Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review
Abstract
:1. Introduction
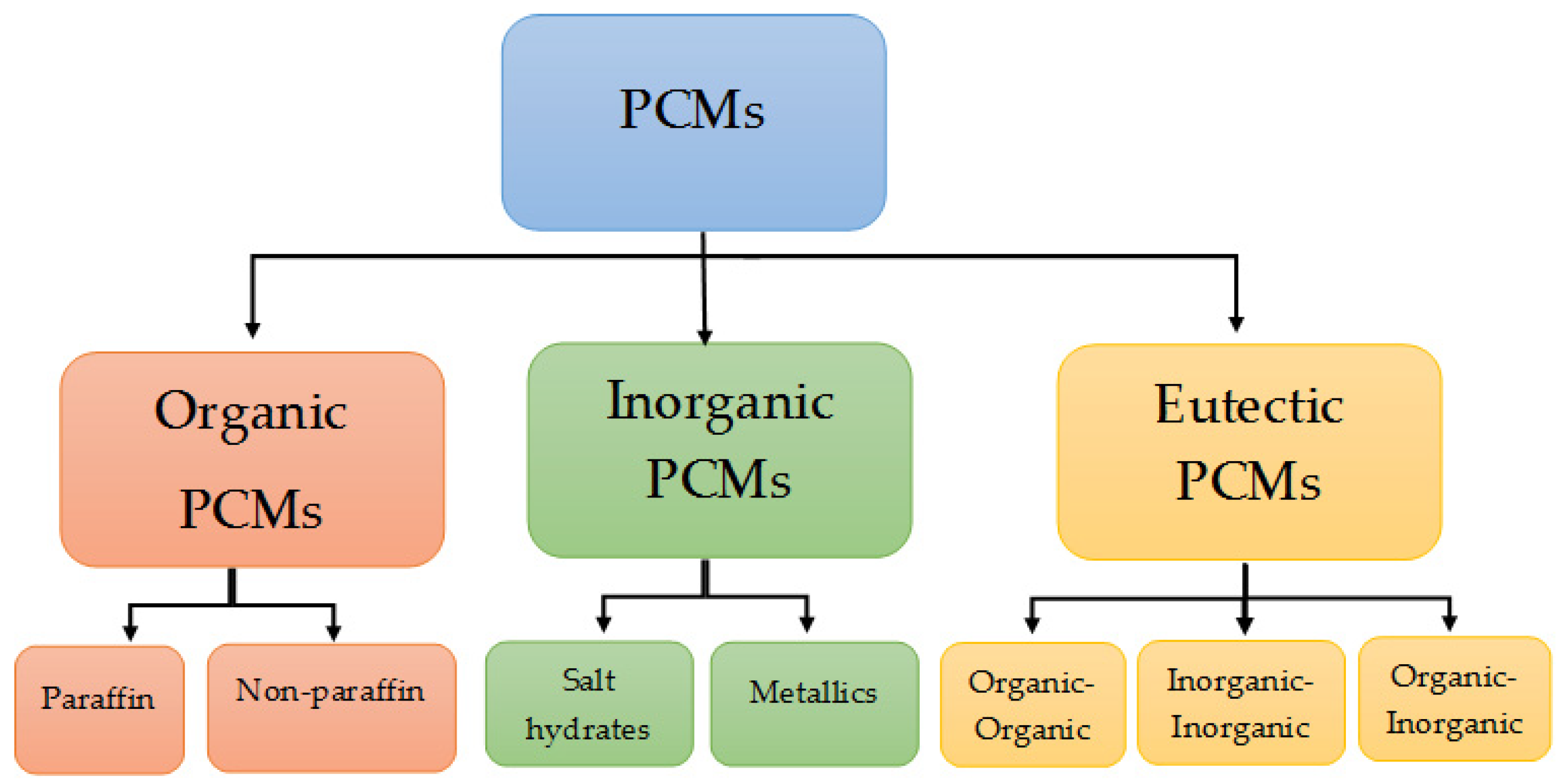
- Organic PCMs: These are typically made up of paraffins, fatty acids, and esters, among other organic substances. They have benefits including inexpensive price, high latent heat, and adaptability to different applications.
- Inorganic PCMs: These are made of inorganic components such metals, non-metals, and salt hydrates. Inorganic PCMs are renowned for their stability and strong heat-storage capacity and frequently exhibit high thermal conductivity.
- Eutectic mixtures are made up of two or more materials that have a melting point that is lower than the sum of their parts. Eutectic PCMs are advantageous due to their high heat-storage capacity and sharp melting point.
2. Nanoencapsulated PCMs (NEPCMs)
- Applications related to heat transfer require the use of suspensions that are stable during pumping and flowing. Therefore, it is more interesting to use nanoencapsulated PCMs, which have higher suspension stability, super-high specific surface area, minimal pump breakage, and promising structures in terms of management and storage of energy.
- PCMs can release or store heat by exchanging it with the surrounding environment. Indeed, these exchanges are more important and faster when the size of the PCM particles is reduced (high surface area to volume ratio).
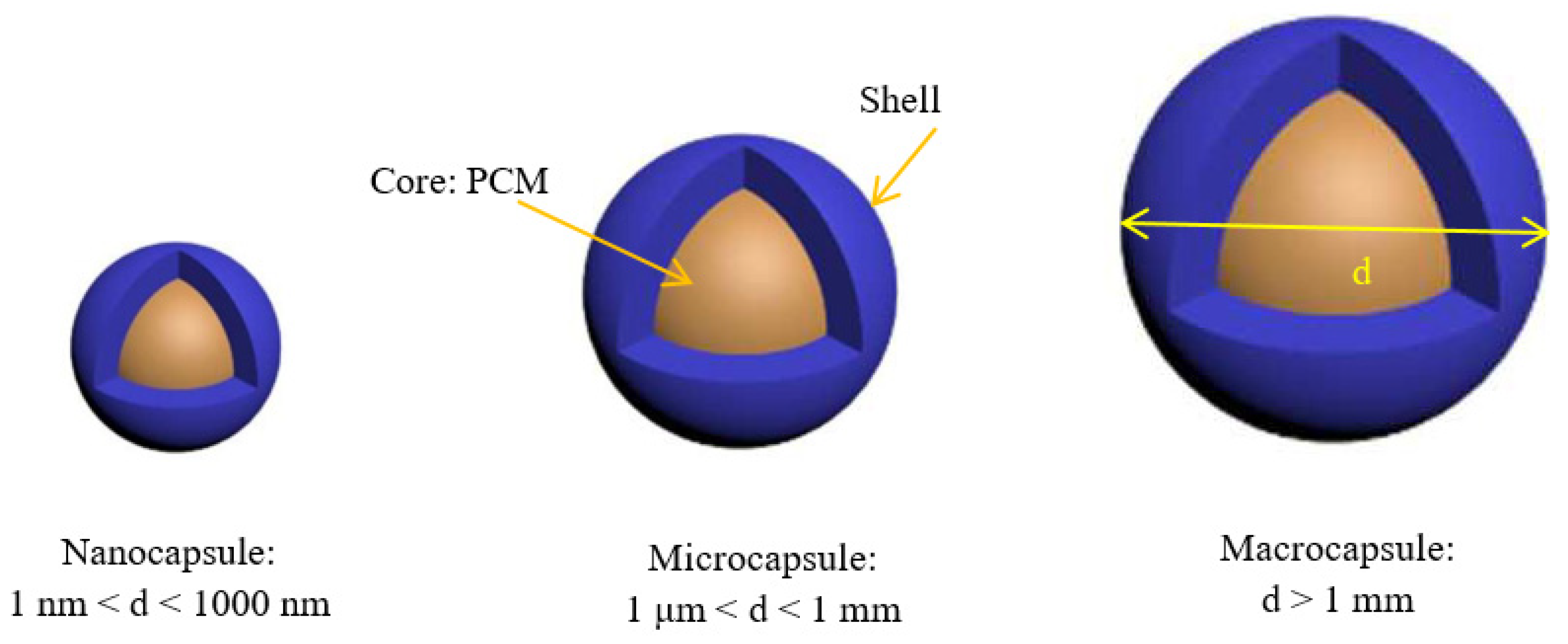
- Compared to the microencapsulation process, the nanoencapsulation of PCMs is expected to result in the fabrication of energy-storage systems with higher characteristics, and the generation of more heat transfer in the system.
2.1. Preparation of NEPCMs
2.1.1. In-Situ Polymerization
Suspension Polymerization
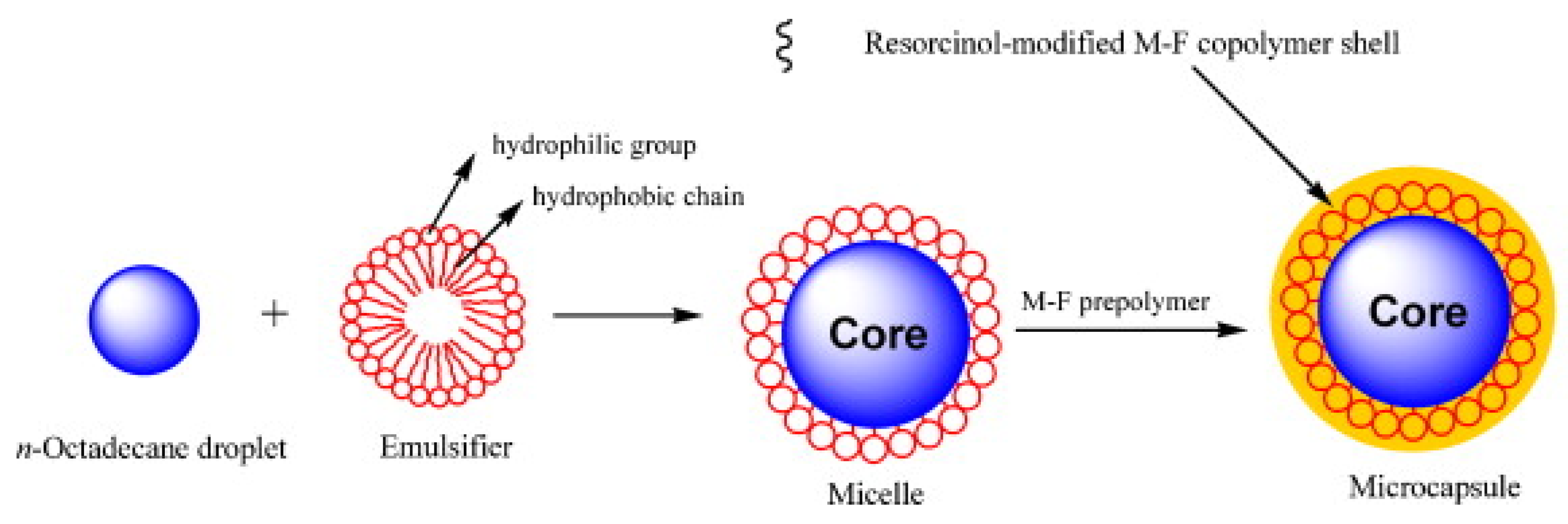
Emulsion/Mini-Emulsion Polymerization
Interfacial Polymerization
2.1.2. Physicochemical Techniques
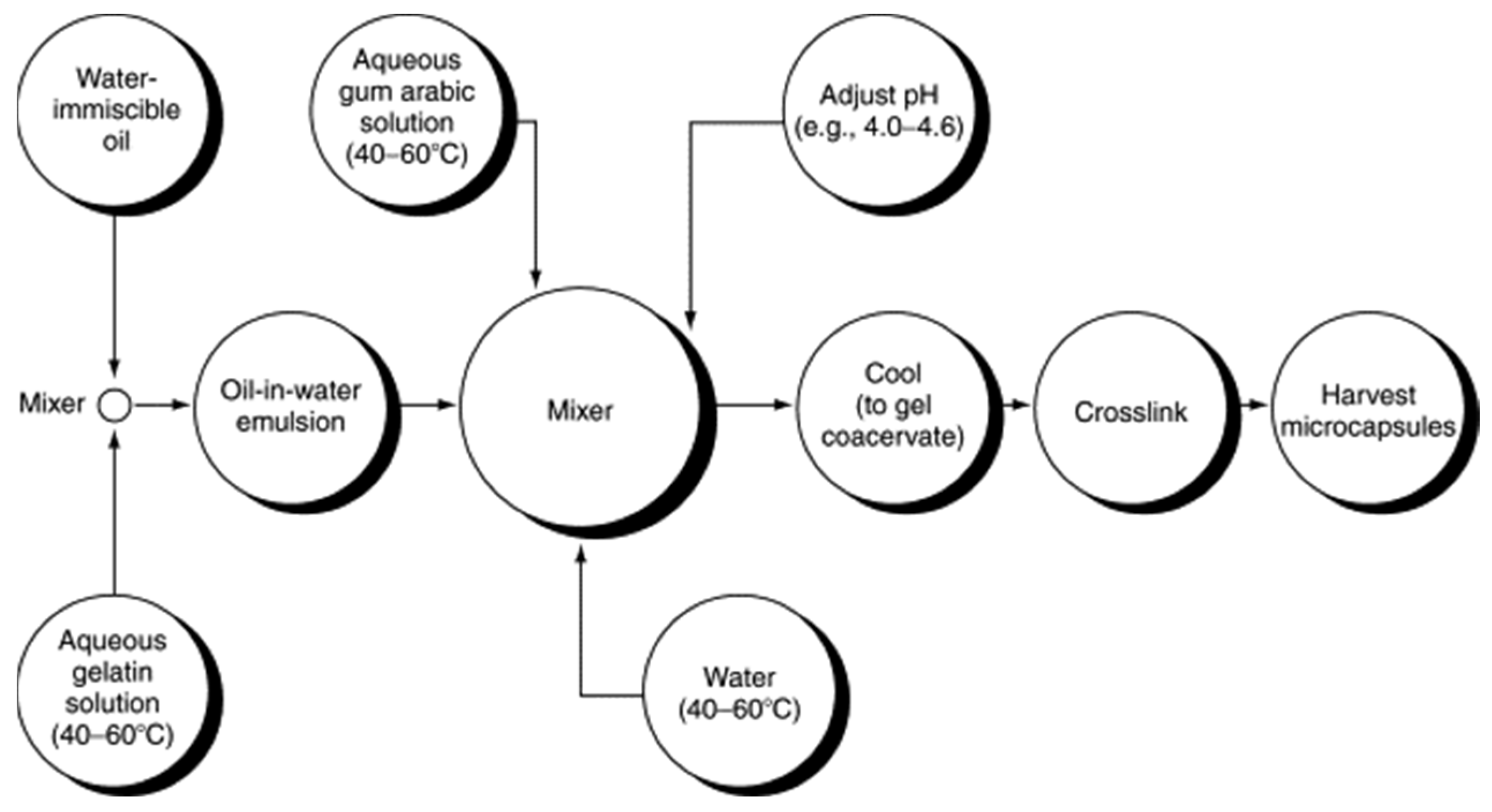
Coacervation
Sol–Gel
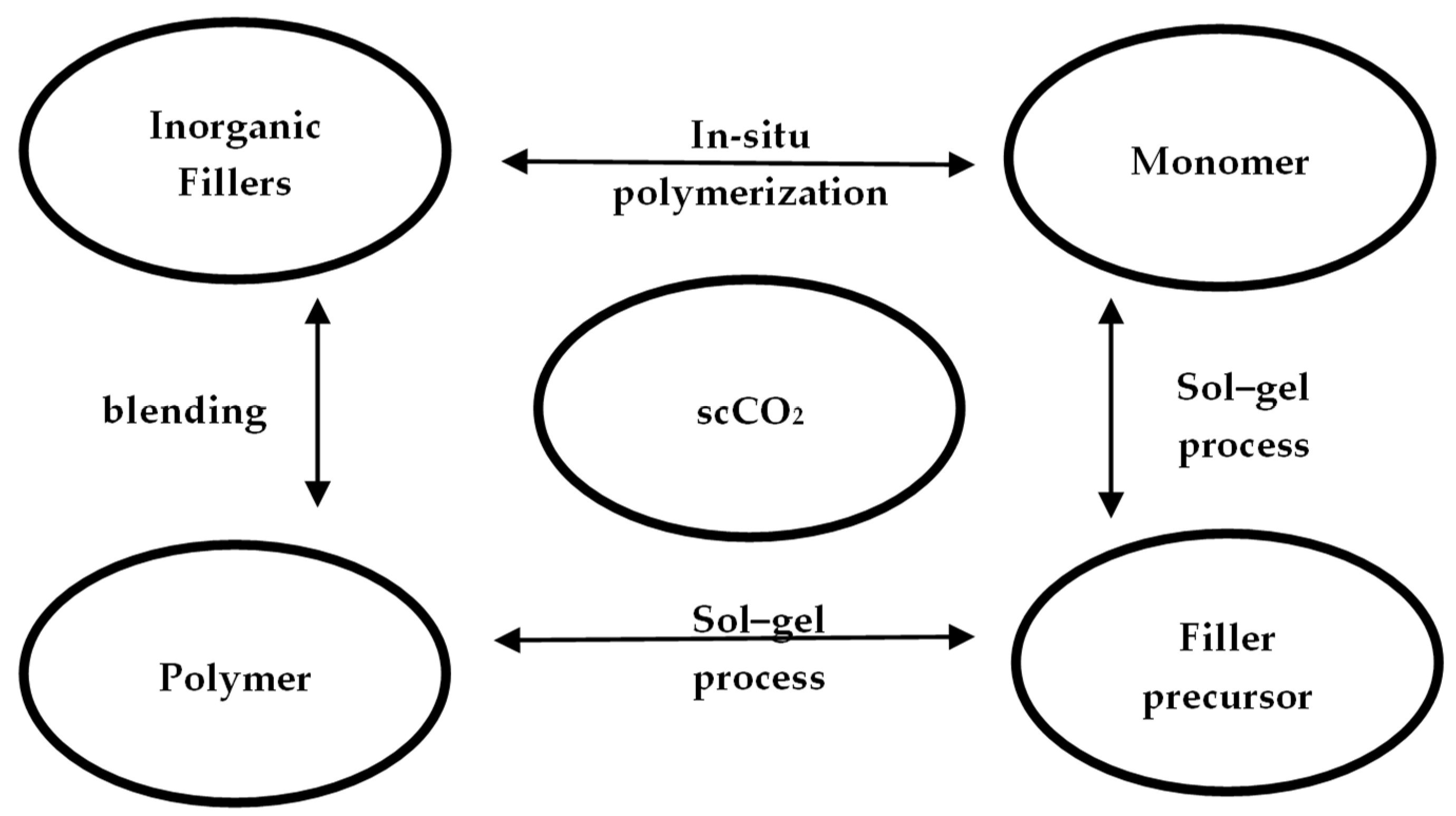
Supercritical CO2-Assisted Encapsulation
2.1.3. Physicomechanical Techniques
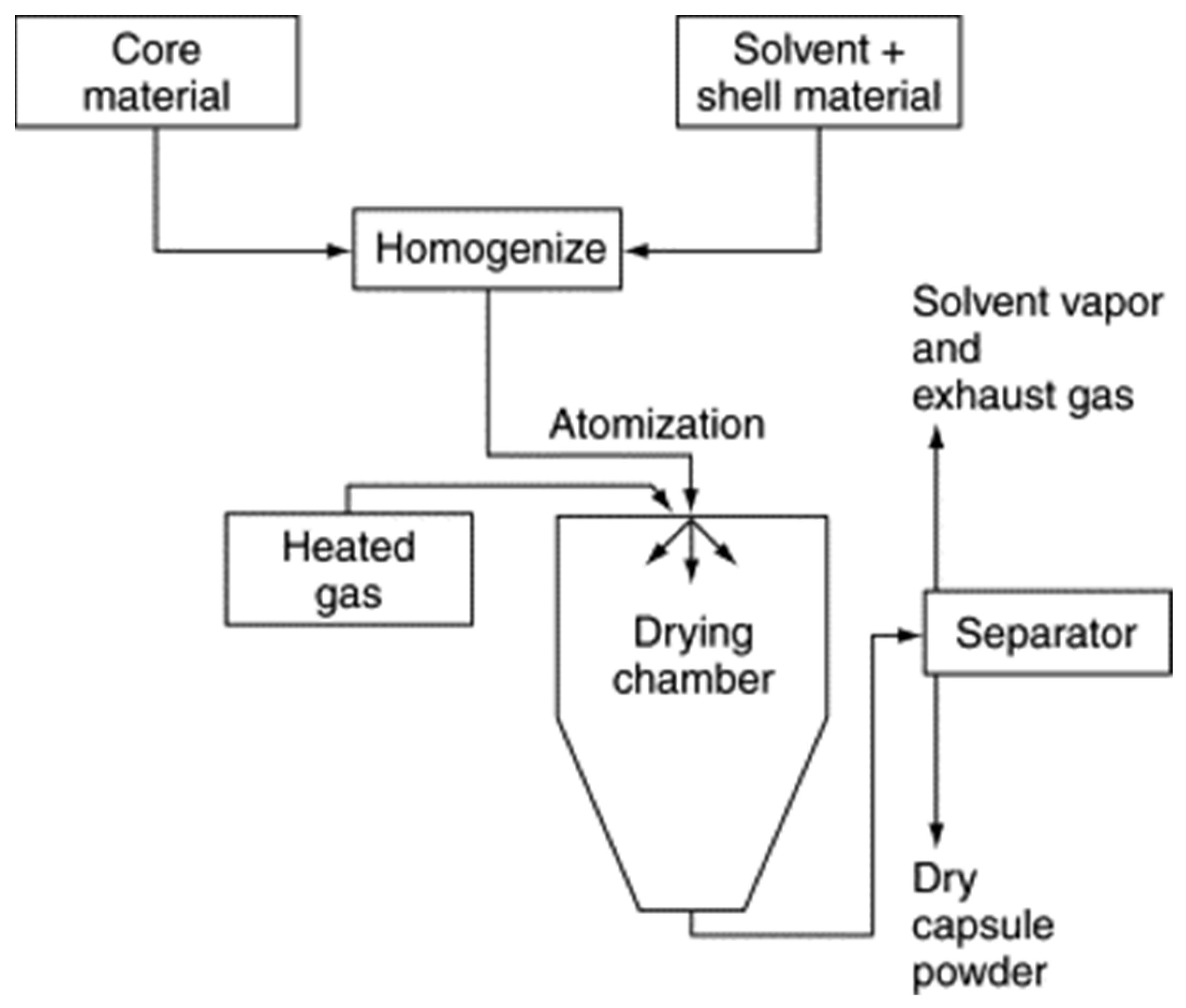
Spray-Drying Techniques
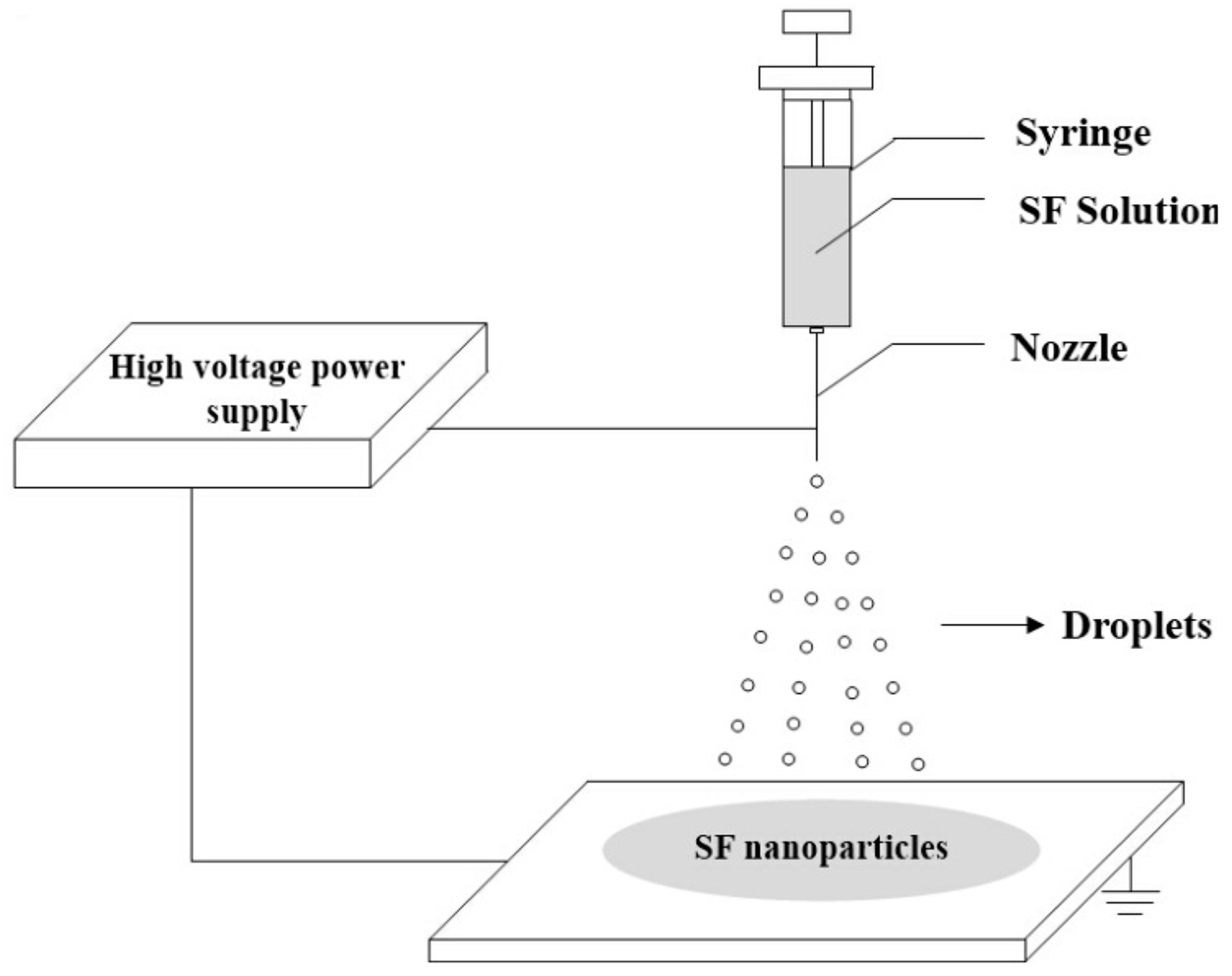
Electrohydrodynamic Process
2.2. Applications of NEPCMs
- Solid-to-liquid phase transition;
- Large amount of energetic changes;
- Stabilization of temperature;
- Variation in thermal conduction during phase transition.
2.2.1. Thermal Management of Electronic Devices
2.2.2. Food Industry
2.2.3. Thermal Storage in Buildings
- Active TES systems: heat transfer is generated by forced convection and, in some cases, also by mass transfer such as with a heat exchanger [81,82]. Active PCM-based systems require mechanical equipment or an additional power source for their operation, such as electricity for pumps or fans. These systems are best suited to situations where greater heat-transfer performance or better application control is required.
- Passive TES systems: the employed PCMs exploit naturally available energy sources (for example, solar power or wind) as well as the architecture of building components to minimize energy requirements [83]. Passive systems reduce the use of mechanical heating or cooling systems. There is no need for additional energy input as heat is charged or discharged when the temperature of the environment rises or falls beyond the phase-change temperature of the PCM. These PCMs can be used in building ceilings; floors; walls; cooling, heating, and hot water systems; etc. [84,85].
2.2.4. Solar Energy Storage
- It is intermittent (day/night);
- It is random (thunderstorms and cloud passages);
- It is diluted and shifted in relation to daily or seasonal energy demands.
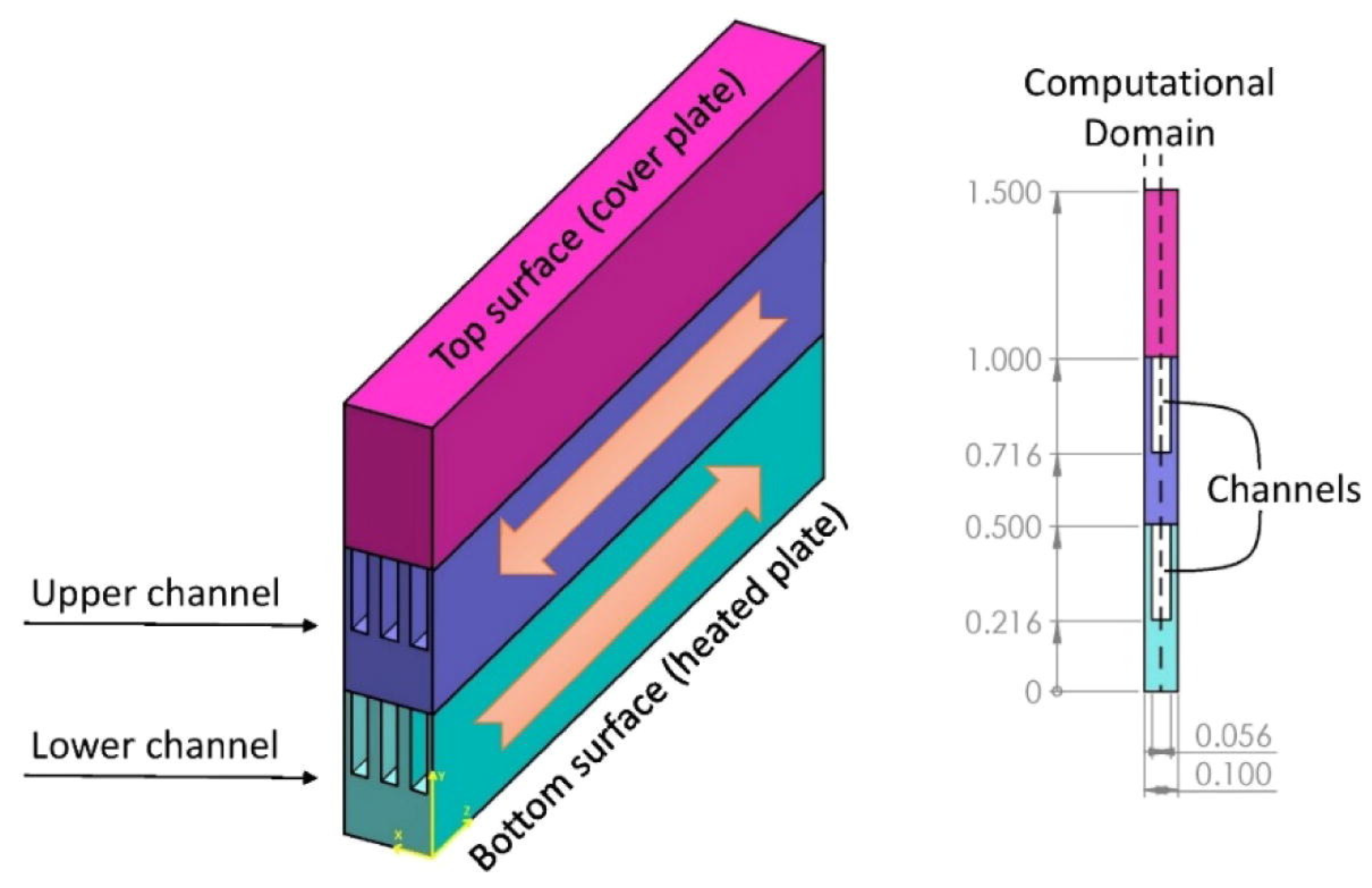
2.2.5. Heat Exchangers
2.2.6. Smarts: Textiles, Clothes, and Footwear
3. Nano-Enhanced PCMs for Thermal Energy Storage Systems
| Authors | Configuration | Used PCM(s) | Used Nanoparticle(s) |
|---|---|---|---|
| Karaagaç et al. [99] |  | paraffin wax | Al2O3 |
| Aqib et al. [106] |  | paraffin wax | Al2O3 and MWCNTs |
| Elarem et al. [107] |  | paraffin wax | copper (Cu) |
| Chen et al. [108] |  | lauric acid | Al2O3 |
| Saeed et al. [109] |  | 2-hydroxypropyl ether cellulose is introduced to stabilize the PCM | nano-graphene platelets (NGPs) |
| Alomair et al. [110] |  | coconut oil biobased in PCM | copper oxide |
| Ali et al. [122] |  | paraffin wax | nano graphene composite |
| Gong et al. [123] |  | 1-octadecanol (OD) | nano-silicon carbide (SiC), expanded graphite (EG) SiC/EG composite |
| Raj et al. [124] | NA: Characterization | manganese organo-metallic SS-PCM | liquid metal gallium |
| Hosseinzadeh et al. [100,101] |  | water | hybrid nanoparticles (HNP) (MoS2–Fe3O4) and (TiO2-Go) |
| Ebadi et al. [119,120] | 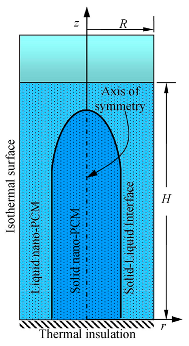 | coconut oil | copper oxide CuO |
| Khan and Khan [118] | 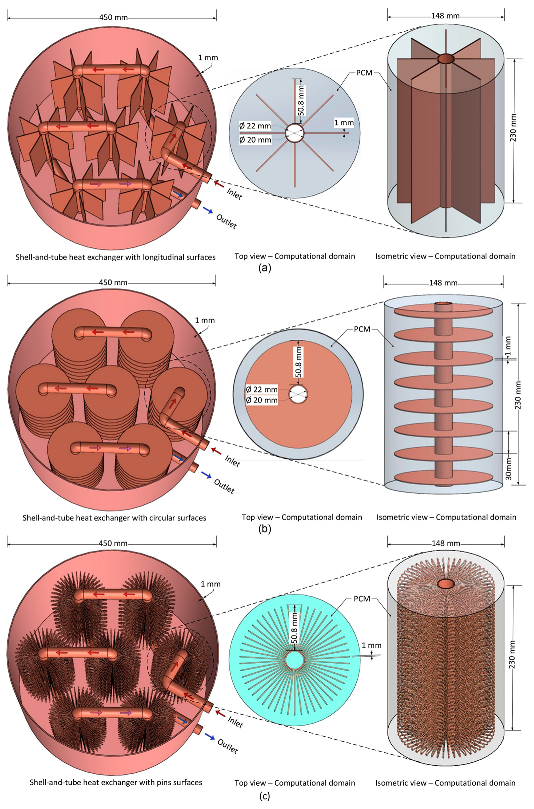 | paraffin | graphene nano-platelets (GNP) |
| Parameshwaran, and Kalaiselvam [116] |  | not specified | silver nanoparticles |
| Al-Jethelah et al. [115] |  | coconut oil | CuO |
| Hosseinzadeh et al. [114] | 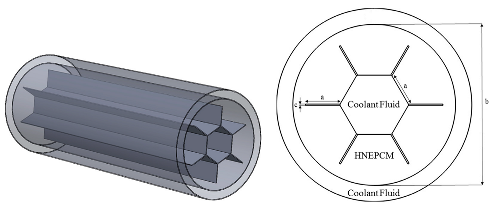 | water | MoS2–TiO2 |
| Kumar and Mylsamy [113] |  | paraffin and NEPCMs | nano-CeO2 |
| Ho et al. [112] | 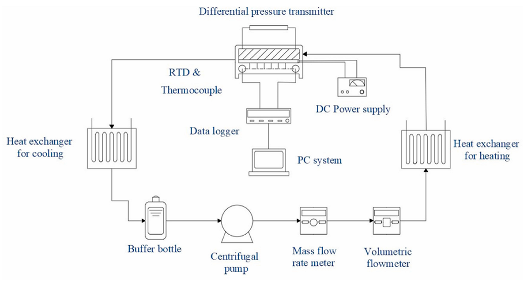 | water | n-eicosane |
| Praveen et al. [111] | 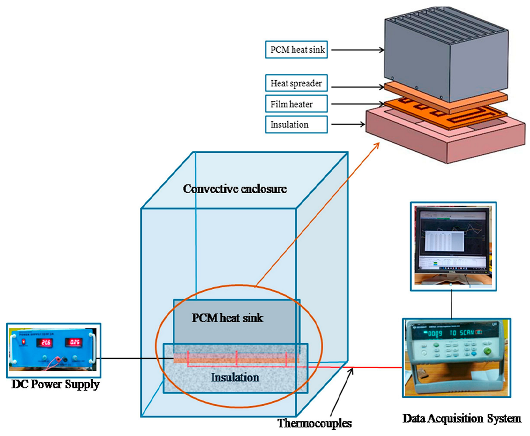 | paraffin (ME/GnP PCM) | graphene nano-platelets (GnP) |
| Li et al. [125] |  | ternary carbonate salt | without nanoparticles: Encapsulated PCM |
| Elbahjaoui et al. [126] | 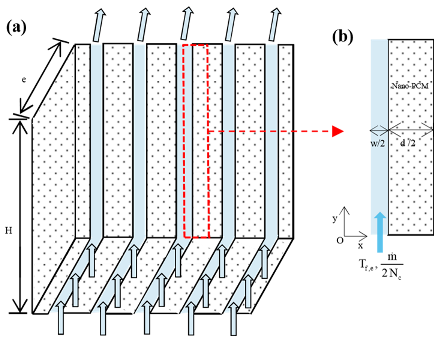 | paraffin wax P116 | copper |
| Kumar et al. [127] |  | PCM | calcium carbonate, silicon carbide, copper |
| Ben Khedher et al. [128] | 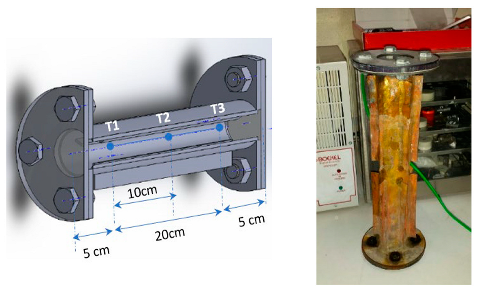 | paraffin wax | CNT, Al2O3 |
| Kolsi et al. [129] |  | Encapsulated Paraffin | CNT |
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | Artificial Neural Network |
| CFD | Computational Fluid Dynamics |
| CPV/TSD | Concentrated Photovoltaic–Thermal Solar Dryer |
| DR | Drying Rate |
| FEM | Finite Element Method |
| GNPs | Graphene Nano-Platelets |
| IEA | International Energy Agency |
| LHS | Latent Heat Storage |
| LHTES | Latent Heat Thermal Energy Storage |
| LTES | Latent Thermal Energy Storage |
| MCHS | Microchannel Heat Sink |
| MePCM | Micro-Encapsulated Phase-Change Material |
| MR | Moisture Ratio |
| MWCNTs | Multiwall Carbon Nanotubes |
| NEPCM | Nanoencapsulated Phase-Change Material |
| NEnPCM | Nano-Enhanced Phase-Change Material |
| NPs | Nanoparticles |
| PCM | Phase-Change Material |
| SEM | Scanning Electron Microscope |
| SF | Silk Fibroin |
| SVM | Support Vector Machines |
| TES | Thermal Energy Storage |
| TESS | Thermal Energy Storage System |
References
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Piselli, C.; Castaldo, V.L.; Pisello, A.L. How to enhance thermal energy storage effect of PCM in roofs with varying solar reflectance: Experimental and numerical assessment of a new roof system for passive cooling in different climate conditions. Sol. Energy 2019, 192, 106–119. [Google Scholar] [CrossRef]
- Sun, X.; Medina, M.A.; Lee, K.O.; Jin, X. Laboratory assessment of residential building walls containing pipe-encapsulated phase change materials for thermal management. Energy 2018, 163, 383–391. [Google Scholar] [CrossRef]
- de Albuquerque Landi, F.F.; Fabiani, C.; Pisello, A.L. Palm oil for seasonal thermal energy storage applications in buildings: The potential of multiple melting ranges in blends of bio-based fatty acids. J. Energy Storage 2020, 29, 101431. [Google Scholar] [CrossRef]
- Mhadhbi, M. Introductory Chapter: Phase Change Material. In Phase Change Materials and Their Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Socaciu, L.G. Thermal energy storage with phase change material. Leonardo Electron. J. Pract. Technol. 2012, 20, 75–98. [Google Scholar]
- Xiao, X.; Zhang, P. Numerical and experimental study of heat transfer characteristics of a shell-tube latent heat storage system: Part I–Charging process. Energy 2015, 79, 337–350. [Google Scholar] [CrossRef]
- Li, S.-F.; Liu, Z.-H.; Wang, X.-J. A comprehensive review on positive cold energy storage technologies and applications in air conditioning with phase change materials. Appl. Energy 2019, 255, 113667. [Google Scholar] [CrossRef]
- Li, B.; Zhai, X.; Cheng, X. Experimental and numerical investigation of a solar collector/storage system with composite phase change materials. Sol. Energy 2018, 164, 65–76. [Google Scholar] [CrossRef]
- Nie, B.; Palacios, A.; Zou, B.; Liu, J.; Zhang, T.; Li, Y. Review on phase change materials for cold thermal energy storage applications. Renew. Sustain. Energy Rev. 2020, 134, 110340. [Google Scholar] [CrossRef]
- Kumar, R.R.; Samykano, M.; Pandey, A.; Kadirgama, K.; Tyagi, V. Phase change materials and nano-enhanced phase change materials for thermal energy storage in photovoltaic thermal systems: A futuristic approach and its technical challenges. Renew. Sustain. Energy Rev. 2020, 133, 110341. [Google Scholar] [CrossRef]
- Khaireldin Faraj, M.K.; Faraj, J.; Hachem, F.; Castelain, C. A Review on Phase Change Materials for Thermal Energy Storage in Buildings: Heating and Hybrid Applications. J. Energy Storage 2021, 33, 101913. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Chang, Y.; Gao, H.; Cheng, P.; Tao, Z.; Lv, J. Toward Tailoring Chemistry of Silica-Based Phase Change Materials for Thermal Energy Storage. Iscience 2020, 23, 101606. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.-N.; Zhou, F. Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Convers. Manag. 2020, 214, 112876. [Google Scholar] [CrossRef]
- Chen, T.; Liu, C.; Mu, P.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Fatty amines/graphene sponge form-stable phase change material composites with exceptionally high loading rates and energy density for thermal energy storage. Chem. Eng. J. 2020, 382, 122831. [Google Scholar] [CrossRef]
- Pakalka, S.; Valančius, K.; Streckienė, G. Experimental comparison of the operation of PCM-based copper heat exchangers with different configurations. Appl. Therm. Eng. 2020, 172, 115138. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Babayan, M.; Chamkha, A. Investigation of using multi-layer PCMs in the tubular heat exchanger with periodic heat transfer boundary condition. Int. J. Heat Mass Transf. 2020, 147, 118970. [Google Scholar] [CrossRef]
- Chen, G.; Sun, G.; Jiang, D.; Su, Y. Experimental and numerical investigation of the latent heat thermal storage unit with PCM packing at the inner side of a tube. Int. J. Heat Mass Transf. 2020, 152, 119480. [Google Scholar] [CrossRef]
- Xu, H.; Wang, N.; Zhang, C.; Qu, Z.; Cao, M. Optimization on the melting performance of triplex-layer PCMs in a horizontal finned shell and tube thermal energy storage unit. Appl. Therm. Eng. 2020, 176, 115409. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Pathak, L.; Trivedi, G.; Parameshwaran, R.; Deshmukh, S.S. Microencapsulated phase change materials as slurries for thermal energy storage: A review. Mater. Today Proc. 2021, 44, 1960–1963. [Google Scholar] [CrossRef]
- Noël, J.A.; White, M.A. Freeze-cast form-stable phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 223, 110956. [Google Scholar] [CrossRef]
- Wu, T.; Hu, Y.; Rong, H.; Wang, C. SEBS-based composite phase change material with thermal shape memory for thermal management applications. Energy 2021, 221, 119900. [Google Scholar] [CrossRef]
- Peng, H.; Wang, J.; Zhang, X.; Ma, J.; Shen, T.; Li, S.; Dong, B. A Review on Synthesis, Characterization and Application of Nanoencapsulated Phase Change Materials for Thermal Energy Storage Systems. Appl. Therm. Eng. 2020, 185, 116326. [Google Scholar] [CrossRef]
- Afgan, S.; Bing, C. Scientometric review of international research trends on thermal energy storage cement based composites via integration of phase change materials from 1993 to 2020. Constr. Build. Mater. 2021, 278, 122344. [Google Scholar] [CrossRef]
- Veismoradi, A.; Ghalambaz, M.; Shirivand, H.; Hajjar, A.; Mohamad, A.; Sheremet, M.; Chamkha, A.; Younis, O. Study of paraffin-based composite-phase change materials for a shell and tube energy storage system: A mesh adaptation approach. Appl. Therm. Eng. 2021, 190, 116793. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.K.; Sharma, V.K. Application of phase change material for thermal energy storage: An overview of recent advances. Mater. Today Proc. 2020, 44, 368–375. [Google Scholar] [CrossRef]
- Sivanathan, A.; Dou, Q.; Wang, Y.; Li, Y.; Corker, J.; Zhou, Y.; Fan, M. Phase change materials for building construction: An overview of nano-/micro-encapsulation. Nanotechnol. Rev. 2020, 9, 896–921. [Google Scholar] [CrossRef]
- Nikpourian, H.; Bahramian, A.R.; Abdollahi, M. On the thermal performance of a novel PCM nanocapsule: The effect of core/shell. Renew. Energy 2020, 151, 322–331. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y. Synthesis and thermal properties of nanoencapsulation of paraffin as phase change material for latent heat thermal energy storage. Energy Built Environ. 2020, 1, 410–416. [Google Scholar] [CrossRef]
- Shchukina, E.; Graham, M.; Zheng, Z.; Shchukin, D. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef] [PubMed]
- Jelle, B.; Kalnæs, S. Phase change materials for application in energy-efficient buildings. Cost-Eff. Energy Effic. Build. Retrofit. 2017, 57–118. [Google Scholar]
- Wen, R.; Zhang, X.; Huang, Y.; Yin, Z.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X. Preparation and properties of fatty acid eutectics/expanded perlite and expanded vermiculite shape-stabilized materials for thermal energy storage in buildings. Energy Build. 2017, 139, 197–204. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Qin, S.-Y.; Tsui, G.C.; Tang, C.-Y.; Ouyang, X.; Liu, J.-h.; Tang, G.N.; Zuo, J.D. Fabrication, morphology and thermal properties of octadecylamine-grafted graphene oxide-modified phase-change microcapsules for thermal energy storage. Compos. Part B Eng. 2019, 157, 239–247. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol. Energy Mater. Sol. Cells 2012, 98, 283–293. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Xu, L.; Xie, H.; Guo, Z. Preparation and thermal characterization of n-octadecane/pentafluorostyrene nanocapsules for phase-change energy storage. J. Energy Storage 2021, 35, 102327. [Google Scholar] [CrossRef]
- Harkins, W.D. A general theory of emulsion polymerization. J. Am. Chem. Soc. 1947, 69, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.T.; Neto, W.S.; Ferreira, G.R.; Glenn, A.F.; Gambetta, R.; Gonçalves, S.B.; Valadares, L.F.; Machado, F. 8—Synthesis of Polymer/Inorganic Hybrids through Heterophase Polymerizations. In Recent Developments in Polymer Macro, Micro and Nano Blends; Visakh, P.M., Markovic, G., Pasquini, D., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 207–235. [Google Scholar]
- Cho, J.-S.; Kwon, A.; Cho, C.-G. Microencapsulation of octadecane as a phase-change material by interfacial polymerization in an emulsion system. Colloid Polym. Sci. 2002, 280, 260–266. [Google Scholar] [CrossRef]
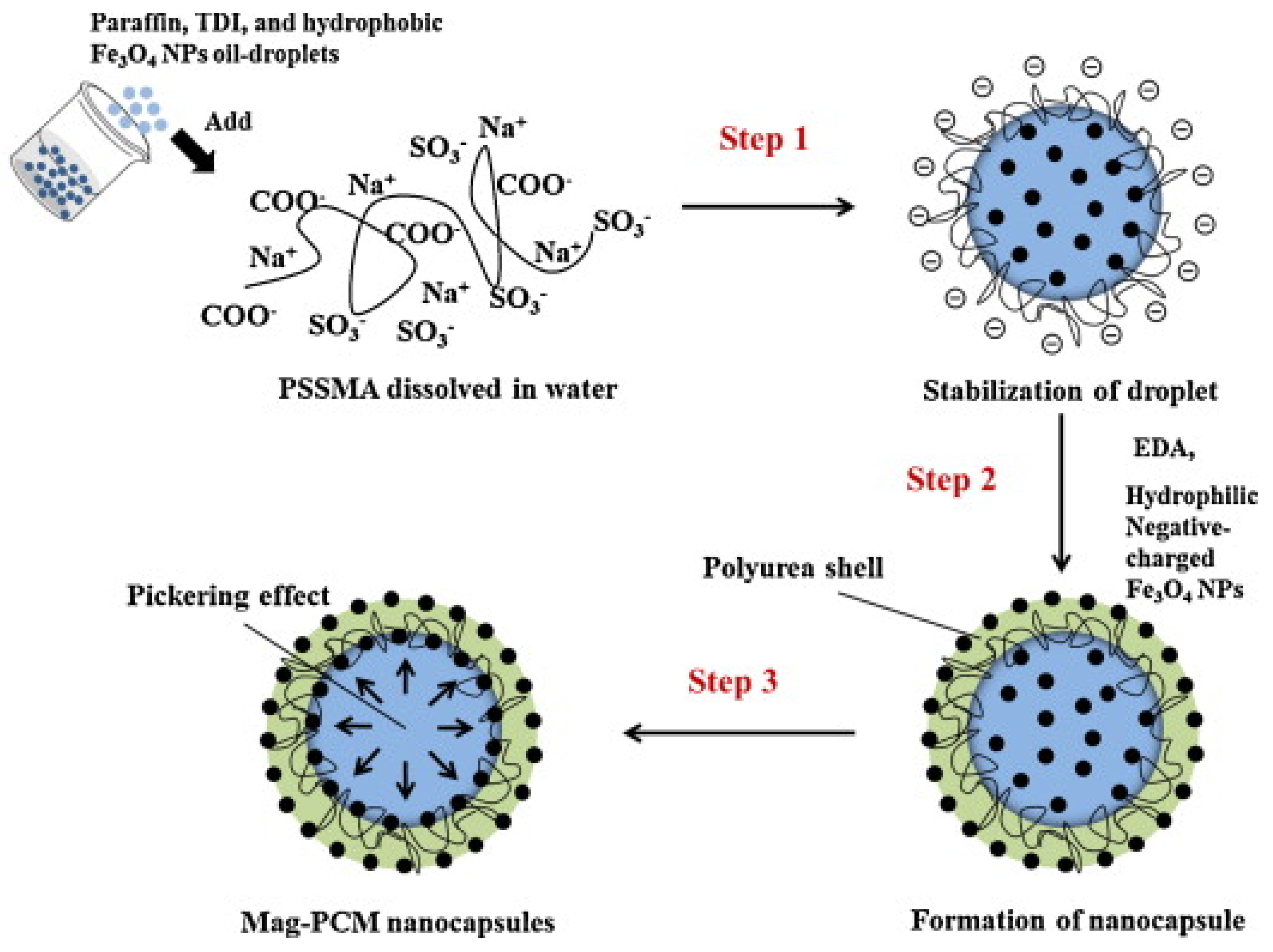
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Barlak, S.; Sara, O.N.; Karaipekli, A.; Yapıcı, S. Thermal conductivity and viscosity of nanofluids having nanoencapsulated phase change material. Nanoscale Microscale Thermophys. Eng. 2016, 20, 85–96. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Thies, C. Microcapsules, Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: New York, NY, USA, 2003; pp. 3892–3903. ISBN 9780122270550. [Google Scholar]
- Yuan, H.; Bai, H.; Lu, X.; Zhao, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol. Energy 2019, 185, 374–386. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Fu, X.; Sun, R. Synthesis and thermal properties of a novel nanoencapsulated phase change material with PMMA and SiO2 as hybrid shell materials. Thermochim. Acta 2015, 617, 90–94. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C.; Henry, M.; Doeuff, S. The chemistry of the sol-gel process. Solid State Ionics 1989, 32–33, 633–638. [Google Scholar] [CrossRef]
- Jessop, P.G.; Leitner, W. Chemical Synthesis Using Supercritical Fluids; Wiley Online Library: Hoboken, NJ, USA, 1999. [Google Scholar]
- Haldorai, Y.; Shim, J.-J.; Lim, K.T. Synthesis of polymer–inorganic filler nanocomposites in supercritical CO2. J. Supercrit. Fluids 2012, 71, 45–63. [Google Scholar] [CrossRef]
- Ghosh, S.K. Functional coatings and microencapsulation: A general perspective. In Functional Coatings: By Polymer Microencapsulation; Wiley: Hoboken, NJ, USA, 2006; pp. 1–28. [Google Scholar]
- Fei, B.; Lu, H.; Qi, K.; Shi, H.; Liu, T.; Li, X.; Xin, J.H. Multi-functional microcapsules produced by aerosol reaction. J. Aerosol Sci. 2008, 39, 1089–1098. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Borreguero, A.; Valverde, J.; Rodríguez, J.; Barber, A.; Cubillo, J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm® RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390. [Google Scholar] [CrossRef]
- Perez-Masia, R.; Lopez-Rubio, A.; Fabra, M.J.; Lagaron, J.M. Biodegradable polyester-based heat management materials of interest in refrigeration and smart packaging coatings. J. Appl. Polym. Sci. 2013, 130, 3251–3262. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of solvents for the encapsulation of a phase change material in polymeric matrices by electro-hydrodynamic processing of interest in temperature buffering food applications. Eur. Polym. J. 2015, 72, 23–33. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Development of polystyrene-based films with temperature buffering capacity for smart food packaging. J. Food Eng. 2015, 164, 55–62. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of phase change materials to develop electrospun coatings of interest in food packaging applications. J. Food Eng. 2017, 192, 122–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Xu, S.; Yu, Y.; Hussain, A.; Shen, Y.; Guo, S. Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. J. Mater. Chem. B 2017, 5, 5464–5472. [Google Scholar] [CrossRef] [PubMed]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Tuckerman, D.; Pease, R. IIIB-8 implications of high performance heat sinking for electron devices. IEEE Trans. Electron Devices 1981, 28, 1230–1231. [Google Scholar] [CrossRef]
- Fleischer, A.S. Thermal Energy Storage Using Phase Change Materials: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Mudawar, I. Assessment of high-heat-flux thermal management schemes. IEEE Trans. Compon. Packag. Technol. 2001, 24, 122–141. [Google Scholar] [CrossRef]
- Rajabifar, B. Enhancement of the performance of a double layered microchannel heatsink using PCM slurry and nanofluid coolants. Int. J. Heat Mass Transf. 2015, 88, 627–635. [Google Scholar] [CrossRef]
- Ho, C.J.; Liu, Y.-C.; Ghalambaz, M.; Yan, W.-M. Forced convection heat transfer of Nano-Encapsulated Phase Change Material (NEPCM) suspension in a mini-channel heatsink. Int. J. Heat Mass Transf. 2020, 155, 119858. [Google Scholar] [CrossRef]
- Krishna, J.; Kishore, P.; Solomon, A.B. Experimental Study of Thermal Energy Storage Characteristics Using Heat Pipe with Nano-Enhanced Phase Change Materials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012058. [Google Scholar]
- Lu, W.; Tassou, S. Characterization and experimental investigation of phase change materials for chilled food refrigerated cabinet applications. Appl. Energy 2013, 112, 1376–1382. [Google Scholar] [CrossRef]
- Oró, E.; Miro, L.; Farid, M.M.; Cabeza, L.F. Thermal analysis of a low temperature storage unit using phase change materials without refrigeration system. Int. J. Refrig. 2012, 35, 1709–1714. [Google Scholar] [CrossRef]
- Oró Prim, E.; Miró, L.; Barreneche Güerisoli, C.; Martorell, I.; Farid, M.M.; Cabeza, L.F. Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 2013, 109, 449–453. [Google Scholar]
- McCann, J.T.; Marquez, M.; Xia, Y. Melt coaxial electrospinning: A versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Lett. 2006, 6, 2868–2872. [Google Scholar] [CrossRef]
- Patil, N.D.; Karale, S. Design and Analysis of Phase Change Material based thermal energy storage for active building cooling: A Review. Int. J. Eng. Sci. Technol. 2012, 4, 2502. [Google Scholar]
- Jelle, B.P.; Hynd, A.; Gustavsen, A.; Arasteh, D.; Goudey, H.; Hart, R. Fenestration of today and tomorrow: A state-of-the-art review and future research opportunities. Sol. Energy Mater. Sol. Cells 2012, 96, 1–28. [Google Scholar] [CrossRef]
- Chan, H.-Y.; Riffat, S.B.; Zhu, J. Review of passive solar heating and cooling technologies. Renew. Sustain. Energy Rev. 2010, 14, 781–789. [Google Scholar] [CrossRef]
- Haillot, D.; Goetz, V.; Py, X.; Benabdelkarim, M. High performance storage composite for the enhancement of solar domestic hot water systems: Part 1: Storage material investigation. Sol. Energy 2011, 85, 1021–1027. [Google Scholar] [CrossRef]
- Kalaiselvam, S.; Parameshwaran, R.; Harikrishnan, S. Analytical and experimental investigations of nanoparticles embedded phase change materials for cooling application in modern buildings. Renew. Energy 2012, 39, 375–387. [Google Scholar] [CrossRef]
- Reddy, V.S.; Kaushik, S.; Ranjan, K.; Tyagi, S. State-of-the-art of solar thermal power plants—A review. Renew. Sustain. Energy Rev. 2013, 27, 258–273. [Google Scholar] [CrossRef]
- Shamshirgaran, S.R.; Assadi, M.K.; Sharma, K.V. Application of nanomaterials in solar thermal energy storage. Heat Mass Transf. 2018, 54, 1555–1577. [Google Scholar] [CrossRef]
- Kibria, M.; Anisur, M.; Mahfuz, M.; Saidur, R.; Metselaar, I. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.; Ni, Z.; Zhang, C.; Lu, C. Synthesis of novel microencapsulated phase change materials with copper and copper oxide for solar energy storage and photo-thermal conversion. Sol. Energy Mater. Sol. Cells 2018, 179, 87–94. [Google Scholar] [CrossRef]
- Hammou, Z.A.; Lacroix, M. A hybrid thermal energy storage system for managing simultaneously solar and electric energy. Energy Convers. Manag. 2006, 47, 273–288. [Google Scholar] [CrossRef]
- Solé, C.; Medrano, M.; Castell, A.; Nogués, M.; Mehling, H.; Cabeza, L. Energetic and exergetic analysis of a domestic water tank with phase change material. Int. J. Energy Res. 2008, 32, 204–214. [Google Scholar] [CrossRef]
- Rupp, J. Interactive textiles regulate body temperature. Int. Text. Bull. 1999, 45, 58–59. [Google Scholar]
- Wang, Y.; Cui, Y.; Shao, Z.; Gao, W.; Fan, W.; Liu, T.; Bai, H. Multifunctional polyimide aerogel textile inspired by polar bear hair for thermoregulation in extreme environments. Chem. Eng. J. 2020, 390, 124623. [Google Scholar] [CrossRef]
- Pan, N.; Sun, G. Functional Textiles for Improved Performance, Protection and Health; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Cabeza, L.; Martorell, I.; Miró, L.; Fernández, A.; Barreneche, C. Introduction to Thermal Energy Storage (TES) Systems. In Advances in Thermal Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–28. [Google Scholar]
- Karthikeyan, M.; Ramachandran, T.; Shanmugasundaram, O. Synthesis, characterization, and development of thermally enhanced cotton fabric using nanoencapsulated phase change materials containing paraffin wax. J. Text. Inst. 2014, 105, 1279–1286. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Karaağaç, M.O.; Ergün, A.; Ağbulut, Ü.; Gürel, A.E.; Ceylan, İ. Experimental analysis of CPV/T solar dryer with nano-enhanced PCM and prediction of drying parameters using ANN and SVM algorithms. Sol. Energy 2021, 218, 57–67. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Moghaddam, M.E.; Asadi, A.; Mogharrebi, A.R.; Jafari, B.; Hasani, M.R.; Ganji, D.D. Effect of two different fins (longitudinal-tree like) and hybrid nano-particles (MoS2-TiO2) on solidification process in triplex latent heat thermal energy storage system. Alex. Eng. J. 2021, 60, 1967–1979. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Moghaddam, M.E.; Asadi, A.; Mogharrebi, A.; Ganji, D. Effect of internal fins along with hybrid nano-particles on solid process in star shape triplex latent heat thermal energy storage system by numerical simulation. Renew. Energy 2020, 154, 497–507. [Google Scholar] [CrossRef]
- Itani, M.; Ghaddar, N.; Ghali, K. Innovative PCM-desiccant packet to provide dry microclimate and improve performance of cooling vest in hot environment. Energy Convers. Manag. 2017, 140, 218–227. [Google Scholar] [CrossRef]
- Khanlari, A.; Güler, H.Ö.; Tuncer, A.D.; Şirin, C.; Bilge, Y.C.; Yılmaz, Y.; Güngör, A. Experimental and numerical study of the effect of integrating plus-shaped perforated baffles to solar air collector in drying application. Renew. Energy 2020, 145, 1677–1692. [Google Scholar] [CrossRef]
- Aktaş, M.; Khanlari, A.; Amini, A.; Şevik, S. Performance analysis of heat pump and infrared–heat pump drying of grated carrot using energy-exergy methodology. Energy Convers. Manag. 2017, 132, 327–338. [Google Scholar] [CrossRef]
- Ceylan, İ.; Gürel, A.E.; Ergün, A.; Tabak, A. Performance analysis of a concentrated photovoltaic and thermal system. Sol. Energy 2016, 129, 217–223. [Google Scholar] [CrossRef]
- Aqib, M.; Hussain, A.; Ali, H.M.; Naseer, A.; Jamil, F. Experimental case studies of the effect of Al2O3 and MWCNTs nanoparticles on heating and cooling of PCM. Case Stud. Therm. Eng. 2020, 22, 100753. [Google Scholar] [CrossRef]
- Elarem, R.; Alqahtani, T.; Mellouli, S.; Aich, W.; Ben Khedher, N.; Kolsi, L.; Jemni, A. Numerical study of an Evacuated Tube Solar Collector incorporating a Nano-PCM as a latent heat storage system. Case Stud. Therm. Eng. 2021, 24, 100859. [Google Scholar] [CrossRef]
- Chen, S.-B.; Saleem, S.; Alghamdi, M.N.; Nisar, K.S.; Arsalanloo, A.; Issakhov, A.; Xia, W.-F. Combined effect of using porous media and nano-particle on melting performance of PCM filled enclosure with triangular double fins. Case Stud. Therm. Eng. 2021, 25, 100939. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Alomair, M.; Alomair, Y.; Tasnim, S.; Mahmud, S.; Abdullah, H. Analyses of bio-based nano-PCM filled concentric cylindrical energy storage system in vertical orientation. J. Energy Storage 2018, 20, 380–394. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S.; Pethurajan, V. Heat transfer performance of graphene nano-platelets laden micro-encapsulated PCM with polymer shell for thermal energy storage based heat sink. Appl. Therm. Eng. 2019, 156, 237–249. [Google Scholar] [CrossRef]
- Ho, C.; Hsu, S.-T.; Yang, T.-F.; Chen, B.-L.; Rashidi, S.; Yan, W.-M. Cooling performance of mini-channel heat sink with water-based nano-PCM emulsion-An experimental study. Int. J. Therm. Sci. 2021, 164, 106903. [Google Scholar] [CrossRef]
- Kumar, P.M.; Mylsamy, K. A comprehensive study on thermal storage characteristics of nano-CeO2 embedded phase change material and its influence on the performance of evacuated tube solar water heater. Renew. Energy 2020, 162, 662–676. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Mogharrebi, A.; Asadi, A.; Paikar, M.; Ganji, D. Effect of fin and hybrid nano-particles on solid process in hexagonal triplex latent heat thermal energy storage system. J. Mol. Liq. 2020, 300, 112347. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S. Energy conservative air conditioning system using silver nano-based PCM thermal storage for modern buildings. Energy Build. 2014, 69, 202–212. [Google Scholar] [CrossRef]
- Zadeh, S.M.H.; Mehryan, S.; Ghalambaz, M.; Ghodrat, M.; Young, J.; Chamkha, A. Hybrid thermal performance enhancement of a circular latent heat storage system by utilizing partially filled copper foam and Cu/GO nano-additives. Energy 2020, 213, 118761. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.A. Role of extended fins and graphene nano-platelets in coupled thermal enhancement of latent heat storage system. Energy Convers. Manag. 2020, 224, 113349. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Melting of nano-PCM inside a cylindrical thermal energy storage system: Numerical study with experimental verification. Energy Convers. Manag. 2018, 166, 241–259. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Geometry and nanoparticle loading effects on the bio-based nano-PCM filled cylindrical thermal energy storage system. Appl. Therm. Eng. 2018, 141, 724–740. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Alsabery, A.I.; Sheremet, M.A.; Kumar, R.; Li, Z.; Bach, Q.-V. Nanoparticle impact on discharging of PCM through a thermal storage involving numerical modeling for heat transfer and irreversibility. Powder Technol. 2020, 376, 424–437. [Google Scholar] [CrossRef]
- Ali, M.A.; Viegas, R.F.; Kumar, M.S.; Kannapiran, R.K.; Feroskhan, M. Enhancement of heat transfer in paraffin wax PCM using nano graphene composite for industrial helmets. J. Energy Storage 2019, 26, 100982. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, X.; Li, Y.; Wang, X.; Wang, Y.; Zhong, H. Effect of nano-SiC on thermal properties of expanded graphite/1-octadecanol composite materials for thermal energy storage. Powder Technol. 2020, 367, 32–39. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Bhavsar, R.; Singh, V.K.; Reddy, S. Effect of nano-gallium capsules on thermal energy storage characteristics of manganese organometallic SS-PCM. Thermochim. Acta 2019, 680, 178341. [Google Scholar] [CrossRef]
- Li, M.-J.; Li, M.-J.; Tong, Z.-X.; Li, D. Optimization of the packed-bed thermal energy storage with cascaded PCM capsules under the constraint of outlet threshold temperature. Appl. Therm. Eng. 2021, 186, 116473. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; El Qarnia, H.; El Ganaoui, M. Numerical study of the melting of Nano-PCM in a rectangular storage unit heated by upward heat transfer fluid. Energy Procedia 2017, 139, 86–91. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kumar, H.A.; Gowtham, P.; Kumar, S.H.S.; Sudhan, R.H. Experimental analysis and increasing the energy efficiency of PV cell with nano-PCM (calcium carbonate, silicon carbide, copper). Mater. Today Proc. 2021, 37, 1221–1225. [Google Scholar] [CrossRef]
- Khedher, N.B.; Bantan, R.A.; Kolsi, L.; Omri, M. Performance Investigation of a vertically configured LHTES via the combination of Nano-enhanced PCM and fins: Experimental and numerical approaches. Int. Commun. Heat Mass Transf. 2022, 137, 106246. [Google Scholar] [CrossRef]
- Kolsi, L.; Hussein, A.K.; Hassen, W.; Said, L.B.; Ayadi, B.; Rajhi, W.; Labidi, T.; Shawabkeh, A.; Ramesh, K. Numerical study of a phase change material energy storage tank working with Carbon Nano Tube-water nanofluid under Ha’il City climatic conditions. Mathematics 2023, 11, 1057. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlissa, F.; Mhadhbi, M.; Aich, W.; Hussein, A.K.; Alhadri, M.; Selimefendigil, F.; Öztop, H.F.; Kolsi, L. Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review. Processes 2023, 11, 3219. https://doi.org/10.3390/pr11113219
Khlissa F, Mhadhbi M, Aich W, Hussein AK, Alhadri M, Selimefendigil F, Öztop HF, Kolsi L. Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review. Processes. 2023; 11(11):3219. https://doi.org/10.3390/pr11113219
Chicago/Turabian StyleKhlissa, Faïçal, Mohsen Mhadhbi, Walid Aich, Ahmed Kadhim Hussein, Muapper Alhadri, Fatih Selimefendigil, Hakan F. Öztop, and Lioua Kolsi. 2023. "Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review" Processes 11, no. 11: 3219. https://doi.org/10.3390/pr11113219
APA StyleKhlissa, F., Mhadhbi, M., Aich, W., Hussein, A. K., Alhadri, M., Selimefendigil, F., Öztop, H. F., & Kolsi, L. (2023). Recent Advances in Nanoencapsulated and Nano-Enhanced Phase-Change Materials for Thermal Energy Storage: A Review. Processes, 11(11), 3219. https://doi.org/10.3390/pr11113219










