Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
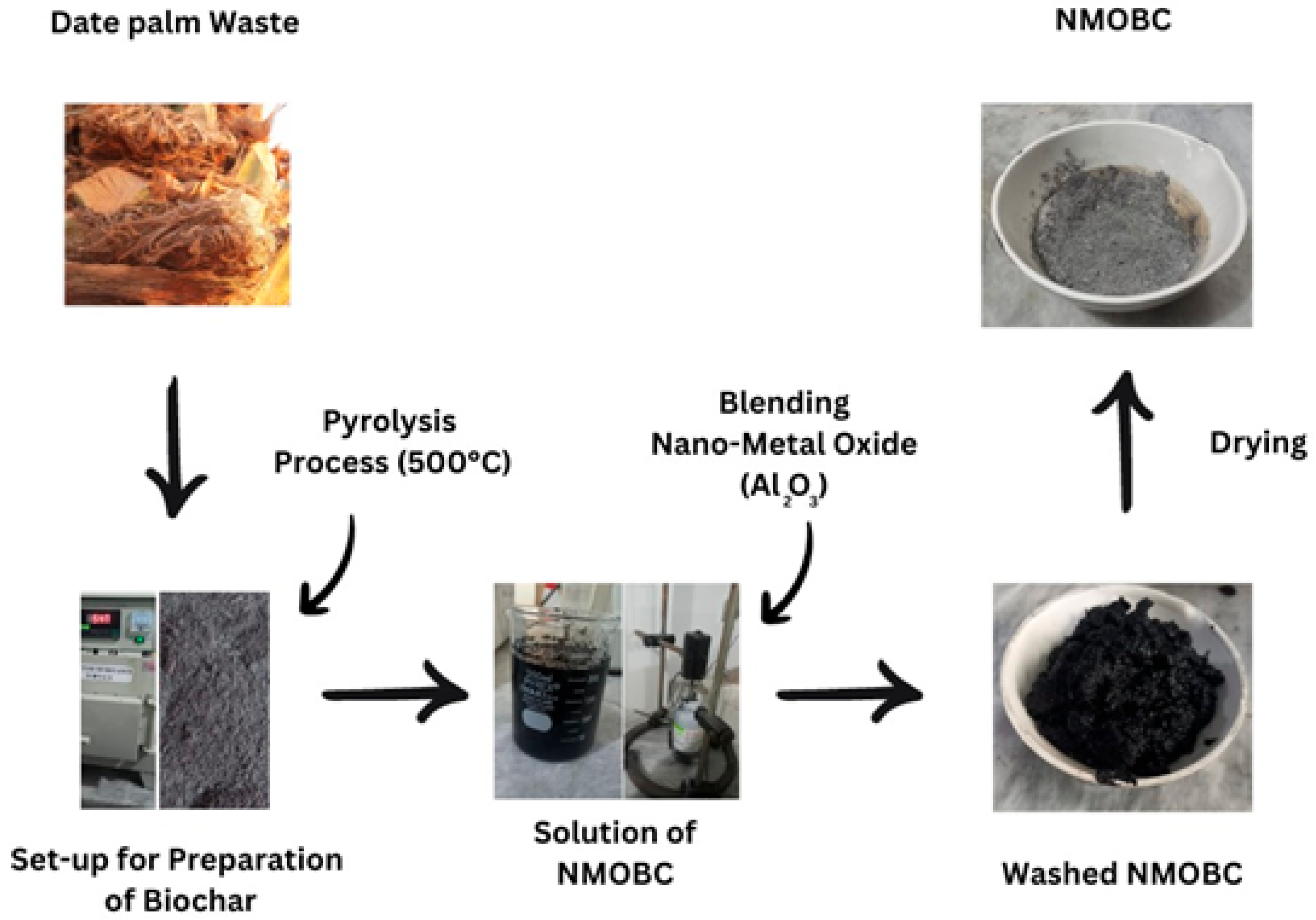
2.2. Synthesis of Biochar Using Date Palm Stems
2.3. Formulation of Nano-Metal Oxide (Al2O3) Blended Biochar
2.4. Formulation of Iron Solutions
2.5. Characterization of BC and NMOBC
2.6. Iron Adsorption Process on BC and NMOBC
2.7. Adsorption Process Modeling
2.7.1. Kinetic Modeling for Iron Removal
2.7.2. Isotherm Modeling
3. Results and Discussion
3.1. FTIR of Raw and Iron Loaded Adsorbents
3.2. SEM/EDX study of Raw and Iron-Loaded Adsorbents
3.3. BET Analysis of BC and NMOBC
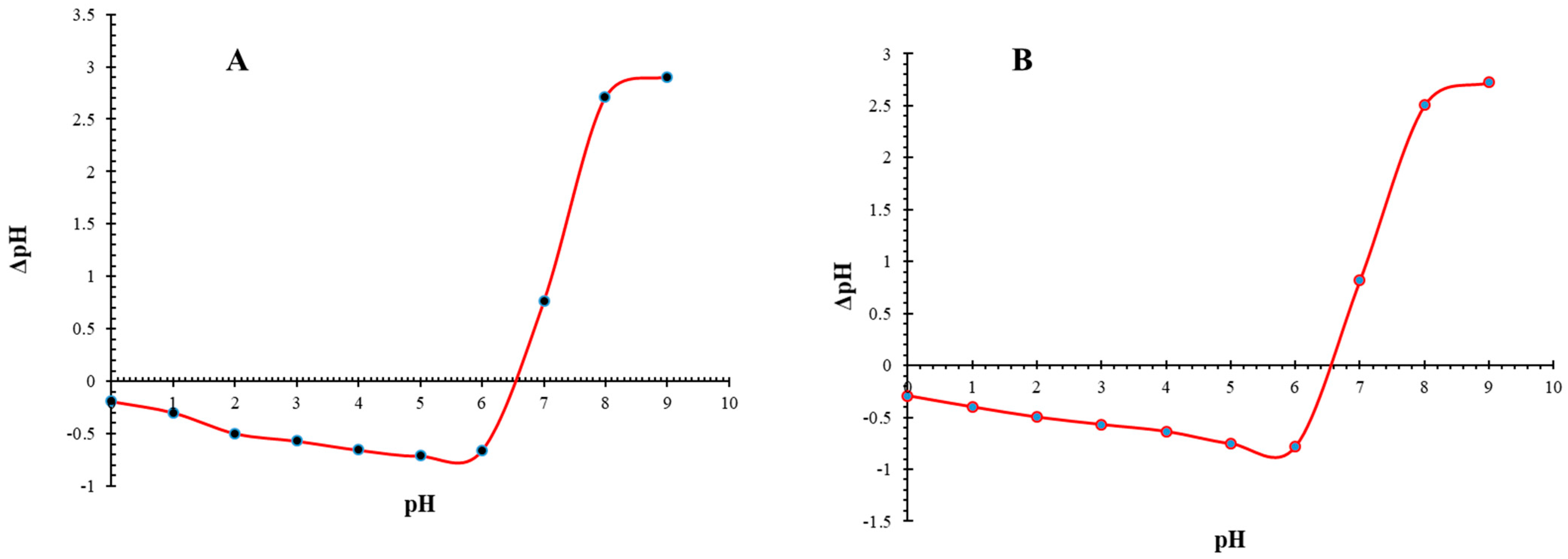
3.4. pHpzc and pH Impact on NMOBC and BC for Iron Adsorption
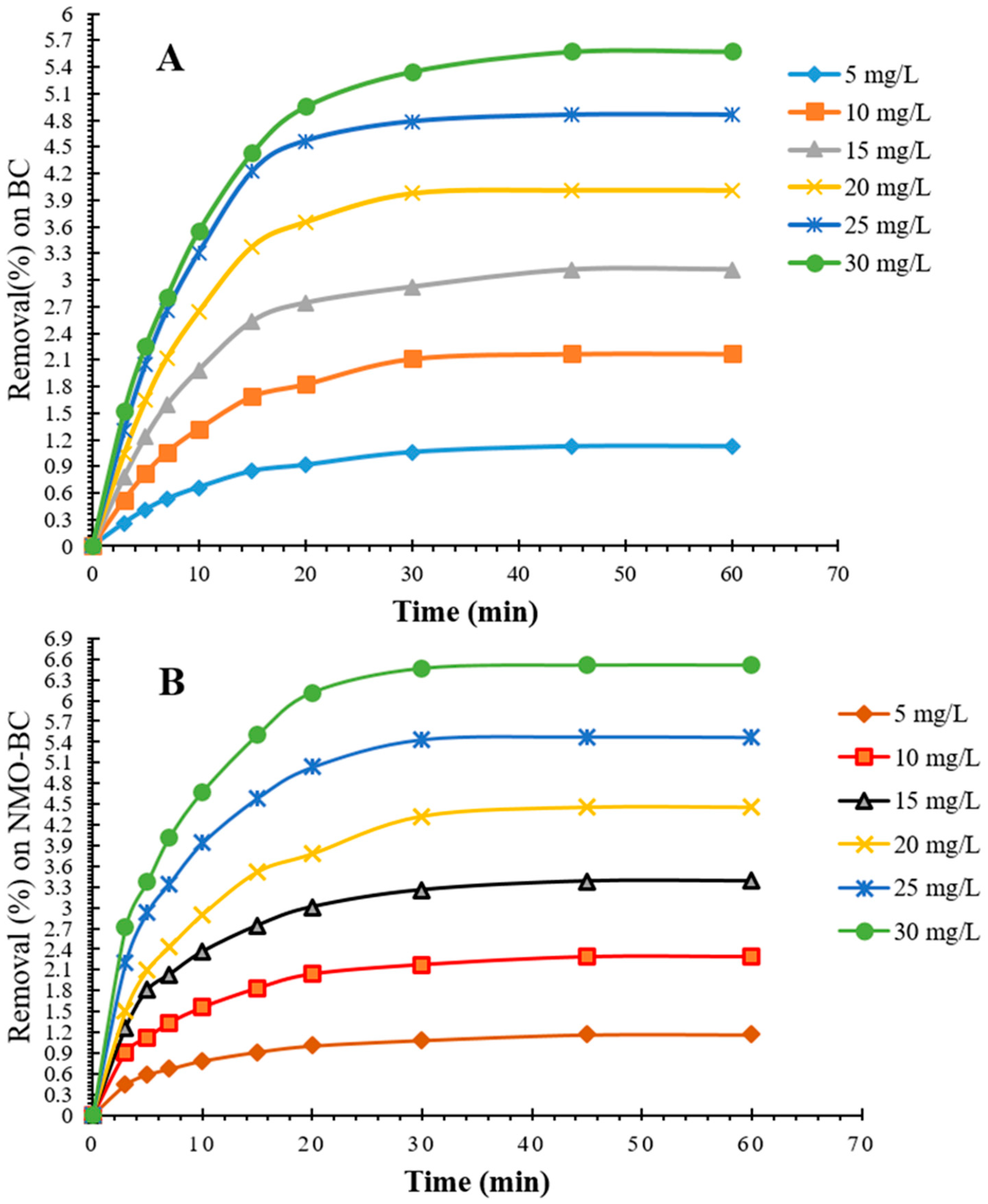
3.5. Influence of Initial Iron Concentration on BC and NMOBC
3.6. Removal Kinetics of Iron onto BC and NMOBC
3.7. Kinetic Modelling of Iron Removal on BC and NMOBC
3.7.1. Insights from Pseudo Order Models for Iron Adsorption on BC and NMOBC
3.7.2. Insights from Elovich Model for Iron Adsorption on BC and NMOBC
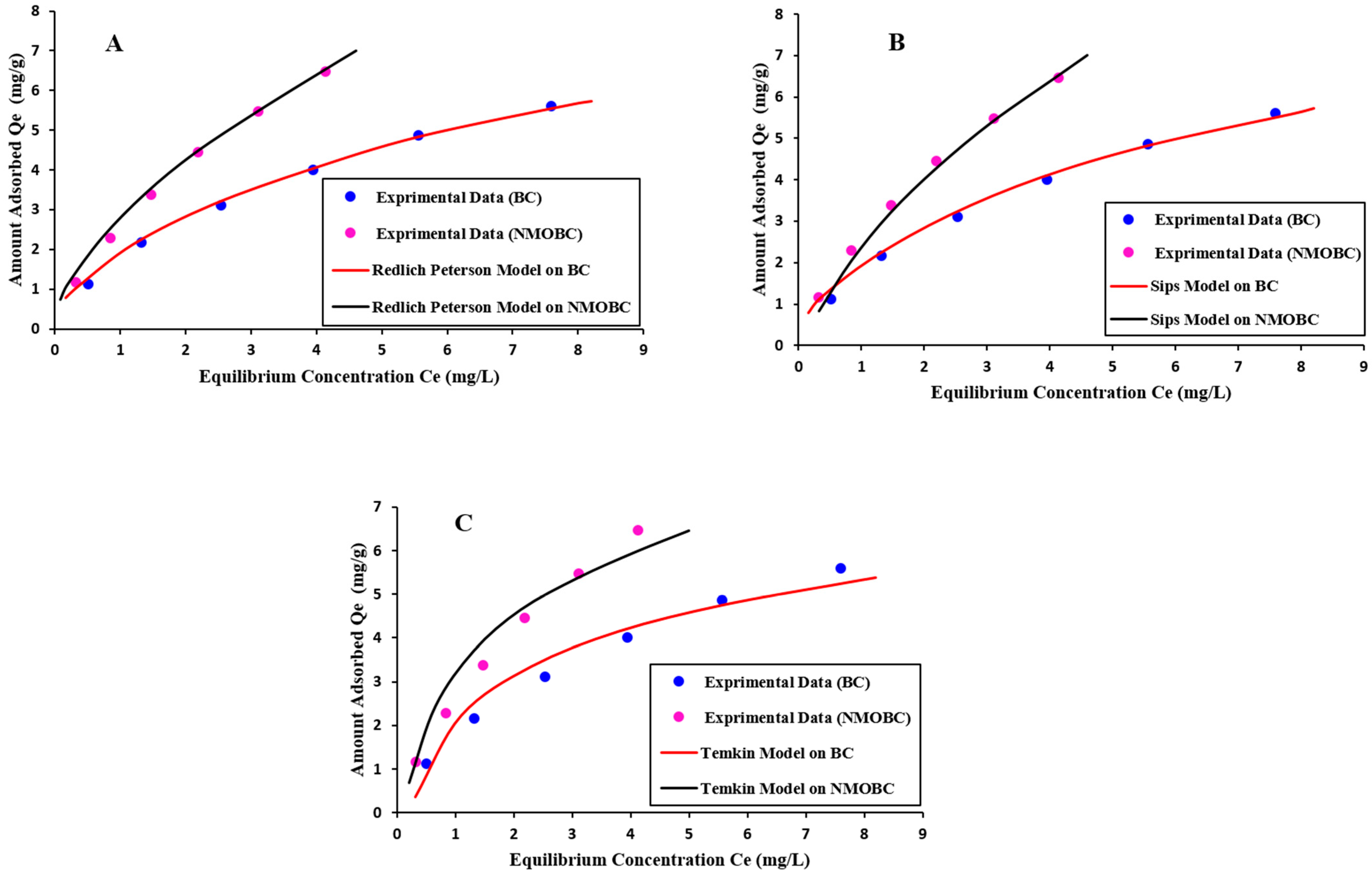
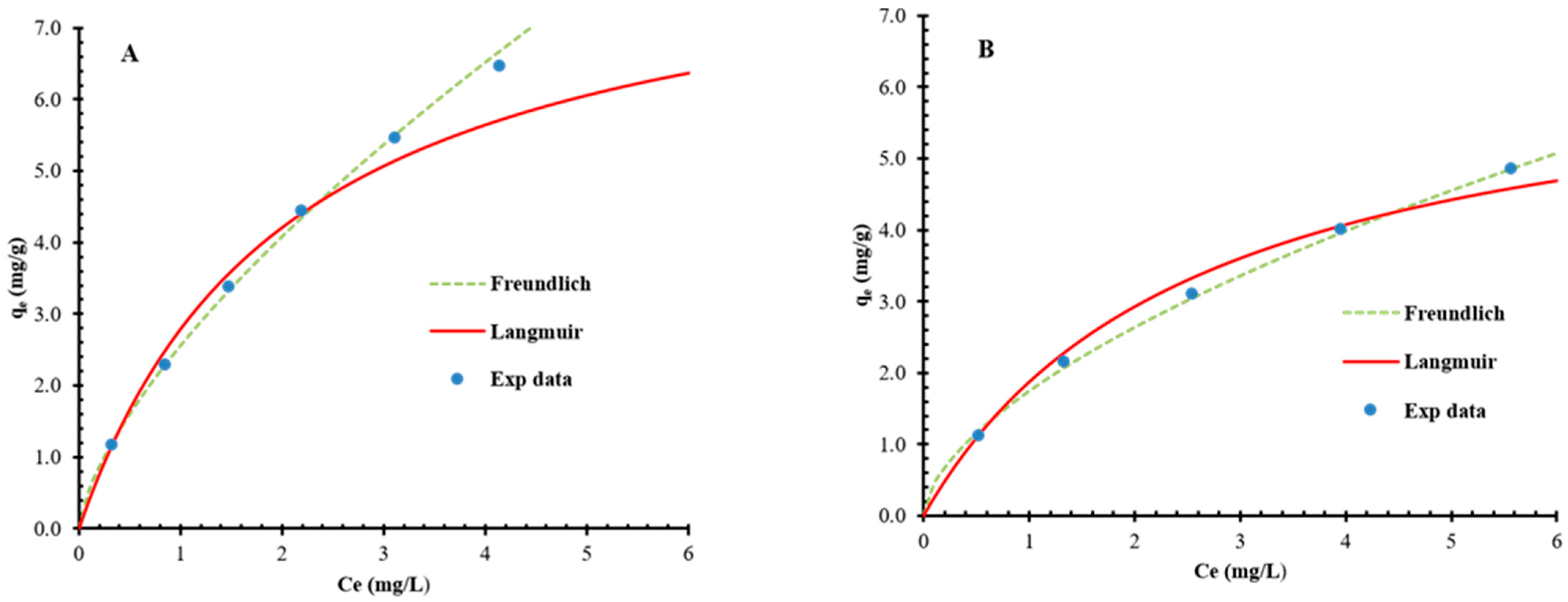
3.8. Evaluation of Isotherm Dynamics of Iron Removal on BC and NMOBC
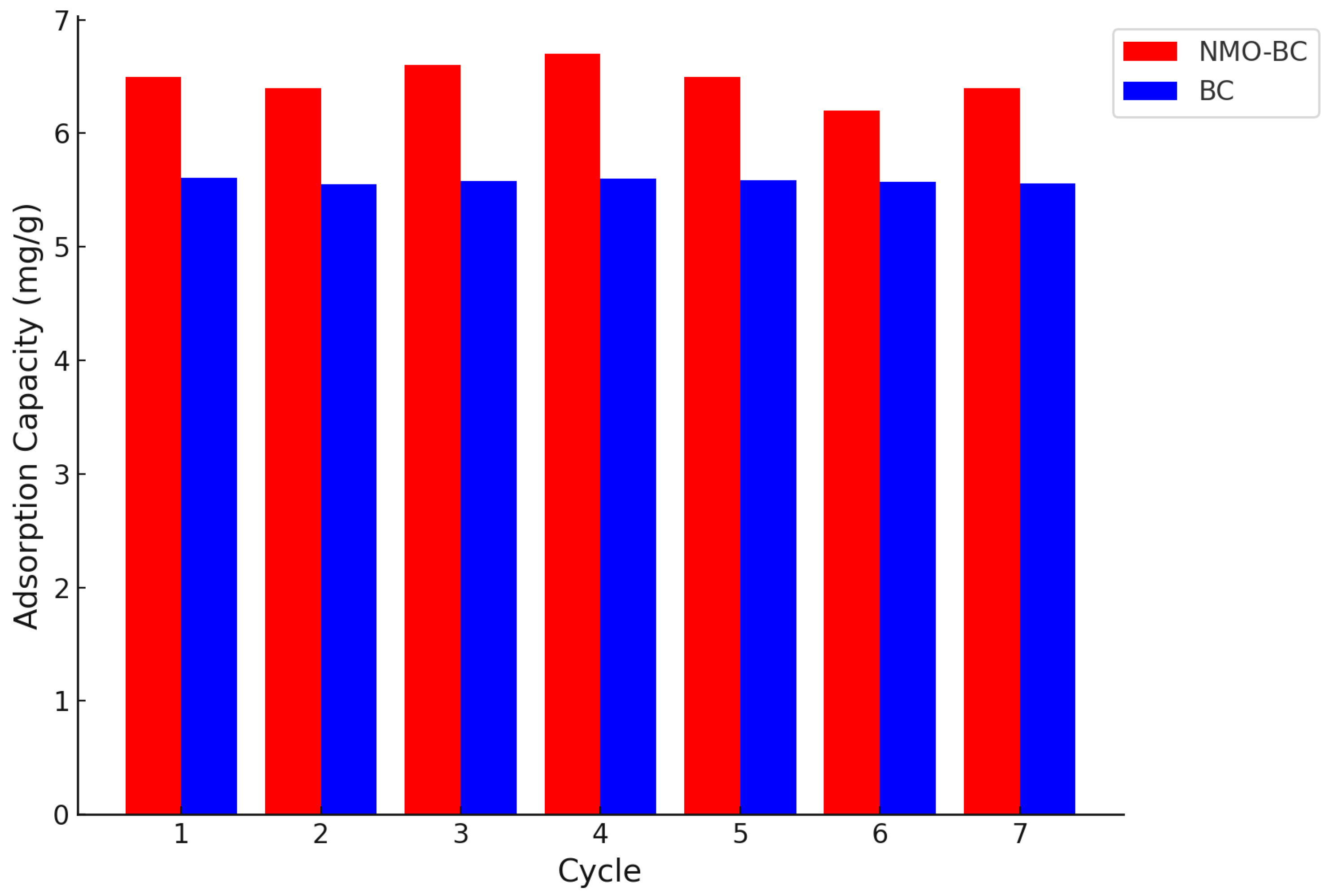
3.9. Regeneration and Reuse of NMOBC and BC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tuli, F.J.; Hossain, A.; Kibria, A.K.M.F.; Tareq, A.R.M.; Mamun, S.M.M.A.; Ullah, A.K.M.A. Removal of Methylene Blue from Water by Low-Cost Activated Carbon Prepared from Tea Waste: A Study of Adsorption Isotherm and Kinetics. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100354. [Google Scholar] [CrossRef]
- Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Khan, M.H.; Alwushayh, B.; Ghazy, A.; Uddin, A.; Iqbal, J. Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water. Water 2023, 15, 3603. [Google Scholar] [CrossRef]
- Wu, P.; Wu, W.; Li, S.; Xing, N.; Zhu, N.; Li, P.; Wu, J.; Yang, C.; Dang, Z. Removal of Cd2+ from Aqueous Solution by Adsorption Using Fe-Montmorillonite. J. Hazard. Mater. 2009, 169, 824–830. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 2 July 2023).
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of Iron-Based Magnetic Nanocomposites and Applications in Adsorption Processes for Water Treatment: A Review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Krivoshapkina, E.; Taheri, P.; Tracey, C.; Al-Fatesh, A.; et al. Advanced Adsorbents for Ibuprofen Removal from Aquatic Environments: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; ISBN 0123456789. [Google Scholar]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent Advances in Hexavalent Chromium Removal from Aqueous Solutions by Adsorptive Methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Beiki, H.; Krivoshapkin, P.; Krivoshapkina, E.; Tracey, C. Insight into the Adsorptive Removal of Ibuprofen Using Porous Carbonaceous Materials: A Review. Chemosphere 2023, 323, 138241. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Kinetics and Equilibrium Studies of Malachite Green Adsorption on Rice Straw-Derived Char. J. Hazard. Mater. 2008, 153, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Lv, Q. Removal Efficiency and Mechanism of Cr (VI) from Aqueous Solution by Maize Straw Biochars Derived at Different Pyrolysis Temperatures. Water 2019, 11, 781. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb (II) and Cd (II) from Water by Adsorption on Peels of Banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Bashir, M.T.; Ali, S.; Idris, A.; Harun, R. Kinetic and Thermodynamic Study of Nitrate Adsorption from Aqueous Solution by Lignocellulose-Based Anion Resins. Desalination Water Treat. 2017, 62, 20136. [Google Scholar] [CrossRef]
- Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Khan, M.A. Synthesis and Performance Evaluation of Olive Fruit Waste Resin for Removal of Fluoride from Aqueous Solution: Batch and Column Modeling. Desalination Water Treat. 2022, 252, 261–275. [Google Scholar] [CrossRef]
- Amdeha, E. Biochar-Based Nanocomposites for Industrial Wastewater Treatment via Adsorption and Photocatalytic Degradation and the Parameters Affecting These Processes. Biomass Convers. Biorefinery 2023, 1–26. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Khan, I.; Hussain, M.; Khan, A.R.; Hamid, Y.; Hussain, S.; Allakhverdiev, S.I.; Zhou, W. Efficacy of Metallic Nanoparticles in Attenuating the Accumulation and Toxicity of Chromium in Plants: Current Knowledge and Future Perspectives. Environ. Pollut. 2022, 315, 120390. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Zeng, G.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Li, J.; Liu, C. Decontamination of Lead and Tetracycline from Aqueous Solution by a Promising Carbonaceous Nanocomposite: Interaction and Mechanisms Insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption Characteristics of Alumina Nanoparticles for the Removal of Hazardous Dye, Orange G from Aqueous Solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Rheima, A.M.; sabri Abbas, Z.; Kadhim, M.M.; Mohammed, S.H.; Alhameedi, D.Y.; Rasen, F.A.; Ramadan, M.F.; Abed, Z.T.; Jaber, A.S.; Hachim, S.K. Aluminum Oxide Nano Porous: Synthesis, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2023, 8, 100428. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.-S.; Angaru, G.K.R.; Karri, R.R.; Yang, J.-K.; Chang, Y.-Y.; Koduru, J.R. Magnetic-Watermelon Rinds Biochar for Uranium-Contaminated Water Treatment Using an Electromagnetic Semi-Batch Column with Removal Mechanistic Investigations. Chemosphere 2022, 286, 131776. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Dai Pang, S.; Kua, H.W. Waste Valorisation Using Biochar for Cement Replacement and Internal Curing in Ultra-High Performance Concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Bakar, A.H.B.A.; Koay, Y.S.; Ching, Y.C.; Abdullah, L.C.; Choong, T.S.Y.; Alkhatib, M.; Mobarekeh, M.N.; Zahri, N.A.M. Removal of Fluoride Using Quaternized Palm Kernel Shell as Adsorbents: Equilibrium Isotherms and Kinetics Studies. BioResources 2016, 11, 4485–4511. [Google Scholar]
- Dehmolaei, A.; Vadi, M. Comparative Study of Isotherms Adsorption of B12 by Single-Wall Carbon Nanotube and Multi-Wall Carbon Nanotube. Orient. J. Chem. 2014, 30, 265. [Google Scholar] [CrossRef][Green Version]
- Noura, A.L.; Abu-Sharar, T.M. Kinetics of Fluoride Adsorption onto Native and Mg(OH)2-Amended Limestone. Appl. Water Sci. 2021, 11, 37. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W. Organic Coating on Biochar Explains Its Nutrient Retention and Stimulation of Soil Fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, Morphology, and Dissolution of Silicon and Carbon in Rice Straw-Derived Biochars under Different Pyrolytic Temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 1493966766. [Google Scholar]
- Ahuja, R.; Kalia, A.; Sikka, R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review. ACS Omega 2022, 7, 45825–45836. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, Characterisation and Methyl Orange Adsorption Capacity of Ferric Oxide–Biochar Nano-Composites Derived from Pulp and Paper Sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B. Removal of Arsenic, Methylene Blue, and Phosphate by Biochar/AlOOH Nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar Supported Nanoscale Zerovalent Iron Composite Used as Persulfate Activator for Removing Trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Cedrún-Morales, M.; Ceballos, M.; Polo, E.; Del Pino, P.; Pelaz, B. Nanosized Metal–Organic Frameworks as Unique Platforms for Bioapplications. Chem. Commun. 2023, 59, 2869–2887. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of Heavy Metals from Aqueous Solution by Biochars Derived from Anaerobically Digested Biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]

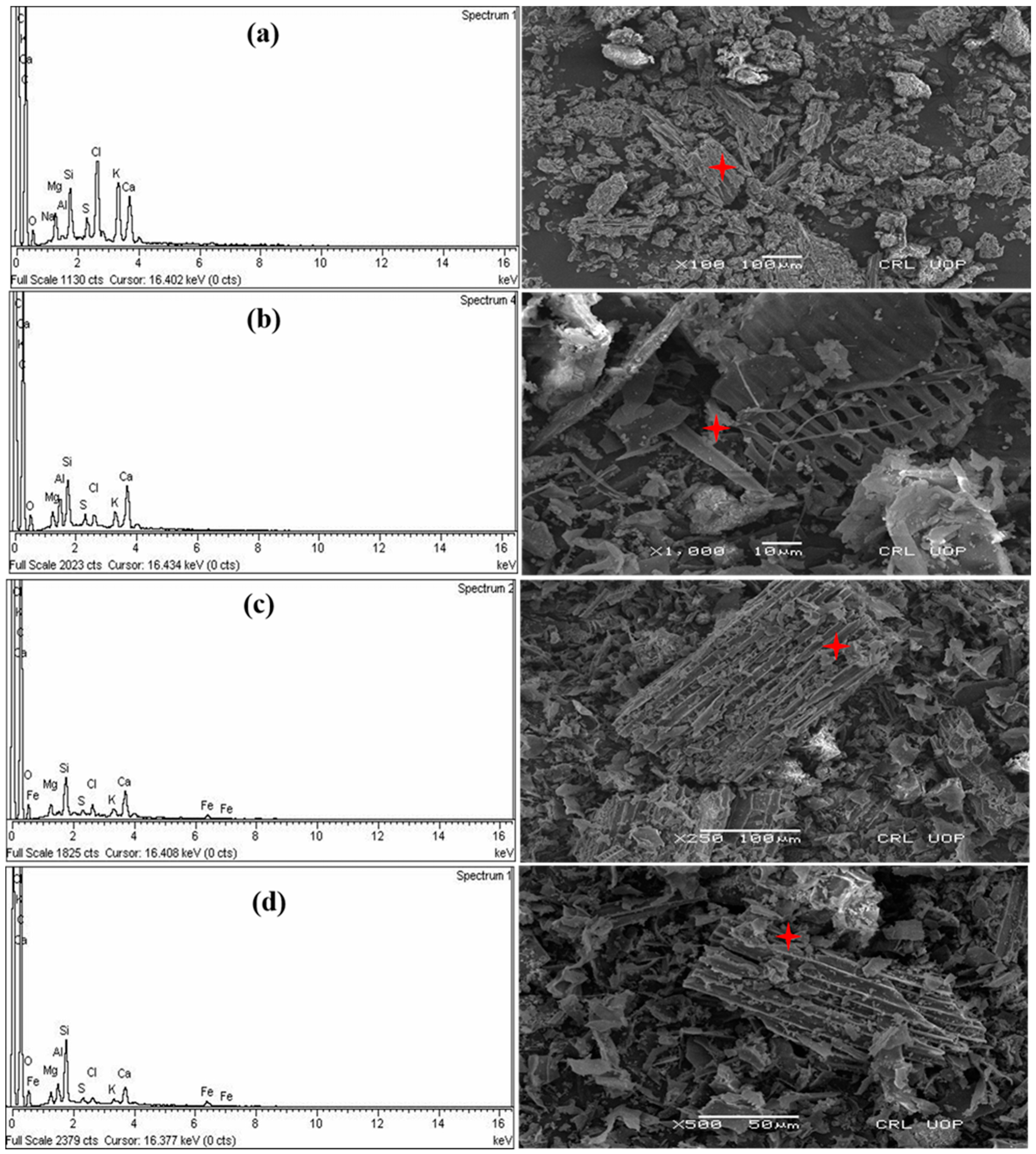

| Adsorbents | C | O | Si | Cl | K | Ca | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| BC | 75.68 | 7.32 | 2.09 | 4.77 | 4.17 | 3.44 | --------- | --------- |
| NMOBC | 77.82 | 11.47 | 1.59 | 1.12 | 1.29 | 4.06 | --------- | 0.33 |
| BC-Fe | 81.48 | 8.95 | 2.43 | 1.38 | 0.81 | 2.86 | 0.90 | --------- |
| NMOBC-Fe | 79.93 | 10.17 | 2.32 | 0.50 | 0.22 | 1.82 | 1.28 | 0.88 |
| Adsorbent | BET Area (m2g−1) | Pore Size (nm) | Pore VAv (cm3g−1) |
|---|---|---|---|
| BC | 31.29 | 5.45 | 42.73 |
| NMOBC | 34.42 | 5.93 | 51.12 |
| Ci (mg/L) | q(mg/L) Exp. | Pseudo—First—Order on NMOBC | Pseudo—First—Order on BC | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/min) | R2 | qe (mg/g) | k1 (1/min) | R2 | ||
| 5 | 1.170 | 1.188 | 0.1634 | 0.894 | 2.052 | 0.1599 | 0.891 |
| 10 | 2.289 | 1.964 | 0.0978 | 0.993 | 2.461 | 0.1664 | 0.907 |
| 15 | 3.384 | 4.662 | 0.1571 | 0.929 | 5.051 | 0.1579 | 0.903 |
| 20 | 4.453 | 5.996 | 0.1928 | 0.927 | 5.252 | 0.1554 | 0.931 |
| 25 | 5.469 | 6.827 | 0.0991 | 0.921 | 5.562 | 0.1432 | 0.996 |
| 30 | 6.513 | 7.469 | 0.1579 | 0.977 | 6.682 | 0.1906 | 0.906 |
| Ci (mg/L) | q(mg/L) Exp. | Pseudo—second—order on NMOBC | Pseudo—second—order on BC | ||||
| qe (mg/g) | k2 (g/mg·min) | R2 | qe (mg/g) | k2 (g/mg·min) | R2 | ||
| 5 | 1.170 | 1.305 | 0.1238 | 0.999 | 1.363 | 0.0687 | 0.999 |
| 10 | 2.289 | 2.548 | 0.0675 | 0.999 | 2.483 | 0.1568 | 0.999 |
| 15 | 3.384 | 3.748 | 0.0489 | 0.999 | 3.671 | 0.0312 | 0.994 |
| 20 | 4.453 | 5.066 | 0.0286 | 0.998 | 4.693 | 0.0271 | 0.991 |
| 25 | 5.469 | 6.028 | 0.0339 | 0.997 | 5.6180 | 0.0248 | 0.990 |
| 30 | 6.513 | 7.168 | 0.0291 | 0.996 | 6.5402 | 0.0207 | 0.995 |
| Ci (mg/L) | q(mg/L) Exp. | Elovich Model on NMOBC | Elovich Model on BC | ||||
| Adsorption rate (α) (mg/g·min) | Surface coverage (β) (g/mg) | R2 | Adsorption rate (α) (mg/g·min) | Surface coverage (β) (g/mg) | R2 | ||
| 5 | 1.170 | 0.524 | 3.870 | 0.981 | 0.258 | 3.216 | 0.977 |
| 10 | 2.289 | 1.082 | 1.983 | 0.969 | 0.531 | 1.671 | 0.964 |
| 15 | 3.384 | 1.721 | 1.359 | 0.965 | 0.842 | 1.202 | 0.955 |
| 20 | 4.453 | 1.616 | 0.942 | 0.972 | 1.165 | 0.933 | 0.934 |
| 25 | 5.469 | 3.108 | 0.852 | 0.948 | 1.528 | 0.785 | 0.918 |
| 30 | 6.513 | 3.749 | 0.716 | 0.94 | 1.554 | 0.678 | 0.957 |
| Isotherms | Langmuir | Freundlich | Redlich-Peterson | Sips | Temkin |
|---|---|---|---|---|---|
| Isotherm Dynamics on NMOBC | |||||
| Parameters | Qmax= 8.56 mg/g | KF = 2.556 mg/g | A = 5.603 mg/g | Qmax = 22.68 mg/g | AT =3.039 |
| b = 0.0.483 L/mg | n = 1.481 | B = 1.135 L/mg | KS =0.1301 L/g | BT = 2.067 mg/g | |
| g = 0.577 | β = 0.7898 | ||||
| R2 | 0.994 | 0.998 | 0.999 | 0.999 | 0.955 |
| RMSE (mg/g) | 0.0972 | 0.0720 | 0.0312 | 0.0239 | 0.3832 |
| Isotherm Dynamics on BC | |||||
| Parameters | Qmax = 6.71 mg/g | KF = 1.747 mg/g | A = 4.0029 mg/g | Qmax = 15.87 mg/g | AT = 1.918 |
| b = 0.386 L/mg | n = 1.679 | B = 1.4132 L/mg | KS = 0.1259 L/g | BT = 1.656 mg/g | |
| g = 0.6250 | β = 0.7246 | ||||
| R2 | 0.998 | 0.996 | 0.999 | 0.999 | 0.971 |
| RMSE (mg/g) | 0.1213 | 0.0805 | 0.0295 | 0.0278 | 0.2615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; Iqbal, J.; Bashir, M.T.; Amin, M.T.; Sikandar, M.A.; Rahman, M.M.; Arifuzzman, M. Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies. Processes 2023, 11, 3423. https://doi.org/10.3390/pr11123423
Khan AA, Iqbal J, Bashir MT, Amin MT, Sikandar MA, Rahman MM, Arifuzzman M. Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies. Processes. 2023; 11(12):3423. https://doi.org/10.3390/pr11123423
Chicago/Turabian StyleKhan, Aftab Ahmad, Javed Iqbal, Muhammad Tariq Bashir, Muhammad Tahir Amin, Muhammad Ali Sikandar, Muhammad Muhitur Rahman, and Md. Arifuzzman. 2023. "Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies" Processes 11, no. 12: 3423. https://doi.org/10.3390/pr11123423
APA StyleKhan, A. A., Iqbal, J., Bashir, M. T., Amin, M. T., Sikandar, M. A., Rahman, M. M., & Arifuzzman, M. (2023). Synthesis, Characterization, and Performance of Nano-Metal-Oxide (Al2O3) Blended Biochar for the Removal of Iron from Contaminated Water for Enhanced Kinetic and Adsorption Studies. Processes, 11(12), 3423. https://doi.org/10.3390/pr11123423










