Sustainable Harnessing of SiO2 Nanoparticles from Rice Husks: A Review of the Best Synthesis and Applications
Abstract
:1. Introduction
Rice Husk as a Source of Bio-Energy and SiO2
2. Overview of General Methods for SiO2 NPs Classical Chemical Synthesis
3. Sustainable Harnessing of SiO2 from Rice Husks
3.1. Traditional Thermochemical Methods: Calcination
3.2. Pyrolysis of Rice Husk for Obtaining Bio-Oil and Bio-Silica in the Literature
3.2.1. Bio-Silica Production
| Material Obtained | SBET (m2/g) | Chemical Pretreatment | Thermal Treatment | Milling | Precipitation/Sol-Gel | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | 164 | - | 100 °C with 1 M HCl for 2 h | washed with distilled water | “pyrolyzed” @700 °C for 2 h | - | - | [6] | ||
| Silica | 352 | washed with water | drying 110 °C | 5 g of RH in 500 mL with 8%wt HCl (2.2 M) @120 °C for 4 h | washed with distilled water | 5 g of RH in tubular furnace and pyrolysis gas; 1 L/min @800 °C for 30 min @ 20 °C/min | calcinated @10 C/min for 2–3 h @ 610 °C | pulverized | - | [45] |
| Biochar | - | - | pyrolysis N2 flow rate 40 L/min | - | - | [59] | ||||
| Silica | 328 | - | pyrolysis 600 °C (50 g) N2 flow rate 0.5 L/min | gasification at 875 °C for 20 min | - | alkali leaching/acidic treatment after pyrolysis to extract the silica and to get a gel | [57] | |||
| Biochar | 228 | washed with water | drying 100 °C | 3 N at 100 °C for 1 h | washed with hot distilled water | pyrolysis N2 flow rate of 30 mL/minramp by 10 °C/min for 2 h until reaching 600 °C | ground with pistol mortar | - | [55] | |
| Silica | - | sieved, washed with water and dried | 20 g of RH acetic acid solution (400 mL) (3%vol) for 2 h | washed with distilled water | pyrolysis at 500 °C for 45 min | - | alkali leaching/acidic treatment after pyrolysis to extract the silica and to get a precipitate | [58] | ||
| Silica | 238 | washed with water | HCl leaching at 100 °C with 10%w/w for 1 h (1 g:15 mL) | washed with distilled water | pyrolysis @550 °C | calcination @350 °C for 12 h; slope 4.5 °C/min | - | - | [60] | |
| Biochar | 230 | - | pyrolysis at 500 °C | - | - | [56] | ||||
3.2.2. Combined Bio-Silica and Bio-Oil Production
4. Tailoring of SiO2 from Rice Husk to Enhance Porosity
4.1. Mesoporous RH-SiO2 Nanoparticles
4.2. Ordered Mesoporous RH-SiO2 Nanoparticles
4.3. Shaping RH-SiO2 NPs into Macroporous Structures
5. RH-SiO2 Applications in the Literature
5.1. RH-SiO2 for Biomedical Applications
5.2. RH-SiO2 for Energy Storage
5.3. RH-SiO2 as Catalyst Support
5.4. RH-SiO2 as Adsorbent for Water Cleaning
5.4.1. Adsorption of Chemicals of Emerging Concern and Other Micropollutants
5.4.2. Adsorption of Metals in Water
6. Conclusions and Future Prospects
- Optimize conditions for pyrolysis of rice husk to obtain porous SiO2 while considering the production of valuable bio-oil and pyrolysis gas;
- Investigate the synthesis of hierarchical silica structures such as monoliths derived from biogenic SiO2 as a precursor and the effect on the adsorptive ability [74];
- To study the effects of organic matter on SiO2 adsorbent and the efficiency of cleaning real waste-, surface- and drinking water. SiO2 has shown high performance in the removal of contaminants in batch or continuous adsorption tests of single pollutants or a simple mixture of pollutants in distilled water;
- Regarding the last point, real contaminated water samples exhibit high complexity. This complexity arises not only from organic matter and salts but also from the multitude and diversity of pollutants present. Consequently, a critical aspect of advancing silica as an adsorbent is its capacity to adapt to shifts in water composition. Investigating how silica can be complemented with other techniques to address the whole complexity of polluted waters will be a key point for its development;
- All syntheses of silica NPs use a direct acidic pretreatment of RH before calcination or, in some other cases, a basic extraction of silica to form sodium aluminate solution to be used in more classical synthesis of silica-based materials. Both methods will generate a large amount of waste solution (acid or alkaline). New strategies are needed to solve this non-sustainable issue. The use of ionic liquid (IL) [108] seems promising as silica NPs from RH featuring 240 m2/g have been obtained after pyrolysis. The use of specific enzymes alone or in a cocktail (cellulase, lignin peroxidase, lytic polysaccharide monooxygenase (LPMOs), etc.) [7] that is capable of promoting dissociation and separation of lignocellulosic components by oxidative or hydrolytic depolymerization [108,109,110,111] could be used alone or in combination with IL to produce silica NPs directly from RH, avoiding thermal treatment to create a more economical and sustainable route than acidic pretreatment or basic extraction.
Author Contributions
Funding
Conflicts of Interest
References
- Imperial College London. Sustainable Biomass Availability in the EU, to 2050. 2021. Available online: https://www.concawe.eu/publication/sustainable-biomass-availability-in-the-eu-to-2050/ (accessed on 27 September 2022).
- An, D.; Guo, Y.; Zhu, Y.; Wang, Z. A green route to preparation of silica powders with rice husk ash and waste gas. Chem. Eng. J. 2010, 162, 509–514. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Tan, K.-H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Microporous Mesoporous Mater. 2015, 204, 204–209. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73. [Google Scholar] [CrossRef]
- European Comission. European Commission|Agri-Food Data Portal|Agricultural Markets|Rice. 2019. Available online: https://agridata.ec.europa.eu/extensions/DataPortal/rice.html (accessed on 23 November 2022).
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanoparticle Res. 2011, 13, 6981–6990. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259. [Google Scholar] [CrossRef]
- Gebretatios, A.G.; Pillantakath, A.R.K.K.; Witoon, T.; Lim, J.-W.; Banat, F.; Cheng, C.K. Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments. Chemosphere 2023, 310, 136843. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Rahman, T.; A Fadhlulloh, M.; Abdullah, A.G.; Hamidah, I.; Mulyanti, B. Synthesis of silica particles from rice straw waste using a simple extraction method. IOP Conf. Ser. Mater. Sci. Eng. 2016, 128, 012040. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Mendes, R.F.; Vilela, A.P.; Farrapo, C.L.; Mendes, J.F.; Tonoli, G.H.D.; Mendes, L.M. Lignocellulosic residues in cement-bonded panels. In Sustainable and Nonconventional Construction Materials Using Inorganic Bonded Fiber Composites; Woodhead Publishing: Sawston, UK, 2017; pp. 3–16. [Google Scholar] [CrossRef]
- Kaniapan, S.; Pasupuleti, J.; Nesan, K.P.; Abubackar, H.N.; Umar, H.A.; Oladosu, T.L.; Bello, S.R.; Rene, E.R. A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment. Int. J. Environ. Res. Public Health 2022, 19, 3427. [Google Scholar] [CrossRef]
- Rao, G.R.; Sastry, A.R.K.; Rohatgi, P.K. Nature and reactivity of silica available in rice husk and its ashes. Bull. Mater. Sci. 1989, 12, 469–479. [Google Scholar] [CrossRef]
- Chen, P.; Gu, W.; Fang, W.; Ji, X.; Bie, R. Removal of metal impurities in rice husk and characterization of rice husk ash under simplified acid pretreatment process. Environ. Prog. Sustain. Energy 2017, 36, 830–837. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.-L.; Zhu, X.-F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Nawaz, S.; Jamil, F.; Akhter, P.; Hussain, M.; Jang, H.; Park, Y.-K. Valorization of lignocellulosic rice husk producing biosilica and biofuels—A review. J. Physics Energy 2023, 5, 012003. [Google Scholar] [CrossRef]
- Mahmad-Toher, A.-S.; Govender, N.; Dorairaj, D.; Wong, M.-Y. Comparative evaluation on calcium silicate and rice husk ash amendment for silicon-based fertilization of Malaysian rice (Oryza sativa L.) varieties. J. Plant Nutr. 2021, 45, 1336–1347. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef]
- Liou, T.-H. Evolution of chemistry and morphology during the carbonization and combustion of rice husk. Carbon 2004, 42, 785–794. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Lee, J.-H.; Cho, E.-B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Anees-Ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Bogush, G.; Tracy, M.; Zukoski, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Crystalline Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Möller, K.; Kobler, J.; Bein, T. Colloidal suspensions of mercapto-functionalized nanosized mesoporous silica. J. Mater. Chem. 2007, 17, 624–631. [Google Scholar] [CrossRef]
- Zhang, A.; Gu, L.; Hou, K.; Dai, C.; Song, C.; Guo, X. Mesostructure-tunable and size-controllable hierarchical porous silica nanospheres synthesized by aldehyde-modified Stöber method. RSC Adv. 2015, 5, 58355–58362. [Google Scholar] [CrossRef]
- Lou, F.; Zhang, A.; Zhang, G.; Ren, L.; Guo, X.; Song, C. Enhanced kinetics for CO2 sorption in amine-functionalized mesoporous silica nanosphere with inverted cone-shaped pore structure. Appl. Energy 2020, 264, 114637. [Google Scholar] [CrossRef]
- Akinjokun, A.I.; Ojumu, T.V.; Ogunfowokan, A.O. Biomass, Abundant Resources for Synthesis of Mesoporous Silica Material. In Microporous and Mesoporous Materials; BoD—Books on Demand: Norderstedt, Germany, 2016; pp. 105–177. [Google Scholar] [CrossRef]
- James, J.; Rao, M. Silica from rice husk through thermal decomposition. Thermochim. Acta 1986, 97, 329–336. [Google Scholar] [CrossRef]
- Kapur, P. Production of reactive bio-silica from the combustion of rice husk in a tube-in-basket (TiB) burner. Powder Technol. 1985, 44, 63–67. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Real, C.; Alcala, M.D.; Criado, J.M. Preparation of Silica from Rice Husks. J. Am. Ceram. Soc. 1996, 79, 2012–2016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Ding, X.; Lei, H.; Chen, X.; An, D.; Li, Y.; Wang, Z. A study on the consecutive preparation of d-xylose and pure superfine silica from rice husk. Bioresour. Technol. 2010, 101, 1263–1267. [Google Scholar] [CrossRef]
- Della, V.; Kühn, I.; Hotza, D. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Matori, K.A.; Haslinawati, M.M. Producing Amorphous White Silica from Rice Husk. Masaum J. Basic Appl. Sci. 2009, 1, 512. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1563650 (accessed on 3 June 2022).
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Russo, B.; Causse, J.; Rey, C.; Lautru, J.; Rebiscoul, D.; Ayral, A. Biosourced adsorbent prepared with rice husk part 1: A complete understanding of the structure of materials, the major role of mineral impurities for metal extraction. Sustain. Mater. Technol. 2023, 36, e00601. [Google Scholar] [CrossRef]
- Gu, S.; Zhou, J.; Yu, C.; Luo, Z.; Wang, Q.; Shi, Z. A novel two-staged thermal synthesis method of generating nanosilica from rice husk via pre-pyrolysis combined with calcination. Ind. Crops Prod. 2015, 65, 1–6. [Google Scholar] [CrossRef]
- Abu Bakar, R.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Castillo, J.; Vargas, V.; Macero, D.; Le Beulze, A.; Ruiz, W.; Bouyssiere, B. One-step synthesis of SiO2 α−Fe2O3/Fe3O4 composite nanoparticles with magnetic properties from rice husks. Phys. B Condens. Matter 2021, 605, 412799. [Google Scholar] [CrossRef]
- Xu, W.T.; Wei, J.; Chen, J.; Zhang, B.; Xu, P.; Ren, J.; Yu, Q. Comparative Study of Water-Leaching and Acid-Leaching Pretreatment on the Thermal Stability and Reactivity of Biomass Silica for Viability as a Pozzolanic Additive in Cement. Materials 2018, 11, 1697. [Google Scholar] [CrossRef]
- Bakdash, R.S.; Aljundi, I.H.; Basheer, C.; Abdulazeez, I. Rice husk derived Aminated Silica for the efficient adsorption of different gases. Sci. Rep. 2020, 10, 19526. [Google Scholar] [CrossRef] [PubMed]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of biogenic silica generated by thermo chemical treatment of rice husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Benassi, L.; Bosio, A.; Dalipi, R.; Borgese, L.; Rodella, N.; Pasquali, M.; Depero, L.E.; Bergese, P.; Bontempi, E. Comparison between rice husk ash grown in different regions for stabilizing fly ash from a solid waste incinerator. J. Environ. Manag. 2015, 159, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Liou, T.-H.; Yang, C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng. B 2011, 176, 521–529. [Google Scholar] [CrossRef]
- Gu, S.; Zhou, J.; Luo, Z.; Wang, Q.; Ni, M. A detailed study of the effects of pyrolysis temperature and feedstock particle size on the preparation of nanosilica from rice husk. Ind. Crops Prod. 2013, 50, 540–549. [Google Scholar] [CrossRef]
- Madduluri, V.R.; Mandari, K.K.; Velpula, V.; Varkolu, M.; Kamaraju, S.R.R.; Kang, M. Rice husk-derived carbon-silica supported Ni catalysts for selective hydrogenation of biomass-derived furfural and levulinic acid. Fuel 2020, 261, 116339. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.; Husni, M.A.; Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Gautam, N.; Athira Merlin Rose, K.V.; Chaurasia, A. Study on chemical kinetics and characterization of nanosilica from rice husk and rice straw in the fixed-bed pyrolysis process. Biomass Convers. Biorefinery 2020, 12, 1435–1448. [Google Scholar] [CrossRef]
- Su, Y.; Liu, L.; Zhang, S.; Xu, D.; Du, H.; Cheng, Y.; Wang, Z.; Xiong, Y. A green route for pyrolysis poly-generation of typical high ash biomass, rice husk: Effects on simultaneous production of carbonic oxide-rich syngas, phenol-abundant bio-oil, high-adsorption porous carbon and amorphous silicon dioxide. Bioresour. Technol. 2020, 295, 122243. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-P.; Huang, A.-N.; Kuo, H.-P. Analysis of the Rice Husk Pyrolysis Products from a Fluidized Bed Reactor. Procedia Eng. 2015, 102, 1183–1186. [Google Scholar] [CrossRef]
- Gómez-Vásquez, R.; Fernández-Ballesteros, E.; Camargo-Trillos, D. Biogenic nanoporous oxides recovery from by-products of bioenergy production: Rice husks and corncob biochars. Biomass Bioenergy 2022, 161, 106455. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Odetoye, T.E.; Titiloye, J.; Ighalo, J.O. A Thermodynamic Study of Rice Husk (Oryza sativa) Pyrolysis. Eur. J. Sustain. Dev. Res. 2019, 3, em0094. [Google Scholar] [CrossRef]
- Manasa, K.; Naresh, G.; Kalpana, M.; Sasikumar, B.; Velisoju, V.K.; Chary, K.V.; Michalkiewicz, B.; Venugopal, A. Improved H2 yields over rice husk derived SiO2 nanoparticles supported Ni catalyst during non-oxidative methane cracking. J. Energy Inst. 2021, 99, 73–81. [Google Scholar] [CrossRef]
- Rajan, R.; Zakaria, Y.; Shamsuddin, S.; Hassan, N.F.N. Robust synthesis of mono-dispersed spherical silica nanoparticle from rice husk for high definition latent fingermark development. Arab. J. Chem. 2020, 13, 8119–8132. [Google Scholar] [CrossRef]
- Porrang, S.; Rahemi, N.; Davaran, S.; Mahdavi, M.; Hassanzadeh, B. Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2021, 163, 105866. [Google Scholar] [CrossRef]
- Schneider, D.; Attallah, A.G.; Wassersleben, S.; Wenzel, M.; Matysik, J.; Krause-Rehberg, R.; Enke, D. Advanced textural characterization of biogenic silica by nitrogen physisorption, positron annihilation lifetime spectroscopy and hyperpolarized 129Xe NMR spectroscopy. Microporous Mesoporous Mater. 2020, 307, 110515. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Integrated Mechanochemical/Microwave-Assisted Approach for the Synthesis of Biogenic Silica-Based Catalysts from Rice Husk Waste. ACS Sustain. Chem. Eng. 2018, 6, 11555–11562. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized Procedure for the Production of High-Grade, Porous Biogenic Silica. Waste Biomass Valorization 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Zareihassangheshlaghi, A.; Dizaji, H.B.; Zeng, T.; Huth, P.; Ruf, T.; Denecke, R.; Enke, D. Behavior of Metal Impurities on Surface and Bulk of Biogenic Silica from Rice Husk Combustion and the Impact on Ash-Melting Tendency. ACS Sustain. Chem. Eng. 2020, 8, 10369–10379. [Google Scholar] [CrossRef]
- Choudhary, P.; Sharma, R.; Kumar, V.; Singh, A.; Sharma, N. Synthesis, Characterization and Catalytic Activity of Bio-MCM-41 for Production of Bio Crude Oil via Pyrolysis of Rice Straw. Waste Biomass Valorization 2023, 14, 4173–4186. [Google Scholar] [CrossRef]
- Alyosef, H.A.; Uhlig, H.; Münster, T.; Kloess, G.; Einicke, W.-D.; Gläser, R.; Enke, D. Biogenic silica from rice husk ash-Sustainable sources for the synthesis of value-added silica. Chem. Eng. Trans. 2014, 37, 667–672. [Google Scholar]
- Costa, J.A.S.; Sarmento, V.H.V.; Romão, L.P.C.; Paranhos, C.M. Adsorption of organic compounds on mesoporous material from rice husk ash (RHA). Biomass Convers. Biorefinery 2020, 10, 1105–1120. [Google Scholar] [CrossRef]
- Kamari, S.; Ghorbani, F. Extraction of highly pure silica from rice husk as an agricultural by-product and its application in the production of magnetic mesoporous silica MCM–41. Biomass Convers. Biorefinery 2021, 11, 3001–3009. [Google Scholar] [CrossRef]
- Zadeh, R.J.; Sayadi, M.H.; Rezaei, M.R. Synthesis of Thiol modified magMCM-41 nanoparticles with rice husk ash as a robust, high effective, and recycling magnetic sorbent for the removal of herbicides. J. Environ. Chem. Eng. 2021, 9, 104804. [Google Scholar] [CrossRef]
- Bahrami, A.; Simon, U.; Soltani, N.; Zavareh, S.; Schmidt, J.; Pech-Canul, M.I.; Gurlo, A. Eco-fabrication of hierarchical porous silica monoliths by ice-templating of rice husk ash. Green Chem. 2017, 19, 188–195. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram. Int. 2015, 41, 275–281. [Google Scholar] [CrossRef]
- Huang, S.-S.; Tung, M.T.; Huynh, C.D.; Hwang, B.-J.; Bieker, P.M.; Fang, C.-C.; Wu, N.-L. Engineering Rice Husk into a High-Performance Electrode Material through an Ecofriendly Process and Assessing Its Application for Lithium-Ion Sulfur Batteries. ACS Sustain. Chem. Eng. 2019, 7, 7851–7861. [Google Scholar] [CrossRef]
- Vijayan, R.; Kumar, G.S.; Karunakaran, G.; Surumbarkuzhali, N.; Prabhu, S.; Ramesh, R. Microwave combustion synthesis of tin oxide-decorated silica nanostructure using rice husk template for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2020, 31, 5738–5745. [Google Scholar] [CrossRef]
- Henao, W.; Jaramillo, L.Y.; López, D.; Romero-Sáez, M.; Buitrago-Sierra, W.A.H. Insights into the CO2 capture over amine-functionalized mesoporous silica adsorbents derived from rice husk ash. J. Environ. Chem. Eng. 2020, 8, 104362. Available online: https://www.sciencedirect.com/science/article/pii/S2213343720307119 (accessed on 2 December 2022). [CrossRef]
- An, D.; Guo, Y.; Zou, B.; Zhu, Y.; Wang, Z. A study on the consecutive preparation of silica powders and active carbon from rice husk ash. Biomass Bioenergy 2011, 35, 1227–1234. [Google Scholar] [CrossRef]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.A.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalination Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Kollarahithlu, S.C.; Balakrishnan, R.M. Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J. Clean. Prod. 2021, 294, 126155. [Google Scholar] [CrossRef]
- Zeb, S.; Ali, N.; Ali, Z.; Bilal, M.; Adalat, B.; Hussain, S.; Gul, S.; Ali, F.; Ahmad, R.; Khan, S.; et al. Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. J. Water Process. Eng. 2020, 38, 101675. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Microscale scavenging of pentachlorophenol in water using amine and tripolyphosphate-grafted SBA-15 silica: Batch and modeling studies. J. Environ. Manag. 2014, 146, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, F.; Huang, D.; Hou, S.; Wang, H.; Wang, M.; Chi, Y.; Zhao, Z. A facile route to magnetic mesoporous core–shell structured silicas containing covalently bound cyclodextrins for the removal of the antibiotic doxycycline from water. RSC Adv. 2018, 8, 31348–31357. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Vargas, V.; Castillo, J.; Ocampo-Torres, R.; Lienemann, C.-P.; Bouyssiere, B. Surface modification of SiO2 nanoparticles to increase asphaltene adsorption. Pet. Sci. Technol. 2018, 36, 618–624. [Google Scholar] [CrossRef]
- Mudhoo, A.; Sillanpää, M. Magnetic nanoadsorbents for micropollutant removal in real water treatment: A review. Environ. Chem. Lett. 2021, 19, 4393–4413. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kumar, A.; Zhang, Y. A novel approach for the removal of Pb2+ and Cd2+ from wastewater by sulfur-ferromagnetic nanoparticles (SFMNs). Chemosphere 2022, 287, 132156. [Google Scholar] [CrossRef]
- Mohmood, I.; Lopes, C.B.; Lopes, I.; Tavares, D.S.; Soares, A.M.; Duarte, A.C.; Trindade, T.; Ahmad, I.; Pereira, E. Remediation of mercury contaminated saltwater with functionalized silica coated magnetite nanoparticles. Sci. Total Environ. 2016, 557–558, 712–721. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Liang, B.; Gu, F.; Xu, Z. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Li, T.; Li, S. Removal of hexavalent chromium in soil and groundwater by supported nano zero-valent iron on silica fume. Water Sci. Technol. 2011, 63, 2781–2787. [Google Scholar] [CrossRef]
- Zheng, T.; Zhan, J.; He, J.; Sunkara, B.; Lu, Y.; McPherson, G.L.; Piringer, G.; Kolesnichenko, V.; John, V.T. Nanostructured Multifunctional Materials for Environmental Remediation of Chlorinated Hydrocarbons. In Environmental Applications of Nanoscale and Microscale Reactive Metal Particles; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 163–179. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini–Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Saha, A.; Gajbhiye, V.T.; Gupta, S.; Kumar, R.; Ghosh, R.K. Simultaneous Removal of Pesticides from Water by Rice Husk Ash: Batch and Column Studies. Water Environ. Res. 2014, 86, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, J.; Guerrero, V.H.; Vizuete, K.; Debut, A. Environmentally friendly synthesis of silicon dioxide nanoparticles and their application for the removal of emerging contaminants in aqueous media. J. Physics Conf. Ser. 2022, 2238, 012005. [Google Scholar] [CrossRef]
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.; Jorfi, S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liang, Y.; Ding, H.; Sun, S. Adsorption Properties and Mechanism of Cd2+ in Water by Zr-containing Silica Residue Purification. Front. Chem. 2018, 6, 556. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A.; Navratil, J.D. Adsorption of Cesium, Strontium, and Cobalt Ions on Magnetite and a Magnetite−Silica Composite. Ind. Eng. Chem. Res. 2001, 40, 1615–1623. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ma, H.T.; Avti, P.; Bashir, M.J.K.; Ng, C.A.; Wong, L.Y.; Jun, H.K.; Ngo, Q.M.; Tran, N.Q. Adsorptive removal of iron using SiO2 nanoparticles extracted from rice husk ash. J. Anal. Methods Chem. 2019, 2019, 6210240. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.; Rashwan, A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. Available online: https://www.sciencedirect.com/science/article/pii/S1319562X21003958 (accessed on 6 December 2022). [CrossRef]
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste—Rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271. [Google Scholar] [CrossRef]
- Priya, A.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef]
- Amirhandeh, S.Z.H.; Salem, A.; Salem, S. Treatment of tannery wastewater by silica nanoparticles produced from rice husk ash via a green route. Environ. Sci. Pollut. Res. 2022, 30, 13039–13047. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Østby, H.; Hansen, L.D.; Horn, S.J.; Eijsink, V.G.H.; Várnai, A. Enzymatic processing of lignocellulosic biomass: Principles, recent advances and perspectives. J. Ind. Microbiol. Biotechnol. 2020, 47, 623–657. [Google Scholar] [CrossRef] [PubMed]
- Wattanasiriwech, S.; Wattanasiriwech, D.; Svasti, J. Production of amorphous silica nanoparticles from rice straw with microbial hydrolysis pretreatment. J. Non-Crystalline Solids 2010, 356, 1228–1232. [Google Scholar] [CrossRef]
- Zemnukhova, L.A.; Skiba, E.A.; Budaeva, V.V.; Panasenko, A.E.; Polyakova, N.V. Composition of inorganic components of oat husks and products of their chemical and enzymatic transformation. Russ. J. Appl. Chem. 2018, 91, 230–234. [Google Scholar] [CrossRef]
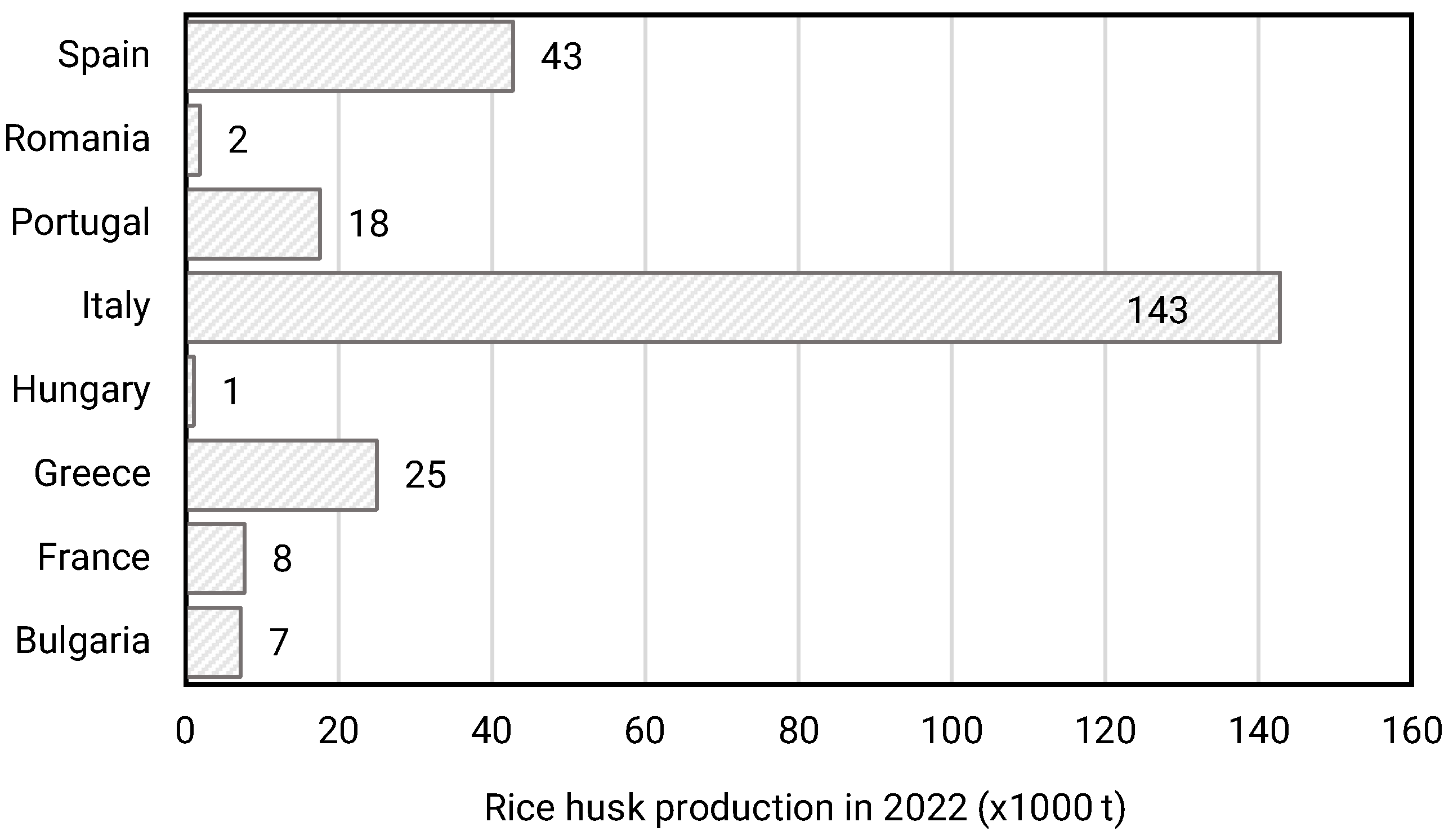
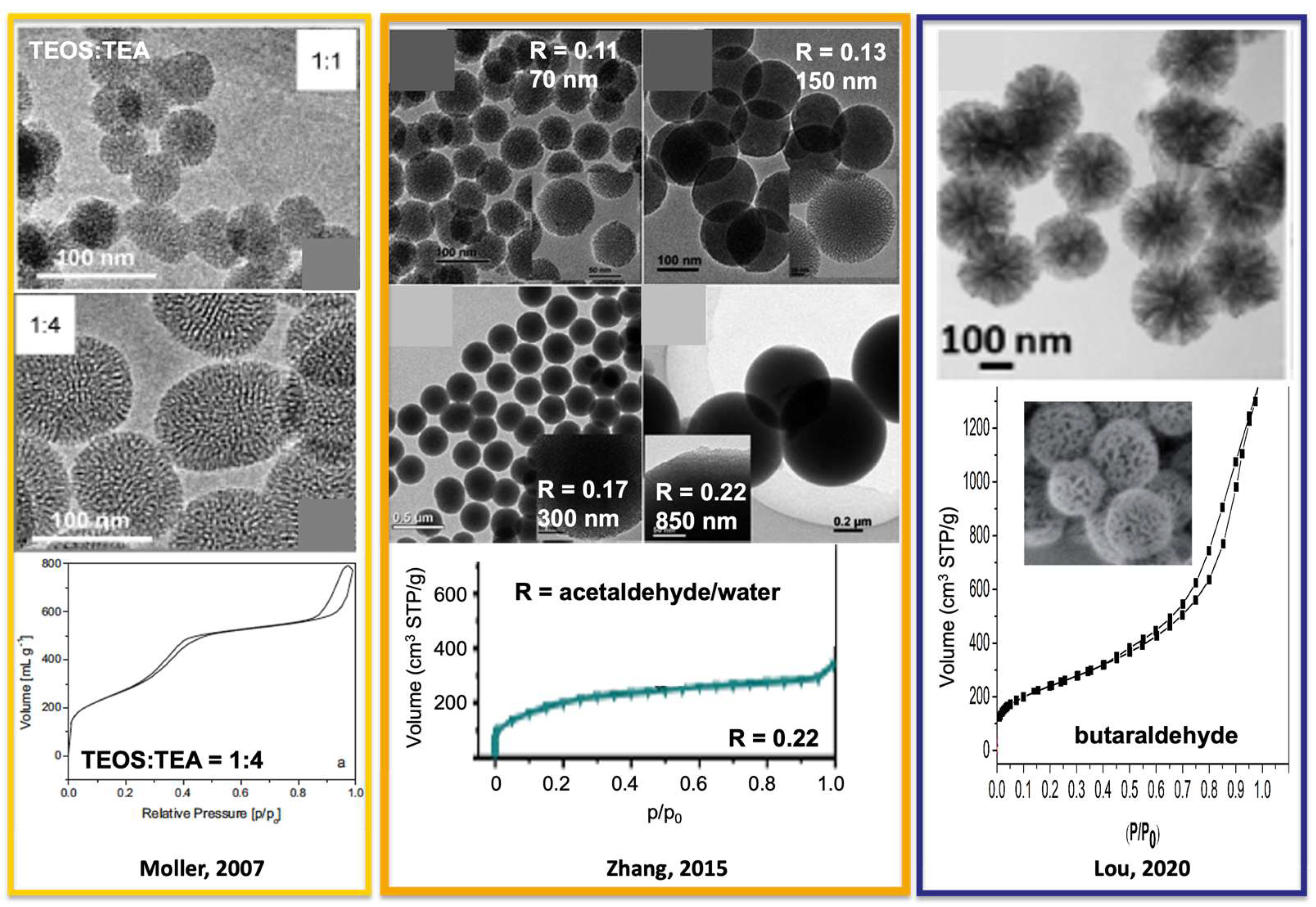
| Agroalimentary Residue | Proximate Analysis (wt%) | Heating Value (MJ/kg) | Silica in Ash (d.b. wt%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Moisture Content | Volatile Matter | Fixed Carbon | Ash Content | ||||
| Rice husk | 6.1–15.0 | 54.4–71.0 | 11.1–25.0 | 10.7–23.0 | 13.0–16.0 | 80.0–99.0 | [9] |
| Rice straw | 8.5–13.0 | 66.8–70.2 | 11.0–14.6 | 6.0–9.2 | 12.1–16.6 | 60.0–80.0 | [10] |
| Wheat husk | 4.4–8.5 | 65.6–69.2 | 12.7–21.0 | 5.0–12.1 | 18.9–19.2 | 74.2–86.0 | [11,12] |
| Sugar cane bagasse | 8.4–10.3 | 75.7–88.5 | 9.4–16.3 | 1.6–2.2 | 16.0–19.2 | 54.9 | [11,12,13] |
| Country | Pretreatment | Acid Washing | Calcination | SBET (m2/g) | Ref. |
|---|---|---|---|---|---|
| China | Drying at 105 °C for 2 h | HCl 0.7 M for 1 h at RT (better than acetic acid) | 600 or 700 °C for 0.5 h Oven directly set to 600 or 700 °C | 210 | [15] |
| H2SO4 0.7 M for 1 h at RT | 240 | ||||
| Malaysia | C12SO4Na2/H2O H2O Drying at 110 °C | HCl 0.5 M at 60 °C for 0.5 h of stirring | 600 °C for 2 h | 218 | [46] |
| H2SO4 0.5 M at 60 °C for 0.5 h | 208 | ||||
| Venezuela | HCl 4 M for 24 h | Sequential: 350 °C for 3 h, 550 °C for 2 h, 700 °C for 3 h Grinding for 12 h | 234 | [47] | |
| China | HCl, H2SO4, HNO3 1,2,3N at RT for 1 or 2.5 h of stirring (best to remove K: HCl 1N) | 600 °C for 2 h (test: 600–1200 °C for 0.25–2 h) Grinding for 10 min | 248 | [48] | |
| India | H2O | H2SO4 1 M | 700 °C for 6 h | 220 | [49] |
| Egypt | H2O Drying at 110 °C Milling | Citric acid (5wt%) 50 °C 3 h + 80 °C 1 h | Sequential (10 °C/min) 310 °C for 1 h, 400 °C for 2 h, 510 °C for 5 h, 600 °C for 0.5 h | 313 | [50] |
| Turkey | H2O Drying at 110 °C for 24 h | Boiling for 2 h HCl (3%v/v) reflux | 600 °C for 4 h (10 °C/min) | 321 | [43] |
| France | H2O | HNO3 2 M 100 °C 1 h, washed at pH 7, dried at 100 °C for 12 h | 700 °C (5 °C/min) | 330 | [44] |
| China | H2O Drying at 110 °C Pulverized in 10–60 Mesh | HCl 8 wt% of 1 g/10 mL at 120 °C for 4 h, washed at pH 7, dried at 110 °C for 3 h | 300 °C for 0.5 h N2 (1 L/min) (20 °C/min) 610 °C for 3 h O2 (1 L/min) (10 °C/min) | 352 | [45] |
| Rice Husk Origin | Silica-Based Adsorbent | Pollutants | pH | qe (mg·g−1) | te (min) | References |
|---|---|---|---|---|---|---|
| Iran | Fe3O4@SiO2@NH2 | Methyl red | 5.24 | 81.39 | - | [94] |
| Brazil | MW-nSiO2 | Methylene blue | 6 | 679.9 | 240 | [95] |
| nSiO2 | 547.5 | |||||
| India | RHA | Pesticides mixture | - | 0.078–0.166 | 120–240 | [96] |
| Ecuador | RH-SiO2 | Caffeine Triclosan | - | 0.75 2.74 | 30 60 | [97] |
| Iran | SHmagMCM-41 | Glyphosate 2,4-D | 5 | 106.38 125.00 | 60 30 | [73] |
| Brazil | MCM-41 | PAHs mixture | 5.6 | 20 | 120 | [71] |
| Metal | SiO2 Extraction Method | pH | qe (mg/g) | te (min) | References |
|---|---|---|---|---|---|
| Ni2+ | Thermochemical from RH | - | 11.7 | (24 h) | [44] |
| Fe2+ | Sol-gel from RHA | 5 | 9.0 | 20 | [103] |
| Pb2+ | Biotransformation | - | 88.0 | (120 h) | [104] |
| Cr3+ Cu2+ | Not extracted, whole RH | 5 | 22.5 30.0 | 30 | [105] |
| Cr3+ | Sol-gel from RHA | 6 | 385.0 | (24 h) | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Otero, A.; Vargas, V.; Galarneau, A.; Castillo, J.; Christensen, J.H.; Bouyssiere, B. Sustainable Harnessing of SiO2 Nanoparticles from Rice Husks: A Review of the Best Synthesis and Applications. Processes 2023, 11, 3373. https://doi.org/10.3390/pr11123373
Rodriguez-Otero A, Vargas V, Galarneau A, Castillo J, Christensen JH, Bouyssiere B. Sustainable Harnessing of SiO2 Nanoparticles from Rice Husks: A Review of the Best Synthesis and Applications. Processes. 2023; 11(12):3373. https://doi.org/10.3390/pr11123373
Chicago/Turabian StyleRodriguez-Otero, Alba, Vicmary Vargas, Anne Galarneau, Jimmy Castillo, Jan H. Christensen, and Brice Bouyssiere. 2023. "Sustainable Harnessing of SiO2 Nanoparticles from Rice Husks: A Review of the Best Synthesis and Applications" Processes 11, no. 12: 3373. https://doi.org/10.3390/pr11123373
APA StyleRodriguez-Otero, A., Vargas, V., Galarneau, A., Castillo, J., Christensen, J. H., & Bouyssiere, B. (2023). Sustainable Harnessing of SiO2 Nanoparticles from Rice Husks: A Review of the Best Synthesis and Applications. Processes, 11(12), 3373. https://doi.org/10.3390/pr11123373









