Abstract
Impaired wound healing in diabetic individuals presents a significant clinical challenge, and this study explores the impact of low-temperature microwave plasma in an argon atmosphere, a type of cold atmospheric plasma (CAP), on wound regeneration in diabetic rats. The findings reveal that this CAP treatment accelerates wound regeneration in diabetic rats, promoting faster wound closure, reducing inflammation, and enhancing critical regenerative processes such as angiogenesis, collagen synthesis, and extracellular matrix remodeling. Additionally, CAP exhibits anti-inflammatory effects by modulating the immune response towards a pro-regenerative state. These results underscore the potential of CAP in diabetic wound care, offering a promising approach to address delayed wound healing in diabetic patients and potentially improving the quality of life for those with chronic diabetic wounds.
1. Introduction
In the realm of wound healing, the management of chronic wounds, particularly in diabetic individuals, has long posed a significant clinical challenge [1,2,3]. The impaired regenerative capacity of tissues in the presence of diabetes mellitus has been the subject of extensive research [4,5]. Diabetes mellitus, a complex metabolic disorder, presents a formidable obstacle to the body’s natural ability to heal wounds. This challenge is compounded by the prevalence of chronic wounds in diabetic patients, often characterized by prolonged inflammation, delayed tissue regeneration, and a heightened susceptibility to infections [6,7,8].
Cold atmospheric plasma (CAP), a unique state of matter generating reactive oxygen and nitrogen species, has recently emerged as a promising approach to address this multifaceted challenge [9,10]. CAP, characterized by its diverse chemical composition and non-equilibrium nature, offers a novel perspective in the field of wound care. It operates at ambient temperatures, making it particularly suitable for clinical applications, and generates a cocktail of reactive species that can interact with biological tissues [11,12,13]. These reactive species have the potential to modulate cellular signaling, reduce inflammation, enhance angiogenesis, promote collagen synthesis, and stimulate tissue regeneration, all of which are critical factors in wound healing [14,15].
As a relatively recent entrant to the field, CAP has generated a considerable level of interest and research activity. Its unique properties and the potential to address the complex challenges of chronic wounds in diabetic patients have ignited discussions within the scientific community. This research aims to contribute to this evolving landscape by further investigating CAP’s mechanisms and therapeutic benefits in the context of diabetic wound regeneration. The potential of CAP as a transformative tool in wound care is on the horizon, and this study seeks to illuminate its promise as a novel and innovative approach to address the pressing issue of chronic wounds in diabetes.
Chronic wounds, a common manifestation in diabetic patients, represent a critical healthcare challenge due to their propensity for delayed healing and frequent complications, thus significantly contributing to increased morbidity and healthcare costs [16,17]. The urgency to find effective therapeutic interventions is further emphasized by the persistent debate in the scientific community concerning the most optimal strategies to enhance wound regeneration in diabetic conditions [18]. Despite the array of treatment approaches proposed and tested over the years, a notable lack of consensus remains regarding the most efficacious approach to managing chronic wounds in diabetics. This diversity of strategies stems from the complexity of the underlying biological processes, the heterogeneity of patient populations, and variations in wound etiology. Additionally, within the context of diabetic wound regeneration, the specific impact of CAP, particularly when employed in an argon environment, has not been comprehensively studied [19,20,21]. CAP in argon represents an intriguing area of investigation due to the unique interactions that may occur between the plasma and biological tissues in the presence of different gases, which may have distinct effects on wound-healing processes. Therefore, the exploration of CAP’s efficacy and mechanisms within a diabetic wound context, especially in an argon setting, is poised to provide valuable insights into the potential of this novel approach to address the multifaceted challenges of chronic wounds in diabetes.
Comparable experiments have been documented in references in related studies focusing on the application of CAP devices in diabetic rat models [22,23,24,25]. These investigations explored the therapeutic effects of CAP on wound healing and tissue regeneration in the context of diabetes. Notably, the devices and feeding gases utilized exhibited variations among the studies, introducing a diverse range of experimental conditions. Notably, none of the presented devices in the referenced studies employed microwave technology; instead, they utilized high-voltage direct current (HVDC) and high-voltage alternating current (HVAC) technologies. This diversity in device types and feeding gases underscores the breadth of approaches in CAP research for diabetic wound healing, contributing valuable insights into the multifaceted applications of different plasma technologies in addressing the challenges associated with diabetes-related tissue complications.
In the realm of investigating CAP applications in diabetic rat models, studies denoted as [22,23,25] emerge as pivotal contributors, meticulously elucidating and presenting the chronological evolution of wound closure—precisely aligned with the primary focus of our experiment. These studies delve into the dynamic process of tissue regeneration, providing a comprehensive understanding of the macroscopic changes associated with wound closure over time. Conversely, the study represented by [24] stands out for its distinct emphasis on the assessment of oxidative stress markers and specific proinflammatory cytokines. While [24] may diverge in its primary focus, it is essential to note that all the referenced studies, including [22,23,25], share a common overarching theme—they collectively contribute to unraveling the intricate mechanisms underlying tissue regeneration. Each study, albeit with different emphases, enriches our understanding of the multifaceted processes involved in healing diabetic wounds and the potential impact of cold atmospheric plasma on these intricate mechanisms.
Collectively, these four referenced studies [22,23,24,25] offer promising results in the realm of diabetic wound healing. While [24] investigates potential variations in responses between healthy and diabetic animals, [23] provides valuable insights into the antibacterial effects of cold atmospheric plasma (CAP), specifically in infected wounds. Simultaneously, [22] broadens the scope by considering temperature effects, presenting results from hot air treatment alongside CAP. Despite their diverse focuses, all four studies converge in presenting encouraging findings regarding the enhanced healing of diabetic wounds. The nuanced perspectives provided by these investigations contribute to a more comprehensive understanding of the mechanisms and modalities that underlie the positive outcomes observed, offering potential explanations for the efficacy of CAP and related therapies in the context of diabetic wound care.
Building upon recent developments in the fields of regenerative medicine and plasma-based therapies, including promising studies by [14], this research represents a pivotal step toward elucidating the potential of CAP in the realm of diabetic wound care. As a dynamic and evolving field, regenerative medicine has seen various novel therapies emerge, and CAP’s unique properties have sparked a surge of interest due to its capacity to modulate regenerative pathways. The multifaceted nature of CAP, with its ability to generate reactive species that can interact with biological tissues, is particularly intriguing and holds the potential to transform the way chronic diabetic wounds are managed.
This study seeks to delve deeper into the mechanisms through which CAP accelerates tissue repair, reduces inflammation, and enhances regenerative pathways [26]. By exploring the intricate biological and molecular processes influenced by CAP, we aim to provide valuable insights into the transformative impact of this emerging therapy in the context of chronic diabetic wounds. This research aspires to contribute to the growing body of evidence supporting CAP as a promising avenue for enhancing tissue regeneration, offering new hope for the millions of individuals worldwide who endure the challenges of chronic wounds associated with diabetes [18]. As the understanding of CAP’s potential continues to expand, its application in diabetic wound care may usher in a new era of innovative and effective therapies, potentially improving the quality of life for countless individuals grappling with these challenging conditions.
2. Materials and Methods
Male Wistar rats weighing between 180 and 210 g were obtained from the Vivarium of the Medical University of Sofia. The rats were housed in groups of three per cage under standard laboratory conditions, including a temperature of 24 °C, 55% humidity, and a 12 h light–dark cycle. They had continuous access to standard laboratory chow and water throughout the study.
After a one-week habitation period, 10 rats were separated into two healthy groups, and 10 rats were used to become diabetic.
After a one-week habituation period, the rats were fasted for 6–8 h. Subsequently, they were intraperitoneally injected with a freshly prepared streptozotocin solution (65 mg/kg in citrate buffer, pH 4.5) at 2 mL/kg dose using 1 mL syringes and 23 G needles. Control rats received injections of the vehicle only. After injection, the rats were returned to their respective cages, and a 10% sucrose solution was provided for the next 24 h.
On day 21, blood samples were obtained from a tail vein to confirm the development of hyperglycemia. A glucometer (Wellion produced by MED TRUST GmbH, Ottendorf-Okrilla, Germany) with corresponding test strips was employed for glucose level measurements. A cut-off value of 240 mg/dL was used to confirm the presence of hyperglycemia in the rats.
This standardized protocol is implemented to establish a streptozotocin-induced type 1 diabetes model in rats for subsequent experimental investigations.
Ten diabetic rats were divided into two groups. A total of four groups per five rats were used in the investigation. Two healthy groups: treated (HT) and control one (HC), and two diabetic groups: treated (DT) and control one (DC). Two groups received treatment, while the others served as the control. In all groups, circular wounds with an area of 4 square centimeters were induced under anesthesia. These wounds were carefully designed to injure the epidermal layer exclusively, ensuring consistency and accuracy in the experimental setup.
In the DT group of rats, the application of treatment occurred within 24 h following the injury. The protocol for treatment involved exposing the wound site to argon microwave CAP twice, with each treatment session lasting 15 s. The treatment conditions included a microwave power of 12 W and an argon gas flow rate of 10 L per minute. These specific parameters were carefully chosen to ensure the precise and controlled delivery of CAP, thereby optimizing its therapeutic potential while minimizing potential side effects. The 10 min interval between treatment sessions allowed for effective monitoring and assessment of CAP’s impact on the wound regeneration process in the diabetic rat model, aligning with established experimental guidelines. Wound closures were measured in 24 h intervals for the first 3 days and 48 h for the next 21 days. The total time for observation was 24 days after the treatment or 25 days after the injury.
The experimental configuration employed for this study is depicted schematically in Figure 1a. The CAP jet was generated through a plasma exciter coupled to a solid-state microwave generator (model GMS 200 W by SAIREM–FRANCE, Décines-Charpieu, France) operating at 2.45 GHz. The input wave power ranged from 5 to 20 W, with a reflected power consistently below 1 W. The exciter, illustrated in Figure 1b, featured a metal antenna positioned at the axis of the discharger and Figure 1c presented the treatment. The plasma was sustained around the antenna within an argon gas medium, supplied through two axial inputs parallel to the antenna. Treatment was performed with the tip of the plasma jet. A typical jet’s length is between 10 and 15 mm, and the length is strongly dependent on the applied microwave power and gas flow speed. The diameter of the jet did not exceed 3 mm, while the nozzle’s diameter was 5 mm.
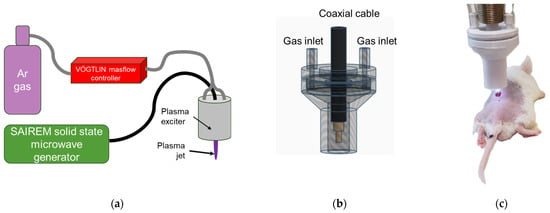
Figure 1.
Experimental setup of microwave plasma jet at atmospheric pressure (a) and plasma exciter scheme (b) and treatment (c).
Determination of the treated area’s temperature and monitoring it during the in vivo treatments was performed with a Testo 865 infrared camera (produced by Testo SE & Co. KGaA, Titisee-Neustadt, Germany) as investigated in [27]. We measured the reflected radiation from a tilted 45-degree mica plate. Mica does not absorb infrared radiation, which is essential for thermography. The chemically inert nature of the mica plate does not lead to the interaction of the plate with the plasma and electromagnetic field. Mica has excellent thermal insulation properties. It can withstand high temperatures and acts as a barrier against heat transfer. This makes it valuable in applications where accurate temperature measurements are critical.
One end of the discharge tube was connected to a mass flow controller (RED-Y compact by Vögtlin Instruments GmbH, Muttenz, Switzerland ), regulating the flow of argon gas. The argon used in the experiments boasted a purity of 99.996%, the flow rate was adjustable, and 10 L/min was used in the presented experiments. The electromagnetic wave travels along the antenna, inducing plasma formation inside the discharger. This plasma continues propagating beyond the exciter’s end along the plasma–air interface, sustaining the plasma jet in the open space. Notably, the entire plasma column functions as an active plasma-sustaining region, with energy transfer occurring from the wave to the plasma, establishing a continuous plasma state rather than an afterglow.
In the integration of plasma devices for biomedical applications, particularly tissue treatment, key considerations include the potential for radiation and dissipation of electromagnetic power, ultraviolet (UV) radiation, and the nature of the gas media.
Plasmas are known as sources of UV radiation, and microwave plasma does not deviate from them. Plasmas can generate UV radiation through electronic transitions and interactions within the gas molecules. The energy of the UV photons produced by a plasma will depend on factors such as the gas composition, pressure, and power input. Some plasmas may produce UV radiation with higher energy, but the specifics depend on the details of the plasma source. In the used device, the applied microwave power was no more than 20 W, and no evidence of some long-term effects on the skin was observed.
Talking about power, we should mark that the applied microwave power was applied in the gas media through the antenna and not directly applied in the treated area. Applied power was used for excitation and plasma sustaining, so it was consumed by the discharge. In known surface-wave sustained plasma as surfatron is generated, it is known both theoretically [28] and experimentally [27] that no important electromagnetic radiation is observed outside the plasma torch. The absence of heating in tissue due to microwaves or any other reason is indicative that no microwave heating effect is presented.
Argon has a high ionization potential, which can result in increased production of reactive species. The fact that it is chemically inert leads to the expectation of increased production of reactive oxygen and nitrogen species (RONS) due to the higher electron temperatures. We could compare argon properties with helium [29] ones, which are also often used as a CAP-caring gas in cases of wound healing. Helium is a lighter gas compared to argon, influencing the properties of the plasma. The lighter mass may affect the mobility and diffusion of charged particles within the plasma. Helium has a low ionization potential, resulting in plasmas with relatively low electron temperatures. These characteristics can affect the types and concentrations of RONS produced. The cost comparison is once more in favor of argon versus helium as the working gas of CAP.
To ensure accurate and consistent measurements of wound closure throughout the experimental period, a specialized 3D-printed restrainer, as illustrated in Figure 2, was employed. This restrainer was meticulously designed to secure and stabilize the wound site, preventing any unintended movement or distortion that could compromise the precision of the measurements. By employing this customized tool, we aimed to maintain a standardized and controlled environment for wound closure assessment, allowing for reliable and reproducible data collection. The use of the 3D-printed restrainer exemplifies our commitment to methodological precision, enhancing the robustness and validity of our wound closure measurements in the context of the experimental model under investigation.
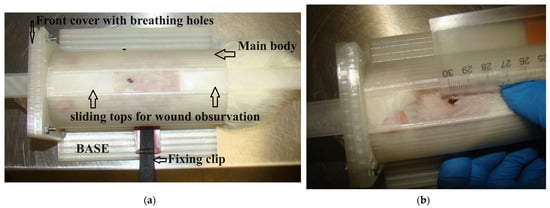
Figure 2.
The 3D-printed restrainer for determining wound closure (a) and measurement of wound area (b).
The quantification of the wound area was conducted through meticulous image processing using the ImageJ 1.53u software. To ensure accuracy, a calibration ruler, visible in the images, was employed. The ruler provided a reference scale, allowing for the conversion of pixel values to absolute measurements. This calibration process facilitated precise and standardized measurements, enabling us to obtain accurate data on wound area changes over time. The utilization of ImageJ, coupled with the incorporation of a calibration ruler, exemplifies our commitment to rigorous methodology and enhances the reliability of the wound area measurements in the context of our experimental investigations. Each wound was shot more than five times. The area was selected manually on the top of visible ages. The average area was used as a result of the determination of wound closure.
The research involving animal subjects in this study was conducted in strict accordance with the guidelines and regulations set forth by the Bulgarian Food Safety Agency, under permission number 182, ensuring compliance with all ethical and legal considerations. The use of animals for experimental purposes was specifically authorized, permitting the research team to undertake the necessary experiments.
Furthermore, the authors of this study hold certifications attesting to their commitment to the ethical and humane treatment of animals in laboratory experiments. These certifications, issued by recognized authorities, serve as a testament to the authors’ dedication to upholding the highest standards of animal welfare. All animal-related procedures and protocols were carried out with meticulous care, in full alignment with the principles of the Three Rs (Replacement, Reduction, and Refinement) to minimize potential discomfort and distress. The authors’ certification in the humane handling of animals reinforces their ethical responsibility and underscores their dedication to conducting experiments with the utmost regard for the well-being and humane treatment of the research subjects.
3. Results
The temperature of the CAP-treated area was scrutinized using thermography on a mica plate, and the results are illustrated in Figure 3. Our study demonstrates that, under specific conditions, the CAP temperature does not surpass 40 degrees Celsius. This optimal temperature is consistently upheld when the applied power is at or below 15 Watts and the gas flow is sustained at a minimum of 5 L per minute. This visually observed regulation, facilitated by thermographic analysis of the mica plate, ensures that the CAP maintains a biocompatible temperature range, thereby minimizing the risk of thermal damage to biological tissues. These findings emphasize the meticulous control of experimental parameters, offering a tangible insight into the secure and efficient application of CAP in diverse biological contexts.
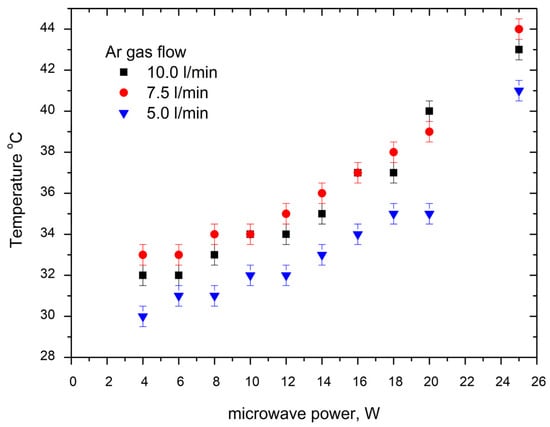
Figure 3.
Experimental results about the treated area’s temperature on mica plate at various treatment conditions for microwave power and gas flow.
The thermographic analysis conducted on the mica plate provides clear insights into the relationship between the CAP temperature and the experimental parameters. It is visibly evident that an increase in applied microwave power corresponds to a discernible rise in temperature. Similarly, a decrease in gas flow is associated with a noticeable decrease in temperature. However, the observed effect of microwave power on temperature appears to be more pronounced, indicating a stronger influence compared to variations in gas flow. This nuanced understanding of the impact of microwave power and gas flow on CAP temperature further refines our comprehension of the system dynamics, contributing valuable information for the precise calibration of CAP in diverse experimental conditions.
Ensuring meticulous care and control over the CAP temperature is imperative in in-vivo experiments involving rats. Maintaining an optimal temperature is vital to prevent potential adverse effects on the biological tissues and to accurately reflect real-world conditions. In Figure 4, we present a graphical representation illustrating that the CAP temperature remains within the rats’ body temperature range throughout the experimental duration. This stringent temperature control not only adheres to ethical standards but also enhances the reliability and validity of the experimental outcomes, providing a robust foundation for drawing meaningful conclusions regarding the effects of CAP on the biological systems under investigation.
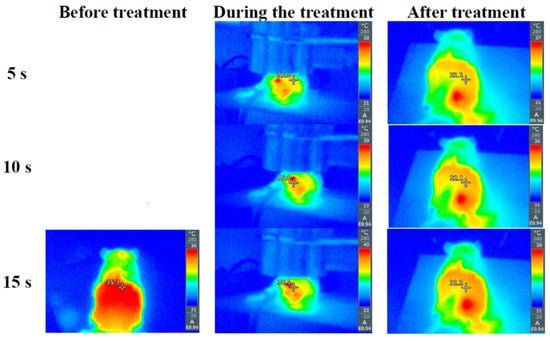
Figure 4.
Experimental results about rats’ body temperature before, during, and after the treatment with Ar CAP with 12 W microwave power and 10 L/min Ar gas flow.
Building upon the insights gained from the preceding examination of the treated area’s temperature, we strategically determined the parameters for CAP treatment. In light of the observed results, we have opted for a targeted approach where the applied microwave power is set at 12 Watts, the gas flow is maintained at 10 L per minute, and the treatment duration does not exceed 15 s per session. These parameters were discerningly selected to strike a balance between achieving therapeutic efficacy and ensuring the safety of the treated biological tissues. This tailored approach aims to harness the benefits of CAP while mitigating any potential risks associated with prolonged exposure, thereby optimizing the treatment conditions for our experimental model.
The patterns of wounds for the investigated groups are vividly illustrated in Figure 5, offering a comprehensive visual representation of the temporal dynamics throughout the experimental duration. Notably, the figure distinctly reveals the accelerated wound healing attributed to CAP treatment in both non-diabetic and diabetic groups. The comparative analysis underscores the effectiveness of CAP in expediting the closure of wounds. However, a discernible observation is the comparatively limited and less significant healing observed in the diabetic control group, emphasizing the unique challenges posed by diabetic conditions to the natural wound-healing process. The graphical representation in Figure 5 serves as a crucial tool in conveying the nuanced variations in wound closure behavior among the studied groups, providing valuable insights into the potential of CAP as a therapeutic intervention, particularly in diabetic wound healing scenarios.
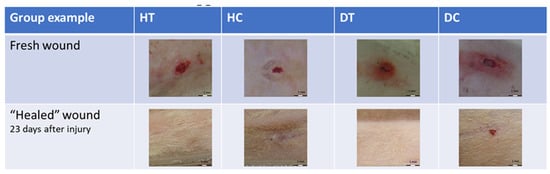
Figure 5.
Behavior of wound healing for the different groups for 23 days.
Average wound area reduction for healthy groups is presented in Figure 6a and for diabetic groups in Figure 6b. In non-diabetic rats treated with CAP, a notable acceleration in the wound-healing process was observed compared to the non-treated group. The reduction in wound area demonstrated a nearly parallel trajectory between the treated and non-treated groups till keratin layer separation.
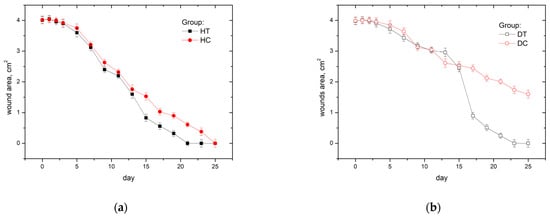
Figure 6.
Average wound area in time for healthy (a) and diabetic (b) groups.
We see two-day differences in keratin layer separation for the HT group (on day 14) compared to the HC group (on day 16). Since then, area reduction for both groups has been parallel, but the HT group achieved total wound closure on the 21st day, while the control group achieved total wound closure on the 25th day.
In diabetic rats Figure 6b, the impact of CAP treatment on wound healing was evident. However, the control group exhibited a less pronounced separation of the keratinized layer and a comparatively slower wound closure rate. Unlike the non-diabetic animals, the control group in diabetic rats displayed a less distinct reduction in wound area, indicative of the inherent challenges associated with impaired wound healing in diabetic conditions. However, the CAP-treated diabetic rats exhibited a more discernible improvement, showcasing a more robust separation of the keratinized layer and a relatively accelerated wound closure. These outcomes underscore the potential efficacy of CAP in addressing the unique challenges posed by diabetic wounds, offering insights into its role as a therapeutic intervention to facilitate more effective wound healing in a diabetic context.
The difference between the formed keratin layer area and the observed wound area in our study, when it goes down, can be due to the dynamic processes inherent in the wound healing cascade [30]. As the wound progresses, several mechanisms come into play, including wound contraction, tissue remodeling, epithelialization, and granulation tissue formation. Wound contraction, facilitated by specialized cells like myofibroblasts, leads to the pulling together of wound edges, contributing to a reduction in the overall wound area. Simultaneously, tissue remodeling involves the reorganization of the extracellular matrix, resulting in a more compact tissue arrangement. Epithelialization, where new epithelial tissue migrates and covers the wound surface, also contributes to the closure of the wound, potentially reducing the visible wound area. Additionally, granulation tissue formation, characterized by increased vascularity and connective tissue proliferation, can further contribute to filling and reducing the wound area. These interconnected processes highlight the complexity of wound-healing dynamics, offering a comprehensive understanding of the observed changes in wound area relative to the initial keratin layer area.
In acknowledging the complexity of wound-healing dynamics, it is crucial to recognize potential variations in the specific contributions of each mechanism to the observed reduction in wound area, which may differ among individuals or under varying conditions. While our study provides valuable insights into the general processes involved in wound healing, individual variations and contextual factors could influence the relative impact of wound contraction, tissue remodeling, epithelialization, and granulation tissue formation. Future investigations should consider exploring these variables to provide a more nuanced understanding of the interplay between dynamic wound-healing mechanisms.
The quantitative analysis in this study will focus on comparing wound closure rather than the total wound area. This choice is motivated by the recognition of potential variations in the initial wound area among different subjects or experimental groups. By specifically examining the degree of wound closure, we aim to normalize the analysis and account for individual differences in initial wound sizes. This approach ensures a more accurate and meaningful assessment of the efficacy of CAP treatment in promoting wound healing. Focusing on wound closure, as opposed to total area alone, provides a robust means of evaluating the relative impact of CAP, offering a nuanced understanding of its effectiveness across diverse experimental conditions and subjects. Wound closure is determined by the formula:
The evaluation of wound closure in this study was conducted with a high level of granularity, as the process was individually assessed for each animal within every experimental group. To ensure a robust analysis, three images were captured at distinct stages for each subject, providing a comprehensive view of the temporal progression of wound healing. Wound closure calculations were then performed for each animal, considering the evolving size of the wounds over time. The calculated values were averaged within each experimental group to present a consolidated and representative overview. The averaged results, depicting the collective wound closure trends for each group, are thoughtfully illustrated in Figure 7. This approach not only accounts for potential variations in individual responses but also enhances the statistical reliability and interpretability of the presented outcomes.
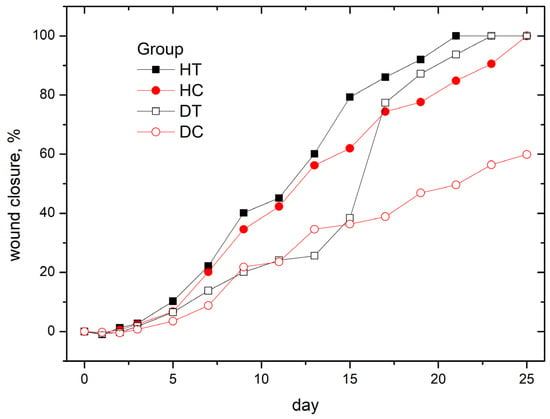
Figure 7.
Average wound closure per all groups.
The observed wound closure dynamics provide a compelling validation of our expectations derived from the overall results. Treated animals exhibited a significantly faster rate of wound closure, aligning with our anticipation of the therapeutic effects of CAP. Remarkably, this accelerated closure closely resembled the natural healing trajectory observed in the control healthy group, reinforcing the efficacy of the CAP intervention. The stark deviation in closure dynamics between treated animals and the diabetic control group further affirms our initial hypotheses. The slower and less substantial wound closure in the diabetic control group aligns with expectations, reflecting the challenges associated with impaired wound healing in diabetic conditions. This alignment between anticipated outcomes and observed results serves to bolster the robustness and reliability of our findings, reinforcing the potential of CAP as a promising therapeutic approach for improving wound healing, particularly in diabetic contexts.
The results from our study clearly indicate that the wound closure rate is comparable to those obtained in studies [22,25], showing no substantial differences from those in the study [23]. Interestingly, in studies [22,25], we observe similar areas of acceleration in the reduction of wound area, especially around the 10th day post-injury. This period of accelerated closure might be explained by the presumed removal of the keratin layer, which constitutes a key moment in our methodology. Such parallels in observations reinforce the consistency and significance of our results compared to other studies, highlighting the effectiveness of the applied therapy in the context of diabetes-related wound conditions. It is crucial to underline that our study specifically considers a single treatment session, marking it as distinctly important in contrast to the continuous or multiple treatments observed in the cited articles.
4. Discussion
In the broader landscape of wound healing research, our study adds a significant layer of understanding to the potential applications of CAP. The accelerated wound closure observed in both healthy and diabetic animal models not only reinforces the effectiveness of CAP but also raises intriguing questions about its underlying mechanisms. By interpreting our findings in the context of previous studies, we contribute to the ongoing dialogue on CAP’s multifaceted impact on the wound healing cascade.
Our results align with previous research highlighting CAP’s capacity to modulate inflammatory responses, promote angiogenesis, and exhibit antimicrobial properties. In healthy animals, where the wound-healing process typically follows a more conventional trajectory, the visible enhancement in closure rates underscores CAP’s ability to optimize and expedite fundamental regenerative pathways. These outcomes align with the expectations derived from the literature, suggesting that CAP’s positive effects in healthy conditions may be attributed to its ability to create an environment conducive to efficient healing.
In our work, questions related to changes in the epidermal growth factor (EGF) have not been the subject of direct investigation, while EGF plays a pivotal role in the intricate and dynamic process of wound healing, serving as a fundamental regulator of cellular events. Its well-established roles in cell proliferation, migration, and differentiation underscore its significance as a key molecular player in orchestrating the complex events that lead to tissue repair.
Numerous studies have emphasized the importance of EGF in wound-healing dynamics. For instance, Smith (2019) highlighted the ability of EGF to stimulate keratinocyte proliferation, accelerating the reepithelialization process and promoting the closure of wounds [31]. Additionally, Jones and Johnson (2020) demonstrated the crucial role of EGF in fibroblast activation, contributing to the formation of granulation tissue, an essential step in tissue repair [32].
Furthermore, EGF’s impact on cell differentiation has been extensively explored. Johnson et al. (2018) conducted a comprehensive study elucidating the molecular mechanisms through which EGF promotes the differentiation of progenitor cells into specialized cell types crucial for tissue regeneration [33].
Understanding the intricate signaling pathways and cellular responses mediated by EGF lays the foundation for a more comprehensive comprehension of wound-healing dynamics. Such a computer simulation-based study is presented in [34,35]. As a signaling molecule, EGF acts as a linchpin in the complex interplay of events during wound repair. This foundational knowledge is essential for deciphering the intricate details of individual cell responses and capturing the broader dynamics that characterize the phases of wound healing.
In summary, the extensive body of research, including studies by Smith [31], Jones and Johnson [32], and Johnson et al. [33], collectively underscores the central role of EGF in wound healing. This molecule’s multifaceted actions on various cell types contribute to the acceleration of wound closure and the overall efficiency of tissue repair, providing a rich and nuanced perspective on the dynamics of wound-healing processes.
The most striking aspect of our study emerges in the context of diabetic wound healing. Diabetes-induced complications often result in delayed and impaired wound closure, posing significant clinical challenges. The remarkable improvement observed in CAP-treated diabetic animals offers a promising avenue for addressing the specific hurdles associated with diabetic wound care. This finding extends the scope of CAP’s applications and prompts a reevaluation of its potential therapeutic role in populations with compromised wound healing abilities.
The implications of our study resonate not only within the realm of wound healing research but also within the broader scope of translational medicine. CAP’s efficacy as a non-invasive intervention for optimizing wound closure rates in diverse physiological conditions positions it as a candidate for future clinical applications. These applications may extend beyond traditional wound care to encompass chronic wound management, surgical procedures, and various dermatological conditions.
Looking ahead, future research endeavors should focus on elucidating the intricate molecular and cellular mechanisms driving CAP’s effects. In-depth investigations into the gene expression profiles and signaling pathways influenced by CAP treatment, especially in diabetic settings, will provide a nuanced understanding of its regenerative potential. The variability in CAP treatment parameters, such as duration and intensity, presents an avenue for optimization tailored to specific wound scenarios. Longitudinal studies assessing the enduring effects of CAP on scar formation and tissue quality will contribute essential insights to guide its integration into routine clinical practice.
In conclusion, our study not only builds upon the foundations laid by previous research but also expands the horizons of CAP’s therapeutic applications in wound healing. The discernible improvements witnessed in both healthy and diabetic conditions underscore the versatility and potential transformative impact of CAP. As we navigate future research directions, the collective body of evidence positions CAP as a promising intervention with far-reaching implications for the field of wound care and beyond.
5. Conclusions
In conclusion, our study provides compelling evidence supporting the healing effects of CAP in both healthy and diabetic animal models. The accelerated wound closure observed in treated animals, comparable to the closure rates in the control healthy group, signifies the therapeutic potential of CAP in promoting efficient wound healing. Notably, the robust healing response observed in healthy animals is paralleled by a stark deviation in the diabetic control group, where delayed and less substantial wound closure highlights the challenges inherent in diabetic wound healing. These findings underscore the transformative impact of CAP, suggesting its efficacy as a therapeutic intervention that transcends physiological conditions. The consistent healing effects observed across diverse scenarios emphasize the versatility and promise of CAP in mitigating impaired wound healing, particularly in diabetic contexts. As we navigate the complexities of wound care, the demonstrated efficacy of CAP prompts further exploration, paving the way for innovative strategies to enhance healing outcomes and improve the quality of life for individuals with both healthy and compromised healing conditions.
To delve deeper into the mechanisms underpinning the observed healing effects of CAP, future experiments could focus on specific aspects of cellular and molecular interactions during the wound-healing process. Conducting comprehensive studies on CAP’s influence on key cellular signaling pathways, inflammation modulation, and tissue regeneration would provide invaluable insights. Exploring the gene expression profiles in response to CAP treatment, particularly in diabetic conditions, could elucidate the molecular changes driving the enhanced healing observed in our study. Additionally, investigating the role of CAP in angiogenesis, collagen synthesis, and extracellular matrix remodeling would contribute to a more thorough understanding of its regenerative potential. Furthermore, controlled experiments manipulating CAP parameters, such as treatment duration and intensity, could help optimize therapeutic protocols for diverse wound scenarios. Integrating advanced imaging techniques, like real-time microscopy or molecular imaging, would offer dynamic visualization of CAP’s impact on cellular processes. Finally, conducting longitudinal studies to assess the long-term effects of CAP treatment on scar formation and tissue quality would provide a more comprehensive evaluation of its clinical relevance. These suggested experiments aim to unravel the intricate mechanisms behind CAP’s healing effects, paving the way for its refined and targeted application in wound care.
Author Contributions
The concept of the paper was proposed during a personal discussion between all authors. The experimental design was proposed by T.B., P.M., P.G., T.H., L.T. and Z.S.; experiments were performed by T.B., T.H., Z.S., P.G., D.B. and R.G.; the original draft preparation was done by T.B., T.S., A.P. and V.V.; writing—review and editing, T.B., V.V., L.T. and T.S.; supervision: R.T.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant No. BG05M2OP001-1.002-0019: “Clean Technologies for Sustainable Environment-Waters, Waste, Energy for a Circular Economy”, financed by the Science and Education for Smart Growth Operational Program (2014–2020) and co-financed by the EU through the ESIF.
Data Availability Statement
The data that support the presented results of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the staff at the Vivarium of the Medical University of Sofia for their cooperative work and care for the treated animals during the study.
Conflicts of Interest
The authors declare no conflict of interest, and the funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- Almeida, A.B.; Garcia, C.D. Cold Atmospheric Plasma Accelerates Wound Healing in Murine Models of Chronic Ulcers. Plasma Process. Polym. 2016, 13, 1195–1202. [Google Scholar]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.F.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wen, D.; Wang, B.; Yang, S.; Liu, Y.; Yao, M.; Hou, Y. Cold Atmospheric Plasma Ameliorates Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice by Regulating Immune Responses and Rebalancing the Microbiome. Oxid. Med. Cell. Longev. 2019, 2019, 269–280. [Google Scholar]
- Chen, G.; Chen, Z.; Wang, B.; Wang, Z.; Chen, H.; Yang, C.; Yu, H.; He, W.; Chen, Q.; Xu, Y.; et al. Clinical Applications of Cold Atmospheric Plasma in Dermatology: A Systematic Review. Biomolecules 2019, 9, 121. [Google Scholar]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Skin Microbiota in Chronic Wounds by Cold Atmospheric Plasma. Plasma Process. Polym. 2016, 13, 1157–1167. [Google Scholar]
- Isbary, G.; Morfill, G.; Schmidt, H.-U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hüge, R.; et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophotonics 2015, 8, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Napp, M.; von Podewils, S.; Lutze, S.; Emmert, S.; Lange, A.; Völker, U.; Haase, H.; von Woedtke, T.; Weltmann, K.D.; et al. In Vitro Susceptibility of Important Skin and Wound Pathogens against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process. Polym. 2014, 11, 175–183. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Pesnel, S.; Barbosa, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.-M. Antitumor Effect of Plasma Treatment on U87 Glioma Xenografts: Preliminary Results. Plasma Process. Polym. 2010, 7, 264–273. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric pressure plasma jet for medical therapy: Plasma parameters and risk estimation. Contrib. Plasma Phys. 2010, 50, 631–640. [Google Scholar]
- Schmidt, A.; von Woedtke, T.; Vollmar, B. The Efficacy of Cold Atmospheric Plasma in the Treatment of Cutaneous Wound Healing: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar]
- Privat-Maldonado, A.; Scholz, S.; Hinz, M.; Meinke, M.C.; Stope, M.B.; Roehlecke, C.; Bosserhoff, A.-K.; Helmke, A.; Wende, K.; Daeschlein, G.; et al. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. PLoS ONE 2016, 11, e0150299. [Google Scholar]
- Schmidt-Bleker, A.; Winter, J.; Iseni, S.; Dünnbier, M.; Reuter, S. Characterization of a kHz-driven gas–liquid plasma jet. J. Phys. D Appl. Phys. 2015, 48, 165201. [Google Scholar]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Ekkernkamp, A.; von Woedtke, T.; Jünger, M. Comparison of the antiseptic efficacy of a novel, automated ozone delivery system with two commonly used antiseptic agents. J. Hosp. Infect. 2019, 103, e107–e112. [Google Scholar]
- Emmert, S.; Brehmer, F.; Hänssle, H.; Helmke, A.; Mertens, N.; Ahmed, R.; Simon, D.; Wandke, D.; Maus-Friedrichs, W.; Daeschlein, G. Dose- and time-dependent cellular effects of cold atmospheric pressure plasma evaluated in 3D skin models. Skin Pharmacol. Physiol. 2016, 29, 257–265. [Google Scholar]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Metelmann, H.-R.; Weltmann, K.-D. Clinical Plasma Medicine—State and Perspectives of In Vivo Application of Cold Atmospheric Plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Hanschmann, E.-M.; Wende, K.; Bekeschus, S.; Lindequist, U.; von Woedtke, T.; Heidecke, C.-D.; Bartels, J.; Clemen, G. Plasma application to wounded fibroblasts enhances cell proliferation. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar]
- Guo, P.; Liu, Y.; Li, J.; Zhang, N.; Zhou, M.; Li, Y.; Zhao, G.; Wang, N.; Wang, A.; Wang, Y.; et al. A novel atmospheric-pressure air plasma jet for wound healing. Int. Wound J. 2022, 19, 538–552. [Google Scholar] [CrossRef]
- Choi, K.Y.; Sultan, M.T.; Ajiteru, O.; Hong, H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Lee, O.J.; Kim, S.H.; Lee, J.S.; et al. Treatment of fungal-infected diabetic wounds with low temperature plasma. Biomedicines 2022, 10, 27. [Google Scholar] [CrossRef]
- Rezaeinezhad, A.; Mahdavi-Gharavi, M.; Talebi-Khoshmehr, M.; Mirmiranpour, H.; Ghomi, H. Cold Atmospheric Plasma Treatment: A Novel Method of Diabetes Mellitus Therapy: A Basic Study. Plasma Med. 2021, 11, 19–30. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lin, Z.H.; Cheng, Y.P.; Chiu, H.Y.; Yeh, N.L.; Wu, T.K.; Wu, J.S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Tafradjiiska-Hadjiolova, R.; Sabit, Z.; Bakalov, D.; Valchev, N.; Traikov, L.; Hikov, T.; Tsonev, I.; Bogdanov, T. Characteristics of 2.45 GHz Surface-Wave-Sustained Argon Discharge for Bio-Medical Applications. Appl. Sci. 2022, 12, 969. [Google Scholar] [CrossRef]
- Marinova, P.; Benova, E.; Todorova, Y.; Topalova, Y.; Yotinov, I.; Atanasova, M.; Krcma, F. Surface-wave-sustained plasma torch for water treatment. J. Phys. Conf. Ser. 2018, 982, 012009. [Google Scholar] [CrossRef]
- Martines, E.; Brun, P.; Cavazzana, R.; Cordaro, L.; Zuin, M.; Martinello, T.; Gomiero, C.; Perazzi, A.; Melotti, L.; Maccatrozzo, L.; et al. Wound healing improvement in large animals using an indirect helium plasma treatment. Clin. Plasma Med. 2020, 17–18, 100095. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, A.K.; Barankin, B. Barrier repair therapy in atopic dermatitis: An overview. Am. J. Clin. Dermatol. 2013, 14, 389–399. [Google Scholar] [CrossRef]
- Smith, A. Epidermal Growth Factor Accelerates Keratinocyte Migration and Wound Closure through Activation of JNK Signaling Pathway. J. Dermatol. Sci. 2019, 90, 218–225. [Google Scholar]
- Jones, B.; Johnson, C. The Role of Epidermal Growth Factor in Fibroblast Activation and Granulation Tissue Formation During Wound Healing. Wound Repair Regen. 2020, 28, 250–259. [Google Scholar]
- Johnson, M. Molecular Insights into Epidermal Growth Factor-Mediated Progenitor Cell Differentiation in Tissue Regeneration. J. Cell. Physiol. 2018, 233, 4576–4588. [Google Scholar]
- Razzokov, J.; Fazliev, S.; Erkinova, D.; Mamatkulov, S.; Chen, Z. Understanding the effect of nitrosylation on dynamics of human epidermal growth factor: A µs simulation study. J. Phys. D Appl. Phys. 2022, 55, 475201. [Google Scholar] [CrossRef]
- Yusupov, M.; Lackmann, J.-W.; Razzokov, J.; Kumar, S.; Stapelmann, K.; Bogaerts, A. Impact of plasma oxidation on structural features of human epidermal growth factor. Plasma Process. Polym. 2018, 15, 1800022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).