Simulation of Biogas Upgrading by Sorption-Enhanced Methanation with CaO in a Dual Interconnected Fluidized Bed System
Abstract
:1. Introduction
2. Materials and Methods
2.1. SEM Modelling
2.1.1. Aspen Flowsheet
2.1.2. Kinetics
2.2. Key Parameters
3. Results and Discussion
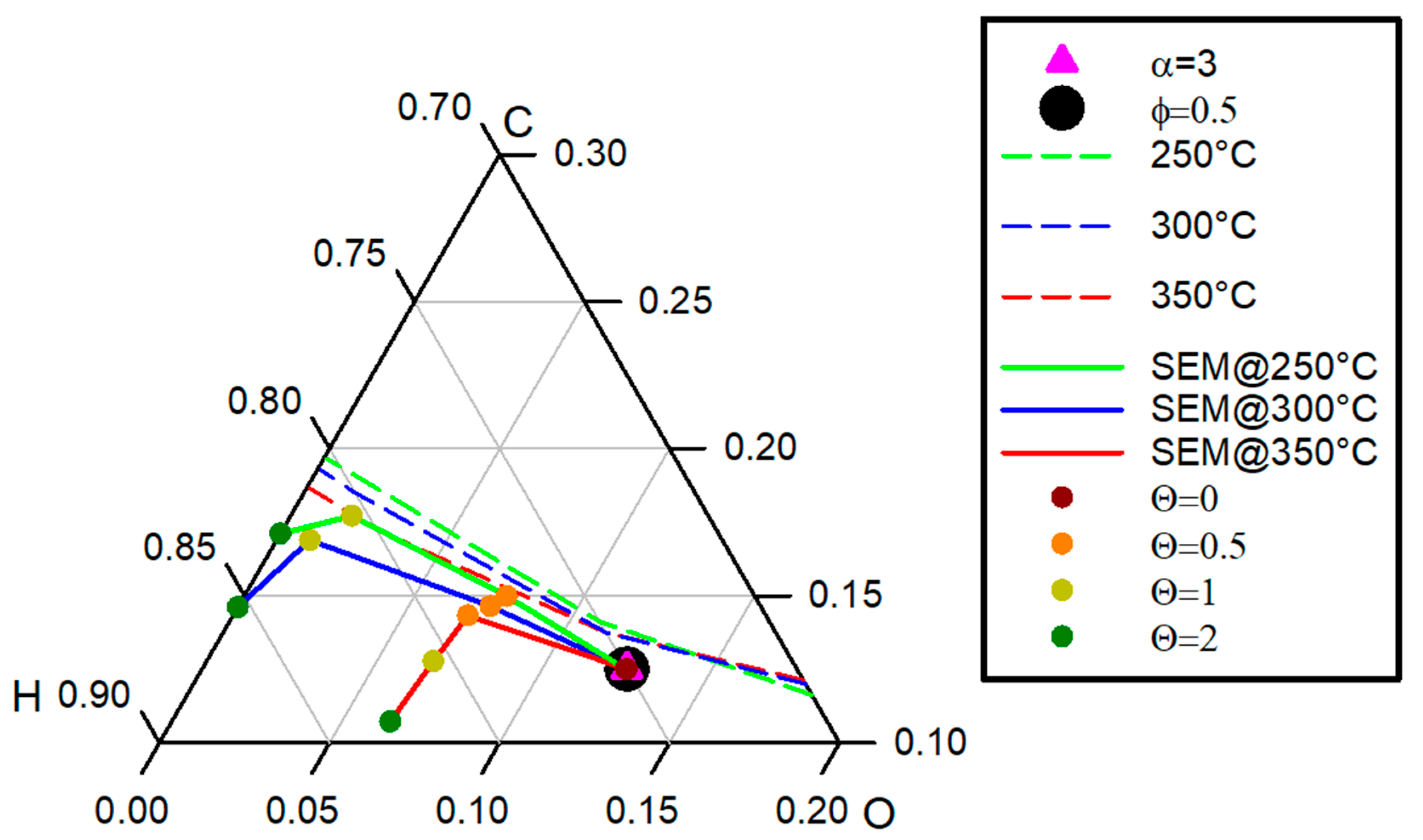
3.1. SEM Performances
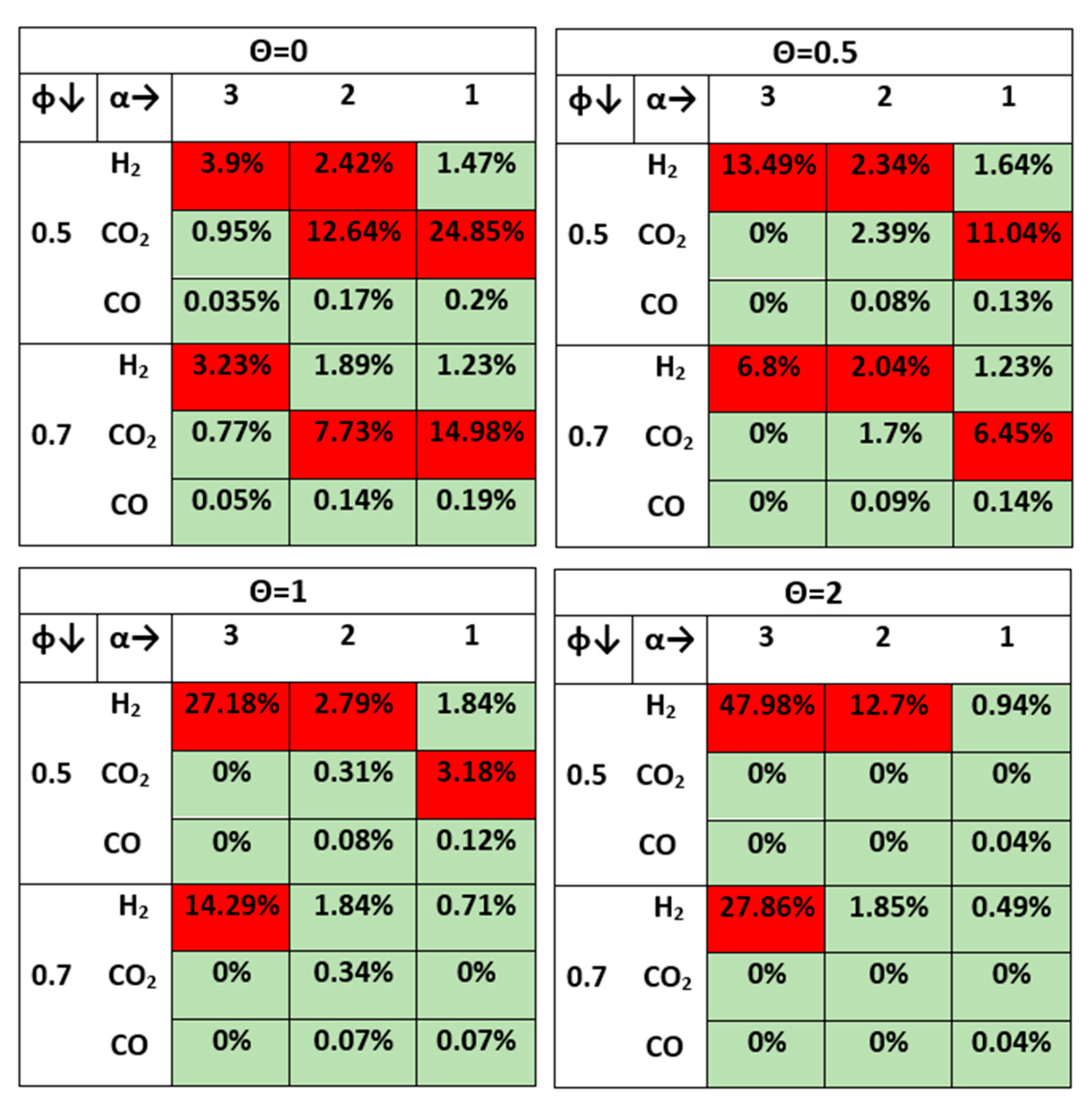
3.1.1. Effect of CaO on the Product Gas Quality
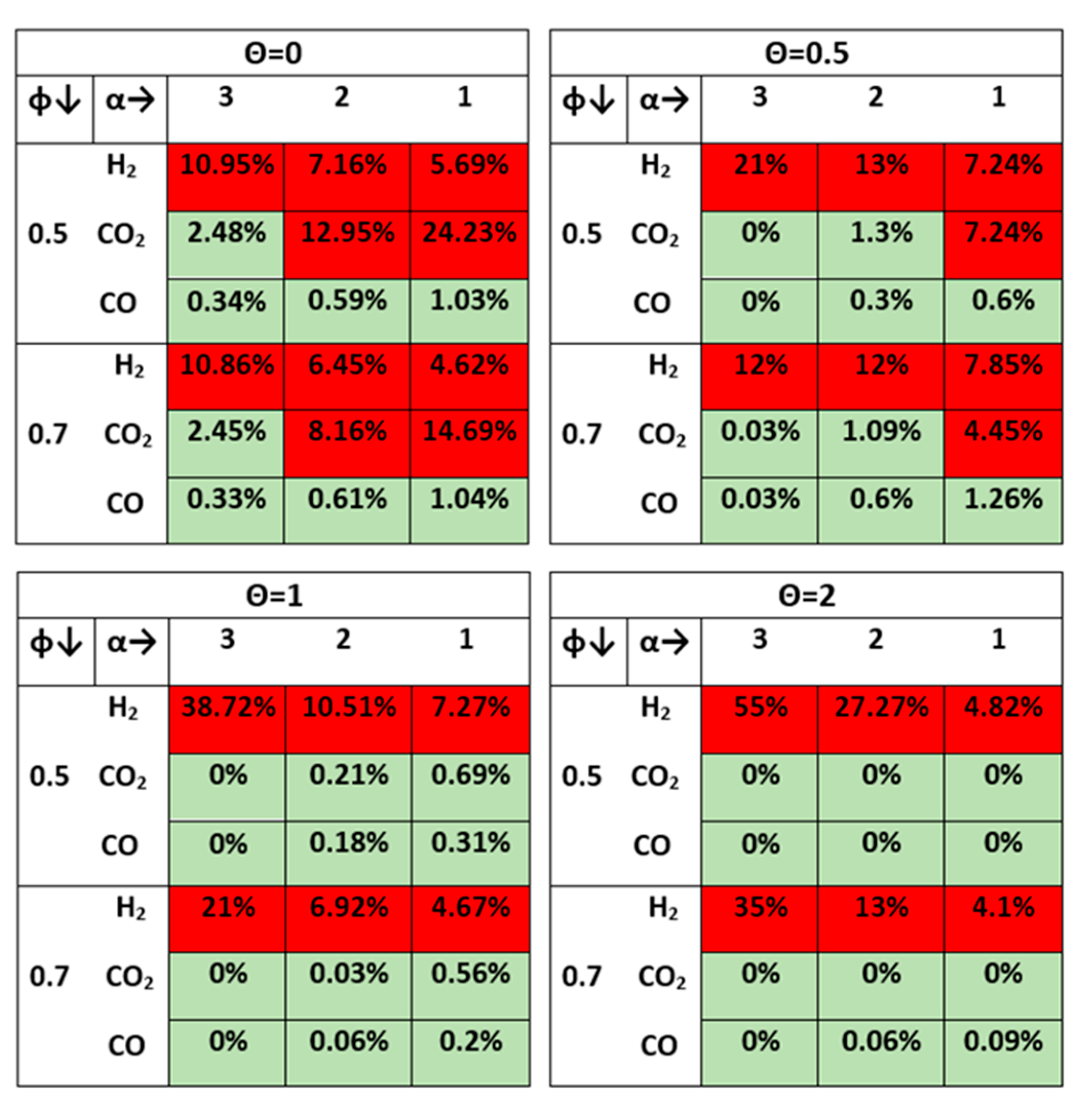
3.1.2. Effect of Temperature on the Product Gas Quality
- CO, which is not a reactant in this biogas upgrading scheme but can be formed by other reaction paths such as the Reverse Water Gas Shift reaction, increases with α decreasing and tends to decrease for the highest values of θ. However, it is always below the limit;
- CO2 is always below the limit except for when the sub-stoichiometric value of CaO (θ = 0.5) and α = 1; under this condition the sorbent fed is still not sufficient to compensate CO2 excess in the feed gas;
- H2 represents, again, the critical species, always being above the limit value even for the best conditions in terms of its consumption, and namely under the limit case with minimum α and maximum θ, where the H2 detected is about 4% for both values of φ.
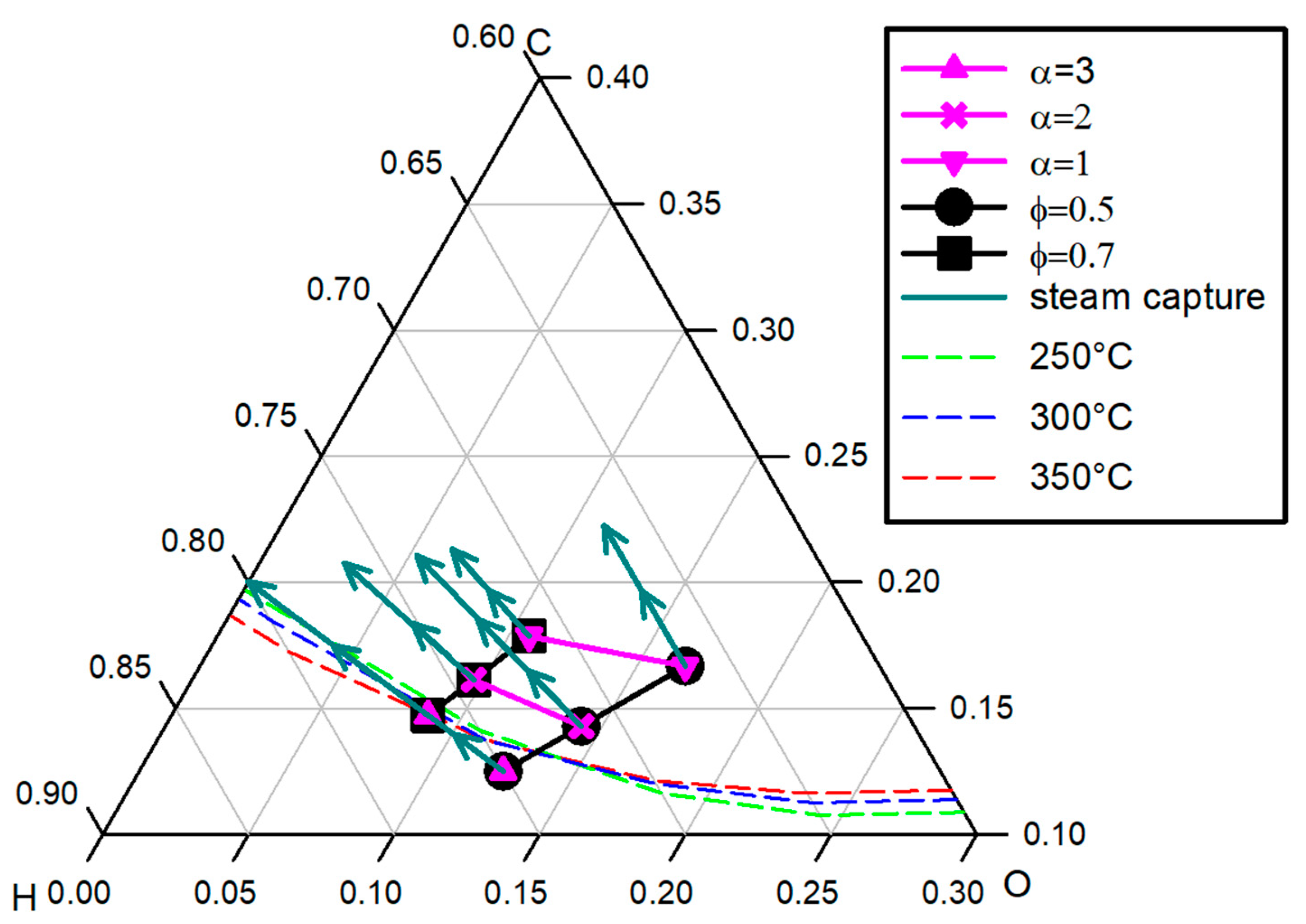
3.2. SEM Thermodynamics and Possible Carbon Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Karellas, S. Equilibrium and kinetic aspects for catalytic methanation focusing on CO2 derived Substitute Natural Gas (SNG). Renew. Sustain. Energy Rev. 2018, 94, 536–550. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohsen, M.; Ismail, S.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Methods to alleviate the inhibition of sludge anaerobic digestion by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 3811–3836. [Google Scholar] [CrossRef]
- Faria, A.C.; Miguel, C.; Madeira, L.M. Thermodynamic analysis of the CO2 methanation reaction with in situ water removal for biogas upgrading. J. CO2 Util. 2018, 26, 271–280. [Google Scholar] [CrossRef]
- Sabatier, P. New synthesis of methane. Comptes Rendus 1902, 134, 514–516. [Google Scholar]
- HaldorTopsoe. From Solid Fuels to Substitute Natural Gas (SNG) Using TREMP; Technical Report; Haldor Topsoe: Lyngby, Denmark, 2009. [Google Scholar]
- Duyar, M.S.; Treviño, M.A.A.; Farrauto, R.J. Dual function materials for CO2 capture and conversion using renewable H2. Appl. Catal. B Environ. 2015, 168–169, 370–376. [Google Scholar] [CrossRef]
- Mills, G.A.; Steffgen, F.W. Catalytic Methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Walspurger, S.; Elzinga, G.D.; Dijkstra, J.W.; Sarić, M.; Haije, W.G. Sorption enhanced methanation for substitute natural gas production: Experimental results and thermodynamic considerations. Chem. Eng. J. 2014, 242, 379–386. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gallandat, N.; Probst, B.; Suter, R.; Callini, E.; Ferri, D.; Arroyo, Y.; Erni, R.; Geerlings, H.; Züttel, A. Sorption enhanced CO2 methanation. Phys. Chem. Chem. Phys. 2013, 15, 9620–9625. [Google Scholar] [CrossRef] [PubMed]
- Delmelle, R.; Duarte, R.; Franken, T.; Burnat, D.; Holzer, L.; Borgschulte, A.; Heel, A. Development of improved nickel catalysts for sorption enhanced CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 20185–20191. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production and reduction of Ni-based oxygen carriers during chemical looping steam reforming of ethanol in a fixed-bed reactor. Int. J. Hydrogen Energy 2017, 42, 26217–26230. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production by sorption-enhanced chemical looping steam reforming of ethanol in an alternating fixed-bed reactor: Sorbent to catalyst ratio dependencies. Energy Convers. Manag. 2018, 155, 243–252. [Google Scholar] [CrossRef]
- Müller, S.; Fuchs, J.; Schmid, J.; Benedikt, F.; Hofbauer, H. Experimental development of sorption enhanced reforming by the use of an advanced gasification test plant. Int. J. Hydrogen Energy 2017, 42, 29694–29707. [Google Scholar] [CrossRef]
- Coppola, A.; Massa, F.; Salatino, P.; Scala, F. Evaluation of two sorbents for the sorption-enhanced methanation in a dual fluidized bed system. Biomass-Convers. Biorefin. 2020, 11, 111–119. [Google Scholar] [CrossRef]
- Agirre, I.; Acha, E.; Cambra, J.; Barrio, V. Water sorption enhanced CO2 methanation process: Optimization of reaction conditions and study of various sorbents. Chem. Eng. Sci. 2021, 237, 116546. [Google Scholar] [CrossRef]
- Seemann, M.; Schildhauer, T.; Biollaz, S.; Stucki, S.; Wokaun, A. The regenerative effect of catalyst fluidization under methanation conditions. Appl. Catal. A Gen. 2006, 313, 14–21. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358. [Google Scholar] [CrossRef]
- Frick, V.; Brellochs, J.; Specht, M. Application of ternary diagrams in the design of methanation systems. Fuel Process. Technol. 2014, 118, 156–160. [Google Scholar] [CrossRef]
- Swapnesh, A.; Srivastava, V.C.; Mall, I.D. Comparative study on thermodynamic analysis of CO2 utilization reactions. Chem. Eng. Technol. 2014, 37, 1765–1777. [Google Scholar] [CrossRef]
- Massa, F.; Coppola, A.; Scala, F. A thermodynamic study of sorption-enhanced CO2 methanation at low pressure. J. CO2 Util. 2019, 35, 176–184. [Google Scholar] [CrossRef]
- Werther, J. Fluidized-bed reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007; ISBN 9783527303854. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering; Butterworth-Heinemann: Boston, MA, USA, 1991. [Google Scholar]
- Yang, W. Handbook of Fluidization and Fluid-Particle Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Coppola, A.; Massa, F.; Scala, F. Simulation of a sorption-enhanced methanation process with CaO in a dual interconnected fluidized bed system. Fuel 2023, 339, 127374. [Google Scholar] [CrossRef]
- Gardner, D.C.; Bartholomew, C.H. Kinetics of Carbon Deposition During Methanation of CO. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 80–87. [Google Scholar] [CrossRef]
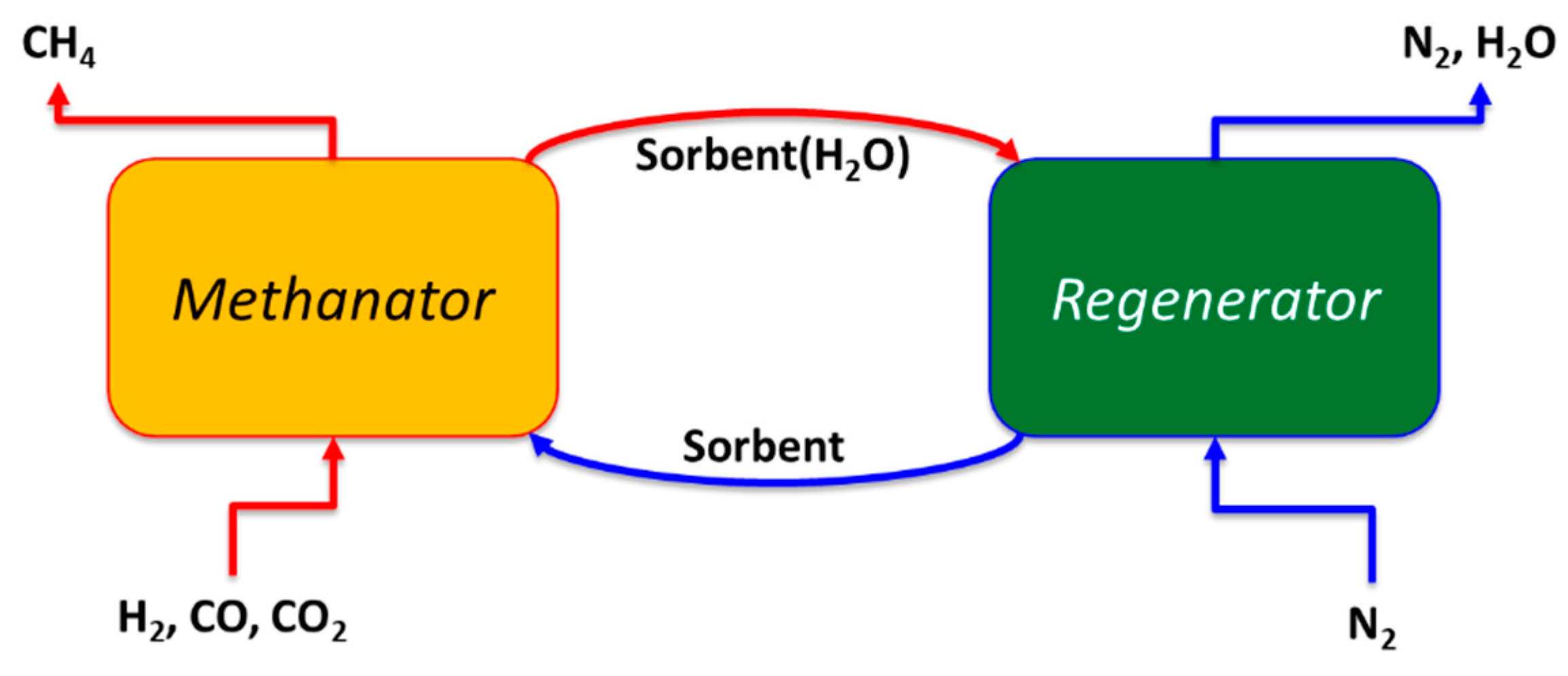


| Parameters | Methanator (METH) | Regenerator (REGEN) |
|---|---|---|
| Height, m | 14 | 8 |
| Internal diameter, m | 0.099–0.147 | 0.33 |
| Solid discharge height, m | 1.4 | 0.64 |
| Voiadge at umf 1, - | 0.5 | 0.5 |
| Geldart classification, - | B | B |
| Reaction | Formula | ∆H298K (kJ/mol) | Description |
|---|---|---|---|
| R1 | CO2 + 4H2 ⇄ CH4 + 2H2O | −165.0 | CO2 methanation |
| R2 | CO + 3H2 ⇄ CH4 + H2O | −206.2 | CO methanation |
| R3 | CO + H2O ⇄ CO2 + H2 | −41.2 | Water-gas shift |
| R4 | CaO + H2O ⇄ Ca(OH)2 | −65 | CaO hydration |
| R5 | CaO + CO2 ⇄ CaCO3 | −178 | CaO carbonation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massa, F.; Scala, F.; Coppola, A. Simulation of Biogas Upgrading by Sorption-Enhanced Methanation with CaO in a Dual Interconnected Fluidized Bed System. Processes 2023, 11, 3218. https://doi.org/10.3390/pr11113218
Massa F, Scala F, Coppola A. Simulation of Biogas Upgrading by Sorption-Enhanced Methanation with CaO in a Dual Interconnected Fluidized Bed System. Processes. 2023; 11(11):3218. https://doi.org/10.3390/pr11113218
Chicago/Turabian StyleMassa, Fiorella, Fabrizio Scala, and Antonio Coppola. 2023. "Simulation of Biogas Upgrading by Sorption-Enhanced Methanation with CaO in a Dual Interconnected Fluidized Bed System" Processes 11, no. 11: 3218. https://doi.org/10.3390/pr11113218
APA StyleMassa, F., Scala, F., & Coppola, A. (2023). Simulation of Biogas Upgrading by Sorption-Enhanced Methanation with CaO in a Dual Interconnected Fluidized Bed System. Processes, 11(11), 3218. https://doi.org/10.3390/pr11113218









