Effects of Surface-Wave-Sustained Argon Plasma Torch Interaction with Liquids
Abstract
:1. Introduction
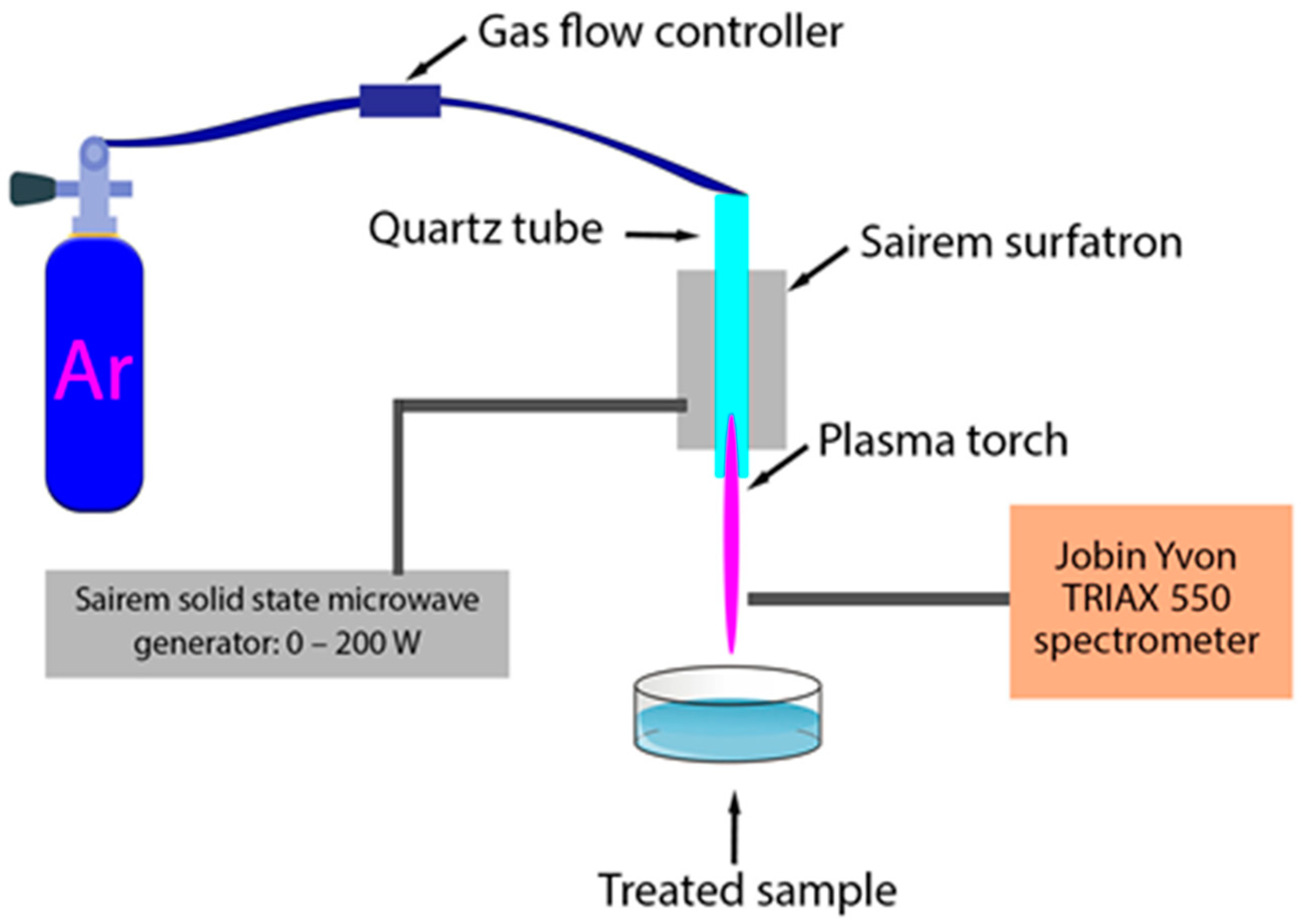
2. Materials and Methods
3. Results
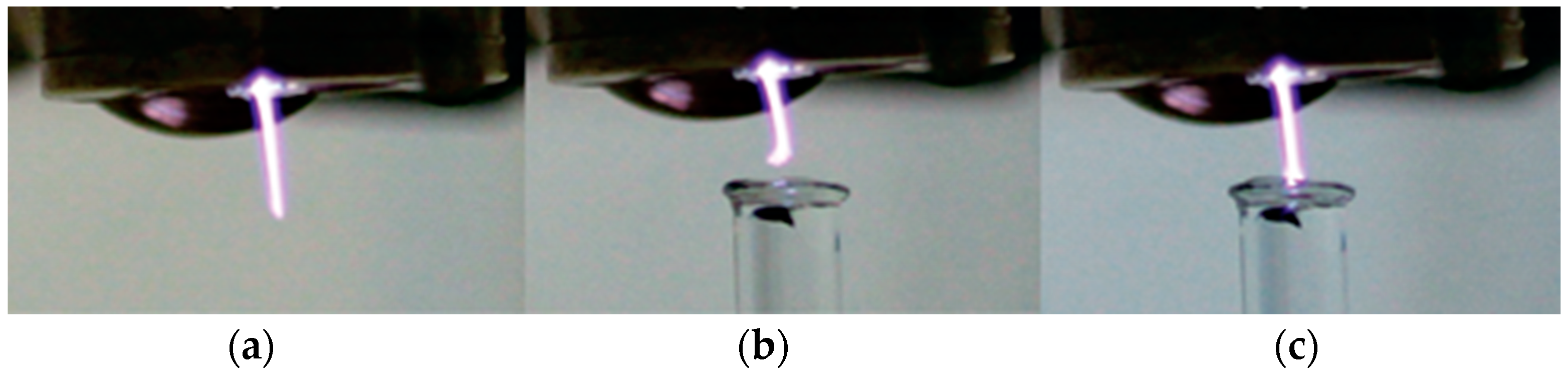
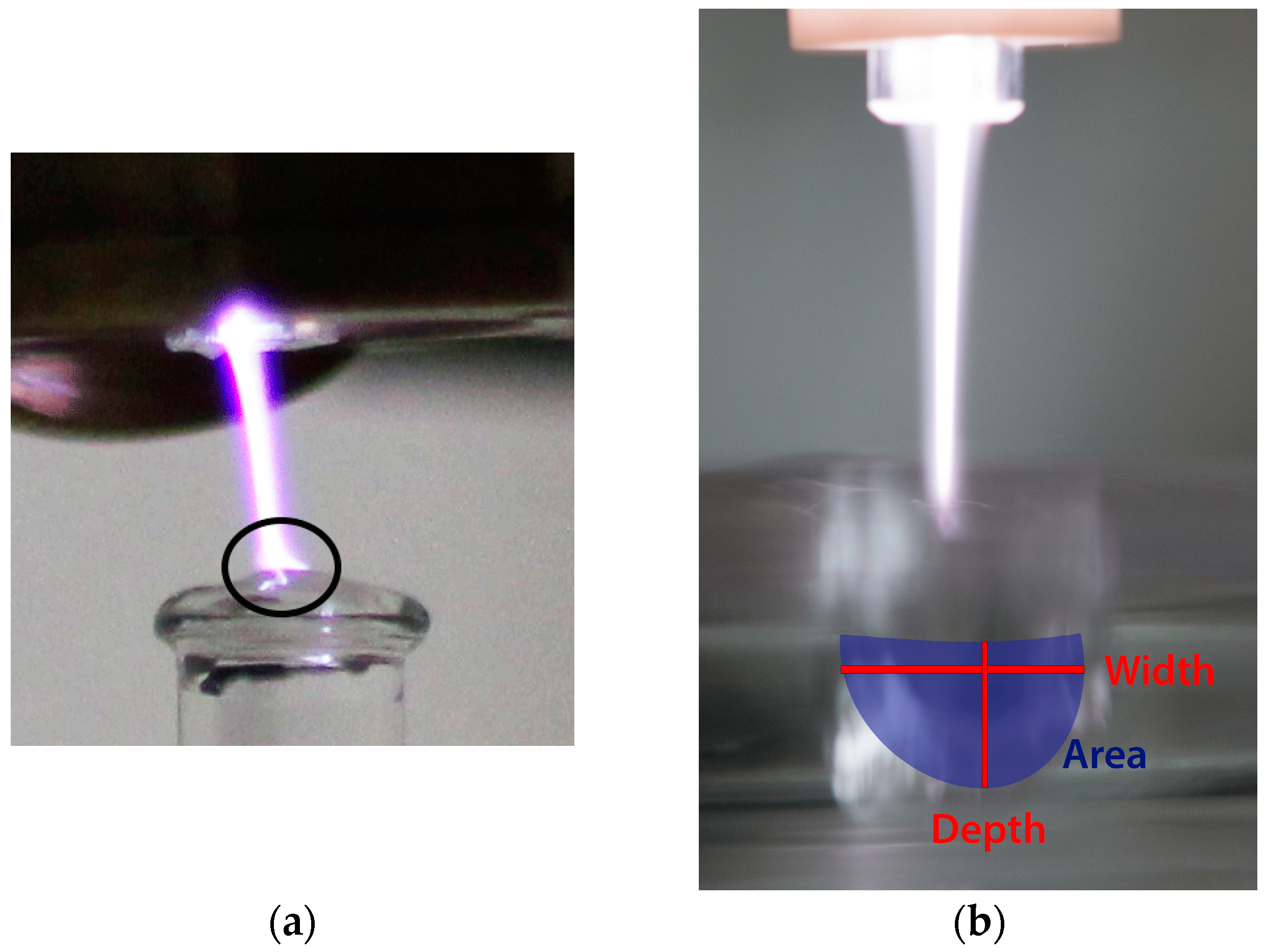
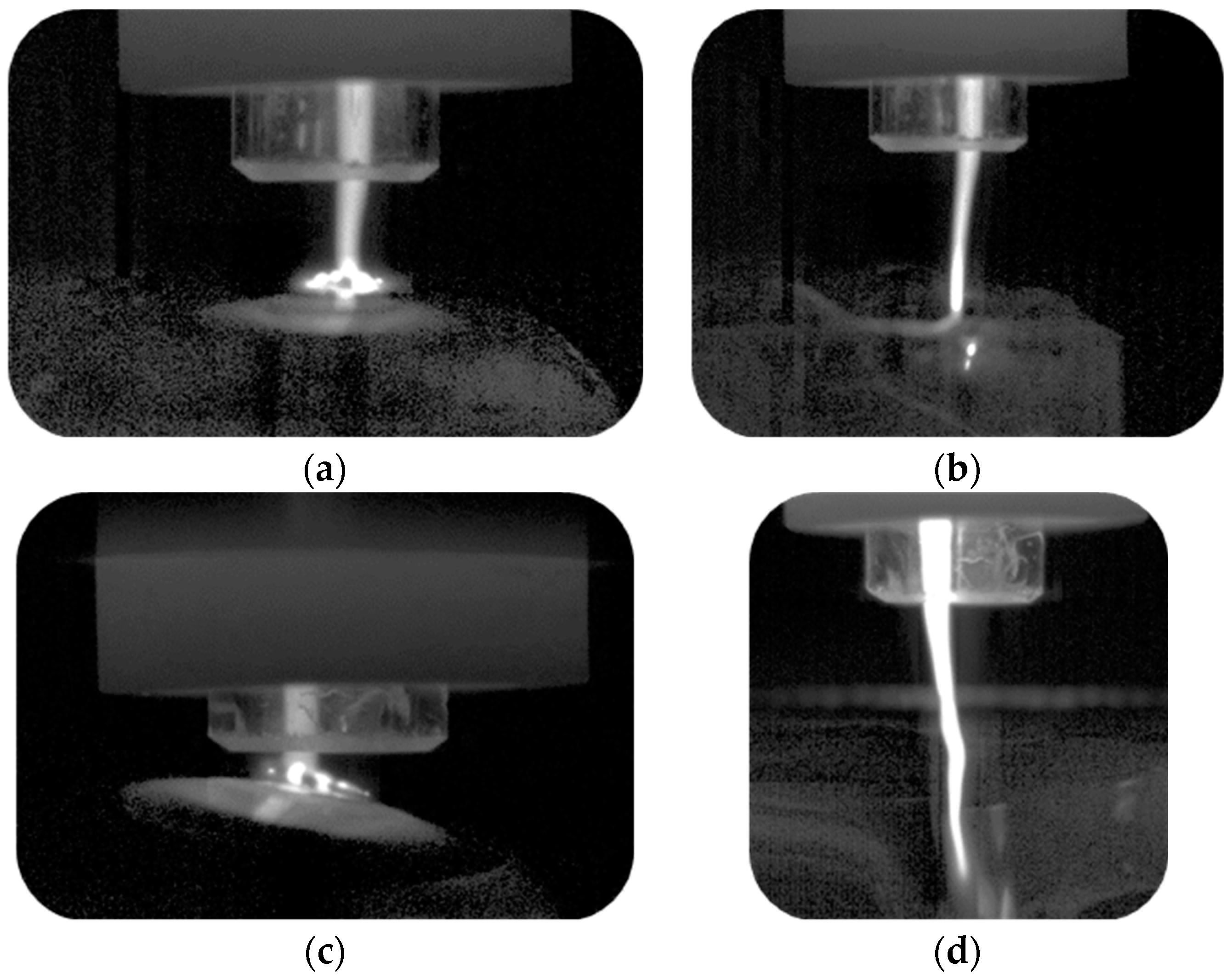
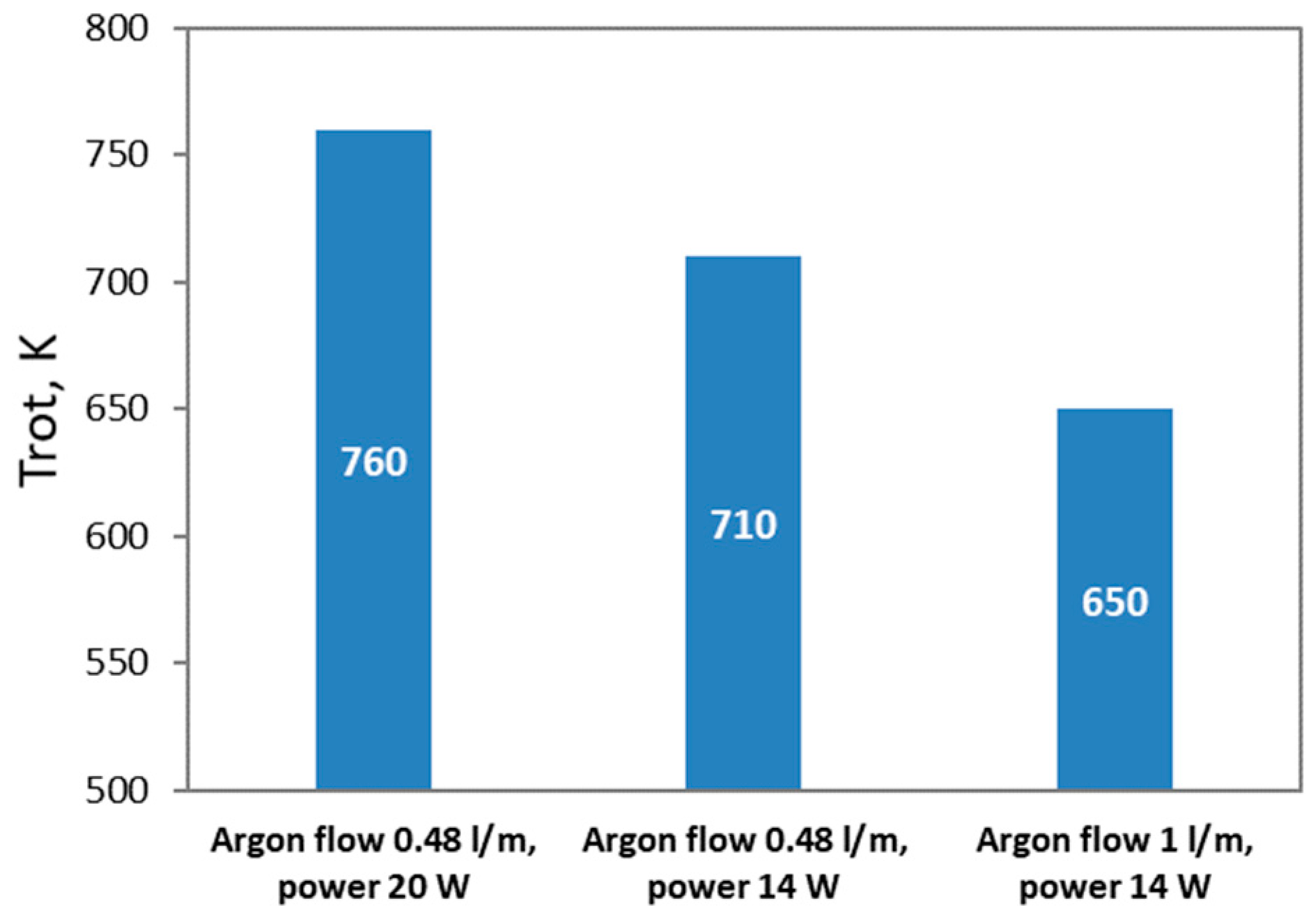
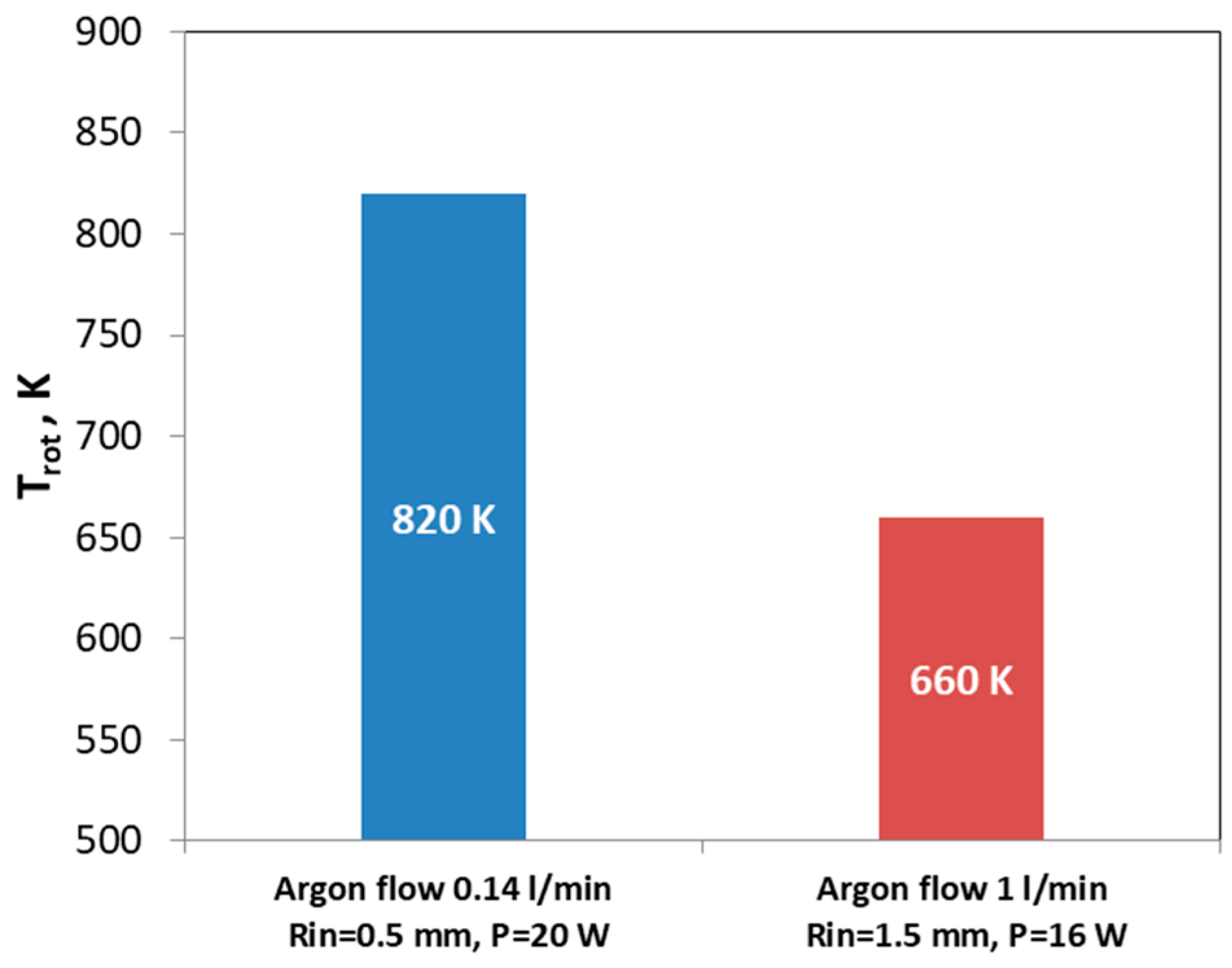
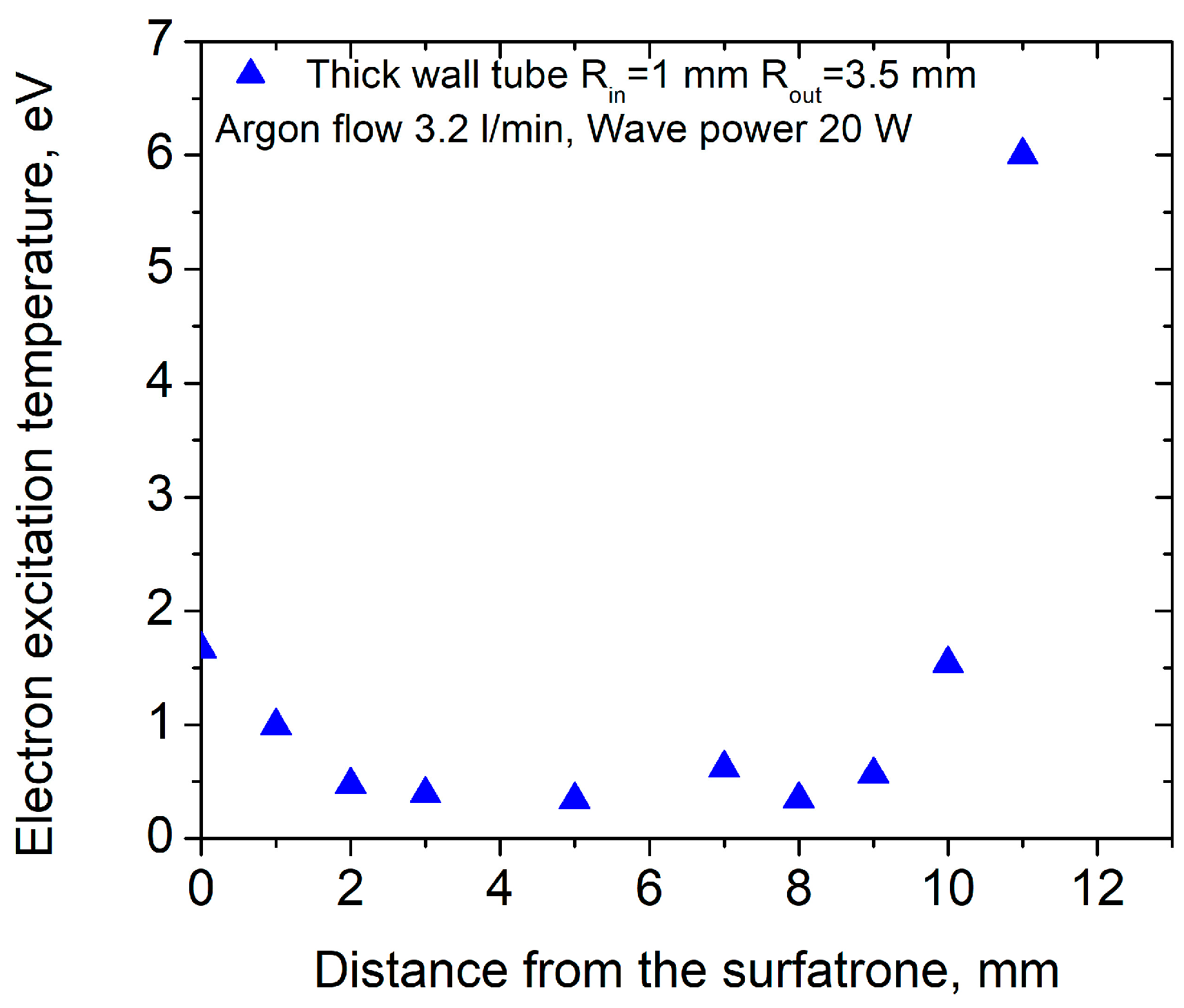
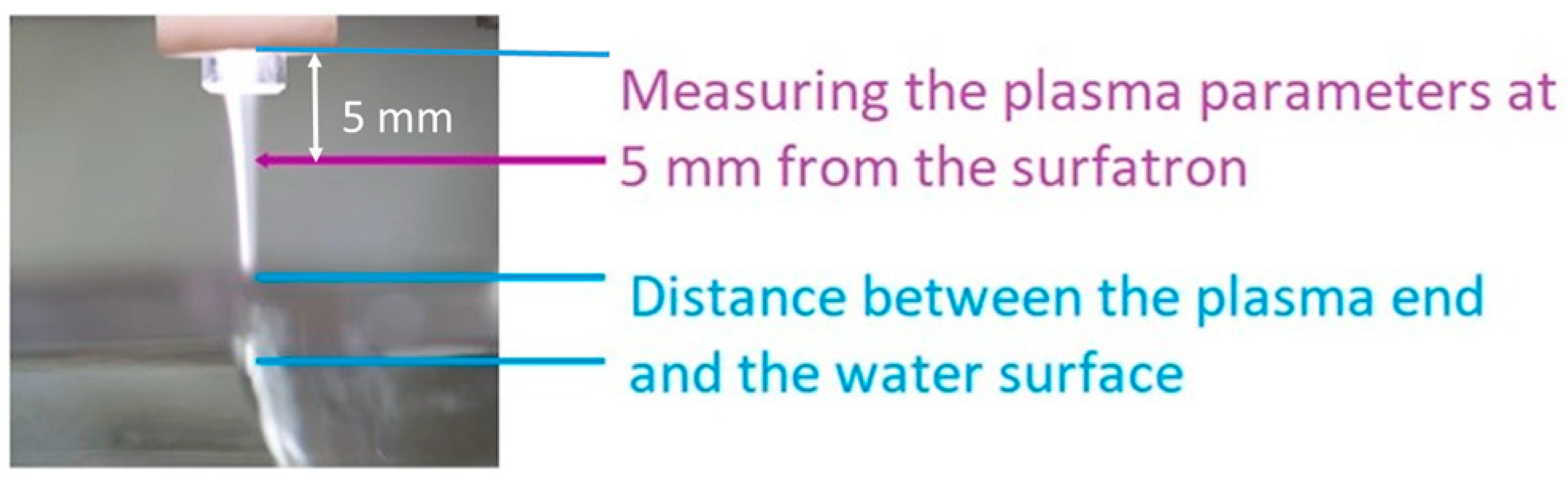
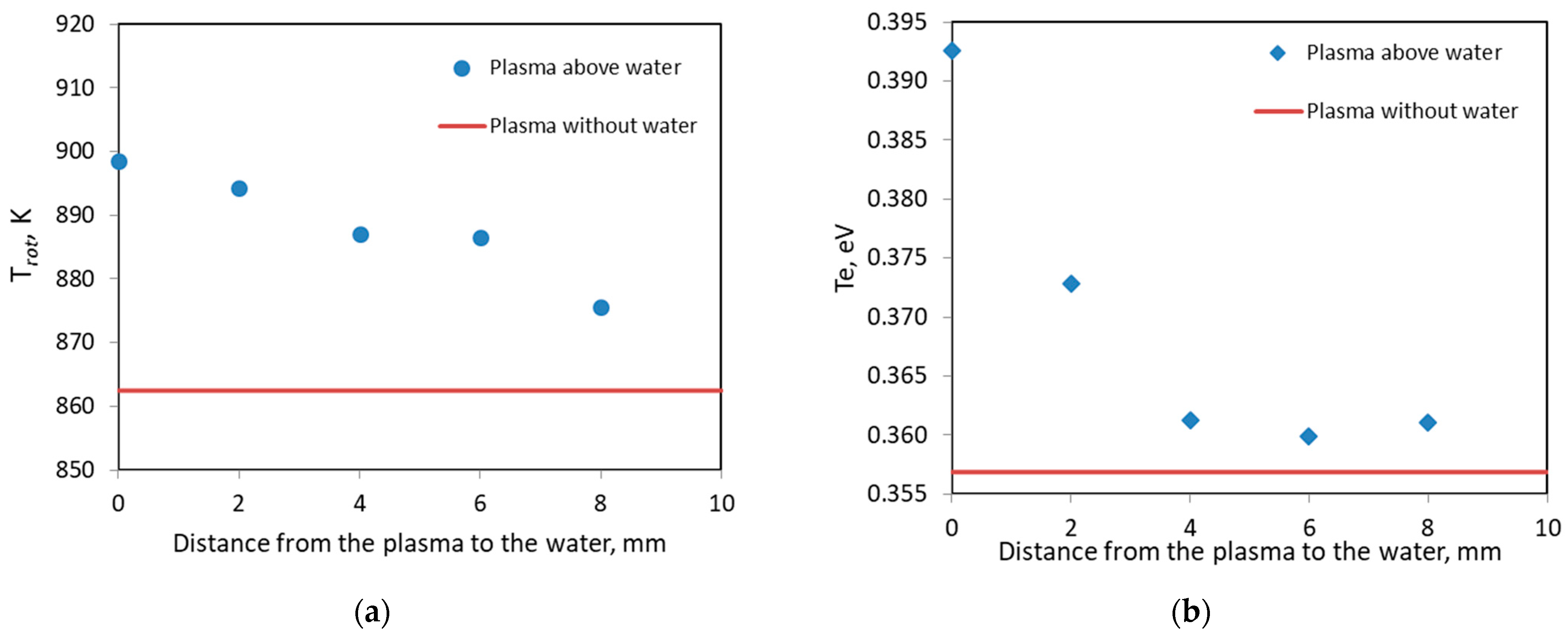
3.1. Interaction of Argon Plasma Torch with Water: Modification of Plasma Characteristics
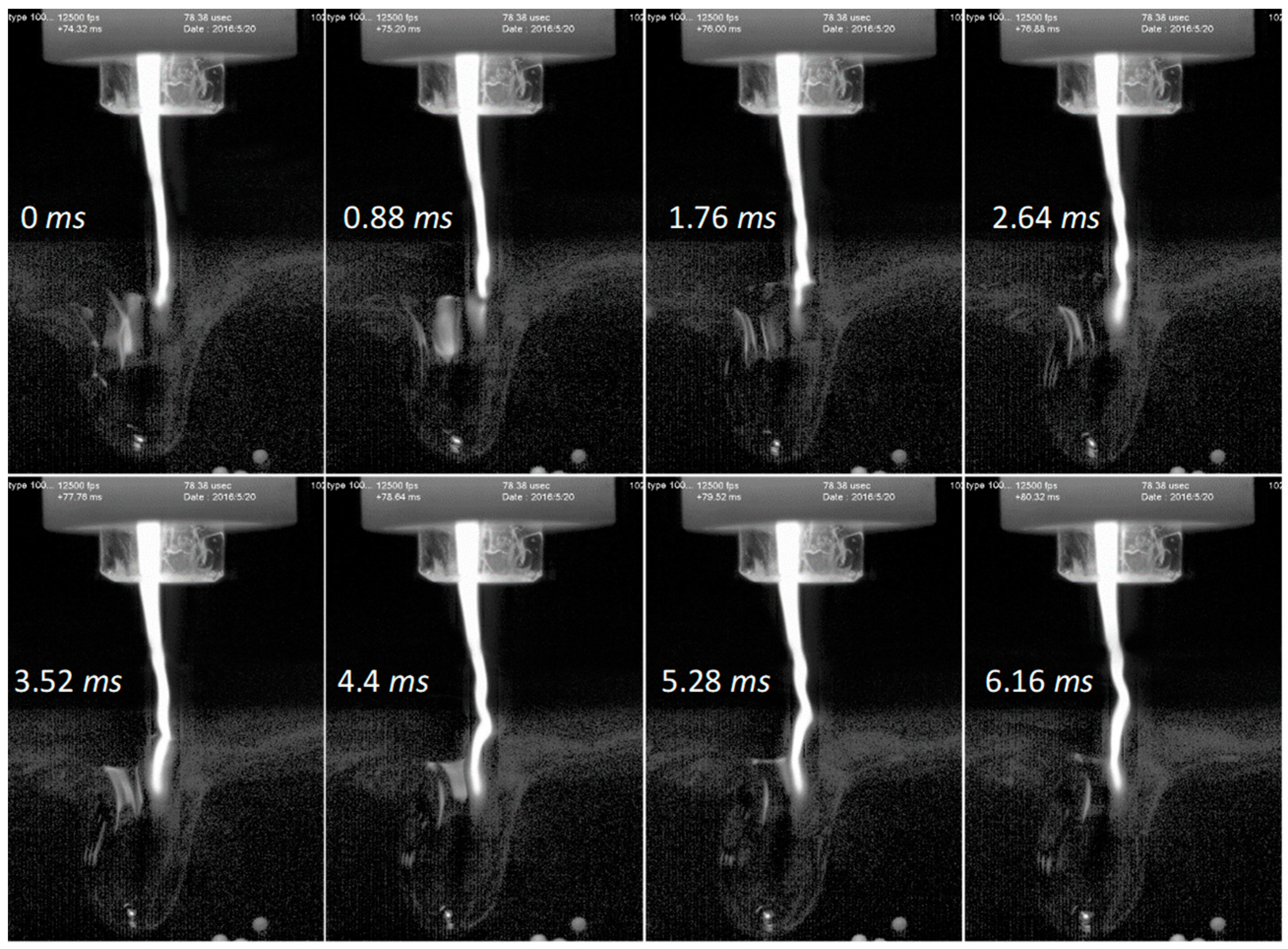
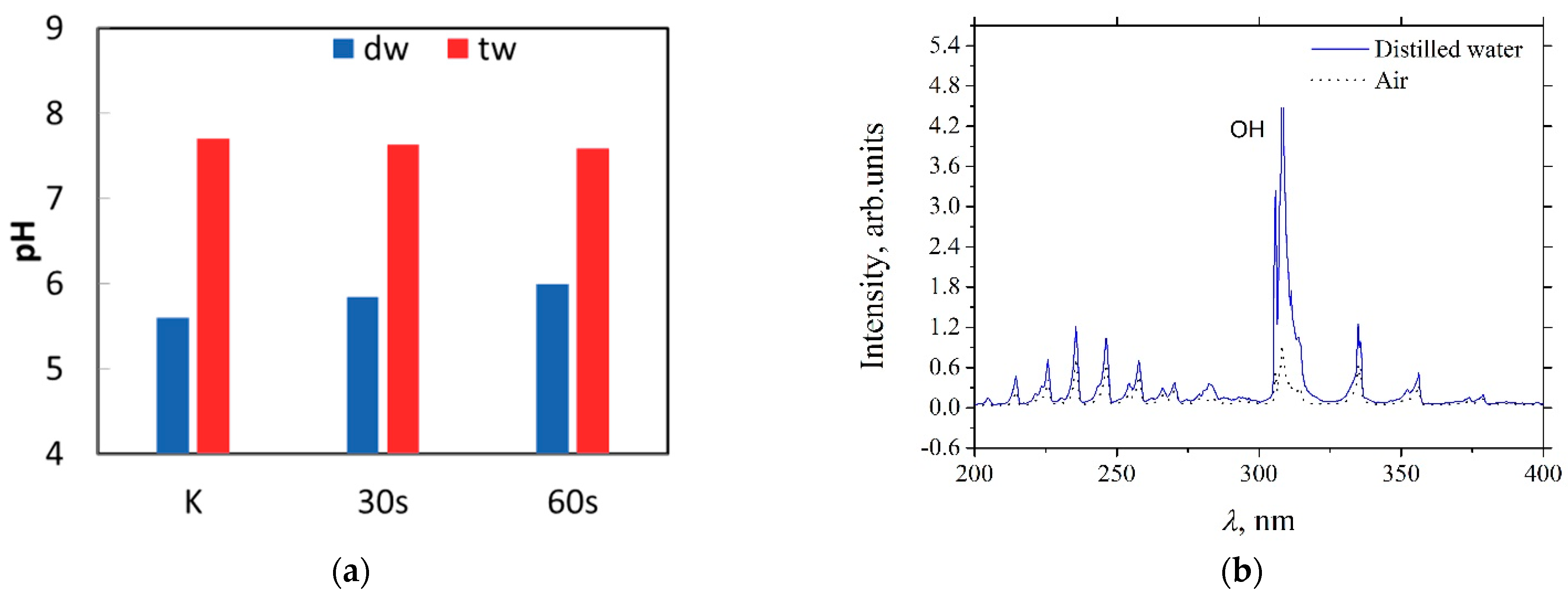
3.2. Interaction of Argon Plasma Torch with Water: Modification of Distilled Water Characteristics
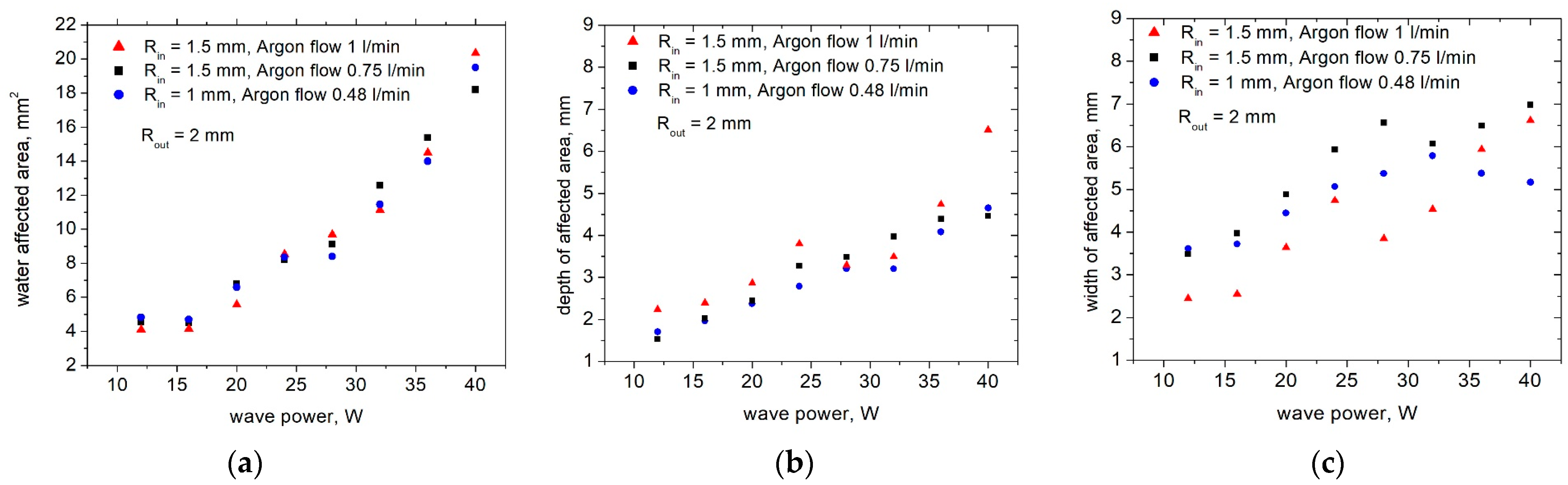
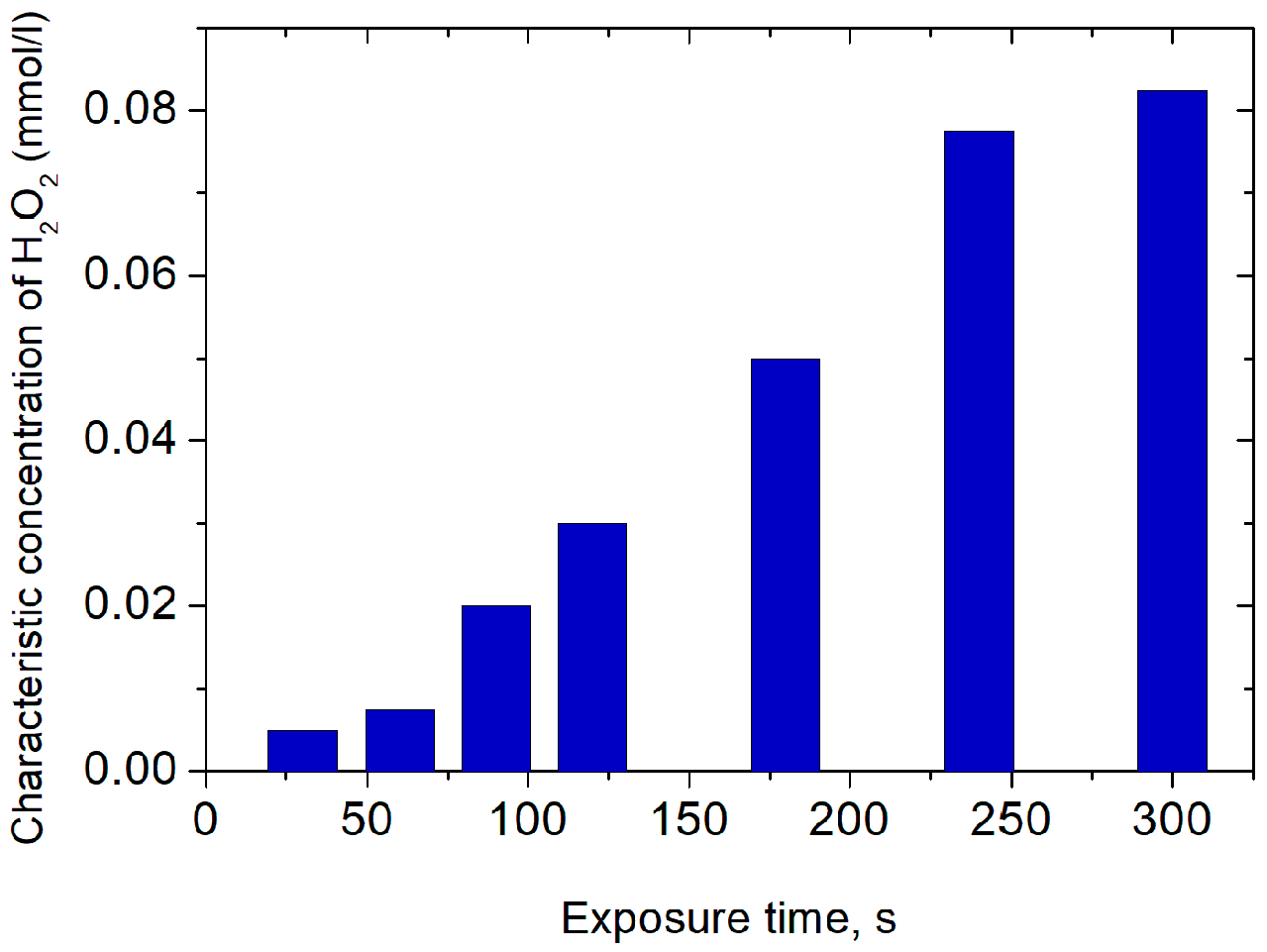
3.3. Surface Wave Sustained Plasma Torch for Model Water Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goriachev, V.L.; Kulishevich, A.I.; Ufimtsev, A.A.; Rutberg, P.G. Electrophysical Properties of Impulse Discharges of Low Power in Water and their use in Ecology. In Progress in Plasma Processingof Materials; Begell House: Danburry, CT, USA, 2001; pp. 827–832. [Google Scholar]
- Locke, B.R.; Sato, M.; Šunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Industr. Eng. Chem. Res. 2006, 45, 892–905. [Google Scholar] [CrossRef]
- Graves, D. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 080901. [Google Scholar] [CrossRef]
- Graves, D.B. Mechanisms of plasma medicine: Coupling plasma physics, biochemistry, and biology. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 281. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomolec. Therap. 2014, 22, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process Polym. 2018, 15, e1700174. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Takeuchi, N.; Yasuoka, K. Review of plasma-based water treatment technologies for the decomposition of persistent organic compounds. Jpn. J. Appl. Phys. 2020, 60, SA0801. [Google Scholar] [CrossRef]
- Matějka, F.; Galář, P.; Khun, J.; Scholtz, V.; Kůsová, K. Mechanisms leading to plasma activated water high in nitrogen oxides. Phys. Scr. 2023, 98, 045619. [Google Scholar] [CrossRef]
- Tulková, T.; Fučík, J.; Kozáková, Z.; Procházková, P.; Krčma, F.; Gargošová, H.Z.; Mravcová, L.; Sovová, K. Impact of various oxidation processes used for removal of sulfamethoxazole on the quality of treated wastewater. Emerg. Contam. 2023, 9, 100231. [Google Scholar] [CrossRef]
- Zambon, Y.; Contaldo, N.; Laurita, R.; Várallyay, E.; Canel, A.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma activated water triggers plant defence responses. Sci. Rep. 2020, 10, 19211. [Google Scholar] [CrossRef]
- Kutasi, K.; Popovic, D.; Krstulovic, N.; Milosevic, S. Tuning the composition of plasma-activated water by a surface-wave microwave discharge and a kHz plasma jet. Plasma Sourc. Sci. Technol. 2019, 28, 095010. [Google Scholar] [CrossRef]
- Weihe, T.; Schnabel, U.; Andrasch, M.; Stachowiak, J.; Tübbecke, F.; Ehlbeck, J. A Plasma-Based Decontamination Process Reveals Potential for an in-Process Surface-Sanitation Method. Plasma 2022, 5, 351–365. [Google Scholar] [CrossRef]
- Todorova, Y.; Benova, E.; Marinova, P.; Yotinov, I.; Bogdanov, T.; Topalova, Y. Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch. Processes 2022, 10, 554. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, D.; Qi, M.; Li, B.; Peng, S.; Li, Q.; Zhang, H.; Liu, D. Plasma-Activated Water Promotes Wound Healing by Regulating Inflammatory Responses. Biophysica 2021, 1, 297–310. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Belmonte, T.; Cardoso, R.P.; Nöel, C.; Henrion, G.; Kosior, F. Microwave plasmas at atmospheric pressure: Theoretical insight and applications in surface treatment. Eur. Phys. J. Appl. Phys. 2008, 42, 41–46. [Google Scholar] [CrossRef]
- Yu, A.L. Microwave discharges: Generation and diagnostics. J. Phys. Conf. Ser. 2010, 257, 012016. [Google Scholar]
- Bruggeman, P.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Chen, C.-J.; Li, S.-Z.; Wu, Y.; Zhang, J. Investigation of role of the discharge tube in pulse modulated surface-wave argon plasma column at atmospheric pressure by optical emission spectroscopy. Phys. Plasmas 2019, 26, 053506. [Google Scholar] [CrossRef]
- Gadonna, K.; Leroy, O.; Silva, T.; Leprince, P.; Boisse-Laporte, C.; Alves, L.L. Hydrodynamic study of a microwave plasma torch. Eur. Phys. J. Appl. Phys. 2011, 56, 24008. [Google Scholar] [CrossRef]
- Sainz, A.; Margot, J.; Garcia, M.C.; Calzada, M.D. Role of dissociative recombination in the excitation kinetics of an argon microwave plasma at atmospheric pressure. J. Appl. Phys. 2005, 97, 113305. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Tafradjiiska-Hadjiolova, R.; Sabit, Z.; Bakalov, D.; Valchev, N.; Traikov, L.; Hikov, T.; Tsonev, I.; Bogdanov, T. Characteristics of 2.45 GHz Surface-Wave-Sustained Argon Discharge for Bio-Medical Applications. Appl. Sci. 2022, 12, 969. [Google Scholar] [CrossRef]
- Benova, E.; Atanasova, M.; Bogdanov, T.; Marinova, P.; Krcma, F.; Mazankova, V.; Dostal, L. Microwave plasma torch at water surface. Plasma Med. 2016, 6, 59. [Google Scholar] [CrossRef]
- Bruggeman, P.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Marinova, P.; Benova, E.; Todorova, Y.; Topalova, Y.; Yotinov, I.; Atanasova, M.; Krcma, F. Surface-wave-sustained plasma torch for water treatment. J. Phys. Conf. Ser. 2018, 982, 012009. [Google Scholar] [CrossRef]
- Moisan, M.; Beaudry, C.; Leprince, P. A New HF Device for the Production of Long Plasma Columns at a High Electron Density. Phys. Lett. 1974, 50, 125. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z.; Pantel, R. The theory and characteristics of an efficient surface wave launcher (surfatron) producing long plasma columns. J. Phys. D Appl. Phys. 1979, 12, 219. [Google Scholar] [CrossRef]
- Moisan, M.; Nowakowska, H. Contribution of surface-wave (SW) sustained plasma columns to the modeling of RF and microwave discharges with new insight into some of their features. A survey of other types of SW discharges. Plasma Sources Sci. Technol. 2018, 27, 073001. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.C.; Kong, M.G.; Leys, C. Is the rotational temperature of OH(A-X) for discharges in and in contact with liquids a good diagnostic for determining the gas temperature? Plasma Proc. Polym. 2009, 6, 751. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko Yu Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (Ver. 5.5.3); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. Available online: https://physics.nist.gov/asd (accessed on 31 March 2018).
- Krčma, F.; Tsonev, I.; Smejkalová, K.; Truchlá, D.; Kozáková, Z.; Zhekova, M.; Marinova, P.; Bogdanov, T.; Benova, E. Microwave micro torch generated in argon based mixtures for biomedical applications. J. Phys. D Appl. Phys. 2018, 51, 414001. [Google Scholar] [CrossRef]
- Locke, B.R.; Shih, K.-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci. Technol. 2011, 20, 034006. [Google Scholar] [CrossRef]
- Barkhudarov, E.; Kossyi, I.A.; Christofi, N.; Misakyan, M. Microwave plasma-chemical reactor based on plasma jet interaction with water. In Proceedings of the 10th International Workshop on Microwave Discharges: Fundamental and Applications, Zvenigorod, Russia, 3–7 September 2018. [Google Scholar]
- Park, S.; Choe, W.; Lee, H.; Park, J.Y.; Kim, J.; Moon, S.Y.; Cvelbar, U. Stabilization of liquid instabilities with ionized gas jets. Nature 2021, 592, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bonch-Bruevich, A.M.; Libenson, M.N.; Makin, V.S.; Trubaev, V.V. Surface electromagnetic waves in optics. Opt. Eng. 1992, 31, 718–730. [Google Scholar] [CrossRef]
- Libenson, M.N.; Makin, V.S.; Shiryaev, V.A.; Soileau, M.J. Surface periodic structures under the optical damage of transparent dielectrics. In Laser-Induced Damage in Optical Materials: 1994; SPIE: Bellingham, WA, USA, 1995; Volume 2428, pp. 676–681. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Atanasova, M.; Petrova, T. Surface-wave-sustained argon plasma kinetics from intermediate to atmospheric pressure. J. Phys. D Appl. Phys. 2018, 51, 474004. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.; González, M.Á.; Rego, R.; Kong, M.G.; Leys, C. Characterization of a direct dc-excited discharge in water by optical emission spectroscopy. Plasma Sources Sci. Technol. 2009, 18, 025017. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]






| Pollutants/Parameters | Value |
|---|---|
| Organic content as COD | 1180.01 mgO2/L |
| 30.702 mg/L | |
| 6.050 mg/L | |
| 1.008 mg/L | |
| pH | 6.4 |
| conductivity | 3.48 mS/cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinova, P.; Benova, E.; Topalova, Y.; Todorova, Y.; Bogdanov, T.; Zhekova, M.; Yotinov, I.; Krcma, F. Effects of Surface-Wave-Sustained Argon Plasma Torch Interaction with Liquids. Processes 2023, 11, 3313. https://doi.org/10.3390/pr11123313
Marinova P, Benova E, Topalova Y, Todorova Y, Bogdanov T, Zhekova M, Yotinov I, Krcma F. Effects of Surface-Wave-Sustained Argon Plasma Torch Interaction with Liquids. Processes. 2023; 11(12):3313. https://doi.org/10.3390/pr11123313
Chicago/Turabian StyleMarinova, Plamena, Evgenia Benova, Yana Topalova, Yovana Todorova, Todor Bogdanov, Maya Zhekova, Ivaylo Yotinov, and Frantisek Krcma. 2023. "Effects of Surface-Wave-Sustained Argon Plasma Torch Interaction with Liquids" Processes 11, no. 12: 3313. https://doi.org/10.3390/pr11123313
APA StyleMarinova, P., Benova, E., Topalova, Y., Todorova, Y., Bogdanov, T., Zhekova, M., Yotinov, I., & Krcma, F. (2023). Effects of Surface-Wave-Sustained Argon Plasma Torch Interaction with Liquids. Processes, 11(12), 3313. https://doi.org/10.3390/pr11123313









