Abstract
This study analyzes the adsorption of two model pesticides, namely, 2,4-dichlorophenoxyacetic acid (2,4-D) and carbofuran on activated carbons obtained by chemical activation with phosphoric acid of peach stones. The effect of the synthesis conditions on the surface area development was analyzed. The highest surface area was obtained with an impregnation time of 5 h, an impregnation ratio equal to 3.5, an activation temperature of 400 °C, and 4.5 h of activation time. Under these conditions, the maximum specific surface area was equal to 1182 m2·g−1 which confirms the high porosity of the activated carbon, predominantly in the form of micropores. The surface chemistry of this activated carbon was also characterized using pH at point of zero charge, scanning electron microscopy, and Fourier transform infrared spectroscopy. Both kinetics and equilibrium adsorption tests were performed. Adsorption kinetics confirmed that 2,4-D adsorption follows a pseudo first-order adsorption kinetic model, while carbofuran adsorption is better described by a pseudo second-order one. Regarding the equilibrium adsorption, a higher adsorption capacity is obtained for 2,4-D than carbofuran (c.a. 500 and 250 mg·g−1, respectively). The analysis of the thermodynamics and characterization after use suggest a predominantly physisorption nature of the process.
1. Introduction
Aquatic ecosystems are the principal receptors of contaminants [1], which are discharged directly or indirectly from diverse natural and human-caused sources, including both industrial and municipal wastewater [2,3]. Their contamination represents a threat to human health due to the potential health dangers produced by many different types of pollutants [4,5]. Among these pollutants, pesticides are worldwide used for the regulation of parasites in agricultural soils and for the control of insects that can cause and disseminate some serious diseases [3]. Due to their persistence, they can bioaccumulate in soils [6] and foods and can pose a threat to animal and human health [7]. Among various pesticides, we selected 2,4-dichlorophenoxyacetic acid (2,4-D) and carbofuran as model pesticide pollutants since they are widely used in the formulation of herbicides and insecticides, respectively. The 2,4-D is an anionic pesticide, highly selective [8], and poorly biodegradable [9,10]. It is a herbicide that kills dicots without affecting monocots and mimics natural auxin at the molecular level. It is widely used, and consequently, it is commonly detected in water lots, and in different soils. The World Health Organization (WHO) establishes the upper allowable concentration for 2,4-D in potable water at 20 μg·L−1 [11]. On the other hand, carbofuran (2,3-dihydro-2,2-dimethyl-7-benzofuranyl), is a highly toxic N-methyl carbamate insecticide [12,13]. It is being widely applied in the agronomic field for its proven efficiency in controlling insects [7]. Carbofuran is an insecticide that poisons arthropods by inhibiting the action of a specific acetylcholine esterase enzyme. In addition, this pesticide is quite persistent in the ground and aqueous ecosystems under neutral or acidic conditions. Therefore, it is considered exceedingly harmful to both birds and aquatic animals [14].
In this context, it is vital to look for effective methods to remove these hazardous products from water [9]. We mention, for example, membrane separation, flocculation, ozonation, coagulation, aerobic and anaerobic treatment [15], chemical precipitation, solvent extraction, ion-exchange capacity, photocatalytic activity [16] and adsorption [17,18]. Compared with the different available technologies for the removal of pesticides, adsorption is the most analyzed due to its flexibility, simplicity, low cost, and easiness of use [9,19,20]. In addition, it is known for its low consumption of energy and relatively high adsorption capacity [21]. Among the different adsorbents [22,23,24], activated carbons have some clear advantages [25,26,27], namely, they can be synthesized from renewable materials (biomass), are of relatively low cost [19,28], and usually show well-developed and tunable porosity [16]. Activated carbon can be obtained through two different activation methods: physical and chemical activation [29]. Physical activations consist of the partial gasification of the carbonaceous precursor using water vapor, carbon dioxide or oxygen. The removal of the carbon atoms during the gasification reaction results in the formation of porosity. Chemical activation is produced through the impregnation of the carbonaceous precursor with an activating agent. During the subsequent heating, the precursor suffers from dehydration and oxidation reactions; therefore, carbonization and activation take place simultaneously. Different chemical activating agents have been used, such as H3PO4, ZnCl2, FeCl3 or KOH. Chemical activation usually results in higher yields. Phosphoric acid activation results in activated carbon with an acidic surface that can be very suitable for some specific applications [30,31,32].
The main objective of this study is to synthesize activated carbons from peach stones through chemical activation with phosphoric acid. The effect of different synthesis parameters, namely, impregnation time, impregnation ratio (mass ratio of activating agent to carbon precursor), activation temperature and activation time, on the surface area of the resulting carbon was analyzed. The activated carbon with the highest surface area was further used for the adsorption of 2,4-D and carbofuran. Different kinetic and thermodynamic models were used to fit the experimental adsorption data.
2. Materials and Methods
2.1. Materials
All chemical products needed were obtained from Sigma Aldrich and employed without any added purification. The phosphoric acid (H3PO4) with 85% of purity was used for activated carbon synthesis. Equally, both pesticides, carbofuran (C12H15NO3) and 2,4-dichlorophenoxyacetic acid (C8H6Cl2O3) were obtained from this company and used for the adsorption studies.
2.2. Preparation of Activated Carbons
Peach stones were collected and washed several times with water to eliminate the impurities. Then, the stones were crushed and sieved (particle size between 100 and 125 mm), and dried for 24 h at 105 °C. The material was subjected to a chemical activation method with phosphoric acid (50%) in a tubular horizontal furnace. The dried stones were physically mixed with a variable amount of phosphoric acid (impregnation ratios between 3 and 5) at different impregnation times (4–6 h). Next, the heterogeneous mixture was introduced in a tubular furnace and heated at 10 °C·min−1 up to the activation temperature (300–600 °C) and maintained at this temperature during the activation time (3–5 h). After cooling down, the samples were washed with distilled water to remove the remains of the activating agent and reaction byproduct and to free the newly developed porous texture. The experimental design provides the possibility of optimizing the parameters and minimizing the error with a reduced number of experiences [33,34]. In the current study, experiments with the specific surface area as a response were designed with the Central Composite Design (CCD) with four, two-level, factors using Design-Expert 13 software with 30 essays.
2.3. Characterization Techniques
Elemental analyses were conducted in a LECO CHNS-932 apparatus to quantify the content of C, N, and H in the activated carbon. N2 adsorption isotherms were acquired at −196 °C with a Micromeritics TriStar II instrument to quantitatively examine the pore structure of the optimized activated carbon. The solid samples (weight between 100 and 150 mg) were degasified at 150 °C during 24 h. The specific surface area was lastly determined by the Brunauer−Emmett−Teller (BET) method. The micropore volume and external surface area were obtained using the t-method applied to the adsorption branch of the isotherm. Finally, the total pore volume was estimated from the amount of N2 adsorbed at relative pressures close to 1 transformed in liquid volume.
Fourier Transform Infrared Spectroscopy (FTIR) analyses were carried out with a Bruker Vertex 70 V spectrophotometer by transmission using KBr pellets in the wavenumber range from 400 to 4000 cm−1.
The pH at the point of zero charge (pHPZC) was quantified using the pH drift method. Briefly, the pH value of several solutions of NaCl was adjusted from 1 to 13 using HCl (0.1 M) or NaOH (0.1 M). The activated carbon (20 mg) was suspended in the different solutions and bubbled with N2 for 5 min to eliminate the gases dissolved in the solutions. After 48 h under stirring, the pH was measured and represented versus the initial one, corresponding to the intersection of the line with the diagonal to the pHPZC value.
Scanning Electron Microscopy (SEM) was performed to identify the morphology of the selected activation carbon. The sample was covered with a 4 nm layer of platinum in Leica EM ACE600 equipment and was measured in a FESEM TESCAN CLARA microscopy with ultra-high resolution at 15 KV and an ET detector.
Thermogravimetric Analyses (TGA/TDA) were investigated for the thermal stability of the best activated carbon in an SDT650 TA instrument, using a 10 °C·min−1 heating rate up to 900 °C under a 100 mL·min−1 continuous air atmosphere.
2.4. Adsorption Tests
The selected activated carbon (that with the highest BET surface area) was used to test the adsorption from aqueous solution of two model pesticides, 2,4-D and carbofuran. Batch equilibrium tests were conducted at different temperatures (25, 45, and 65 °C) with a volume equal to 250 mL of aqueous solution at various initial concentrations of the pesticide ranging from 5 to 100 mg⋅L−1 with 50 mg·L−1 of the activated carbon. After 24 h of controlled temperature and agitation (200 rpm), the solutions were filtered and finally, the remaining concentration of the corresponding pesticide in the liquid solution phase was analyzed by UV-Vis. Also, adsorption kinetic experiments were performed in the same batch system with an initial pesticide concentration of 20 mg⋅L−1 with the same solution volume, activated carbon concentration, and temperatures mentioned previously for the equilibrium trials.
3. Results and Discussion
3.1. Characterization of Activated Carbons
The optimization of the activated carbon preparation procedure from peach stones was carried out. The selection of the best activated carbon was made based on the maximization of the specific surface area, as a response, to the experiences of the CCD plan. Table 1 shows some results for the textural properties of activated carbons prepared at different conditions. As can be seen, in all cases, the specific surface area was high, achieving the sample AC5_4.5_400_4.5, that synthesized with an impregnation time of 5 h, at an impregnation ratio of 4.5, an activation temperature of 400 °C and activation time of 4.5 h, the highest total surface area (1182 m2·g−1). This value was higher than the values found in the literature for activated carbons prepared from the same precursor. For example, Pérez-Rodríguez et al. [35] obtained a specific surface area value of 846 m2·g−1 for activated carbon obtained from peach stones through physical activation with water vapor, and the values presented by Kozyatnyk et al. [36] did not exceed 500 m2·g−1 when activating peach stones with CO2. Since AC5_4.5_400_4.5 showed the highest surface area and the most developed porous texture, it was selected for the following adsorption tests and a complete characterization.

Table 1.
Porous texture parameters.
Figure 1 depicts the N2 adsorption–desorption isotherm of the ACs with highest surface areas. The isotherm showed the typical shape of type I according to the UIPAC classification [37]. This type of isotherm corresponds to a predominantly microporous solid with some minor contribution of mesoporosity. This is confirmed by the low value of external (Sext) compared to the BET surface areas as well as the higher proportion of micropore (Vmicro) in the total pore volume (Vpore) [37,38,39], as can be seen in Table 1.
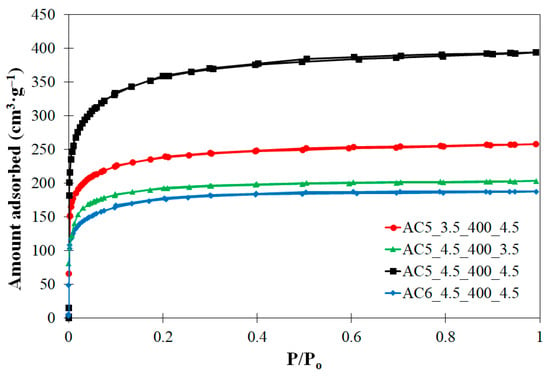
Figure 1.
N2 adsorption–desorption isotherm at 77 K.
The results of the elemental analysis of peach stone precursor and AC5_4.5_400_4.5 are collected in Table 2. After activation of raw material, the carbon content increases and becomes close to 80%, while the percentages of hydrogen, oxygen, and nitrogen decrease. This can be justified by the removal of the molecules of water and the decomposition/devolatilization of the precursor during the activation stage. The ash content increased slightly with activation, although it remains very low (lower than 5%). These results agree with others found in the literature for activated carbons obtained from the same raw material [40,41,42,43,44].

Table 2.
Elemental analysis of peach precursor and AC5_4.5_400_4.5 activated carbon.
Thermogravimetric analyses for both activated carbon and raw material were conducted in the temperature range from 30 to 900 °C under an air atmosphere (Figure 2). Three major steps were observed in the case of the raw peach stone. The first one, from 30 to 100 °C corresponds to the lowest reduction in weight (less than 7%), and is associated with the removal of water molecules (dehydration). The highest value of weight reduction was found during the second step for temperatures ranging from 100 to 500 °C and it can be explained by the oxidation of cellulose and hemicellulose in the peach stone. Finally, the last part of the curve is expected to correspond to the oxidation of lignin, the most stable biopolymer in biomass. In the case of the activated carbon, the TG profile is clearly displaced to very significantly higher temperatures due to the previous devolatilization of part of the organic matter during the activation step at 400 °C. Only a negligible mass loss (most likely associated with the loss of humidity) is obtained below the activation temperature. Above 500 °C, a dramatic mass loss is observed due to the fast combustion of the organic matter in the carbon structure. It is worth mentioning the high oxidation resistance of this activated carbon with no significant mass loss up to temperatures close to 500 °C. This is a consequence of the formation of different phosphate groups on the activated carbon surface that are responsible for this enhancement of the oxidation resistance as confirmed by previous studies [45,46].
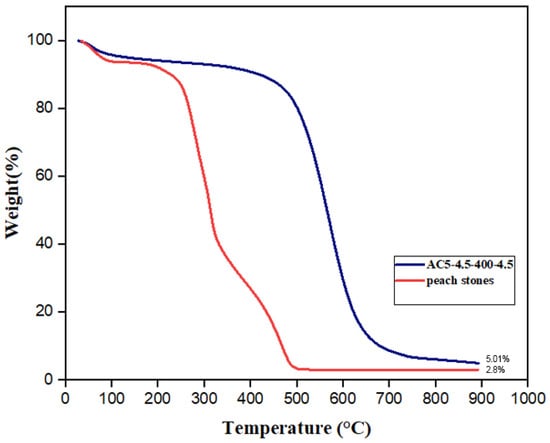
Figure 2.
Thermogravimetric analysis of peach stones and AC5_4.5_400_4.5.
Figure 3 shows the morphology of the activated carbon at different resolutions. SEM images highlighted the presence of many cavities that would allow for faster access to the internal porosity of the activated carbon improving the kinetics of the adsorption process. This morphology is characteristic of activated carbons obtained from biomass precursors, with high cavities coming from the original structure of the biomass precursor [47,48].
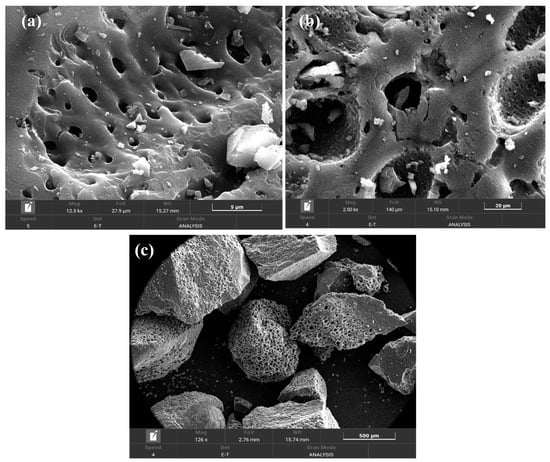
Figure 3.
SEM images of AC5_4.5_400_4.5 (a) = 5 µm; (b) = 20 µm; (c) = 500 µm.
Besides the morphological and textural properties, the surface charge and functional groups are significant for the understanding of the adsorption mechanism. Figure 4 represents the evolution of the final pH of the solution versus the initial value for the activated carbon. The pHPZC of the activated carbon was 4.3 characteristic of an acidic surface. It is expected that the activation with phosphoric acid resulted in an acidic carbon surface. Therefore, in aqueous solutions at a pH higher than 4.3, the activated carbon surface would be charged negatively, while it would be charged positively for a pH lower than this value. The pKa value of 2,4-D was approximately equal to 2.8, resulting in the speciation diagram represented in Figure 5a. Consequently, at pH values lower than 2.8, the 2,4-D pesticide molecules are in a molecular state, and therefore no electrostatic interactions between the positively charged surface of the carbon and 2,4-D molecules are expected [49]. In contrast, at pH values higher than 4.3, a certain electrostatic repulsion would be expected for the 2,4-D adsorption. In the case of carbofuran, the pKa of this pesticide is 11.9 [50]; therefore, carbofuran is a non-ionic pesticide, without strong acidic or basic functionalities and it does not dissociate in the solution (except at a very basic pH higher than 11.9, see speciation diagram in Figure 5b) indicating that no electrostatic interactions would exist between carbofuran and the activated carbon surface.
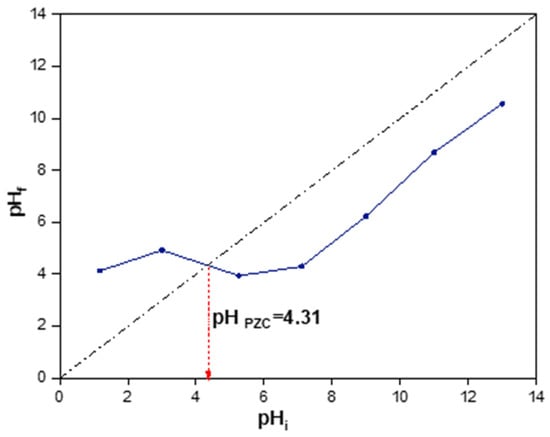
Figure 4.
pHPZC of AC5_4.5_400_4.5.
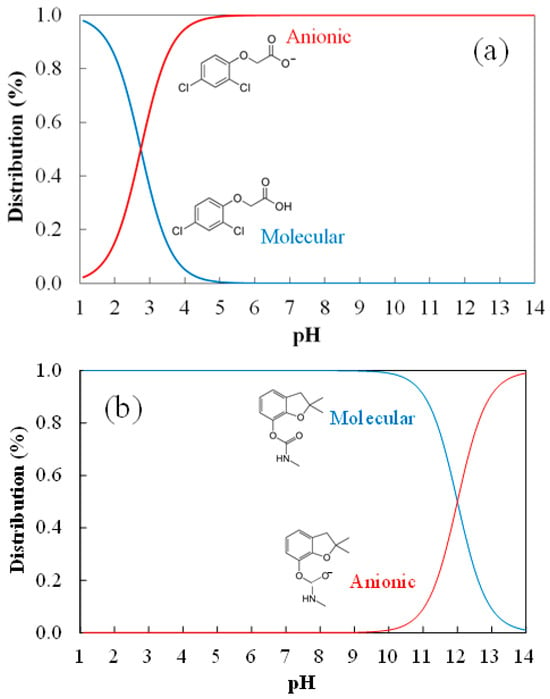
Figure 5.
Speciation diagram of (a) 2,4-dichlorophenoxyacetic acid and (b) carbofuran.
Figure 6 represents the FTIR spectra of the original activated carbon and after its use in 2,4-D and carbofuran adsorption. The spectrum of the original carbon shows a limited number of bands. This is expected since the increase in the temperature during the activation step removes part of the functional groups in the form of volatile matter. The string band centered at around 3390 cm−1 is associated with the stretching vibrations of the O–H bonds, usually due to the presence of surface hydroxyl groups or adsorbed water molecules. The asymmetry of this band at lower wavenumbers is characteristic of the presence of strong hydrogen bonds. The broad band at 1570 cm−1 corresponds to C–C vibrations in aromatic rings. The last broad band at 1161 cm−1 can be ascribed to C–O stretching in acids, alcohols, phenols, ethers, and/or esters groups [51]. However, it is also characteristic of phosphorous, and phosphor carbonaceous compounds present in the phosphoric acid-activated carbons, such as the stretching vibration of hydrogen-bonded P=O, to O–C stretching vibrations in P–O–C (aromatic) linkage, and to P=OOH [52]. The band at around 1700 cm−1 is related to the C═O stretching vibrations of carboxyl or carbonyl groups, while those at 1430 and 1380 cm−1 are characteristic of the C═C vibrations of the aromatic rings [53]. The band centered at 1050 cm−1 may be ascribed to the asymmetrical stretch vibrations of C–O–C groups [54]. In the low wavenumber region, the bands at 870 and 750 cm−1 can be related to the out-of-plane bending of C–H groups in the aromatic rings [55].
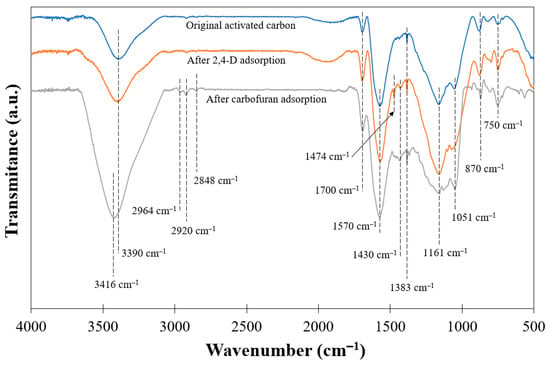
Figure 6.
FTIR diagrams for AC5_4.5_400_4.5 before and after adsorption.
3.2. Adsorption Isotherms
Figure 7 and Figure 8 represent the adsorption isotherms at three different temperatures (25, 45, and 65 °C) of 2,4-D and carbofuran, respectively. The tests were performed at natural pH (c.a. 6.0). The isotherms correspond to type L according to Giles classification [56] characteristics of a strong interaction between the molecules of adsorbate and adsorbent and weak interaction between the molecules of adsorbates [57]. In this type of adsorption isotherm, the initial curvature shows that as more sites in the substrate are filled, it becomes increasingly difficult for the adsorbate molecules to find vacant sites. It can also be observed that the increase in the adsorption temperature results in an increase in the amounts adsorbed for both pesticides, suggesting an endothermic character of the adsorption process. If we compare the adsorption capacities of the two pesticides, it is concluded that those of 2,4-D are significantly higher than the corresponding ones of carbofuran in agreement with previous studies [5,58]. This can be explained on the basis of donor–acceptor mechanism between the negatively charged oxygen of the carbonyl groups of 2,4-D molecule (which is in anionic state at the natural pH used in the tests, while carbofuran is in molecular form, see Figure 5) and some surface oxygenated groups of the activated carbon surface.
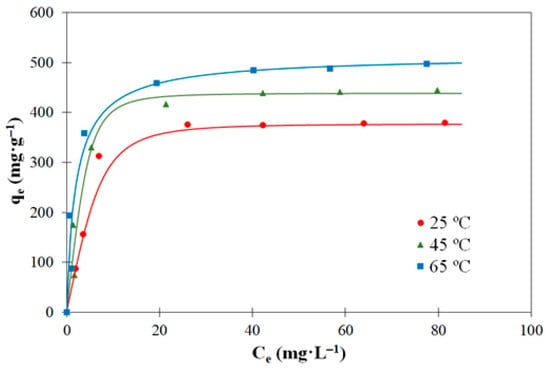
Figure 7.
Experimental data (symbols) and Toth model’s fitting (solid lines) for the adsorption equilibrium of 2,4-D on the activated carbon (C0 = 250 mg·L−1; V = 250 mL).
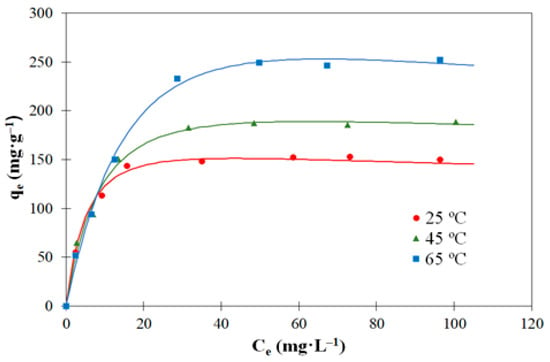
Figure 8.
Experimental data (symbols) and Redlich–Peterson model’s fitting (solid lines) for the adsorption equilibrium of carbofuran on the activated carbon (V = 250 mL; C0 = 250 mg·L−1).
The adsorption capacities of both pesticides have been compared with those obtained in previous studies with different adsorbents. Hameed et al. [10] reported a 2,4-D adsorption capacity of 238.1 mg·g−1 at 30 °C on an activated carbon from KOH chemical activation of date stones. Lazarotto et al. [59] achieved an adsorption capacity of 250.8 mg·g−1 at 35 °C and pH 4 on mushroom-derived activated carbon synthesized by chemical activation with zinc chloride. Other different studies can be found in the literature, most of them reporting 2,4-D adsorption capacities significantly lower than those obtained in the current study (between 350 and 500 mg·g−1, depending on the adsorption temperature). In the case of carbofuran, Njoku et al. [48] found a maximum adsorption capacity of 198.4 mg·g−1 when using activated carbon from coconuts activated with phosphoric acid. This value is similar to the adsorption capacity of carbofuran achieved in this work. However, other authors reported significantly lower adsorption capacities for the same compound. For example, J.M. Salman et al. reported values of 96.1 mg·g−1 [5] and 137.0 mg·g−1 [60] using a commercial granular activated carbon and a date seed activated carbon, respectively. The most plausible explanation for the higher adsorption capacities obtained by our activated carbon is related to its high total surface area (close to 1200 m2·g−1), higher than those of the adsorbents used in the studies.
For the aim of investigating the relationship between equilibrium adsorbate and adsorbent concentrations, the experimental data were fitted with five empirical models (with two or three parameters), namely, Langmuir, Freundlich, Sips, Toth, and Redlich–Peterson. Table 3 shows their non-linear equations with the coefficients of correlation and the corresponding fitting parameters for both pesticides.

Table 3.
Adsorption isotherms modeling for the 2,4-D and carbofuran pesticides.
In the case of 2,4-D, Langmuir and Freundlich models presented high deviations with respect to the experimental data (specially Freundlich), which suggests that the ideal hypothesis of preferential monolayer coverage and energetic homogeneity of adsorption sites must be discarded. Toth model is the most adequate for the description of the experimental data as justified by the highest value of the correlation coefficient, although any of the three parameter models fit properly the experimental adsorption data. Toth model is typical of the adsorption of gas or organic compounds [61] and indicates that the adsorbent surface is heterogenous [62]. In the case of carbofuran adsorption, deviations were lower than those of 2,4-D, being Toth and Redlich–Peterson models the most suitable for the description of the experimental adsorption equilibrium by the activated carbon. As it is mentioned in the literature [61,62], the Redlich–Peterson model can be used to describe both heterogeneous and homogeneous systems. Figure 7 and Figure 8 represent the fitting of the 2,4-D and carbofuran adsorption experimental data (points) to Toth and Redlich–Peterson models (solid lines), respectively. As can be seen, these models fit the experimental results properly.
3.3. Adsorption Kinetics
Figure 9 and Figure 10 represent the adsorption kinetic curves of 2,4-D and carbofuran at different temperatures (25, 45, and 65 °C), respectively. Adsorption was slow for both pesticides, requiring around 200 min to achieve equilibrium.
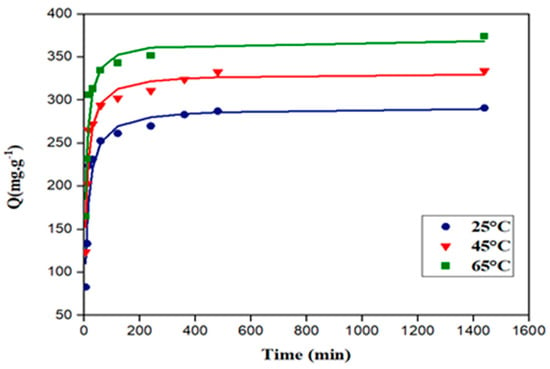
Figure 9.
Kinetic curves of adsorption of 2,4-D on the activated carbon at 25, 45, and 65 °C. Symbols: experimental data; solid lines: fitting to pseudo first-order model (C0 = 20 mg·L−1; V = 250 mL).
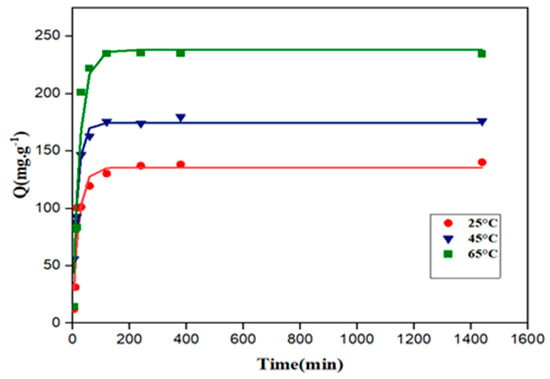
Figure 10.
Kinetic curves of adsorption of 2,4-D on the activated carbon at 25, 45, and 65 °C. Symbols: experimental data; solid lines: fitting to second-order model (C0 = 20 mg·L−1; V = 250 mL).
To understand the adsorption mechanisms and the rate-limiting step, and to identify the kinetic parameters, numerous models were employed in the literature [5,63,64]. In the current study, four models were checked, namely, pseudo-first order, pseudo-second order, Elovich, and intraparticle diffusion. Table 4 summarizes the non-linear form of each equation with the regression coefficients and the corresponding parameters obtained from the fitting of the models to the experimental adsorption kinetic data.

Table 4.
Adsorption kinetics modeling for the 2,4-D and carbofuran pesticides.
According to the results, the pseudo first-order model yielded the best fitting for both 2,4-D and carbofuran adsorption on the activated carbon. Aksu et al. [64] found similar results for the adsorption of the 2,4-D on a commercial activated carbon. Meththika Vithanage et al. [65] confirmed that both pseudo first-order and pseudo second-order models described properly the kinetics of carbofuran adsorption. The intraparticle diffusion model does not fit properly with the experimental data (very low values of the correlation coefficients), suggesting that intraparticle diffusion is not the limiting step for the adsorption of both pollutants [66].
3.4. Thermodynamics
Temperature is a key parameter in adsorption since the change in temperature can modify the adsorption process, and consequently, the adsorption uptake. The thermodynamic parameters, standard free energy (ΔG⁰), standard enthalpy (ΔH⁰), and standard entropy (ΔS⁰) were calculated for the following equations:
where KL is the Langmuir constant in (L·mol−1) [67], T is the absolute temperature in kelvins, and R is the gas constant (8.314 J·mol−1·K−1). Table 5 summarizes the values of the thermodynamic parameters obtained for the adsorption of both pesticides. The negative values of the standard free energy for both adsorbates are characteristic of spontaneous processes, as usual in adsorption.
ΔG⁰ = −R·T·ln (KL)
ΔG⁰ = ΔH⁰ − T·ΔS⁰

Table 5.
Thermodynamic parameters of the 2,4-D and carbofuran pesticide adsorption.
In the case of 2,4-D adsorption, the standard enthalpy is 16.9 kJ·mol−1. The positive value indicates that the adsorption process of this compound is endothermic, and the relatively low value is characteristic of predominantly physical adsorption. The standard entropy is −0.146 kJ·mol−1·K−1. The negative value is indicative of a decrease in the randomness of the molecules at the solid−liquid interface and a higher ordering of the 2,4-D molecules after being adsorbed on the activated carbon surface, although it should be considered that the value is very low, and consequently, indeed implies no significant change in entropy during adsorption. These results agree with other previous reports. In this sense, Kamaraj et al. [68] reported values of 0.13 kJ·mol−1 and 0.412 kJ·mol−1·K−1 for standard enthalpy and entropy, respectively, when analyzing the 2,4-D adsorption on metal hydroxides. A very similar value of the standard enthalpy 15.6 kJ·mol−1 was obtained by Ding et al. [69] when analyzing the adsorption of 2,4-D on a magnetic ion-exchange resin, although they reported a low positive value of the standard entropy (0.076 kJ·mol−1·K−1). In the case of carbofuran, values of −22.4 kJ·mol−1 and 0.016 kJ·mol−1·K−1 were obtained for the standard enthalpy and entropy, respectively. The negative value of the standard enthalpy implies an exothermic adsorption process and again predominantly physical adsorption. The value of the standard entropy is very low, suggesting that the adsorption does not produce significant changes in the ordering of carbofuran molecules. The value of the standard enthalpy agrees with that reported by Mayakaduwa et al. [70] (−27.85 kJ·mol−1) for the carbofuran adsorption on an extruded activated carbon. Although, it should be noticed that this same study obtained positive standard enthalpy values for the adsorption of this same compound on a carbon black and a granular activated carbon (9.71 and 8.72 kJ·mol−1, respectively).
3.5. Adsorption Mechanism
At the adsorption pH used (c.a. 6.0), the activated carbon surface is negatively charged (see Figure 4) and 2,4-D and carbofuran molecules are in an anionic and molecular state, respectively. Considering this, in the case of 2,4-D, some repulsive interaction is expected between the carbon surface and the pesticide molecules, despite which high 2,4-D adsorption capacities have been observed. In the case of carbofuran, no significant electrostatic interaction is expected. This suggests that the adsorption mechanism of both pesticides is not governed by electrostatic forces. Most likely, a specific interaction between the oxygenated surface groups of the carbon surface and some groups of the molecules of the pesticides would take place, in addition to a physical adsorption process as suggested by the relatively low values of the standard adsorption enthalpies for both pollutants. Figure 6 represents the FTIR spectra of the activated carbon after adsorption of 2,4-D and carbofuran. In the case of 2,4-D adsorption, a new band is observed at 1474 cm−1 that can be associated with the corresponding C=C vibration of the aromatic ring of the molecule, confirming the presence of the component of the carbon surface. However, no additional bands or significant modifications are observed which support the predominant physisorption of the 2,4-D molecules. The carbofuran adsorption results in the appearance of bands at 2964, 2920, and 2848 cm−1 related to the stretching vibrations of C–H bonds in the pesticide molecule. In addition, a broadening and displacement of the stretching vibrations of the O–H bonds up to 3416 cm−1 can be observed, which suggest the participation of alcohols, phenols, and/or carboxylic acid groups in carbofuran adsorption via hydrogen bonding or dipole interactions [70].
4. Conclusions
The chemical activation of peach stones with phosphoric acid resulted in carbons with well-developed surface area, obtaining the highest surface area (1182 m2·g−1) with an impregnation time of 5 h, an impregnation ratio equal to 3.5, an activation temperature of 400 °C, and 4.5 h of activation time. This carbon showed promising behavior for the adsorption of two model pesticides, 2,4-D and carbofuran. Adsorption kinetics confirmed that 2,4-D adsorption follows a pseudo first-order adsorption kinetic model, while carbofuran adsorption is better described by a pseudo second-order one. Regarding the equilibrium adsorption, a higher adsorption capacity is obtained for 2,4-D than carbofuran (c.a. 500 and 250 mg·g−1, respectively). The analysis of the thermodynamics and characterization after use suggest a predominantly physisorption nature of the process. It should be considered that all the adsorption tests were performed with only one pollutant, future work should be performed in the evaluation of the performance of these carbon-based adsorbents in a more complex mixture of pollutants, and in the presence of other components usually found in natural waters. In addition, fixed-bed adsorption tests can be analyzed in the future to assess the interest of the proposed adsorbents in real practical applications.
Author Contributions
Conceptualization, C.B. and J.B.; methodology, S.H., A.Á.-M. and A.G.-A.; validation, S.H., A.Á.-M. and A.G.-A.; formal analysis, M.B., C.B. and J.B.; resources, C.B. and J.B.; writing—original draft preparation, S.H. and M.B.; writing—review and editing, A.Á.-M. and J.B.; supervision, S.G., C.B. and J.B.; project administration, S.G., C.B. and J.B.; funding acquisition, C.B. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish “Agencia Estatal de Investigación” from “Ministerio de Ciencia e Innovación” proyect number TED2021-129948B-I00.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salomón, Y.L.D.O.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. High-Performance Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide in Water Using Activated Carbon Derived from Queen Palm Fruit Endocarp (Syagrus romanzoffiana). J. Environ. Chem. Eng. 2021, 9, 104911. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Mini, J.; Munuswamy, N. Effects of Heavy Metals on Antioxidants and Expression of HSP70 in Different Tissues of Milk Fish (Chanos chanos) of Kaattuppalli Island, Chennai, India. Ecotoxicol. Environ. Saf. 2013, 98, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Harabawy, A.S.A.; Ibrahim, A.T.A. Sublethal Toxicity of Carbofuran Pesticide on the African Catfish Clarias Gariepinus (Burchell, 1822): Hematological, Biochemical and Cytogenetic Response. Ecotoxicol. Environ. Saf. 2014, 103, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Binh, Q.A.; Nguyen, H.H. Investigation the Isotherm and Kinetics of Adsorption Mechanism of Herbicide 2,4-Dichlorophenoxyacetic Acid (2,4-D) on Corn Cob Biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar] [CrossRef]
- Salman, J.M.; Hameed, B.H. Adsorption of 2,4-Dichlorophenoxyacetic Acid and Carbofuran Pesticides onto Granular Activated Carbon. Desalination 2010, 256, 129–135. [Google Scholar] [CrossRef]
- Cotillas, S.; Sáez, C.; Cañizares, P.; Cretescu, I.; Rodrigo, M.A. Removal of 2,4-D Herbicide in Soils Using a Combined Process Based on Washing and Adsorption Electrochemically Assisted. Sep. Purif. Technol. 2018, 194, 19–25. [Google Scholar] [CrossRef]
- Ibrahim, K.E.A.; Şolpan, D. Removal of Carbofuran in Aqueous Solution by Using UV-Irradiation/Hydrogen Peroxide. J. Environ. Chem. Eng. 2019, 7, 102820. [Google Scholar] [CrossRef]
- Nejati, K.; Davary, S.; Saati, M. Study of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Removal by Cu-Fe-Layered Double Hydroxide from Aqueous Solution. Appl. Surf. Sci. 2013, 280, 67–73. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective Photodegradation of 2,4-D Pesticide from Water by Molecularly Imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872. [Google Scholar] [CrossRef]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption Isotherm and Kinetic Modeling of 2,4-D Pesticide on Activated Carbon Derived from Date Stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef]
- Goswami, B.; Mahanta, D. Polyaniline-Fe3O4 and Polypyrrole-Fe3O4 magnetic Nanocomposites for Removal of 2,4-Dichlorophenoxyacetic Acid from Aqueous Medium. J. Environ. Chem. Eng. 2020, 8, 103919. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran Toxicity and Its Microbial Degradation in Contaminated Environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Ferrarini, A.M.T.; Rezende, K.F.O.; Martinez, D.S.T.; Alves, O.L. Effects of Multiwalled Carbon Nanotubes and Carbofuran on Metabolism in Astyanax Ribeirae, a Native Species. Fish Physiol. Biochem. 2019, 45, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Mansano, A.S.; Moreira, R.A.; Pierozzi, M.; Oliveira, T.M.A.; Vieira, E.M.; Rocha, O.; Regali-Seleghim, M.H. Effects of Diuron and Carbofuran Pesticides in Their Pure and Commercial Forms on Paramecium Caudatum: The Use of Protozoan in Ecotoxicology. Environ. Pollut. 2016, 213, 160–172. [Google Scholar] [CrossRef]
- Taher, T.; Munandar, A.; Mawaddah, N.; Syamsuddin Wisnubroto, M.; Siregar, P.M.S.B.N.; Palapa, N.R.; Lesbani, A.; Wibowo, Y.G. Synthesis and Characterization of Montmorillonite—Mixed Metal Oxide Composite and Its Adsorption Performance for Anionic and Cationic Dyes Removal. Inorg. Chem. Commun. 2023, 147, 110231. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent Advances in Activated Carbon Modification Techniques for Enhanced Heavy Metal Adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Brishti, R.S.; Kundu, R.; Habib, M.A.; Ara, M.H. Adsorption of Iron(III) from Aqueous Solution onto Activated Carbon of a Natural Source: Bombax Ceiba Fruit Shell. Results Chem. 2023, 5, 100727. [Google Scholar] [CrossRef]
- Subba Reddy, Y.; Matangi, R.; Rotte, N.K. Sustainable Mesoporous Graphitic Activated Carbon as Biosorbent for Efficient Adsorption of Acidic and Basic Dyes from Wastewater: Equilibrium, Kinetics and Thermodynamic Studies. SSRN Electron. J. 2022, 9, 100214. [Google Scholar] [CrossRef]
- Sellaoui, L.; Yazidi, A.; Taamalli, S.; Bonilla-Petriciolet, A.; Louis, F.; El Bakali, A.; Badawi, M.; Lima, E.C.; Lima, D.R.; Chen, Z. Adsorption of 3-Aminophenol and Resorcinol on Avocado Seed Activated Carbon: Mathematical Modelling, Thermodynamic Study and Description of Adsorbent Performance. J. Mol. Liq. 2021, 342, 116952. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by Magnetic Activated Carbon and Its Mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Xu, L.; Liu, Z. Removal of Toluene from Waste Gas by Adsorption-Desorption Process Using Corncob-Based Activated Carbons as Adsorbents. Ecotoxicol. Environ. Saf. 2018, 165, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, Y.H.; Pei, R. Recent progress on adsorption of cadmium ions from water systems using metal-organic frameworks (MOFs) as an efficient class of porous materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef] [PubMed]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Nabipour, H.; Mansoorianfar, M.; Hu, Y. Carboxymethyl cellulose-coated HKUST-1 for baclofen drug delivery in vitro. Chem. Pap. 2022, 76, 6557–6566. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; García-Lovera, R.; Escalona, N.; Sepúlveda, C.; Sotelo, J.L.; García, J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 2015, 279, 788–798. [Google Scholar] [CrossRef]
- Mohammad, S.G.; El-Sayed, M.M.H. Removal of imidacloprid pesticide using nanoporous activated carbons produced via pyrolysis of peach stone agricultural wastes. Chem. Eng. Commun. 2021, 208, 1069–1080. [Google Scholar] [CrossRef]
- Sanz-Santos, E.; Álvarez-Torrellas, S.; Larriba, M.; García, J. Activated carbons derived from biomass for the removal by adsorption of several pesticides from water. In Advanced Materials for Sustainable Environmental Remediation; Giannakoudakis, D., Meili, L., Anastopoulos, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 565–583. ISBN 978032390485. [Google Scholar]
- Bedia, J.; Belver, C.; Ponce, S.; Rodriguez, J.; Rodriguez, J.J. Adsorption of Antipyrine by Activated Carbons from FeCl3-Activation of Tara Gum. Chem. Eng. J. 2018, 333, 58–65. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C—J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef]
- Hosseinzaei, B.; Hadianfard, M.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Heating Rate and H3PO4 as Catalyst on the Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2022, 168, 105724. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of SO2 Removal over Lignin-Based Activated Carbon. Chem. Eng. J. 2017, 307, 707–721. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Hemp-Derived Activated Carbon Fibers by Chemical Activation with Phosphoric Acid. Fuel 2009, 88, 19–26. [Google Scholar] [CrossRef]
- Kousha, M.; Tavakoli, S.; Daneshvar, E.; Vazirzadeh, A.; Bhatnagar, A. Central Composite Design Optimization of Acid Blue 25 Dye Biosorption Using Shrimp Shell Biomass. J. Mol. Liq. 2015, 207, 266–273. [Google Scholar] [CrossRef]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Alipanahpour Dil, E.; Javadian, H. RSM-CCD Design of Malachite Green Adsorption onto Activated Carbon with Multimodal Pore Size Distribution Prepared from Amygdalus Scoparia: Kinetic and Isotherm Studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Sebastián, D.; Alegre, C.; Tsoncheva, T.; Petrov, N.; Paneva, D.; Lázaro, M.J. Biomass Waste-Derived Nitrogen and Iron Co-Doped Nanoporous Carbons as Electrocatalysts for the Oxygen Reduction Reaction. Electrochim. Acta 2021, 387, 138490. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Oesterle, P.; Wurzer, C.; Mašek, O.; Jansson, S. Removal of Contaminants of Emerging Concern from Multicomponent Systems Using Carbon Dioxide Activated Biochar from Lignocellulosic Feedstocks. Bioresour. Technol. 2021, 340, 125561. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Calzaferri, G.; Gallagher, S.H.; Brühwiler, D. Multiple Equilibria Describe the Complete Adsorption Isotherms of Nonporous, Microporous, and Mesoporous Adsorbents. Microporous Mesoporous Mater. 2022, 330, 111563. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pinto, O.; Izquierdo, M.T.; Segura, C.; Poon, P.S.; Celzard, A.; Matos, J.; Fierro, V. Upgrading of Pine Tannin Biochars as Electrochemical Capacitor Electrodes. J. Colloid Interface Sci. 2021, 601, 863–876. [Google Scholar] [CrossRef]
- Martins, A.F.; Villetti, M.A.; Mortari, S.R.; Pedroso, G.B.; Saldanha, L.F.; Rambo, M.K.D. Detoxification of Fermentable Broth with Activated Biocarbon Resulting from Pyrolysis of Agroforestry Residues. Water Environ. Res. 2021, 93, 1445–1454. [Google Scholar] [CrossRef]
- Gerçel, Ö.; Özcan, A.; Özcan, A.S.; Gerçel, H.F. Capacity of Activated Carbon Derived from Peach Stones by K2CO3 in the Removal of Acid, Reactive, and Direct Dyes from Aqueous Solution. Environ. Eng. 2009, 135, 919–932. [Google Scholar] [CrossRef]
- Demiral, İ.; Samdan, C.; Demiral, H. Enrichment of the Surface Functional Groups of Activated Carbon by Modification Metho. Surf. Interfaces 2021, 22, 100873. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of Straw Activated Carbon and Its Application in Wastewater Treatment: A Review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd Supported on Mesoporous Activated Carbons with High Oxidation Resistance as Catalysts for Toluene Oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorption of Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Njoku, V.O.; Islam, M.A.; Asif, M.; Hameed, B.H. Preparation of Mesoporous Activated Carbon from Coconut Frond for the Adsorption of Carbofuran Insecticide. J. Anal. Appl. Pyrolysis 2014, 110, 172–180. [Google Scholar] [CrossRef]
- Calisto, J.S.; Pacheco, I.S.; Freitas, L.L.; Santana, L.K.; Fagundes, W.S.; Amaral, F.A.; Canobre, S.C. Adsorption Kinetic and Thermodynamic Studies of the 2, 4—Dichlorophenoxyacetate (2,4-D) by the [Co–Al–Cl] Layered Double Hydroxide. Heliyon 2019, 5, e02553. [Google Scholar] [CrossRef]
- Samy, M.; Gar Alalm, M.; Fujii, M.; Ibrahim, M.G. Doping of Ni in MIL-125(Ti) for Enhanced Photocatalytic Degradation of Carbofuran: Reusability of Coated Plates and Effect of Different Water Matrices. J. Water Process Eng. 2021, 44, 102449. [Google Scholar] [CrossRef]
- Zawadzki, J. Infrared Spectroscopy in Surface Chemistry of Carbons. Chem. Phys. Carbon 1989, 21, 147–386. [Google Scholar]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic Carbons Activated with Phosphoric—Acid I. Surface Chemistry and Ion Binding Properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Feng, P.; Li, J.; Wang, H.; Xu, Z. Biomass-Based Activated Carbon, and Activators: Preparation of Activated Carbon from Corncob by Chemical Activation with Biomass Pyrolysis Liquids. ACS Omega 2020, 5, 24064–24072. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Huang, L.; Xu, X.; Li, Q. Comparative Study on Characterization and Adsorption Properties of Activated Carbons with H3PO4 and H4P2O7 Activation Employing Cyperus Alternifolius as Precursor. Chem. Eng. J. 2012, 181–182, 790–797. [Google Scholar] [CrossRef]
- Giles, C.H.; Silva, A.P.D.; Easton, I.A. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Belver, C.; Rodriguez, J.J.; Bedia, J. Equilibrium, Kinetics and Breakthrough Curves of Acetaminophen Adsorption onto Activated Carbons from Microwave-Assisted FeCl3-Activation of Lignin. Sep. Purif. Technol. 2022, 278, 119654. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Suhas; Saini, V.K. Adsorption of 2,4-D and Carbofuran Pesticides Using Fertilizer and Steel Industry Wastes. J. Colloid Interface Sci. 2006, 299, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Preparation of Activated Carbon from the Residues of the Mushroom (Agaricus bisporus) Production Chain for the Adsorption of the 2,4-Dichlorophenoxyacetic Herbicide. J. Environ. Chem. Eng. 2021, 9, 106843. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Bentazon and Carbofuran Adsorption onto Date Seed Activated Carbon: Kinetics and Equilibrium. Chem. Eng. J. 2011, 173, 361–368. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Orduz, A.E.; Acebal, C.; Zanini, G. Activated Carbon from Peanut Shells: 2,4-D Desorption Kinetics Study for Application as a Green Material for Analytical Purposes. J. Environ. Chem. Eng. 2021, 9, 104601. [Google Scholar] [CrossRef]
- Aksu, Z.; Kabasakal, E. Batch Adsorption of 2,4-Dichlorophenoxy-Acetic Acid (2,4-D) from Aqueous Solution by Granular Activated Carbon. Sep. Purif. Technol. 2004, 35, 223–240. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, Thermodynamics and Mechanistic Studies of Carbofuran Removal Using Biochars from Tea Waste and Rice Husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.A.; Yusup, S.; Hameed, B.H. Kinetic Studies on Carbon Dioxide Capture Using Lignocellulosic Based Activated Carbon. Energy 2013, 61, 440–446. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Kamaraj, R.; Davidson, D.J.; Sozhan, G.; Vasudevan, S. Adsorption of 2,4-Dichlorophenoxyacetic Acid (2,4-D) from Water by in Situ Generated Metal Hydroxides Using Sacrificial Anodes. J. Taiwan Inst. Chem. Eng. 2014, 45, 2943–2949. [Google Scholar] [CrossRef]
- Ding, L.; Lu, X.; Deng, H.; Zhang, X. Aqueous Solutions Using MIEX Resin. Ind. Eng. Chem. Res. 2012, 51, 11226–11235. [Google Scholar] [CrossRef]
- Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D.; Vithanage, M. Insights into Aqueous Carbofuran Removal by Modified and Non-Modified Rice Husk Biochars. Environ. Sci. Pollut. Res. 2017, 24, 22755–22763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).