NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. NADES Development and Characterization
2.2.1. NADES Development
2.2.2. NADES Characterization
2.3. NADES-Based Extraction of Bioactive Compounds from Cork Powder
2.4. Extractives Characterization
2.5. Incorporation of the Extracts into Commercial Cosmetic Formulations
Characterization and Stability of Formulations
2.6. Dyeing/Coloration of Cotton Fabrics with the NADES-Based Cork Extracts
2.7. Surface Characterization of Dyed Cotton Fabrics by Scanning Electron Microscopy (SEM)
2.8. DPPH Radical-Scavenging Activity of Dyed Fabrics
2.9. Antimicrobial Activity of Coated Cotton Fabrics
3. Results and Discussion
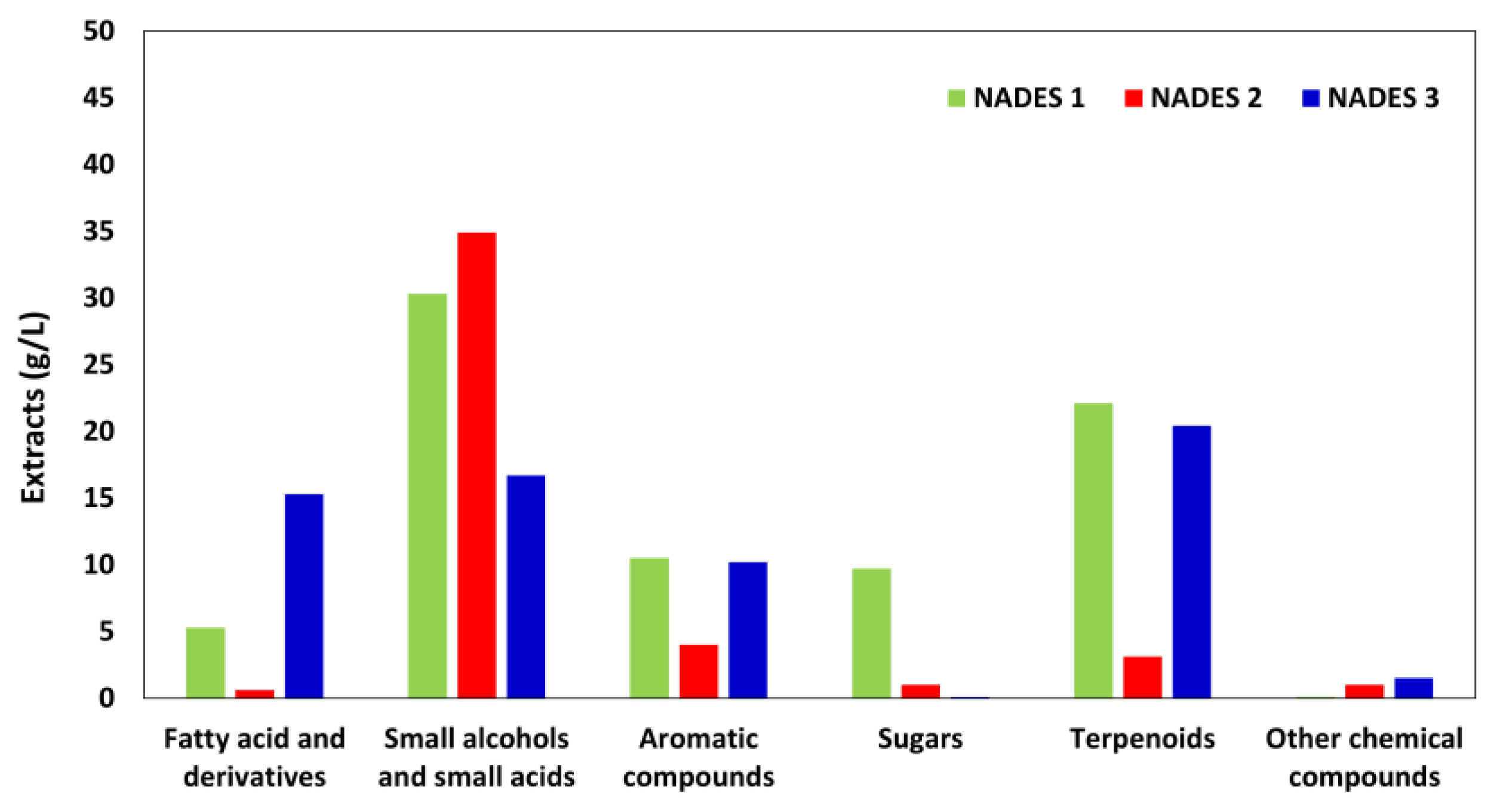
3.1. Physical and Chemical Characterization of Cork Extracts
3.2. Phenolic Content and Antioxidant Activity of Cork Extracts
3.3. Transdermal Permeation of Cork Extract
3.3.1. Through Cellulose Acetate Membranes
3.3.2. Through Pig Skin
3.4. Cytotoxicity of Cork Extracts
3.5. Characterization and Stability of Cosmetic Formulations Containing Cork Extracts
3.5.1. Organoleptic Properties
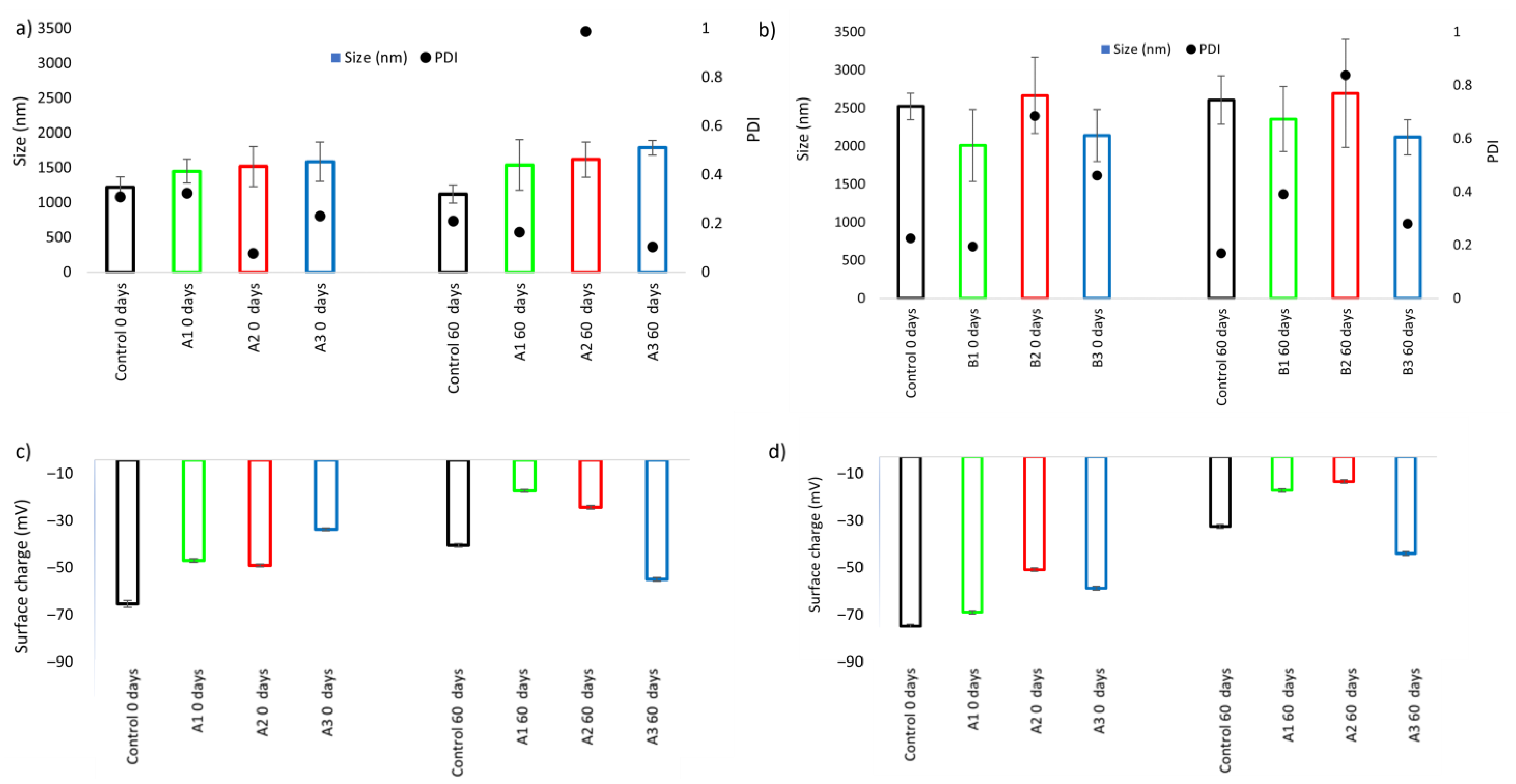
3.5.2. Particle Size and Surface Charge
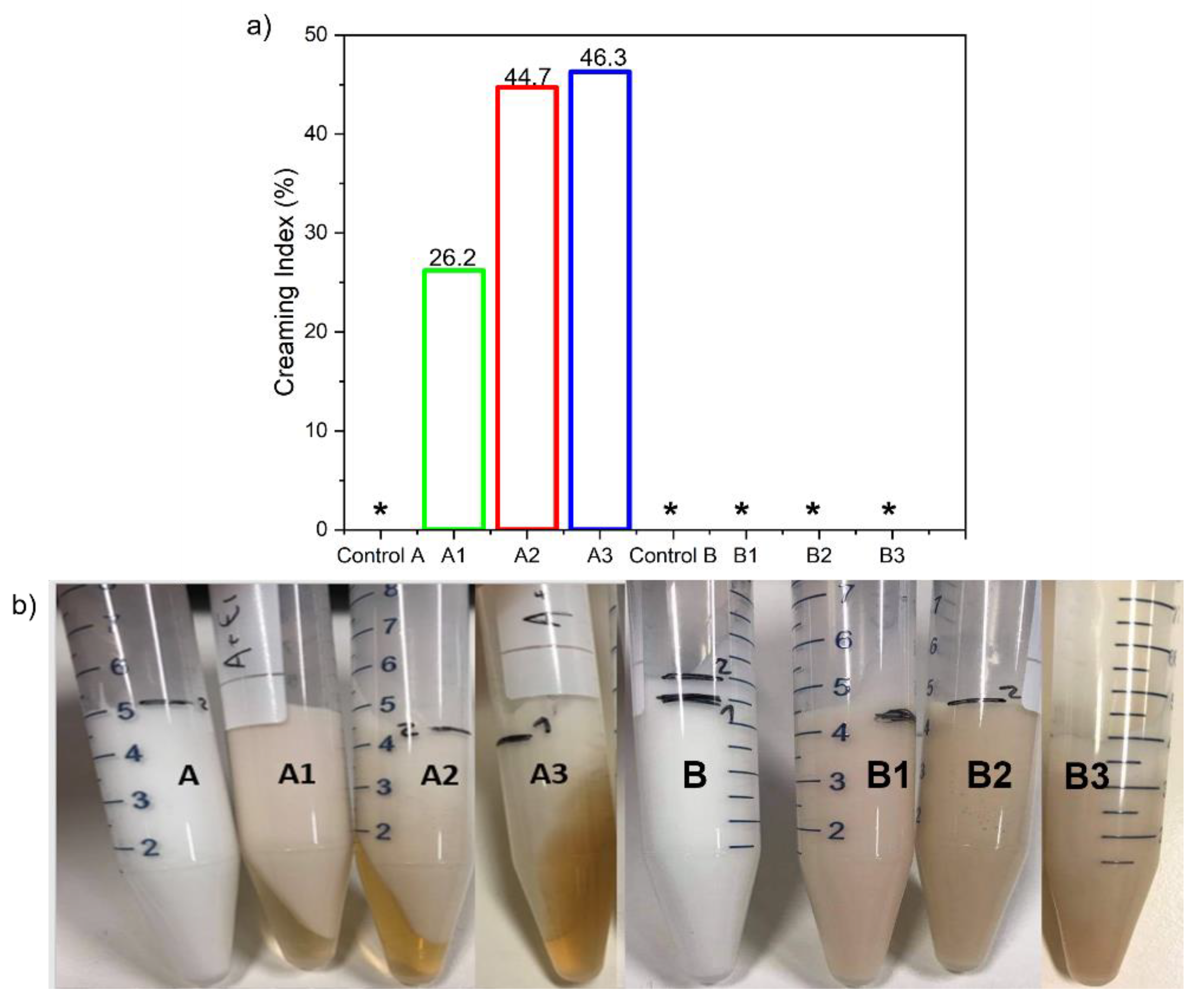
3.5.3. Creaming Index
3.5.4. Rheologic Behavior
3.5.5. Determination of Antioxidant Activity of Formulations Containing the Extracts
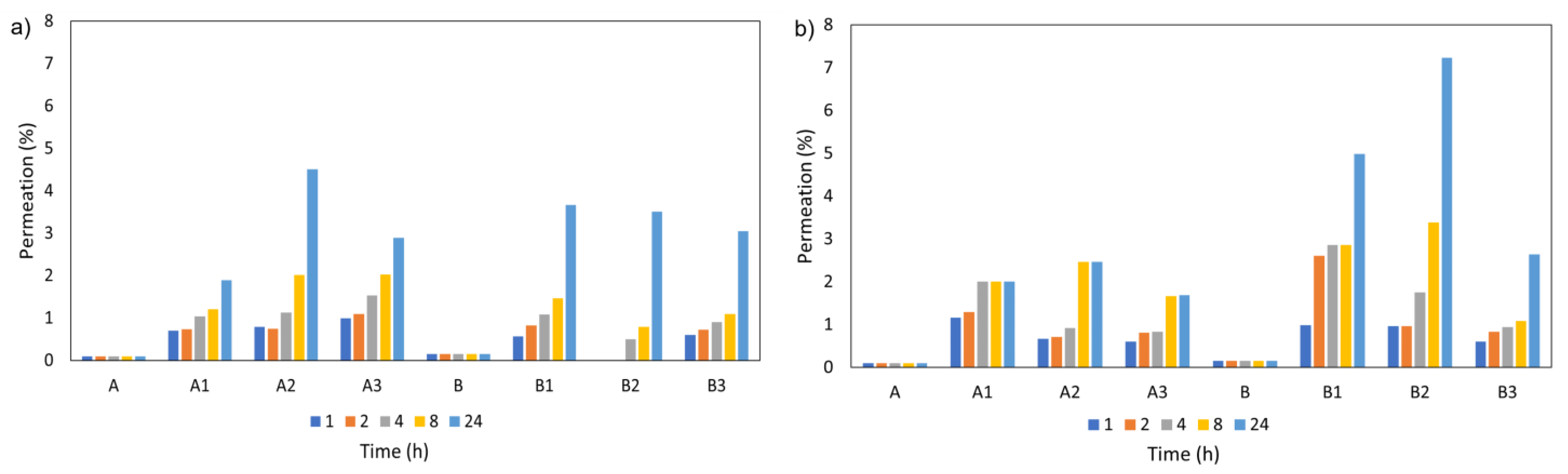
3.5.6. Transdermal Permeation of Formulations Containing Cork Extracts
Through Cellulose Acetate Membranes
Through Pig Skin
3.6. Dyeing/Coloration of Cotton Fabrics with the NADES-Based Cork-Colored Extracts
3.7. Surface Characterization of Dyed Cotton Fabrics by Scanning Electron Microscopy (SEM)
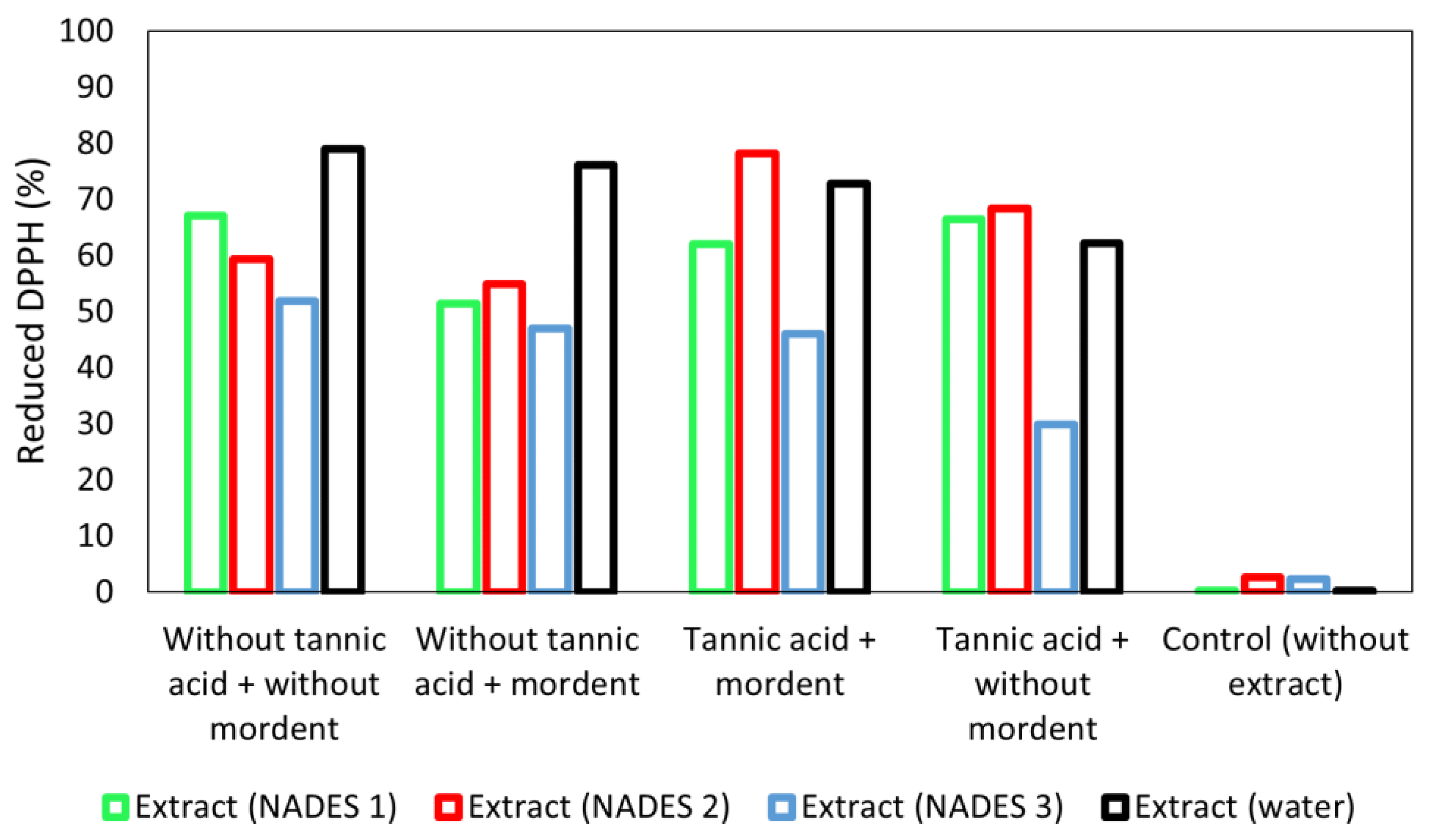
3.8. DPPH Radical-Scavenging Activity of Coated Fabrics
3.9. Antimicrobial Activity of Coated Cotton Fabrics
4. Conclusions
4.1. Cork Extracts
4.2. Cosmetic Formulations Incorporating NADES-Based Cork Extracts
4.3. Textiles Dyeing/Coloration of Cotton Fabrics with NADES-Based Cork Extracts
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Branco, D.G.; Santiago, C.; Lourenço, A.; Cabrita, L.; Evtuguin, D.V. Structural Features of Cork Dioxane Lignin from Quercus suber L. J. Agric. Food Chem. 2021, 69, 8555–8564. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araújo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current Technological Developments and Future Perspectives for this Natural, Renewable, and Sustainable Material. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Gandini, A.; Pascoal Neto, C.; Silvestre, A.J.D. Suberin: A promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 2006, 31, 878–892. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The polyphenolic profile of Oak (Quercus) species: A phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic Potential and Bioactivity of Cork Stoppers and Cork By-Products. Foods 2020, 9, 133. [Google Scholar] [CrossRef]
- Lopes, M.H.; Gil, A.M.; Silvestre, A.J.D.; Neto, C.P. Composition of Suberin Extracted upon Gradual Alkaline Methanolysis of Quercus suber L. Cork. J. Agric. Food Chem. 2000, 48, 383–391. [Google Scholar] [CrossRef]
- Sousa, A.F.; Gandini, A.; Silvestre, A.J.; Pascoal Neto, C.J.C.C. Synthesis and characterization of novel biopolyesters from suberin and model comonomers. ChemSusChem 2008, 1, 1020–1025. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Sousa, A.F.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Pascoal Neto, C. Triterpenic and Other Lipophilic Components from Industrial Cork Byproducts. J. Agric. Food Chem. 2006, 54, 6888–6893. [Google Scholar] [CrossRef] [PubMed]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and Characterization of a New Quercus Suber-Based Pickering Emulsion for Topical Application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.S.; Rocha, D.; Castro, T.G.; Noro, J.; Castro, V.I.B.; Teixeira, M.A.; Reis, R.L.; Cavaco-Paulo, A.; Silva, C. Green Extraction of Cork Bioactive Compounds Using Natural Deep Eutectic Mixtures. ACS Sustain. Chem. Eng. 2022, 10, 7974–7989. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 5. [Google Scholar] [CrossRef]
- Karabulut, K.; Atav, R. Dyeing of Cotton Fabric with Natural Dyes without Mordant Usage Part I: Determining the Most Suitable Dye Plants for Dyeing and UV Protective Functionalization. Fibers Polym. 2020, 21, 1773–1782. [Google Scholar] [CrossRef]
- Arora, J.; Agarwal, P.; Gupta, G. Rainbow of Natural Dyes on Textiles Using Plants Extracts: Sustainable and Eco-Friendly Processes. Green Sustain. Chem. 2017, 7, 13. [Google Scholar] [CrossRef]
- Ayele, M.; Tesfaye, T.; Alemu, D.; Limeneh, M.; Sithole, B. Natural dyeing of cotton fabric with extracts from mango tree: A step towards sustainable dyeing. Sustain. Chem. Pharm. 2020, 17, 100293. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Yablon, D.; Schilowitz, A. Solvatochromism of Nile Red in Nonpolar Solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef]
- Ogihara, W.; Aoyama, T.; Ohno, H. Polarity Measurement for Ionic Liquids Containing Dissociable Protons. Chem. Lett. 2004, 33, 1414–1415. [Google Scholar] [CrossRef]
- Yeo, Y.-H.; Hsu, F.-L.; Chen, Y.-L.; Chang, T.-C. Evaluation of the extracts from the renewable parts in Garcinia subelliptica as natural sunscreen additives. Ind. Crops Prod. 2022, 186, 115214. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Rahman, M.M.; Koh, J.; Hong, K.H. Coloration and multi-functionalization of cotton fabrics using different combinations of aqueous natural plant extracts of onion peel, turmeric root, and pomegranate rind. Ind. Crops Prod. 2022, 188, 115562. [Google Scholar] [CrossRef]
- Bayer, A.W.; Kirby WM, M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Dugoni, G.C.; Di Pietro, M.E.; Ferro, M.; Castiglione, F.; Ruellan, S.; Moufawad, T.; Moura, L.; Costa Gomes, M.F.; Fourmentin, S.; Mele, A. Effect of Water on Deep Eutectic Solvent/β-Cyclodextrin Systems. ACS Sustain. Chem. Eng. 2019, 7, 7277–7285. [Google Scholar] [CrossRef]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Balboa, E.M.; Soto, M.L.; Nogueira, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crops Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- Masson, P. The Contribution of the European Cosmetics Directive Towards International Harmonization: Impact on the Evaluation of Safety and Efficacy. In Cosmetics: Controlled Efficacy Studies and Regulation; Elsner, P., Maibach, H.I., Merk, H.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 20–35. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoon, J.-W.; Kim, C.-T.; Lim, S.-T. Antioxidant activity of phenylpropanoid esters isolated and identified from Platycodon grandiflorum A. DC. Phytochemistry 2004, 65, 3033–3039. [Google Scholar] [CrossRef]
- Dönmez, İ.E.; Hemming, J.; Willför, S. Bark Extractives and Suberin Monomers from Arbutus andrachne and Platanus orientalis. BioResources 2016, 11, 2809–2819. [Google Scholar] [CrossRef]
- Pisitsak, P.; Hutakamol, J.; Thongcharoen, R.; Phokaew, P.; Kanjanawan, K.; Saksaeng, N. Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind. Crops Prod. 2016, 79, 47–56. [Google Scholar] [CrossRef]
- Rattanaphani, S.; Chairat, M.; Bremner, J.B.; Rattanaphani, V. An adsorption and thermodynamic study of lac dyeing on cotton pretreated with chitosan. Dye. Pigment. 2007, 72, 88–96. [Google Scholar] [CrossRef]
- Souissi, M.; Guesmi, A.; Moussa, A. Valorization of natural dye extracted from date palm pits (Phoenix dactylifera) for dyeing of cotton fabric. Part 2: Optimization of dyeing process and improvement of colorfastness with biological mordants. J. Clean. Prod. 2018, 204, 1143–1153. [Google Scholar] [CrossRef]
- Phan, K.; Van Den Broeck, E.; Van Speybroeck, V.; De Clerck, K.; Raes, K.; De Meester, S. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dye. Pigment. 2020, 176, 108180. [Google Scholar] [CrossRef]
- Farha, S.; Gamal, M.; Sallam, H.; Mahmoud, G.; Ismail, L.F.M. Sodium Edate and Sodium Citrate as an Exhausting and Fixing Agents for Dyeing Cotton Fabric with Reactive Dyes and Reuse of Dyeing Effluent. J. Am. Sci. 2010, 6, 109–127. [Google Scholar] [CrossRef]
- Zerin, I.; Foisal, A. Effect of Mordanting Process on Cotton Dyeing with Acacia catechu. In Proceedings of the 3rd Textile Research Conference (TRC), Dhaka, Bangladesh, 29 October 2016. [Google Scholar]
- Sun, S.-S.; Xing, T.; Tang, R.-C. Simultaneous Coloration and Functionalization of Wool, Silk, and Nylon with the Tyrosinase-Catalyzed Oxidation Products of Caffeic Acid. Ind. Eng. Chem. Res. 2013, 52, 8953–8961. [Google Scholar] [CrossRef]
- Baaka, N.; El Ksibi, I.; Mhenni, M.F. Optimisation of the recovery of carotenoids from tomato processing wastes: Application on textile dyeing and assessment of its antioxidant activity. Nat. Prod. Res. 2017, 31, 196–203. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Nateri, A.S.; Mortazavi, S.M.; Abedi, D.; Mokhtari, J. The effect of mordant salts on antibacterial activity of wool fabric dyed with pomegranate and walnut shell extracts. Color. Technol. 2012, 128, 473–478. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]





| NADES | Components (HBD:HBA) | Molar Ratio | Water (%) | |
|---|---|---|---|---|
| 1 |  DL-Lactic Acid (LA) |  Glycerol (GLY) | 1:1 | ≈11% |
| 2 | 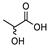 DL-Lactic Acid (LA) | 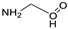 Glycine (GLYC) | 5:1 | ≈13% |
| 3 |  DL-Lactic Acid (LA) | 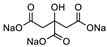 Sodium Citrate (tribasic) (SC) | 4:1 | ≈31% |
| Formulation A | Formulation B |
|---|---|
| Water | Water |
| Glycerol | Glycerol |
| Cetearyl alcohol | Cetearyl alcohol |
| Panthenol (B vitamin) | Panthenol (vitamin B) |
| Dimethicone | Dimethicone |
| Lanolin | Phenoxyethanol |
| Glyceryl stearate | Glyceryl stearate |
| Polyglyceryl-3 methyglucose distearate | Benzyl alcohol |
| Decyl oleate | Lanolin |
| Lactic acid | Urea |
| Coumarin | Ethylhexyl stearate |
| Citronellol | Citronellol |
| Parfum | Caprylic/Capric triglyceride |
| Linalool | Citric acid |
| Alpha-isomethyl ionone | Niacinamide |
| Butylphenyl methylpropional | Xanthan gum |
| Geraniol | Geraniol |
| Hexyl cinnamal | Isopropyl palmitate |
| Caprylyl glycol, decylene glycol | Parfum |
| Limonene | |
| Sodium stearoyl glutamate | |
| Caprylyl glycol, decylene glycol |
| Control | Extract 1 | Extract 2 | Extract 3 | |
|---|---|---|---|---|
| Formulation A: Moisturizer ATL | A | A1 | A2 | A3 |
| Formulation B: Cien Urea 10% | B | B1 | B2 | B3 |
| NADES | Ratio | m.p. (°C) | pH | ρ (g/mL) (MD) | η (Pa.s) (MD) | σ (μS.cm−1) | nD | ENR (kcal/mol) (λ nm) | Surface Tension (mN/m) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1:1 | <−50 | 0.9 | 1.24 (1.19) | 1.0 (0.295) | 0.9 | 1.457 | 48.542 (589) | 44.210 |
| 2 | 5:1 | 52 | 2.3 (a) | 1.23 (a) | 0.081 (a) | 1548 (a) | 1.438 (a) | 48.500 (a) | 44.003 (a) |
| 3 | 4:1 | −12 | 4.0 | 1.39 | 10.1 (b) | 346.8 (b) | 1.438 | 50.612 (565) | 45.877 (c) |
| %Permeation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose Acetate Membrane | Pig Skin | |||||||||
| 1 h | 2 h | 4 h | 8 h | 24 h | 1 h | 2 h | 4 h | 8 h | 24 h | |
| Extract E1 | 0.44 | 0.31 | 33.34 | 65.30 | 66.36 | 4.14 | 5.97 | 5.97 | 6.01 | 6.09 |
| Extract E2 | 0 | 0 | 0 | 67.60 | 67.60 | 0.25 | 0.88 | 1.55 | 2.59 | 3.63 |
| Extract E3 | 17.21 | 22.83 | 41.30 | 41.30 | 41.30 | 2.19 | 3.37 | 3.80 | 3.98 | 4.66 |
| Color | Odor | Appearance | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 22 °C | 37 °C | 4 °C | 22 °C | 37 °C | 4 °C | 22 °C | 37 °C | ||
| Control A | Normal | Normal | Homogeneous | 6–7 | ||||||
| A1 | N | N | N | N | N | N | H | H | H | 6–7 |
| A2 | N | N | N | N | N | M | S- | S- | S- | 5 |
| A3 | N | N | M | N | M | M | S | S | M | 4–5 |
| Control B | Normal | Normal | Homogeneous | 7 | ||||||
| B1 | N | N | M | N | N | M | H | H | M | 7 |
| B2 | N | N | M | N | N | M | H | H | M | 5 |
| B3 | N | N | N | N | N | N | H | H | H | 5–6 |
| E1 | K/S | E2 | K/S | E3 | K/S | Control | K/S | |
|---|---|---|---|---|---|---|---|---|
| Without tannic acid + without mordant |  | 0.56 |  | 0.85 |  | 2.43 |  | 1.60 |
| Without tannic acid + mordant |  | 0.55 |  | 0.73 |  | 1.21 |  | 1.68 |
| Tannic acid + mordant |  | 2.56 |  | 0.92 |  | 1.92 |  | 1.50 |
| Tannic acid + without mordant |  | 1.38 |  | 0.74 |  | 1.26 |  | 1.17 |
| E1 (Cork Extract + NADES 1) | E3 (Cork Extract + NADES 3) | |||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| Without tannic acid + without mordant |  |  |  |  |
| Inhibition zone | 0 mm | 0 mm | 14 mm | 12 mm |
| Tannic acid + mordant |  |  |  |  |
| Inhibition zone | 12 mm | 11 mm | 15 mm | 15 mm |
| Tannic acid + without mordant |  |  |  |  |
| Inhibition zone | 10 mm | 13 mm | 0 mm | 0 mm |
| Controls |  |  |  |  |
| Inhibition zone | 0 mm | 0 mm | 0 mm | 0 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, D.; Freitas, D.S.; Magalhães, J.; Fernandes, M.; Silva, S.; Noro, J.; Ribeiro, A.; Cavaco-Paulo, A.; Martins, M.; Silva, C. NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes 2023, 11, 309. https://doi.org/10.3390/pr11020309
Rocha D, Freitas DS, Magalhães J, Fernandes M, Silva S, Noro J, Ribeiro A, Cavaco-Paulo A, Martins M, Silva C. NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes. 2023; 11(2):309. https://doi.org/10.3390/pr11020309
Chicago/Turabian StyleRocha, Diana, David S. Freitas, Jéssica Magalhães, Mariana Fernandes, Sónia Silva, Jennifer Noro, Artur Ribeiro, Artur Cavaco-Paulo, Madalena Martins, and Carla Silva. 2023. "NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles" Processes 11, no. 2: 309. https://doi.org/10.3390/pr11020309
APA StyleRocha, D., Freitas, D. S., Magalhães, J., Fernandes, M., Silva, S., Noro, J., Ribeiro, A., Cavaco-Paulo, A., Martins, M., & Silva, C. (2023). NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes, 11(2), 309. https://doi.org/10.3390/pr11020309











