Phenolic Profile and Antioxidant Potential of Beverages from Buckwheat and Side Streams after Beverages Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Preparation of Buckwheat Beverage
2.3. Extraction Procedure
2.4. Total Phenolic Content Determination
2.5. Analysis of the Phenolic Profile Using RP-HPLC-DAD
2.6. Determination of Antiradical Capacity towards ABTS•+
2.7. Determination of Ferric-Reducing Antioxidant Power
2.8. Determination of DPPH Radical Scavenging Capacity
2.9. Determination of Chain-Breaking Antioxidant Activity
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-based milk alternatives: Types, processes, benefits, and characteristics. Food Rev. Int. 2023, 39, 2320–2351. [Google Scholar] [CrossRef]
- Pieczyńska, K.; Rzymski, P. Health Benefits of Vegetarian and Mediterranean Diets: Narrative Review. Pol. J. Food Nutr. Sci. 2022, 72, 327–346. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Vakima, H.; Kaleda, A.; Rosend, J.; Rosenvald, S. Market mapping of plant-based milk alternatives by using sensory (RATA) and GS analysis. Future Food 2021, 4, 100049. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Func. Food 2020, 70, 103975. [Google Scholar] [CrossRef]
- Galanakis, C. Recovery of high-added value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals derived from agricultural residues and their valuable properties and applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Farooq, S.; Rehman, R.; Pirzadah, T.B.; Malik, B.; Dar, F.A.; Tahir, I. Cultivation, agronomic practices, and growth performance of buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 299–313. [Google Scholar]
- Christa, K.; Soral-Śmietana, M. Buckwheat grains and buckwheat products-Nutritional and prophylactic value of their components—A review. Czech J. Food Sci. 2008, 6, 153–162. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H.; Li, W.D. Buckwheat. In Encyclopedia of Grain Science; Wrigley, C., Corke, H., Walker, C.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 120–128. [Google Scholar]
- Watanabe, M.; Ohshita, Y.; Tsushida, T. Antioxidant compounds from buckwheat (Fagopyrum esculentum Moench) hulls. J. Agric. Food Chem. 1997, 45, 1039–1044. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat–the source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive compounds in different buckwheat species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Govindhaswamy Krishnaswamy, G.; Parameshwari, S. A concise review on buckwheat materials based ready to serve and ready to eat food products. Mater. Today-Proc. 2022, 66, 783–788. [Google Scholar] [CrossRef]
- Fernandez, C.G.; Sonawake, S.K.; Arya, S.S. Cereal based functional beverages. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 914–919. [Google Scholar] [CrossRef]
- Mousavi, M.-H.; Gharekhani, M.; Aliirezalu, K.; Roufegarinejad, L.; Azadmard-Damirchi, S. Production and characterization of nondairy gluten-free fermented beverage based on buckwheat and lentil. Food Sci. Nutr. 2023, 11, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Ziarno, M. Characterization of buckwheat beverages fermented with lactic acid bacterial cultures and bifidobacterial. Foods 2020, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Tanashkina, T.V.; Peregoedova, A.A.; Semenyuta, A.A.; Boyarova, M.D. Gluten-free buckwheat kvass with aromatic raw materials. Food Process Tech. Technol. 2020, 50, 70–78. [Google Scholar] [CrossRef]
- Kokwar, A.; Arya, S.S.; Bhat, M.S. A cereal-based nondairy probiotic functional beverage: An insight into the improvement in quality characteristics, sensory profile, and shelf-life. J. Food Process Pres. 2022, 46, e16147. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. 2020, 60, 3005–3023. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Drabińska, N. Crossroad of tradition and innovation—The application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products—A Review. Pol. J. Food Nutr. Sci. 2021, 71, 107–134. [Google Scholar] [CrossRef]
- Xiao, C.W. Functional soy products. In Functional Foods, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 534–556. [Google Scholar] [CrossRef]
- Janas, K.M.; Amarowicz, R.; Zielińska-Tomaszewska, J.; Kosińska, A.; Posmyk, M.M. Induction of phenolic compounds in two dark-grown lentil cultivars with different tolerance to copper ions. Acta Physiol. Plant. 2009, 31, 587–595. [Google Scholar] [CrossRef]
- Sulewska, K.; Rybarczyk-Płońska, A.; Karamać, M. Antioxidant capacity of lentil flour hydrolysates obtained with pancreatin. Pol. J. Food Nutr. Sci. 2022, 72, 381–391. [Google Scholar] [CrossRef]
- Gai, F.; Janiak, M.A.; Sulewska, K.; Peiretti, P.G.; Karamać, M. Phenolic compound profile and antioxidant capacity of flax (Linum usitatissimum L.) harvested at different growth stages. Molecules 2023, 28, 1807. [Google Scholar] [CrossRef]
- Herman, M.; Janiak, M.A.; Sadlik, J.K.; Piekoszewski, W.; Amarowicz, R. Iron, zinc, copper, manganese and chromium in green teas, their transfer to extracts and correlations between contents of elements and bioactive compounds. Pol. J. Food Nutr. Sci. 2022, 72, 421–429. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1999, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Karamac, M.; Kancheva, V.; Amarowicz, R. Antioxidant activity of flaxseed extracts in lipid systems. Molecules 2016, 21, 17. [Google Scholar] [CrossRef]
- Yanishlieva, N.; Popov, A.; Marinova, E. Eine modifizierte jodometrische methode zur bestimmung der peroxidzahl in kleinen lipidproben. Comptes Rend. Acad. Bulg. Sci. 1978, 31, 869–871. [Google Scholar]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Janiak, M.A.; Slavova-Kazakova, A.; Kancheva, V.D.; Ivanova, M.; Tsrunchev, T.; Karamać, M. Effects of γ-irradiation of wild thyme (Thymus serpyllum L.) on the phenolic compounds profile of its ethanolic extract. Pol. J. Food Nutr. Sci. 2017, 67, 309–315. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- Quettier-Deleu, C. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Möench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.; Lee, Y.C.; Eskin, N.A.M. Antioxidant and radical-scavenging activities of buckwheat seed components. J. Am. Oil Chem. Soc. 1998, 75, 1595–1601. [Google Scholar] [CrossRef]
- Karamać, M.; Biskup, I.; Kulczyk, A. Fractionation of buckwheat seed phenolics and analysis of their antioxidant activity. Pol. J. Food Nutr. Sci. 2015, 65, 243–249. [Google Scholar] [CrossRef]
- Dietrych-Szostak, D.; Oleszek, W. Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum Möench) grain. J. Agric. Food Chem. 1999, 47, 4384–4387. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Mazza, G. Flavonoids and antioxidative activities in buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Vollmannová, A.; Musilová, J.; Lidiková, J.; Árvay, J.; Šnirc, M.; Tóth, T.; Bojňanská, T.; Čičová, I.; Kreft, I.; Germ, M. Concentrations of phenolic acids are differently genetically determined in leaves, flowers, and grain of common buckwheat (Fagopyrum esculentum Moench). Plants 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Zieliński, H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011, 124, 672–678. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography–electrospray ionization-time of flight-mass spectrometry (RP-HPLC–ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Karamać, M. Antioxidant activity of tannin fractions isolated from buckwheat seeds and groats. J. Am. Oil Chem. Soc. 2010, 87, 559–566. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Z.; Liu, Z.; Liu, J.; Piao, C.; Liu, D. Purification and identification of buckwheat hull flavonoids and its comparative evaluation on antioxidant and cytoprotective activity in vitro. Food Sci. Nutr. 2020, 8, 3882–3892. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–354. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, C.; Xu, H.; Zhang, J.; Zhang, L. Study on the change of flavonoid glycosides to aglycones during the process of steamed bread containing Tartary buckwheat flour and and antioxidant, α-glucosidase inhibitory activities evaluation in vitro. LWT-Food Sci. Technol. 2021, 145, 111527. [Google Scholar] [CrossRef]
- Kancheva, V.; Taskova, R.; Totseva, I.; Hanjieva, N. Antioxidant activity of extracts, fractions and flavonoid constituents from Carthamus lanatus L. Riv. Ital. Sostanze Gr. 2007, 84, 77–86. [Google Scholar]
- Rúa, J.; de Arriaga, D.; García-Armesto, R.M.; Busto, F.; del Valle, P. Binary combinations of natural phenolic compounds with gallic acid or with its alkyl esters: An approach to understand the antioxidant interactions. Eur. Food Res. Technol. 2017, 243, 1211–1217. [Google Scholar] [CrossRef]
- Arslan, A.; Haros, C.M.; Yalçın, E.; Güneş, A. Wet milling of buckwheat cultivars and some quality properties of the fractions. Food Sci. Technol. Int. 2022, 28, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-Dairy Plant Based Milk Substitutes and Fermented Dairy Type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Streimikyte, P.; Balciunaitiene, A.; Liapman, T.D.; Streimikyte-Mockeliune, Z.; Puzeryte, V.; Borkertas, S.; Viskelis, P.; Viskelis, J. Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars. Appl. Sci. 2022, 12, 8210. [Google Scholar] [CrossRef]
- Znamirowska, A.; Sajnar, K.; Kowalczyk, M.; Kluz, M.; Buniowska, M. Effect of addition of spelt and buckwheat hull on selected properties of yoghurt. JMBFS 2020, 10, 296–300. [Google Scholar] [CrossRef]
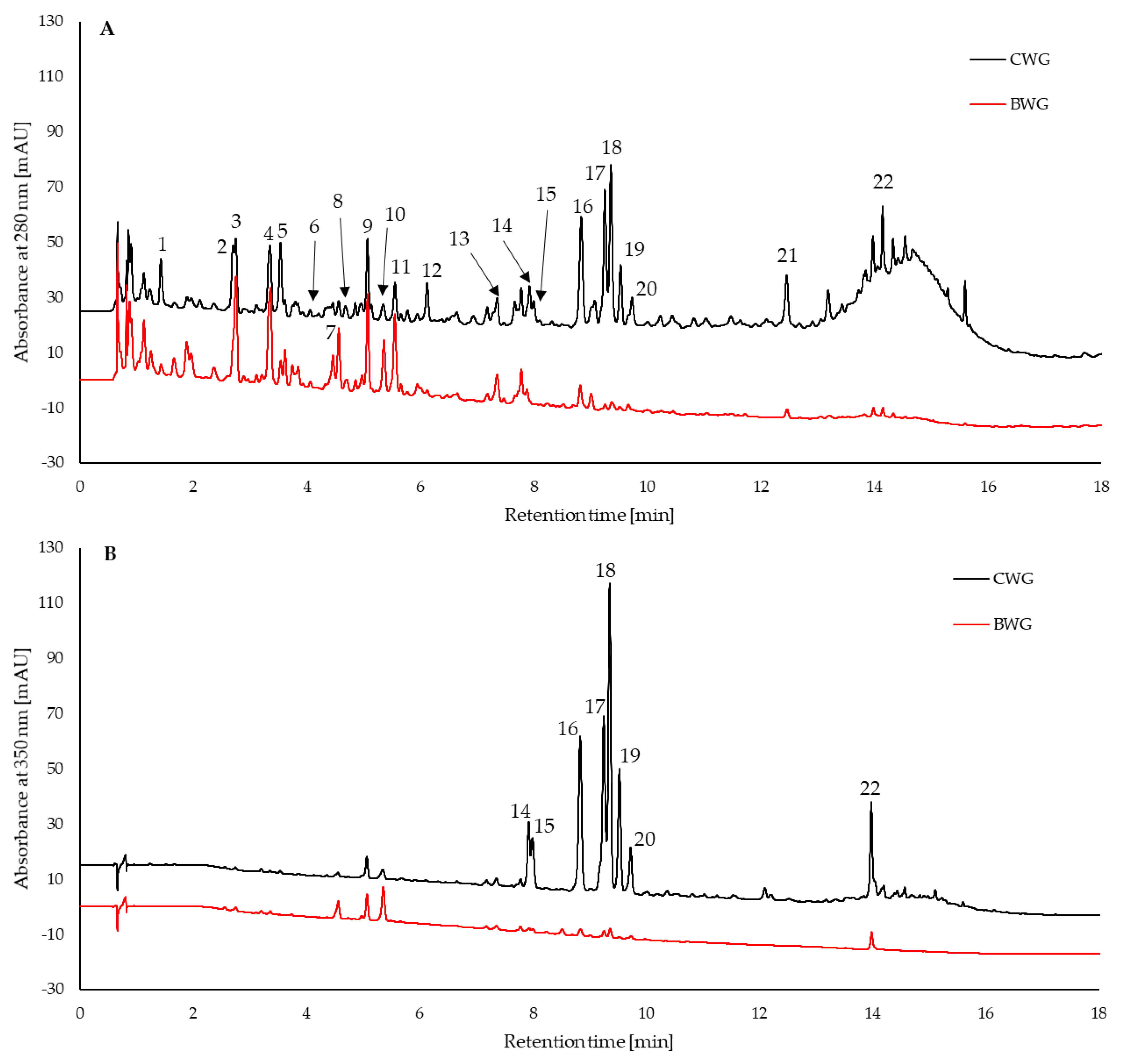
| Sample | Total Phenolic Content | ABTS Assay | FRAP | DPPH Assay |
|---|---|---|---|---|
| [mg catechin/100 mL] | [mmol Trolox/100 mL] | [mmol Fe2+/100 mL] | [mmol Trolox/100 mL] | |
| BDH | 44.7 ± 7.0 | 0.349 ± 0.049 | 418 ± 52 | 0.162 ± 0.029 |
| BWG | 43.7 ± 2.9 | 0.331 ± 0.029 | 385 ± 61 | 0.148 ± 0.018 |
| [mg catechin/100 g DW] | [mmol Trolox/100 g DW] | [mmol Fe2+/100 g DW] | [mmol Trolox/100 g DW] | |
| CDH | 681 ± 83 * | 5.70 ± 0.79 | 6591 ± 964 * | 1.349 ± 0.105 * |
| CWG | 840 ± 47 * | 5.30 ± 0.34 | 8157 ± 494 * | 1.915 ± 0.187 * |
| No. | Compound | BDH | BWG | CDH | CWG |
|---|---|---|---|---|---|
| [mg/100 mL] | [mg/g DW] | ||||
| 1 | Gallic acid | 0.153 ± 0.026 | 0.148 ± 0.033 | 1.57 ± 0.32 * | 2.4 ± 0.14 * |
| 2 | Protocatechuic acid | - | 0.317 ± 0.110 | - | 3.62 ± 0.62 |
| 3 | Phenolic acid 1 | 0.333 ± 0.054 | 0.280 ± 0.008 | 2.84 ± 0.62 ** | 1.14 ± 0.11 ** |
| 4 | Phenolic acid 1 | 0.655 ± 0.120 | 0.558 ± 0.069 | 5.88 ± 1.1 ** | 2.29 ± 0.30 * |
| 5 | Flavanol 2 | 0.386 ± 0.065 | 0.428 ± 0.069 | 5.37 ± 0.91 *** | 12.1 ± 0.89 *** |
| 6 | p-Hydroxybenzoic acid | 0.032 ± 0.007 * | 0.0837 ± 0.024 * | 0.29 ± 0.09 ** | 0.534 ± 0.051 ** |
| 7 | Phenolic acid 1 | 0.106 ± 0.020 ** | 0.299 ± 0.063 ** | 0.61 ± 0.09 | 0.612 ± 0.16 |
| 8 | (+)-Catechin | 0.503 ± 0.083 * | 0.309 ± 0.065 * | 6.05 ± 1.36 ** | 1.48 ± 0.198 ** |
| 9 | Phenolic acid 1 | 0.522 ± 0.103 * | 0.311 ± 0.030 * | 4.75 ± 0.88 ** | 1.48 ± 0.24 ** |
| 10 | Caffeic acid | 0.062 ± 0.007 * | 0.379 ± 0.166 * | 0.47 ± 0.26 | 0.72 ± 0.26 |
| 11 | Phenolic acid 1 | 0.447 ± 0.076 | 0.472 ± 0.090 | 3.26 ± 0.88 * | 1.15 ± 0.18 * |
| 12 | (–)-Epicatechin | 1.001 ± 0.149 ** | 0.354 ± 0.173 ** | 15.80 ± 2.65 ** | 7.71 ± 0.18 ** |
| 13 | p-Coumaric acid | 0.037 ± 0.006 * | 0.085 ± 0.023 * | 0.40 ± 0.10 | 0.32 ± 0.09 |
| 14 | Homoorientin | - | - | - | 2.86 ± 0.13 |
| 15 | Orientin | - | - | - | 2.83 ± 0.027 |
| 16 | Flavonoid 3 | - | 0.091 ± 0.019 | - | 11.30 ± 0.20 |
| 17 | Flavonoid 3 | - | 0.076 ± 0.012 | - | 11.21 ± 0.44 |
| 18 | Rutin | 0.082 ± 0.018 | 0.043 ± 0.004 | 10.40 ± 1.53 * | 7.72 ± 0.82 * |
| 19 | Hyperoside | - | - | - | 19.02 ± 2.38 |
| 20 | Flavonoid 3 | - | - | - | 1.83 ± 0.22 |
| 21 | Flavanol 2 | 0.656 ± 0.0987 ** | 0.209 ± 0.059 ** | 11.3 ± 0.63 | 12.5 ± 0.96 |
| 22 | Quercetin | 0.463 ± 0.071 ** | 0.179 ± 0.07 ** | 1.14 ± 0.33 *** | 6.66 ± 0.18 *** |
| Sum of phenolic acids | 2.35 | 2.93 | 20.1 | 14.3 | |
| Sum of flavonoids | 3.09 | 1.69 | 50.1 | 97.2 | |
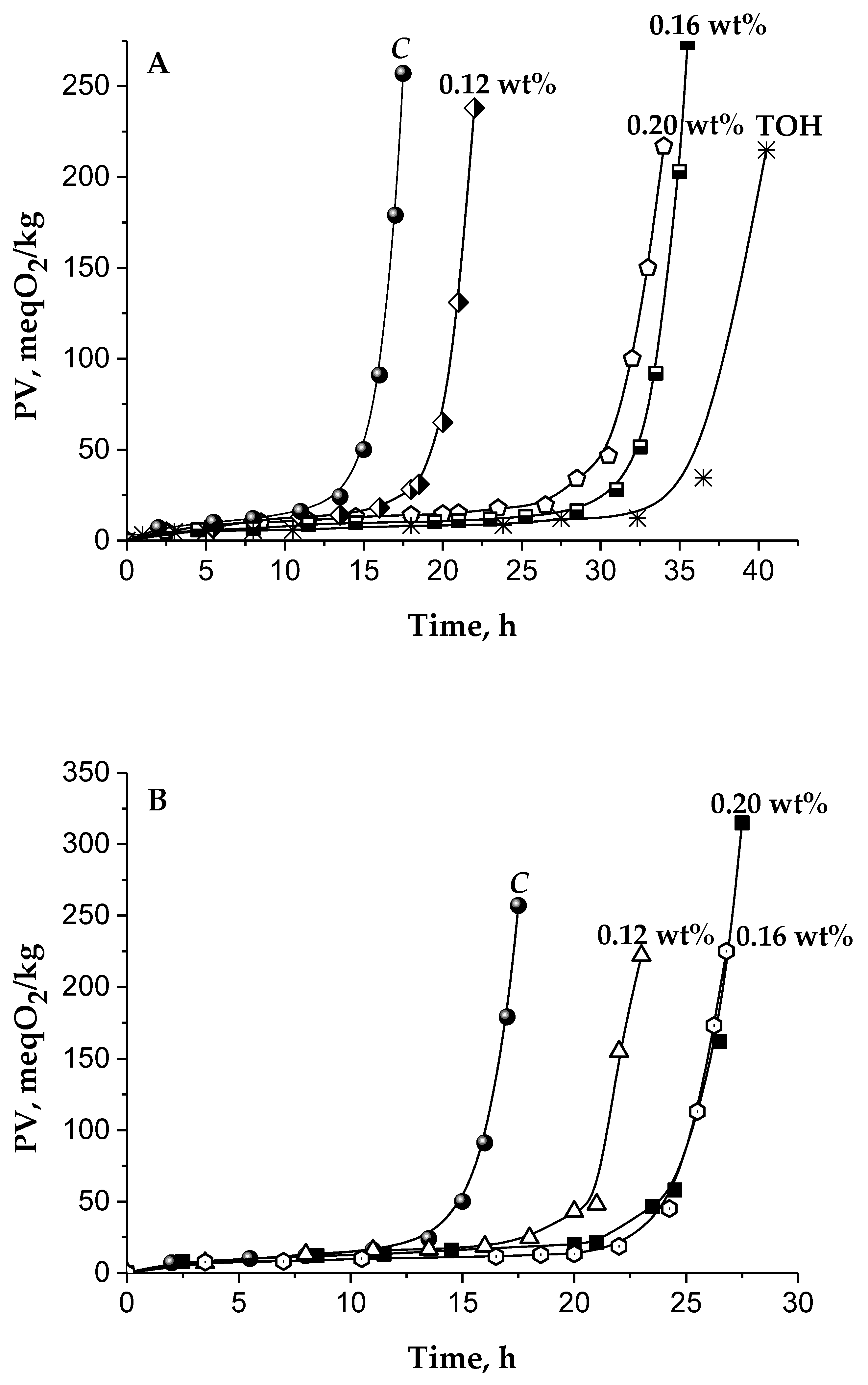
| Cake Extracts | The Main Kinetic Parameters during TGSO Autoxidation | ||||
|---|---|---|---|---|---|
| Abbr. | Concentr. wt% | IPA h | PF - | RA, 10−7 Ms−1 | ID - |
| CDH | 0.12 | 20.5 ± 1.5 | 1.2 | 1.56 ± 0.08 | 0.9 |
| 0.16 | 24.7 ± 1.5 | 1.5 | 0.97 ± 0.06 | 1.4 | |
| 0.2 | 25.5 ± 1.5 | 1.5 | 1.21 ± 0.09 | 1.1 | |
| CWG | 0.12 | 20.0 ± 1.5 | 1.2 | 1.49 ± 0.09 | 0.9 |
| 0.16 | 33.0 ± 2.0 | 1.9 | 0.76 ± 0.04 | 1.8 | |
| 0.2 | 32.0 ± 2.0 | 1.9 | 0.69 ± 0.06 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiak, M.A.; Karamać, M.; Sulewska, K.; Amarowicz, R.; Denev, P.; Slavova-Kazakova, A. Phenolic Profile and Antioxidant Potential of Beverages from Buckwheat and Side Streams after Beverages Production. Processes 2023, 11, 3205. https://doi.org/10.3390/pr11113205
Janiak MA, Karamać M, Sulewska K, Amarowicz R, Denev P, Slavova-Kazakova A. Phenolic Profile and Antioxidant Potential of Beverages from Buckwheat and Side Streams after Beverages Production. Processes. 2023; 11(11):3205. https://doi.org/10.3390/pr11113205
Chicago/Turabian StyleJaniak, Michał Adam, Magdalena Karamać, Katarzyna Sulewska, Ryszard Amarowicz, Petko Denev, and Adriana Slavova-Kazakova. 2023. "Phenolic Profile and Antioxidant Potential of Beverages from Buckwheat and Side Streams after Beverages Production" Processes 11, no. 11: 3205. https://doi.org/10.3390/pr11113205
APA StyleJaniak, M. A., Karamać, M., Sulewska, K., Amarowicz, R., Denev, P., & Slavova-Kazakova, A. (2023). Phenolic Profile and Antioxidant Potential of Beverages from Buckwheat and Side Streams after Beverages Production. Processes, 11(11), 3205. https://doi.org/10.3390/pr11113205










