Computational Fluid Dynamics Simulation of Combustion and Selective Non-Catalytic Reduction in a 750 t/d Waste Incinerator
Abstract
:1. Introduction
2. Models and Simulation Method
2.1. Parameters of the Waste Incinerator
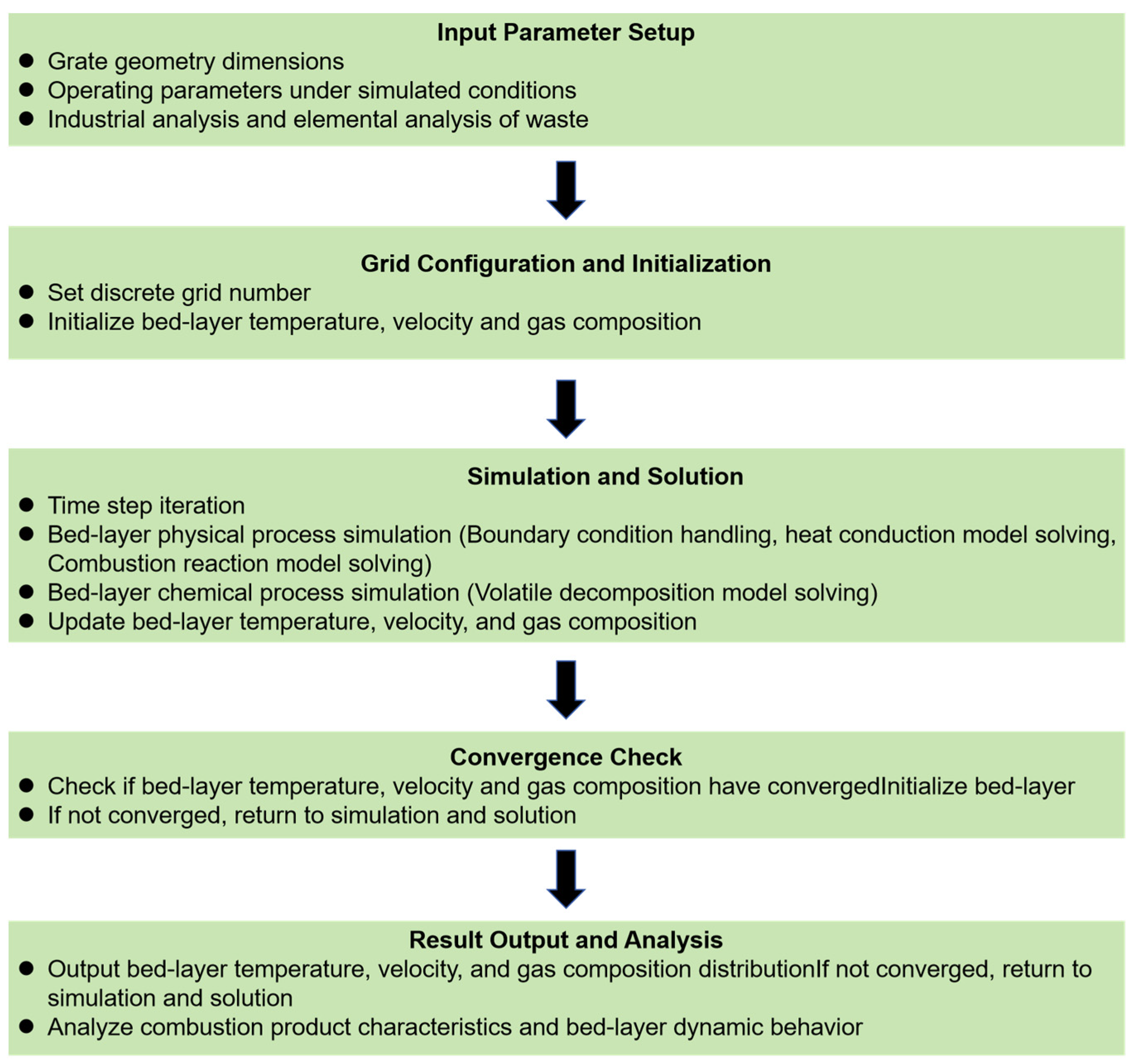
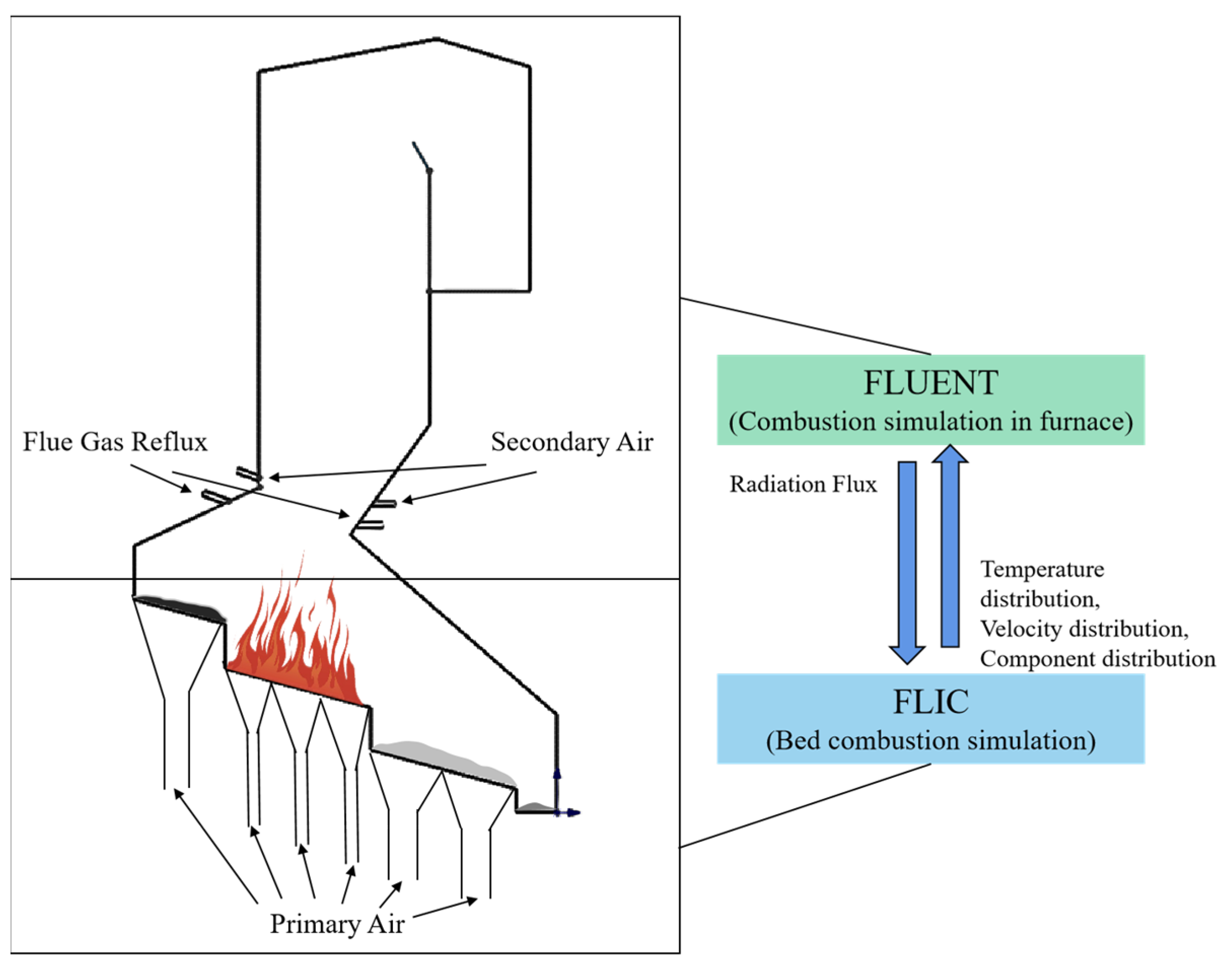
2.2. Simulation Method
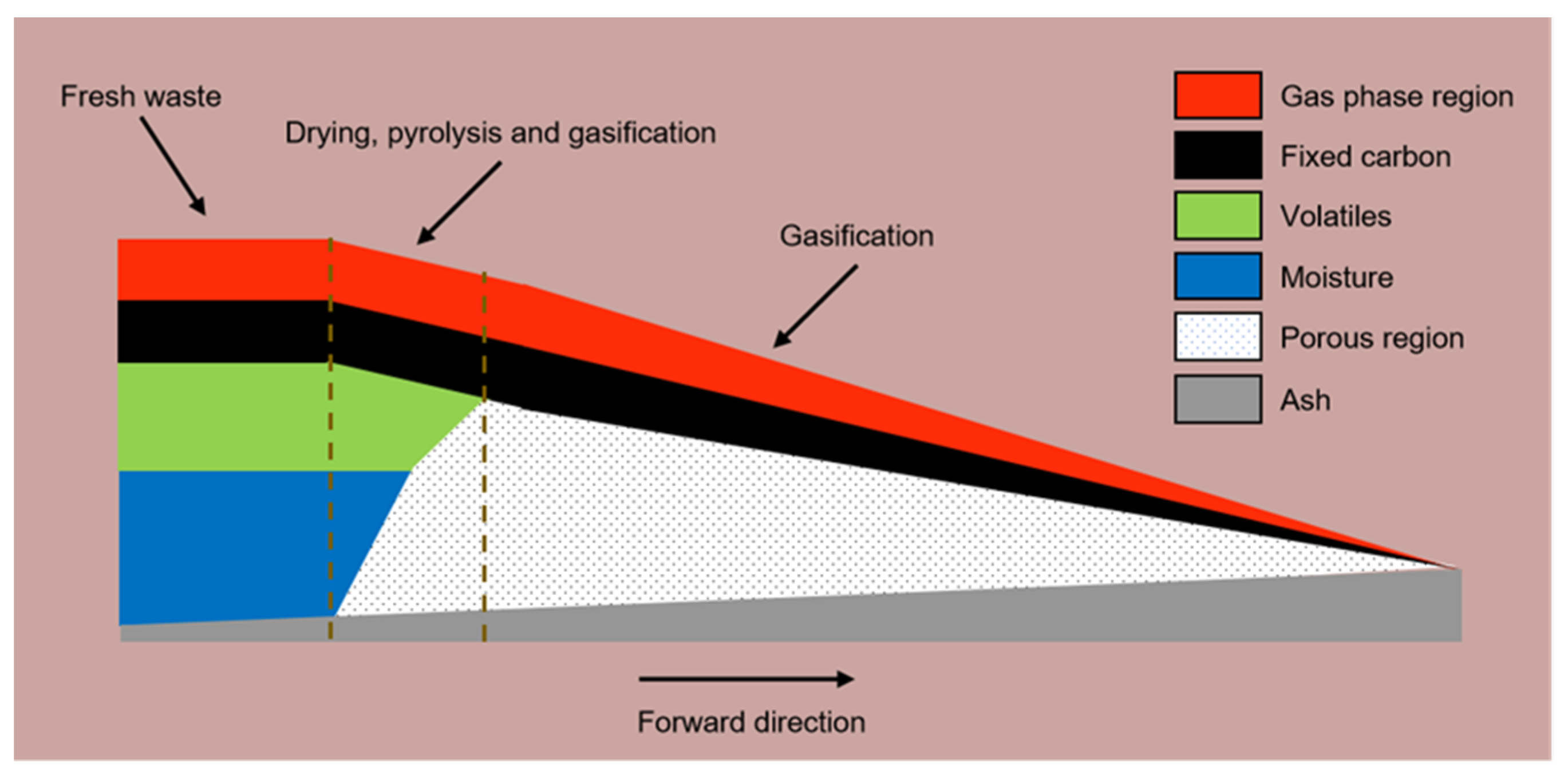
2.2.1. Bed Combustion Simulation
Water Evaporation
Volatilization Analysis
Volatile Combustion
Char Combustion
2.2.2. Combustion Simulation in Furnace
3. Results
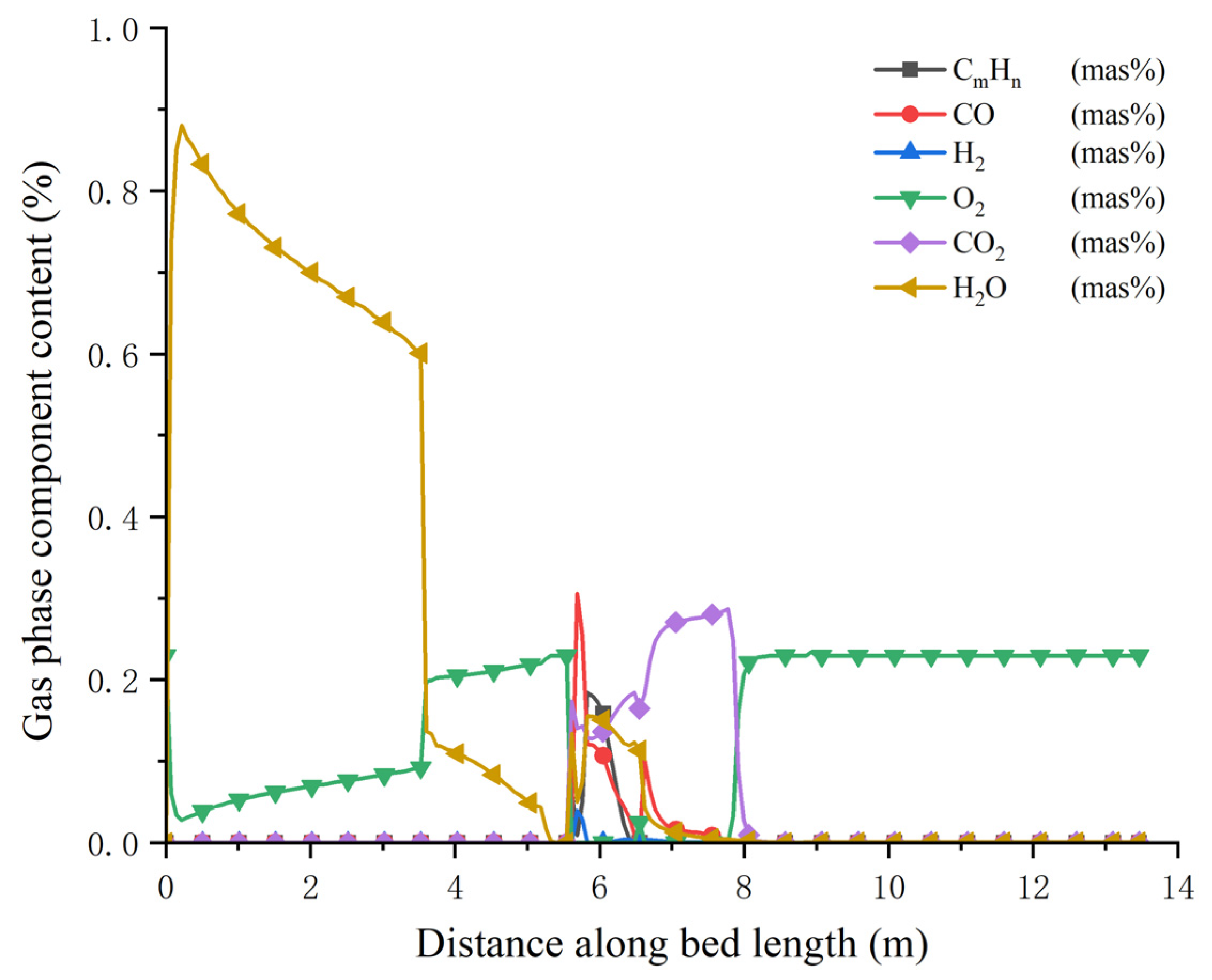
3.1. Simulation Results of Bed Combustion
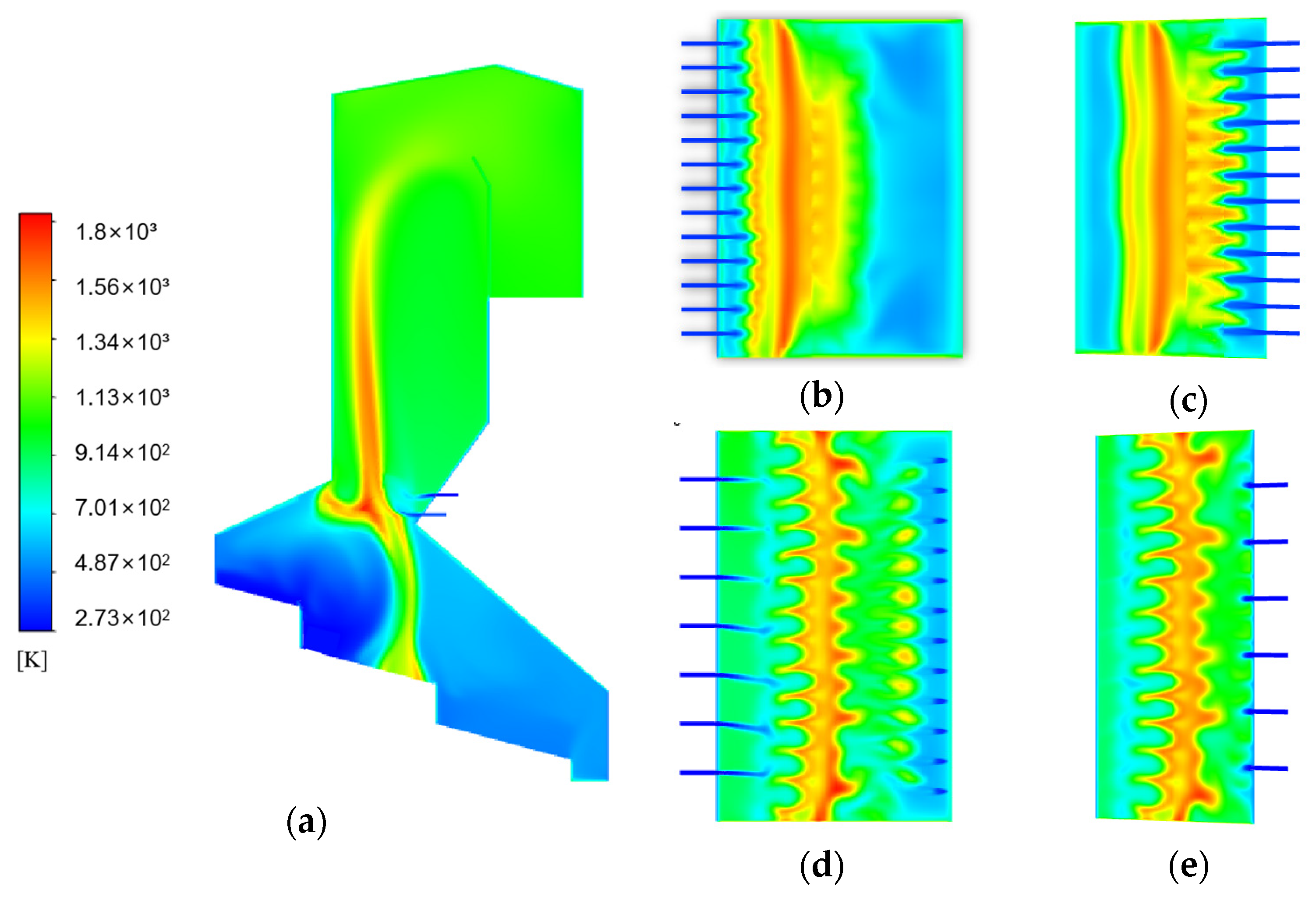
3.2. Simulation Results of Gas Combustion
3.3. Validating Model Accuracy
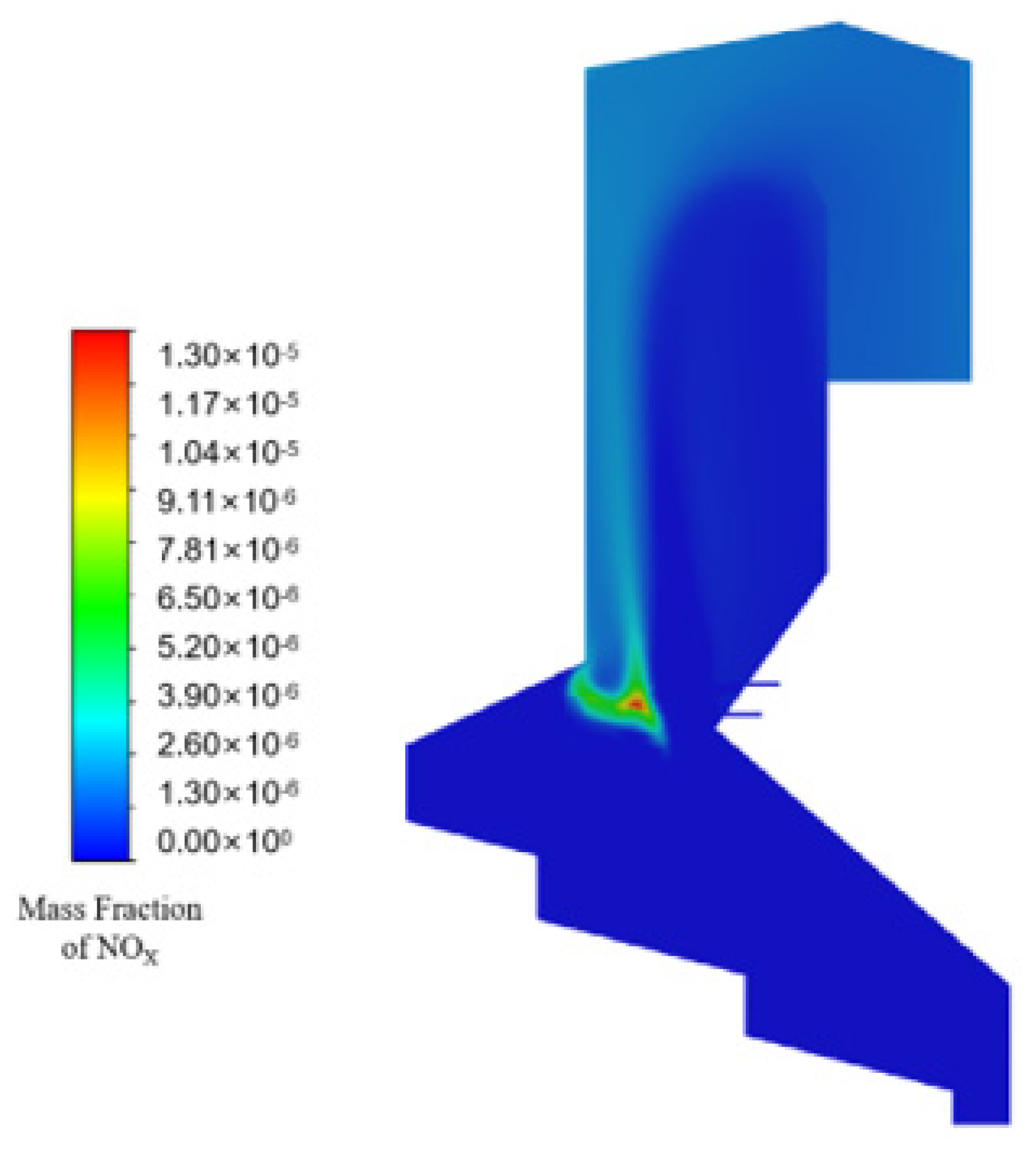
3.4. NOx Distribution in the Furnace without SNCR Addition
3.5. NOx Distribution in Furnace after Using SNCR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | predigital factor, s−1 |
| As | particle surface area, m2 |
| Avol | pre-exponential factor from volatilization analysis, 1/s |
| Cfuel | volume fraction of combustible gas, % |
| Cmix | empirical constant of gas mixing |
| CO2 | volume fraction of oxygen, % |
| Cvol,s | volatile matter concentration (solid phase), kg/m3 |
| Cvol,g | volatile matter concentration (gas phase), kg/m3 |
| Cw,s | water concentration (solid phase), kg/m3 |
| Cw,g | water concentration (gas phase), kg/m3 |
| D | mass diffusion coefficients, m2/s |
| Dg | gas phase diffusion coefficient, m2/s |
| dp | particle diameter, m |
| E | activation energy, J/mol |
| Evol | volatile activation energy, J/mol |
| Hevap | latent heat of water evaporation, J/kg |
| Hg | gas phase enthalpy, J/kg |
| Hs | solid phase enthalpy, J/kg |
| hs | convective mass transfer coefficient, W/(m2·K) |
| h’ | convective heat transfer coefficient, W/(m2·K) |
| convective heat transfer coefficient between solid and gas, W/(m2·K) | |
| thermal effects of reactions or kth processes, W/m3 | |
| k | reaction rate |
| kvol | volatilization analyzed rate constant, 1/s |
| pg | gas pressure, Pa |
| R | general gas constant, J/(mol·K) |
| Rkin | kinetic reaction rate of volatile combustion, kmol/(m3·s) |
| RM | the rate of water evaporation, kg/(m3·s) |
| Rmix | mixing rate of volatile matter, kmol/(m3·s) |
| Rvol | volatilization analytical rate, kg/(m3·s) |
| T | flame temperature, k |
| Tenv | radiation temperature in furnace, K |
| Tg | gas temperature, K |
| Ts | solid temperature, K |
| Sa | particle surface area, m2/m3 |
| Sfuel | stoichiometric coefficient of combustible gases |
| Sjg | mass source term of gaseous species, kg/m3s |
| Sjs | mass sources due to evaporation, devolatilization and combustion, kg/m3s |
| Sis | source term, kg/m3s |
| SO2 | stoichiometric coefficient of oxygen |
| Ss | mass loss rate from solid, kg m−3 s−1 |
| Ub | bed movement speed, m/s |
| Ug | gas velocity in the direction of the parallel grate component, m/s |
| Vs | perpendicular to bed movement speed, m/s |
| Vg | gas velocity in the direction of the vertical grate component, m/s |
| x | grate length direction, m |
| y | perpendicular to the length of the grate, m |
| Yis | mass fractions of particle compositions (moisture, volatile, fixed carbon and ash) |
| Yjg | mass fractions for gaseous species |
| t | time, s |
| ρsb | solid bulk density in the bed, kg/m3 |
| ρg | gas density, kg/m3 |
| ρM | the density of remaining water in a solid, kg/m3 |
| ρvol | residual volatile matter density, kg/m3 |
| ϕ | bed porosity |
| λ | thermal conductivity, W/(m·K) |
| εs | material particle emissivity |
| σb | Stefan-Boltzmann constant, 5.67 × 10−8 W/(m2·K4) |
References
- Sharma, K.D.; Jain, S. Municipal solid waste generation, composition, and management: The global scenario. Soc. Responsib. J. 2020, 16, 917–948. [Google Scholar] [CrossRef]
- Xu, J.; Liao, Y.; Yu, Z.; Cai, Z.; Ma, X.; Dai, M.; Fang, S. Co-combustion of paper sludge in a 750t/d waste incinerator and effect of sludge moisture content: A simulation study. Fuel 2018, 217, 617–625. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Nasrollahi, V.R.; Darajeh, N.; Lotfi Ghahroud, M.; Mehranzamir, K. Review on Waste-to-Energy Approaches toward a Circular Economy in Developed and Developing Countries. Processes 2023, 11, 2566. [Google Scholar] [CrossRef]
- Makarichi, L.; Jutidamrongphan, W.; Techato, K.A. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821. [Google Scholar] [CrossRef]
- Wang, J.; Lou, H.H.; Yang, F.; Cheng, F. Numerical simulation of a decoupling and Re-burning combinative Low-NOx coal grate boiler. J. Clean. Prod. 2018, 188, 977–988. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Wang, J.; Zuo, X.; Kao, H. Numerical Simulation of Selective Non-Catalytic Reduction Denitrification Process in Precalciner and the Effect of Natural Gas Injection on Denitrification. Processes 2023, 11, 1377. [Google Scholar] [CrossRef]
- Gehrmann, H.-J.; Jaeger, B.; Wirtz, S.; Scherer, V.; Aleksandrov, K.; Hauser, M.; Stapf, D.; Pollmeier, G.; Danz, P. Oscillating Combustion—Primary Measure to Reduce Nitrogen Oxide in a Grate Furnace–Experiments and Simulations. Processes 2021, 9, 2210. [Google Scholar]
- Hu, Z.; Jiang, E.; Ma, X. Numerical simulation on operating parameters of SNCR process in a municipal solid waste incinerator. Fuel 2019, 245, 160–173. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liao, Y.; Ma, X.; Zhou, J. Effects of air supply optimization on NOx reduction in a structurally modified municipal solid waste incinerator. Appl. Therm. Eng. Des. Process. Equip. Econ. 2022, 201, 117706. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Tan, Y.; Yang, Y.; Yang, J.; Tan, D. Fluid-induced vibration evolution mechanism of multiphase free sink vortex and the multi-source vibration sensing method. Mech. Syst. Signal Process. 2023, 189, 110058. [Google Scholar] [CrossRef]
- Chen, J.; Ge, M.; Li, L.; Zheng, G. Material Transport and Flow Pattern Characteristics of Gas–Liquid–Solid Mixed Flows. Processes 2023, 11, 2254. [Google Scholar] [CrossRef]
- Hu, Z.; Jiang, E.; Ma, X. Numerical simulation on NOX emissions in a municipal solid waste incinerator. J. Clean. Prod. 2019, 233, 650–664. [Google Scholar] [CrossRef]
- Yan, M.; Wang, J.; Hantoko, D.; Kanchanatip, E. Numerical investigation of MSW combustion influenced by air preheating in a full-scale moving grate incinerator. Fuel 2021, 285, 119193. [Google Scholar] [CrossRef]
- Yang, Y.B.; Goh, Y.R.; Zakaria, R.; Nasserzadeh, V.; Swithenbank, J. Mathematical modelling of MSW incineration on a travelling bed. Waste Manag. 2002, 22, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Li, J.; Wu, T.; Chen, C.; Zhang, X. CFD simulation of MSW combustion and SNCR in a commercial incinerator. Waste Manag. 2014, 34, 1609–1618. [Google Scholar] [CrossRef]
- Qi, X.; Ma, X.Q.; Yu, Z.S.; Huang, Z.G.; Teng, W. Numerical simulation of municipal waste and food digestate blending combustion and NOx reduction under oxygen-enriched atmospheres. Fuel 2023, 345, 128115. [Google Scholar] [CrossRef]
- Fluent. 6.3, Manual; Fluent Inc.: Lebanon, NH, USA, 2005. [Google Scholar]
- Yang, Y.B.; Newman, R.; Sharifi, V. Mathematical modelling of straw combustion in a 38MWe power plant furnace and effect of operating conditions. Fuel 2007, 86, 129–142. [Google Scholar] [CrossRef]
- Yang, Y.B.; Sharifi, V.N.; Swithenbank, J. Converting moving-grate incineration from combustion to gasification—Numerical simulation of the burning characteristics. Waste Manag. 2007, 27, 645–655. [Google Scholar] [CrossRef]
- Costa, M.; Massarotti, N.; Mauro, A.; Arpino, F.; Rocco, V. CFD modelling of a RDF incineration plant. Appl. Therm. Eng. 2016, 101, 710–719. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, A.; Xu, M.; Yang, W.; Siah, K.B.; Subbaiah, P. NOx reduction in a 40 t/h biomass fired grate boiler using internal flue gas recirculation technology. Appl. Energy 2018, 220, 962–973. [Google Scholar] [CrossRef]

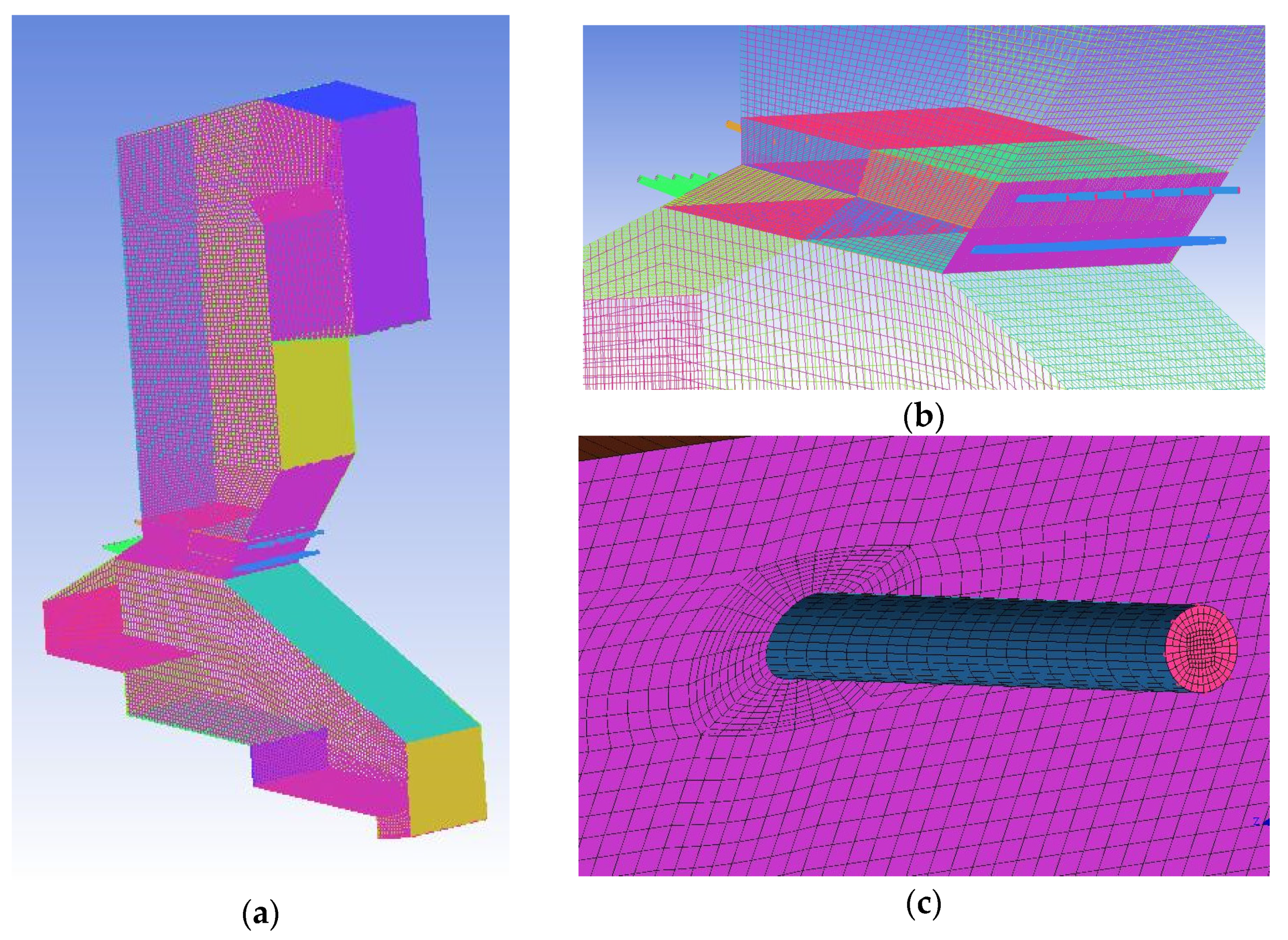




| Ultimate Analysis (wt%) | MSW | Proximate Analysis (wt%) | MSW |
|---|---|---|---|
| Carbon | 56.9 | Moisture | 45.32 |
| Hydrogen | 8.75 | Volatile | 25.36 |
| Oxygen | 32.37 | Fixed carbon | 11.46 |
| Nitrogen | 0.38 | Ash | 17.86 |
| Sulfur | 0.41 | LHVar | 7.98 MJ/kg |
| Ultimate Analysis (wt%) | Mcr Case | Proximate Analysis (wt%) | MCR Case |
|---|---|---|---|
| Carbon | 55.39 | Moisture | 49.2 |
| Hydrogen | 10.98 | Volatile | 27.16 |
| Oxygen | 30.59 | Fixed carbon | 5.43 |
| Nitrogen | 1.66 | Ash | 18.21 |
| Sulfur | 1.35 | LHVar | 7536 kJ/kg |
| Combustion Model in the Furnace | Solution Method | |
|---|---|---|
| Turbulence | Realizable k-ε | SIMPLE |
| Radiation | P-1 | |
| Interaction between turbulence and chemicals | FR/ED | Second-order upwind |
| Point | Item | Simulation Results (K) | Actual Result (K) | Error (%) |
|---|---|---|---|---|
| T1 | Flue gas temperature in the lower part of the furnace | 1151.02 | 1162.43 | 0.98 |
| T2 | Flue gas temperature in the middle of the furnace | 990.69 | 972.07 | 1.92 |
| T3 | Flue gas temperature at the top of the furnace | 1021.33 | 1031.90 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Jin, Y.; Song, X.; Wang, Z.; Liu, B.; Wu, Y. Computational Fluid Dynamics Simulation of Combustion and Selective Non-Catalytic Reduction in a 750 t/d Waste Incinerator. Processes 2023, 11, 2790. https://doi.org/10.3390/pr11092790
Cao H, Jin Y, Song X, Wang Z, Liu B, Wu Y. Computational Fluid Dynamics Simulation of Combustion and Selective Non-Catalytic Reduction in a 750 t/d Waste Incinerator. Processes. 2023; 11(9):2790. https://doi.org/10.3390/pr11092790
Chicago/Turabian StyleCao, Hai, Yan Jin, Xiangnan Song, Ziming Wang, Baoxuan Liu, and Yuxin Wu. 2023. "Computational Fluid Dynamics Simulation of Combustion and Selective Non-Catalytic Reduction in a 750 t/d Waste Incinerator" Processes 11, no. 9: 2790. https://doi.org/10.3390/pr11092790
APA StyleCao, H., Jin, Y., Song, X., Wang, Z., Liu, B., & Wu, Y. (2023). Computational Fluid Dynamics Simulation of Combustion and Selective Non-Catalytic Reduction in a 750 t/d Waste Incinerator. Processes, 11(9), 2790. https://doi.org/10.3390/pr11092790





