan was obtained, starting from 10 g of a dry agro-fiber crop (Arundo donax L.) treated for 30 min | Recovery and reuse of chemicals Develop methods to add value to lignin High capital and operating costs | 4–6 | [30] |
| Dilute acid hydrolysis | 1.46 kWh/kg of glucose | 10–90 | Residual acids | Yes | About 220.7 g of glucose kg−1 biomass was obtained after 30 min | Developing micro-organisms more tolerant to inhibitors | 5–7 | [31] |
| Laccases | - | 4320 | No | No | About 73.75–76.6% of glucose was produced, starting from 2.5 g of corn stover | Development of robust micro-organisms | 3–4 | [32] |
| Steam explosion | 12.03 kWh/kg of fermentable carbohydrates | 1–3 | No | Yes | 76% of glucose was obtained, starting from 100 g of cardoon after 10 min. | Development of new catalysts Developing micro-organisms more tolerant of inhibitors | 6–8 | [33] |
| Milling | 0.50–2.15 kWh/kg of glucose extracted | 30 | No | No | About 24.45–59.67% of glucose was obtained, starting from 250 g of Douglas fir forestry residues treated for 7–30 min. | Integration of the process, combined with mild chemical treatments | 5–6 | [34] |
| Alkaline treatment | 0.71 kWh/kg of glucose | 10–90 | Residual alkaline solution | Yes | 440.6 g of glucose kg−1 biomass was obtained after 30 min. | Recovery and reuse of chemicals | 5–7 | [35] |
| Pretreatment | Energy | Average Time (min) | Wastes | Production of Fermentation Inhibitors | Results | Limitations to Improvement | TRL | Reference |
|---|---|---|---|---|---|---|---|---|
| Non-thermal plasma | 60 kWh/kg of biomass | 60–420 | No | No | 51.3% of glucose was obtained from 10 g of dry raw sugarcane bagasse after 120 min | Homogeneous and heterogeneous catalysts should be used to reduce the process the time. The reactor’s configurations should be optimized to achieve high pretreatment efficiency | 2–4 | [43] |
| Ultrasound | 75 kW/kg of biomass 12.5 kHz/g of biomass | 10–80 | No | No | Around 59.56% of reducing sugar was released from 2 g of dry garden biomass after 60 min of treatment | Reducing the energy loss by using a proper configuration | 2–4 | [44] |
| Hydrodynamic cavitation | 55 kW/kg of biomass | 10–50 | No | No | 67.61% of glucose was released from 20 g of dry sugarcane bagasse after 10 min of treatment | Optimizing the operating conditions such as the liquid’s flow rate, the inlet pressure, and the number of recirculation passes across the cavitation zone | 2–4 | [45] |
| Microwave irradiation | 58.47 kW/kg of biomass | 5–30 | No | Yes | Around 75.4% of reducing sugars was obtained from 5.13 g of maize stillage after 10 min of treatment | Reducing high-capital investments through the use of an optimal system to generate microwaves | 2-4 | [46] |
| Gamma irradiation | 200 kGy/mg of biomass | 10–80 | No | No | 75.4% of reducing sugars was released from 5 mg of poplar bark. | The dosage of gamma rays should be optimized to achieve high pretreatment efficiency | 2–4 | [47] |
| Substrate | Non-Thermal Plasma | Sequential Pretreatments | Main Results | References | |
|---|---|---|---|---|---|
| Acid Hydrolysis | Enzymatic Hydrolysis | ||||
| Wheat straw | The dielectric barrier discharge was driven by an alternating current (AC) power supply (adjustable between 10 and 40 kHz). The frequency was set to 18.4 kHz | - | The enzymatic activity of Celluclast was 108 filter paper units (FPU) per cubic centimeter Enzymatic conversion of plasma-pretreated solids (not washed and washed) was performed | The amount of glucose that was released was between 20 g/100 g (6 h of enzymatic hydrolysis of samples that had been pretreated for 1 h) and 30 g/100 g (48 h of enzymatic hydrolysis of samples that had been pretreated for 7 h). | [61] |
| Wheat straw | The dielectric barrier discharge was driven by an alternating current (AC) power supply (adjustable between 10 and 40 kHz). The frequency was set to 18.4 kHz | Dried and milled samples (160 mg) were treated with 72% (w/w) H2SO4 (1.5 mL) at 30 °C | - | Dried and milled samples (160 mg) were treated with 72% (w/w) H2SO4 (1.5 mL) at 30 °C | [61] |
| Spent coffee waste | Pre-treatment was performed in a dielectric barrier discharge plasma reactor. The coffee waste samples were subjected to non-thermal plasma in triplicate for 2 min, 4 min, and 6 min at three discrete voltages of 60 kV, 70 kV, and 80 kV | - | Cellulose enzymes with an enzyme activity of 77 FPU/mL were used | 268.68 mg of reducing sugar/g of spent coffee waste were obtained after 80 kV and 4 min of NTP pre-treatment | [71] |
| Sugarcane bagasse | The reactor consisted of a glass container and a Teflon cover supporting four electrodes. The reactor was powered by an alternating high-voltage power supply (14 kV), with frequency of 60 Hz and current of 30 mA | - | The enzymatic cocktail used in the experiments was a commercial cellulolytic enzyme complex (Accelerase 1500, Genencor, CA, USA) | Sugar extraction yields were around 13%. | [43] |
| Wasted grain brewer | Pretreatment was performed in a submerged dielectric barrier discharge (DBD) plasma reactor. Three voltages (22 kV, 25 kV, and 28 kV) were tested for different durations (5, 10, and 15 min) | - | Cellulose and hemicellulose enzymes with an enzyme activity of 77.08 FPU/mL and 72.23 U/mL. respectively, were used. | 162.9 mg of reducing sugars/g of biomass were obtained after NTP pre-treatment at 28 kV for 10 min | [72] |
| Miscanthus grass | The reactor incorporated a DBD plasma module which consisted of two electrodes. The electrical discharge was driven by a custom-built full-bridge resonant power supply that delivered a sinusoidal voltage of 16.4 kVRMS at 21.2 kHz | - | 7.5–30 filter paper units (FPU)/g glucan of Cellic CTec2 cellulase and 100 U/g of SEB xylanase (Advanced Enzymes technology LTD) were used | 26% of sugar was released after 3 h | [73] |
| Ball-milled wooden lignin (MWL) derived from corncobs and poplar | Atmospheric dielectric barrier discharge (DBD) plasma (air was the feed gas), 4.5 kW | [16] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meoli, C.M.; Iervolino, G.; Procentese, A. Non-Thermal Plasma as a Biomass Pretreatment in Biorefining Processes. Processes 2023, 11, 536. https://doi.org/10.3390/pr11020536
Meoli CM, Iervolino G, Procentese A. Non-Thermal Plasma as a Biomass Pretreatment in Biorefining Processes. Processes. 2023; 11(2):536. https://doi.org/10.3390/pr11020536
Chicago/Turabian StyleMeoli, Carmen Maria, Giuseppina Iervolino, and Alessandra Procentese. 2023. "Non-Thermal Plasma as a Biomass Pretreatment in Biorefining Processes" Processes 11, no. 2: 536. https://doi.org/10.3390/pr11020536
APA StyleMeoli, C. M., Iervolino, G., & Procentese, A. (2023). Non-Thermal Plasma as a Biomass Pretreatment in Biorefining Processes. Processes, 11(2), 536. https://doi.org/10.3390/pr11020536










