Enzymatic Hydrolysis of Complex Carbohydrates and the Mucus in a Mathematical Model of a Gut Reactor
Abstract
1. Introduction
2. Model Formulation
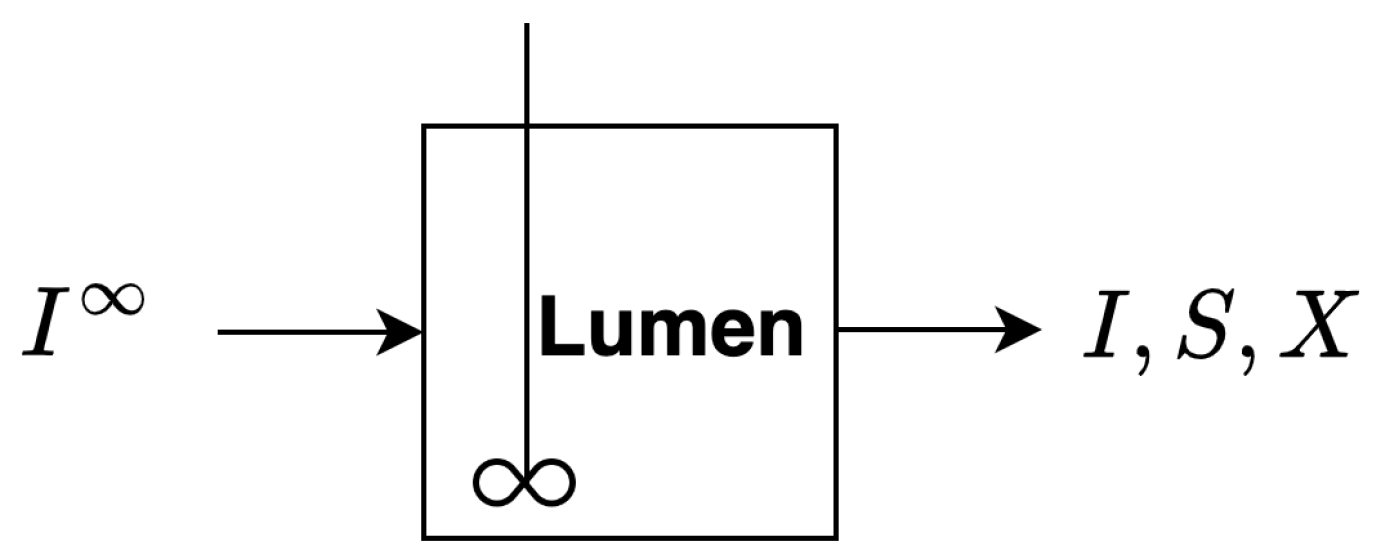
2.1. Single Compartment Model
2.1.1. Basic Model Assumptions
- A1
- The reactor is fully mixed and contains fiber, sugar and bacteria. There is constant inflow of a fiber into the reactor and constant outflow of fiber, sugar and biomass from the reactor.
- A2
- Fiber is exogenously broken down into digestible monosaccharide sugar by the bacteria.
- A3
- Sugar is a growth limiting substrate consumed by the bacteria. The growth of bacteria is proportional to the uptake of sugar.
- A4
- Bacteria die through first-order decay.
2.1.2. Governing Equations
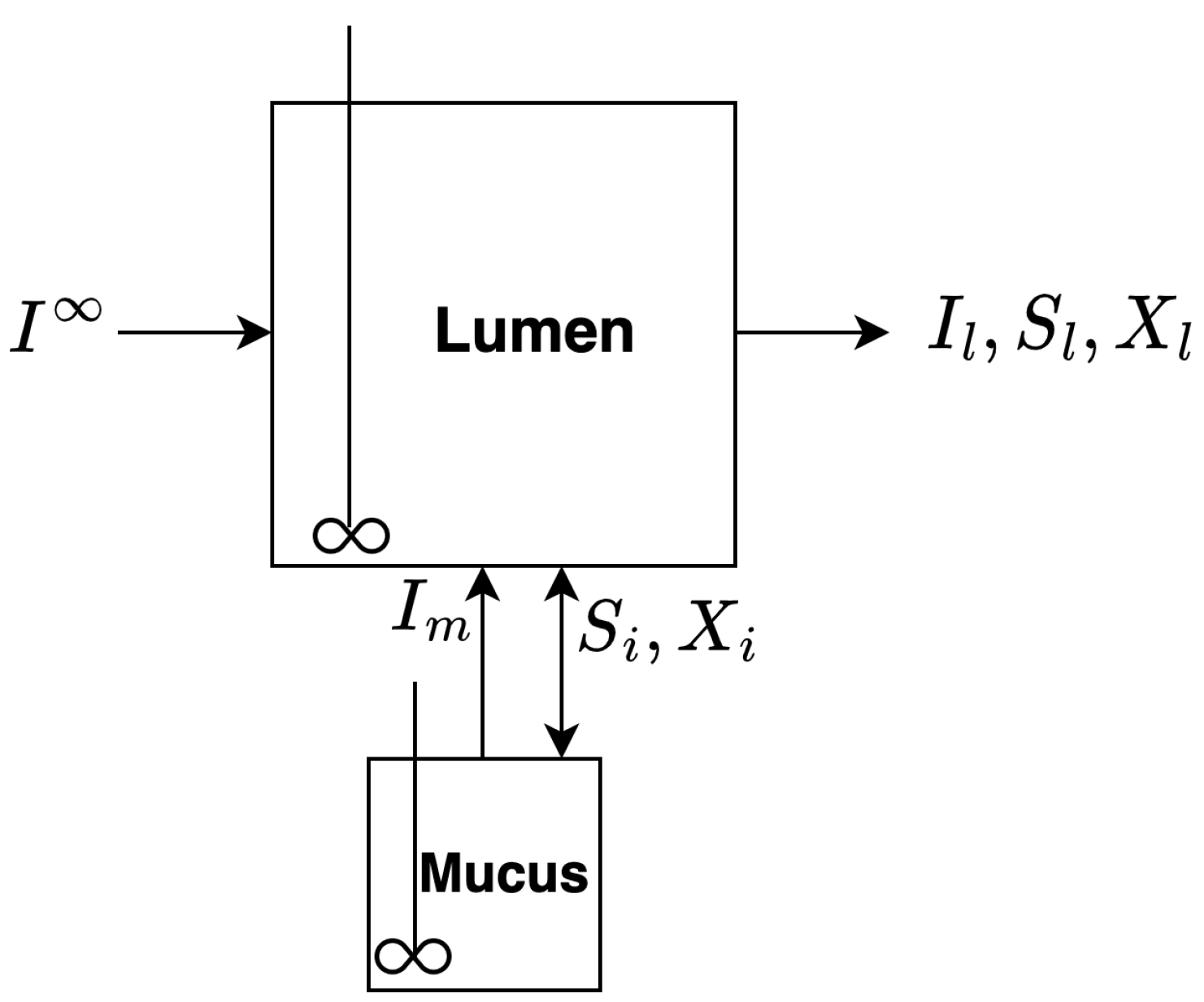
2.2. Dual Compartment Model
2.2.1. Basic Model Assumptions
- A1
- The reactor consists of two compartments of different volumes. Both reactor compartments are fully mixed and contain fiber, sugar and bacteria. The main compartment of the reactor represents the lumen of the colon. The second compartment of the reactor represents the mucus lining of the colon. There is constant inflow of a fiber into the lumen compartment and constant outflow of fiber, sugar and biomass from the lumen compartment. There is transport through the mucus compartment.
- A2
- Fiber is exogenously broken down into digestible monosaccharide sugar by the bacteria.
- A3
- Sugar is a growth limiting substrate consumed by the bacteria. The growth of bacteria is proportional to the uptake of sugar.
- A4
- Bacteria die through first-order decay.
- A5
- Mucins, which are analogous to fiber, are endogenously produced by the host in the mucus compartment.
- A6
- There is bi-directional exchange of all components between the lumen and mucus, excluding fiber. Mucins in the mucus compartment are sloughed into the lumen compartment, but there is no exchange of fiber from the lumen to the mucus compartment. Exchange is linear and donor-controlled.
2.2.2. Governing Equations
3. Model Analysis
3.1. Single Compartment Analysis
3.2. Dual Compartment Analysis
4. Numerical Results
4.1. Single Compartment
4.1.1. Typical Simulations
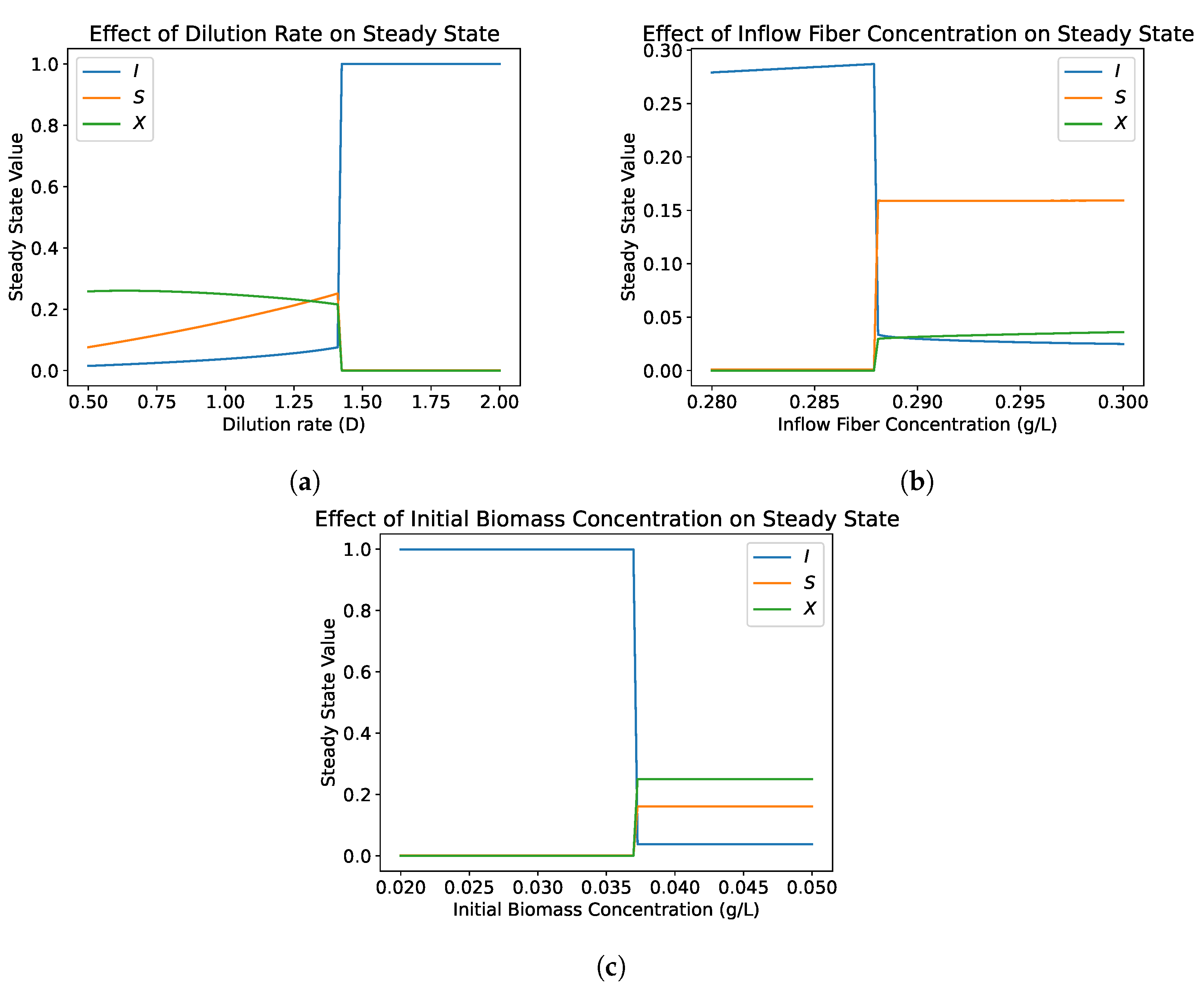
4.1.2. Bifurcation Analysis
4.2. Dual Compartment
4.2.1. Typical Simulations
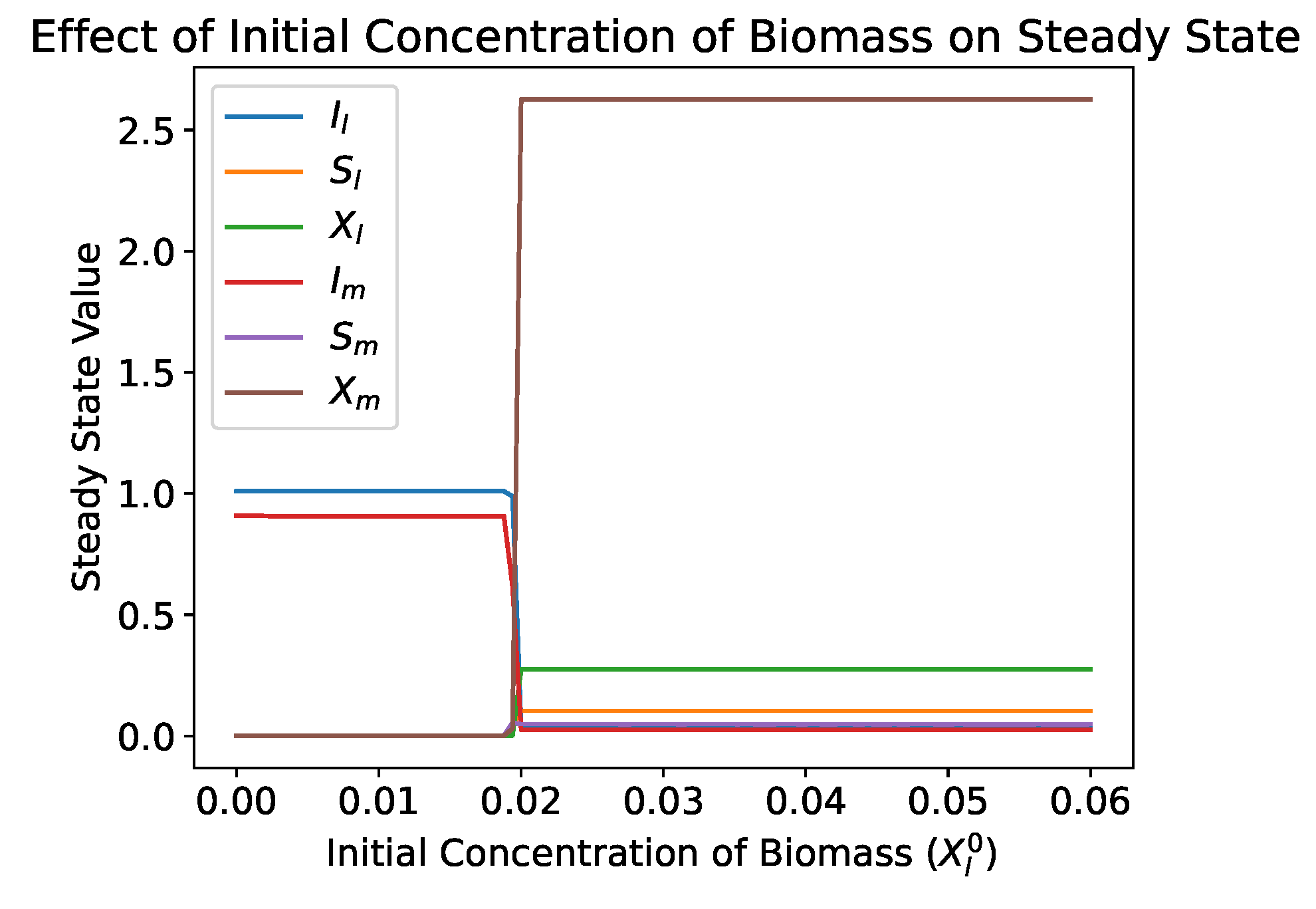
4.2.2. Role of Mucus Compartment
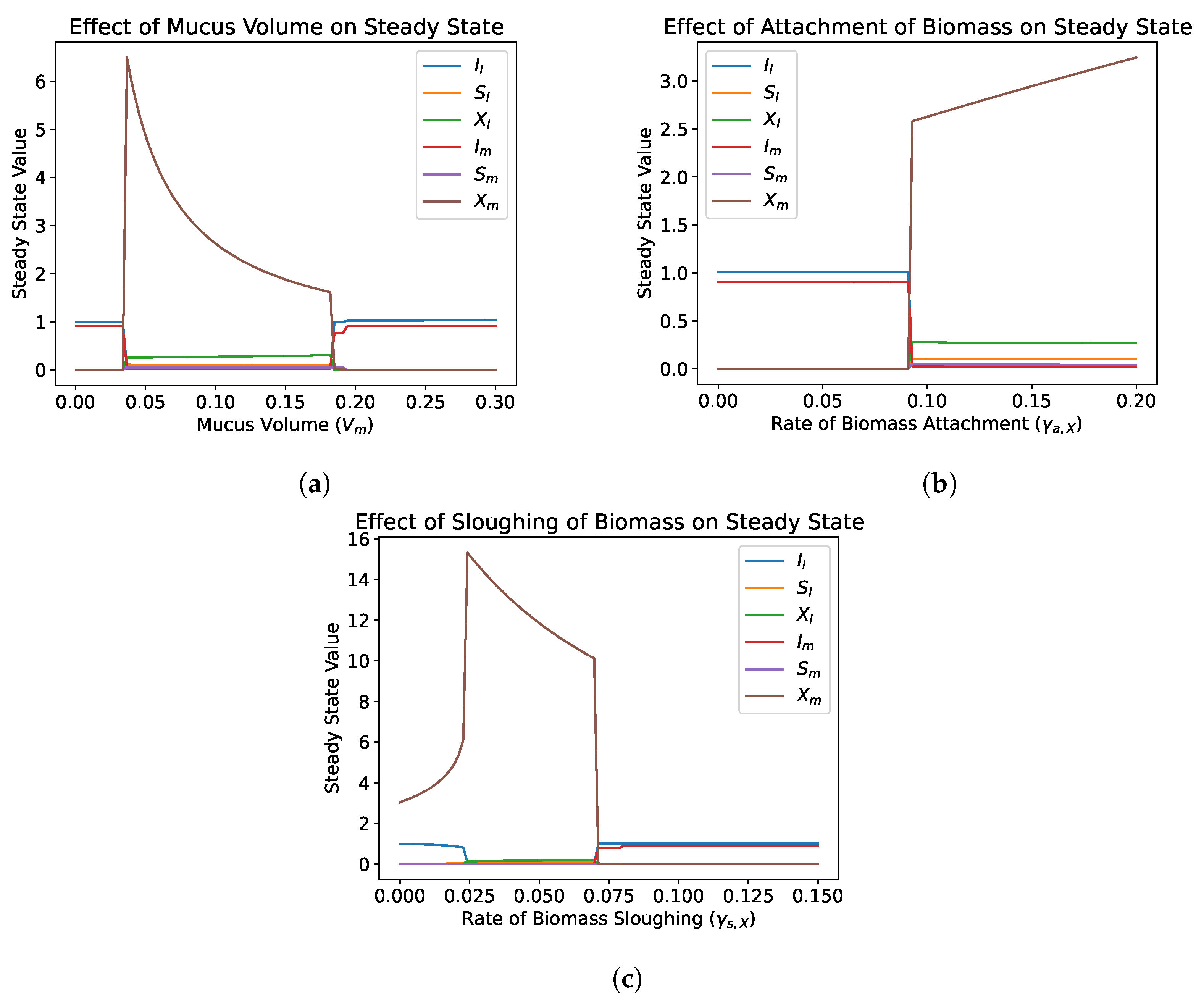
4.2.3. Role of Transfer Parameters
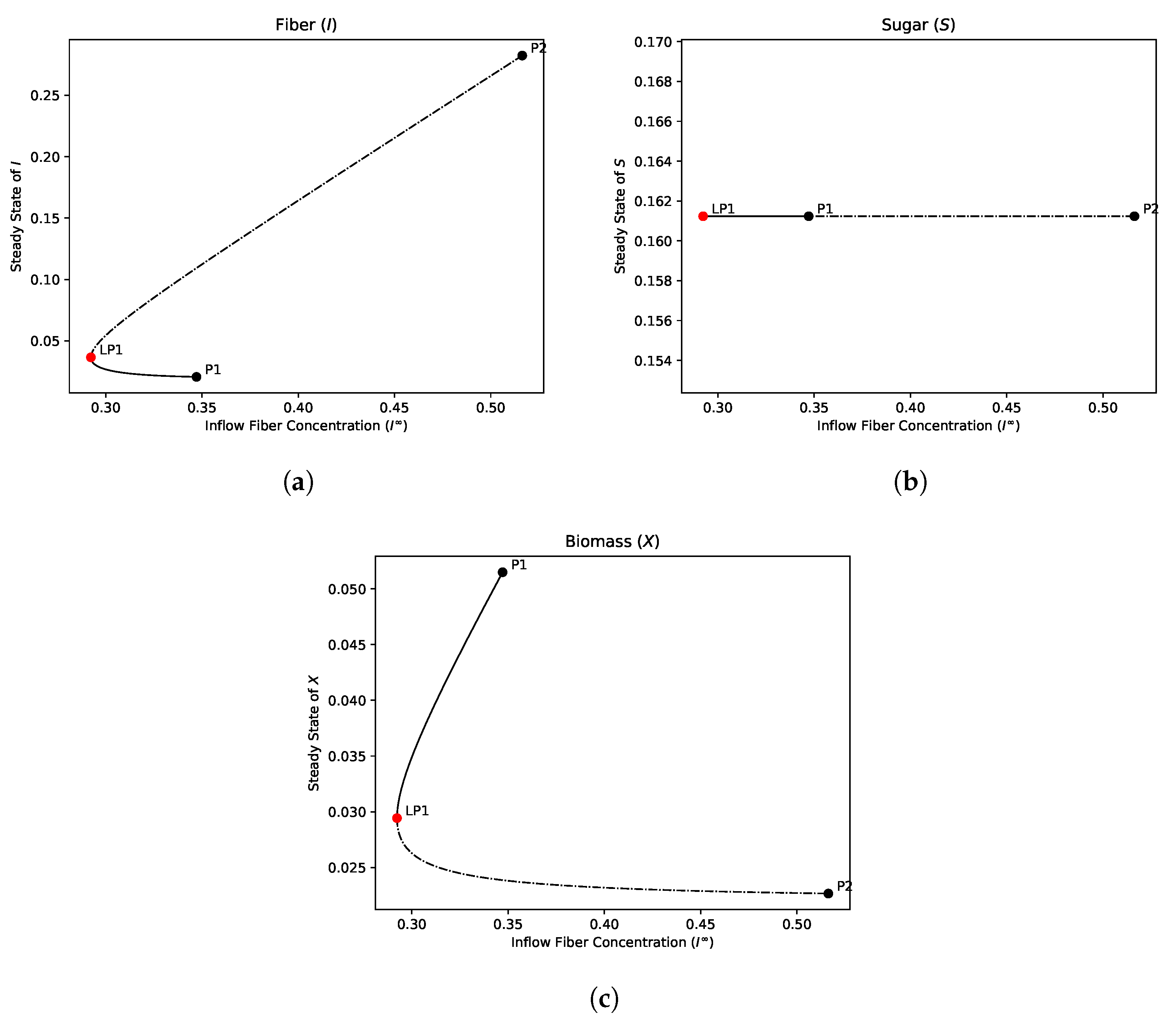
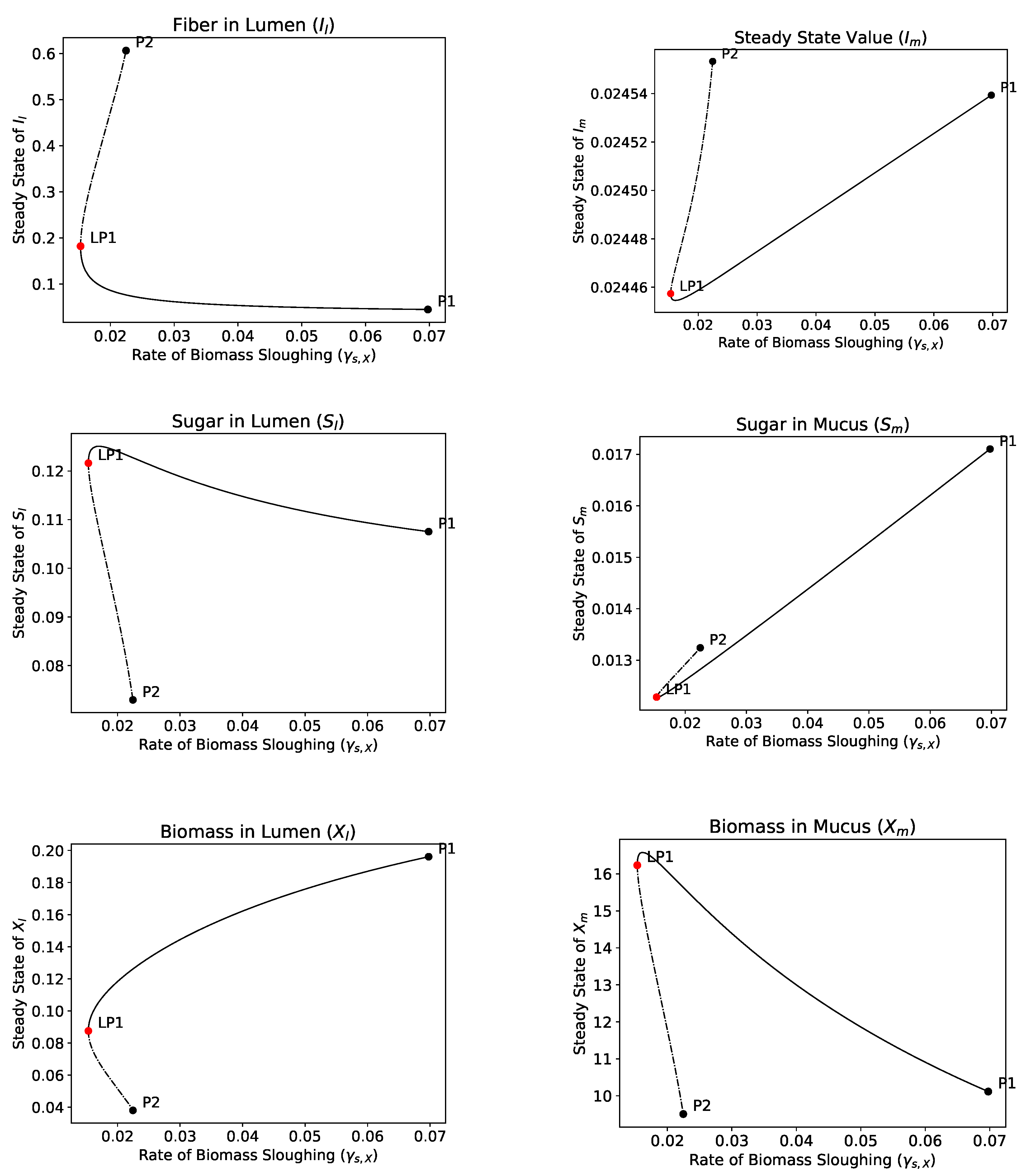
4.2.4. Bifurcation Analysis
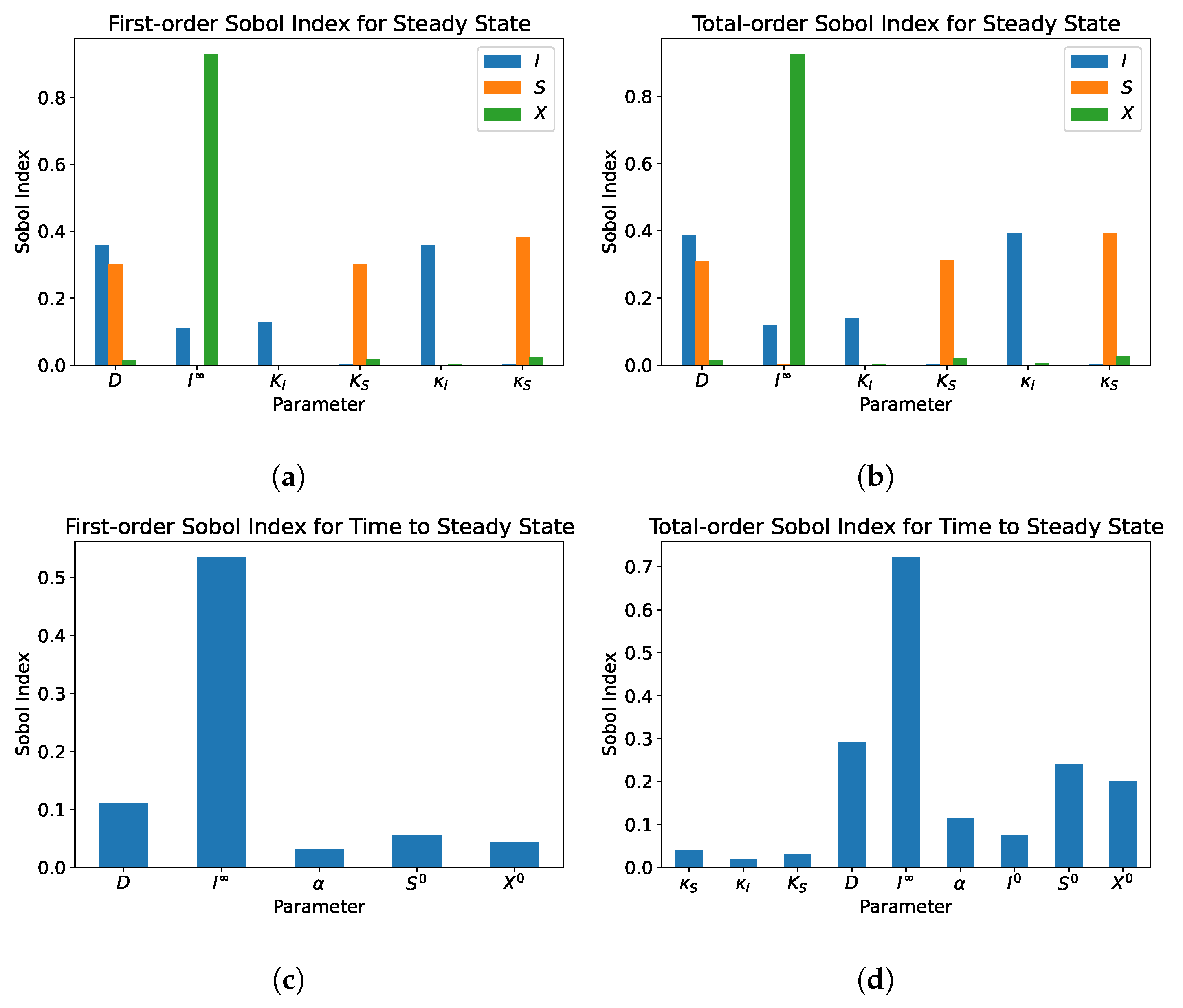
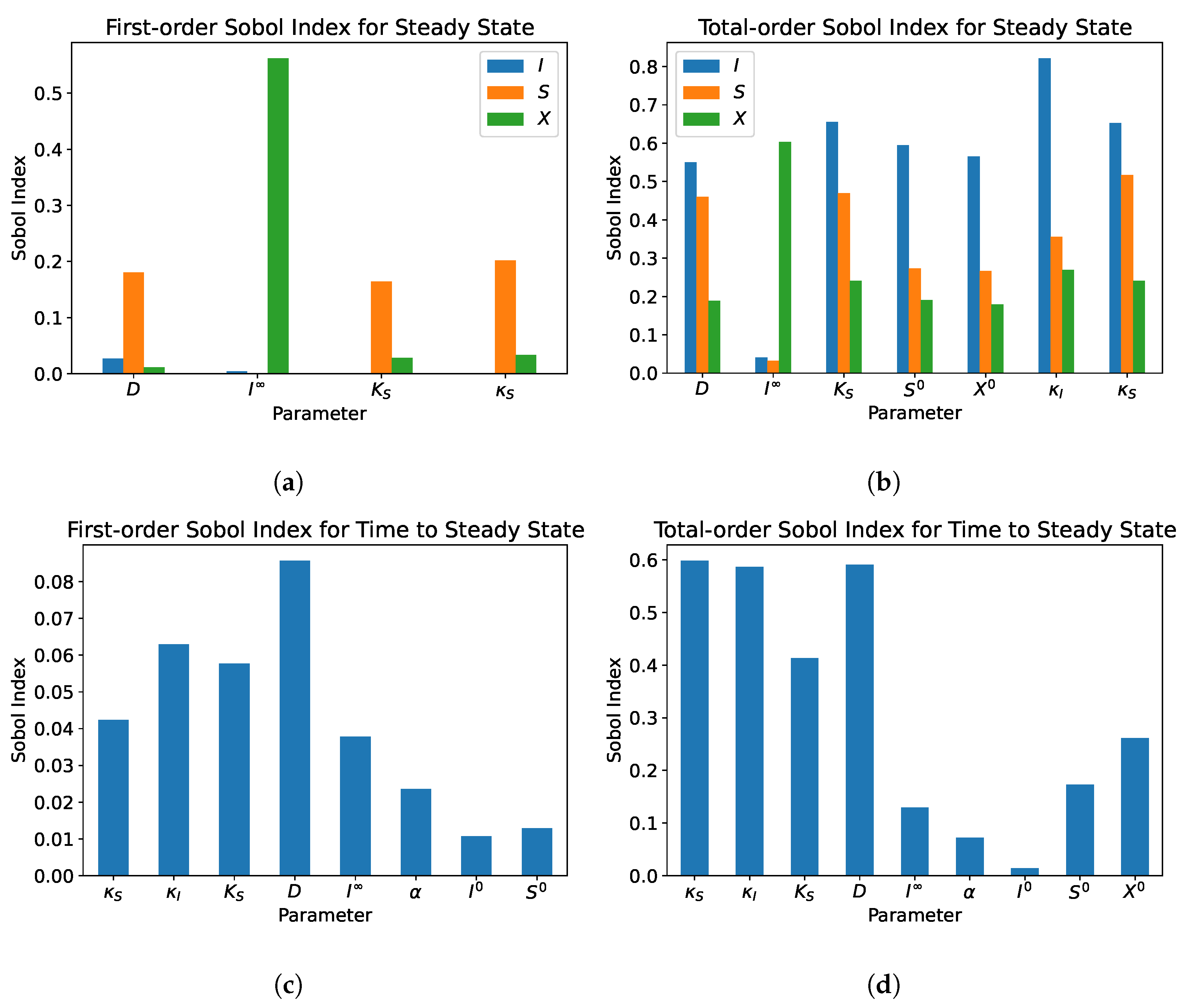
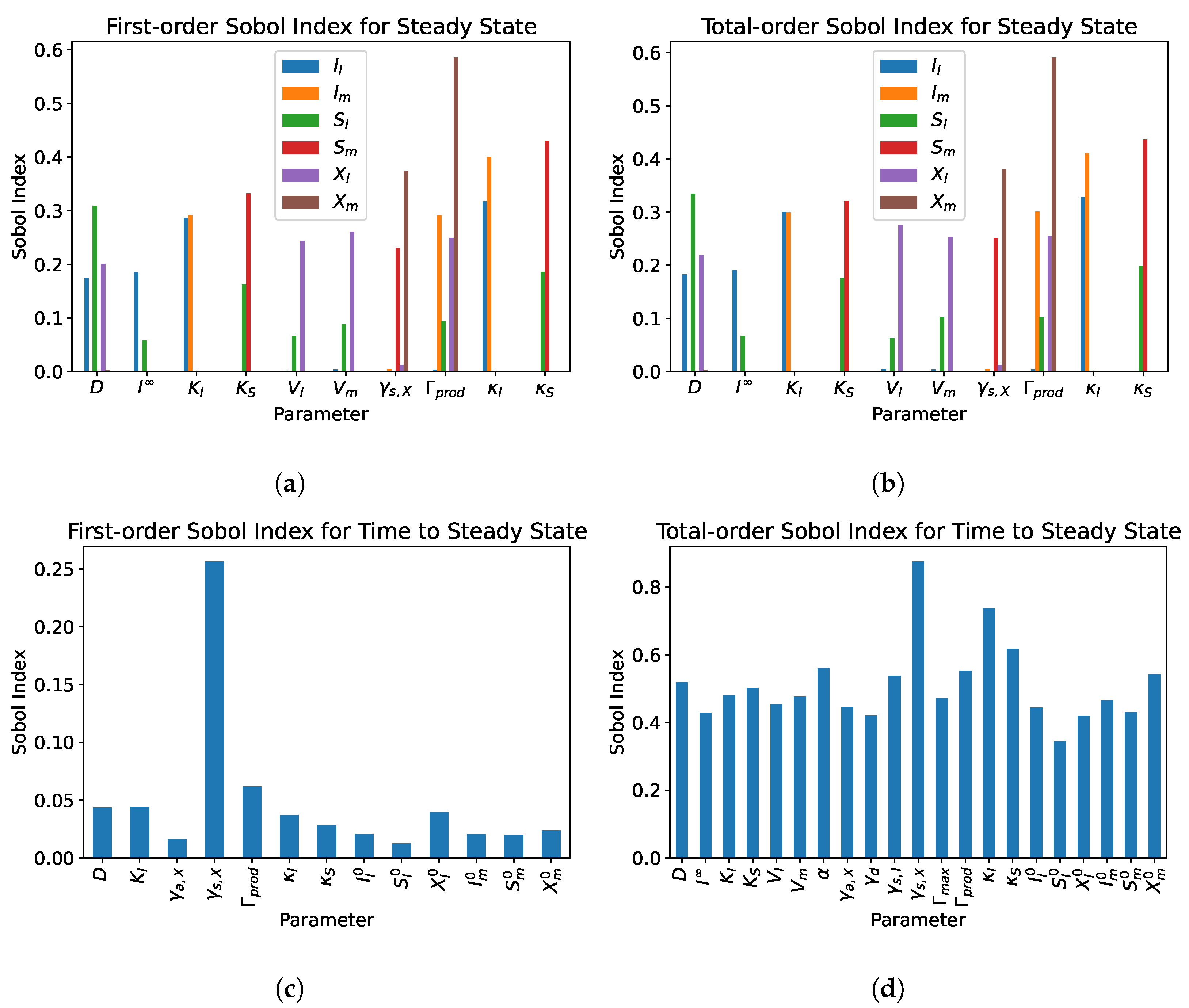
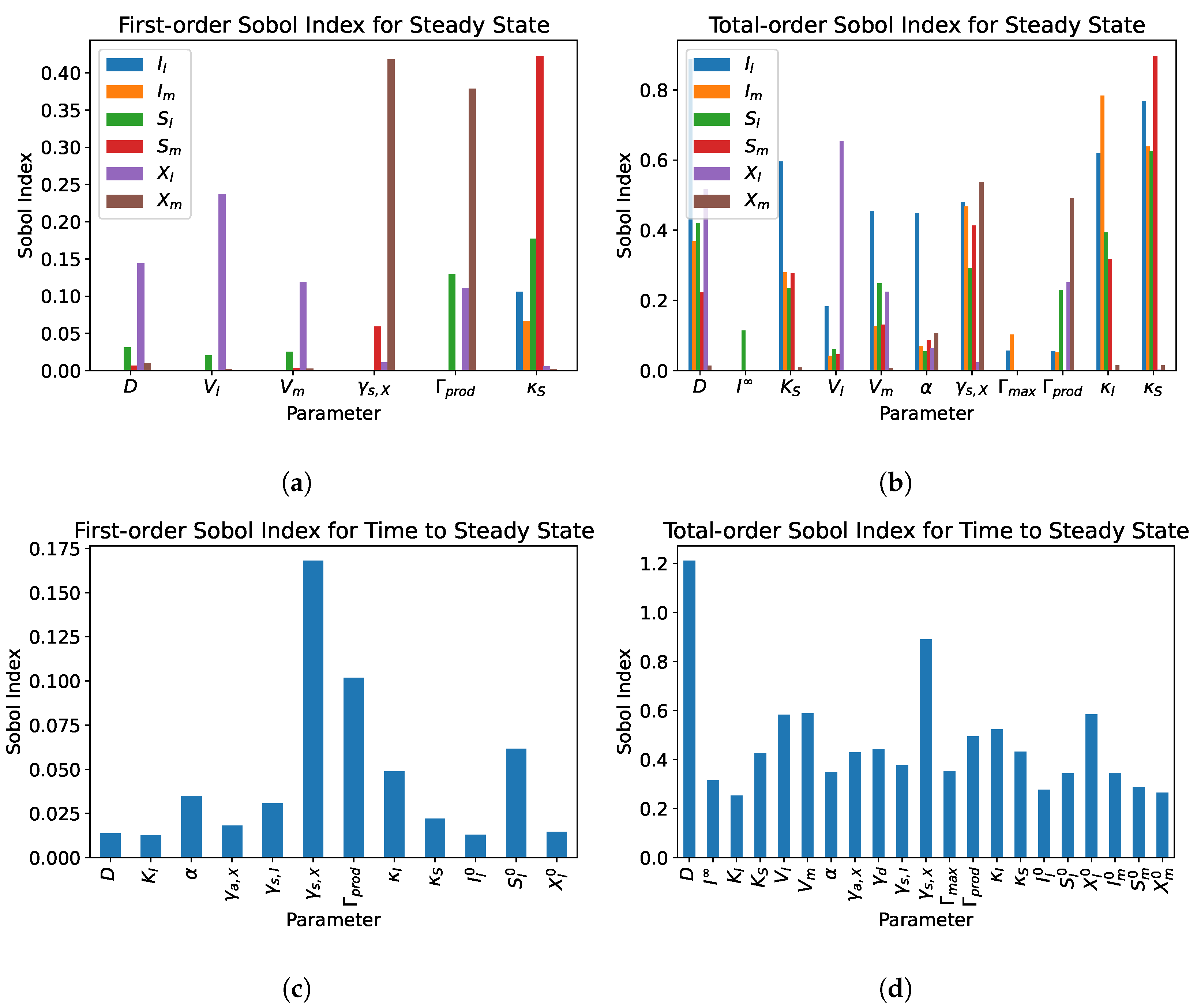
5. Sensitivity Analysis
5.1. Single Compartment
5.2. Dual Compartment Model
6. Discussion
6.1. Primary Degradation
6.2. Mucus Compartment
6.3. Importance of Parameters
6.4. Implications for Mathematical Models of the Gut
7. Conclusions
- In both the single and dual compartment models, the washout equilibrium is unconditionally asymptotically stable. If the biomass concentration is within the basin of attraction of the washout equilibrium, this will result in the washout of the biomass species. This can be due to a perturbation, such as antibiotic treatment, or it can be due to inoculating the reactor with too low of an initial concentration of biomass. This is contrary to the standard single species chemostat model in which the washout equilibrium is unstable when a nontrivial equilibrium exists and survival does not depend on the initial concentration of the biomass.
- The hydrolysis step results in bistability in both the single and dual compartment model. A saddle-node bifurcation appears when the positive equilibrium emerges. This process should not be neglected in mathematical models of the gut and experimental gut reactor systems, particularly in studies of dysbiosis in the gut.
- Numerical simulations show that the addition of the lateral diffusive chamber allows for the survival of microbial species under conditions that would normally result in washout. This depends primarily on the transfer parameters, which can be optimized to maximize the regions that allow for survival. The inclusion of a representation of the mucus layer should be considered in mathematical gut reactor models and experimental gut reactor models.
- Fiber and mucins were considered to be metabolically equivalent in terms of the microbial species that degrade them and the yield and type of monomeric sugars produced. It was also assumed that all primary degraders in the gut can be simplified into a single microbial group that can degrade all complex carbohydrates and reside in both the lumen and mucus environments. In the human gut, there are both generalist and specialist species, each with varying degrees of substrate and environment preferences. For example, abundant generalist species such as Bacteroides thetaiotaomicron are able to shift from dietary polysaccharides to mucus glycans in the absence of fiber, while lower abundance species such as Akkermansia muciniphila can be considered mucus specialists [7]. Additionally, low fiber diets can result in shifts in microbial composition and mucus defects. Future mathematical models should consider the representation of multiple primary degraders, multiple polysaccharides and a dynamic mucus layer to study this interplay.
- From the sensitivity analysis of the single and dual compartment models, different parameters are of importance depending on the desired outcome and should be considered when including therapeutics in simulation design and experiments, e.g., increasing abundance of a target species or preventing the washout of a species.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADM1 | Anaerobic Digestion Model 1 |
| CSTR | Continuous stirred-tank reactor |
| SCFA | Short-chain fatty acid |
References
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forana, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Crost, E.H.; Le Gall, G.; Laverde-Gomez, J.A.; Mukhopadhya, I.; Flint, H.J.; Juge, N. Mechanistic insights into the cross-feeding of Ruminococcus gnavus and Ruminococcus bromii on host and dietary carbohydrates. Front. Microbiol. 2018, 9, 2558. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Li, H.; Limenitakis, J.P.; Fuhrer, T.; Geuking, M.B.; Lawson, M.A.; Wyss, M.; Brugiroux, S.; Keller, I.; Macpherson, J.A.; Rupp, S.; et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 2015, 6, 8292. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.A.; Schroeter, K.; Fuentes, S.; Heikamp-deJong, I.; Khursigara, C.M.; de Vos, W.M.; Allen-Vercoe, E. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J. Microbiol. Methods 2013, 95, 167–174. [Google Scholar] [CrossRef]
- Sauvaitre, T.; Etienne-Mesmin, L.; Sivignon, A.; Mosoni, P.; Courtin, C.M.; Van de Wiele, T.; Blanquet-Diot, S. Tripartite relationship between gut microbiota, intestinal mucus and dietary fibers: Towards preventive strategies against enteric infections. FEMS Microbiol. Rev. 2021, 45, fuaa052. [Google Scholar]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Ex. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef]
- Roupar, D.; Berni, P.; Martins, J.T.; Caetano, A.C.; Teixeira, J.A.; Nobre, C. Bioengineering approaches to simulate human colon microbiome ecosystem. Trends Food Sci. Technol. 2021, 112, 808–822. [Google Scholar] [CrossRef]
- De Weirdt, R.; Coenen, E.; Vlaeminck, B.; Fievez, V.; Van den Abbeele, P.; Van de Wiele, T. A simulated mucus layer protects Lactobacillus reuteri from the inhibitory effects of linoleic acid. Benef. Microbes 2013, 4, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation in vitro models to study food-induced gut microbiota shift: An updated review. FEMS Microbiol. Lett. 2020, 367, fnaa097. [Google Scholar] [CrossRef] [PubMed]
- Allen-Vercoe, E. Bringing the gut microbiota into focus through microbial culture: Recent progress and future perspective. Curr. Opin. Microbiol. 2013, 16.5, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tamayo, R.; Laroche, B.; Walter, E.; Doré, J.; Leclerc, M. Mathematical modelling of carbohydrate degradation by human colonic microbiota. J. Theor. Biol. 2010, 266, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.A.; Vavilin, V.A. The IWA anaerobic digestion model no 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Motelica-Wagenaar, A.M.; Nauta, A.; van den Heuvel, E.G.; Kleerebezem, R. Flux analysis of the human proximal colon using anaerobic digestion model 1. Anaerobe 2014, 28, 137–148. [Google Scholar] [CrossRef]
- Kettle, H.; Louis, P.; Holtrop, G.; Duncan, S.H.; Flint, H.J. Modelling the emergent dynamics and major metabolites of the human colonic microbiota. Environ. Microbiol. 2015, 17, 1615–1630. [Google Scholar] [CrossRef]
- Moorthy, A.S.; Brooks, S.P.; Kalmokoff, M.; Eberl, H.J. A spatially continuous model of carbohydrates digestion and transport processes in the colon. PLoS ONE 2015, 10, e0145309. [Google Scholar] [CrossRef]
- Kettle, H.; Holtrop, G.; Louis, P.; Flint, H.J. micropop: Modelling microbial populations and communities in R. MEE 2018, 9, 399–409. [Google Scholar] [CrossRef]
- Moorthy, A.S.; Eberl, H.J. compuGUT: An in silico platform for simulating intestinal fermentation. SoftwareX 2017, 6, 237–242. [Google Scholar] [CrossRef]
- Cremer, J.; Arnoldini, M.; Hwa, T. Effect of water flow and chemical environment on microbiota growth and composition in the human colon. Proc. Natl. Acad. Sci. USA 2017, 114, 6438–6443. [Google Scholar] [CrossRef]
- Labarthe, S.; Polizzi, B.; Phan, T.; Goudon, T.; Ribot, M.; Laroche, B. A mathematical model to investigate the key drivers of the biogeography of the colon microbiota. J. Theor. Biol. 2019, 462, 552–581. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wolkowicz, G. Mathematical models of microbial growth and competition in the chemostat regulated by cell-bound extracellular enzymes. J. Math. Biol. 1992, 31, 1–23. [Google Scholar] [CrossRef]
- Smith, H.L.; Waltman, P. The Theory of the Chemostat: Dynamics of Microbial Competition, 1st ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 1–14. [Google Scholar]
- Crespo, M.; Rapaport, A. Analysis and Optimization of the Chemostat Model with a Lateral Diffusive Compartment. J. Optim. Theory Appl. 2020, 185, 597–621. [Google Scholar] [CrossRef]
- Jegatheesan, T.; Eberl, H.J. Modelling the Effects of Antibiotics on Gut Flora Using a Nonlinear Compartment Model with Uncertain Parameters. ICCS 2020, 12137, 399–412. [Google Scholar]
- Walter, W. Ordinary Differential Equations, 1st ed.; Springer Science & Business Media: New York, NY, USA, 1998; pp. 148–157. [Google Scholar]
- Herman, J.; Usher, W. SALib: An open-source Python library for Sensitivity Analysis. J. Open Source Softw. 2017, 2, 97. [Google Scholar] [CrossRef]
- Saltelli, A. Making best use of model evaluations to compute sensitivity indices. Comput. Phys. Commun. 2002, 145, 280–297. [Google Scholar] [CrossRef]
- Saltelli, A.; Annoni, P. How to avoid a perfunctory sensitivity analysis. Environ. Model. Softw. 2010, 25, 1508–1517. [Google Scholar] [CrossRef]
- Sobol’, I.M. Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math. Comput. Simul. 2001, 55, 271–280. [Google Scholar] [CrossRef]
- Saltelli, A.; Annoni, P.; Azzini, I.; Campolongo, F.; Ratto, M.; Tarantola, S. Variance based sensitivity analysis of model output. Design and estimator for the total sensitivity index. Comput. Phys. Commun. 2010, 181, 259–270. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Wade, M.J. Not Just Numbers: Mathematical Modelling and Its Contribution to Anaerobic Digestion Processes. Processes 2020, 8, 888. [Google Scholar] [CrossRef]
- Fekih-Salem, R.; Abdellatif, N.; Sari, T.; Harmand, J. On a three step model of anaerobic digestion including the hydrolysis of particulate matter. IFAC Proc. Vol. 2012, 45, 671–676. [Google Scholar] [CrossRef]
- Hanaki, M.; Harmand, J.; Mghazli, Z.; Sari, T.; Rapaport, A.; Ugalde, P. Mathematical study of a two-stage anaerobic model when the hydrolysis is the limiting step. Processes 2021, 9, 2050. [Google Scholar] [CrossRef]
- Ng, K.M.; Aranda-Díaz, A.; Tropini, C.; Frankel, M.R.; Treuren, W.V.; O’Loughlin, C.T.; Merrill, B.D.; Yu, F.B.; Pruss, K.M.; Oliveira, R.A.; et al. Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context and Environmental Resevoirs. Cell Host Microbe 2019, 25, 650–665. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Symbol | Default Value | Unit |
|---|---|---|---|
| Death rate | 0.1 | [gL] | |
| Dilution rate | D | 1.007 | [d] |
| Inflow fiber concentration | 1.0 | [gL] | |
| Max specific growth rate of X from S | 12.627 | [d] | |
| Max specific hydrolysis rate | 10.619 | [d] | |
| Half-saturation coefficient for growth of X | 0.468 | [gL] | |
| Half-saturation coefficient | 0.265 | dimensionless | |
| Yield coefficient for X using S | 0.342 | dimensionless | |
| Yield coefficient for hydrolysis | 1.0 | dimensionless |
| Parameter | Symbol | Default Value | Units |
|---|---|---|---|
| Death rate | 0.1 | [gL] | |
| Dilution rate | D | 1.007 | [d] |
| Inflow fiber concentration | 1.0 | [gL] | |
| Max specific growth rate of X from S | 12.627 | [d] | |
| Max specific hydrolysis rate | 10.619 | [d] | |
| Half-saturation coefficient for growth of X | 0.468 | [gL] | |
| Half-saturation coefficient | 0.265 | dimensionless | |
| Yield coefficient for X using S | 0.342 | dimensionless | |
| Yield coefficient for hydrolysis | 1.0 | dimensionless | |
| Diffusion rate | 3.9 | [Ld] | |
| Attachment rate of X (Lumen to mucus) | 0.1 | [Ld] | |
| Sloughing rate of I (Mucus to lumen) | 0.1 | [Ld] | |
| Sloughing rate of X (Mucus to lumen) | 0.4 | [Ld] | |
| Maximum mucus amount | 500.0 | [gL] | |
| Mucus production rate | 50.0 | [g(Ld)] | |
| Volume of lumen | 1.0 | [L] | |
| Volume of mucus | 1.0 | [L] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jegatheesan, T.; Moorthy, A.S.; Eberl, H.J. Enzymatic Hydrolysis of Complex Carbohydrates and the Mucus in a Mathematical Model of a Gut Reactor. Processes 2023, 11, 370. https://doi.org/10.3390/pr11020370
Jegatheesan T, Moorthy AS, Eberl HJ. Enzymatic Hydrolysis of Complex Carbohydrates and the Mucus in a Mathematical Model of a Gut Reactor. Processes. 2023; 11(2):370. https://doi.org/10.3390/pr11020370
Chicago/Turabian StyleJegatheesan, Thulasi, Arun S. Moorthy, and Hermann J. Eberl. 2023. "Enzymatic Hydrolysis of Complex Carbohydrates and the Mucus in a Mathematical Model of a Gut Reactor" Processes 11, no. 2: 370. https://doi.org/10.3390/pr11020370
APA StyleJegatheesan, T., Moorthy, A. S., & Eberl, H. J. (2023). Enzymatic Hydrolysis of Complex Carbohydrates and the Mucus in a Mathematical Model of a Gut Reactor. Processes, 11(2), 370. https://doi.org/10.3390/pr11020370







