Perovskite Type B-CaTiO3 Coupled with Graphene Oxide as Efficient Bifunctional Composites for Environmental Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
2.2.1. Synthesis of CaTiO3 and B-CaTiO3
2.2.2. Synthesis of Graphene Oxide
2.2.3. Synthesis of B-CaTiO3/GO Composites
2.2.4. Material Characterization
2.3. Photocatalytic Activity Study
3. Results and Discussions
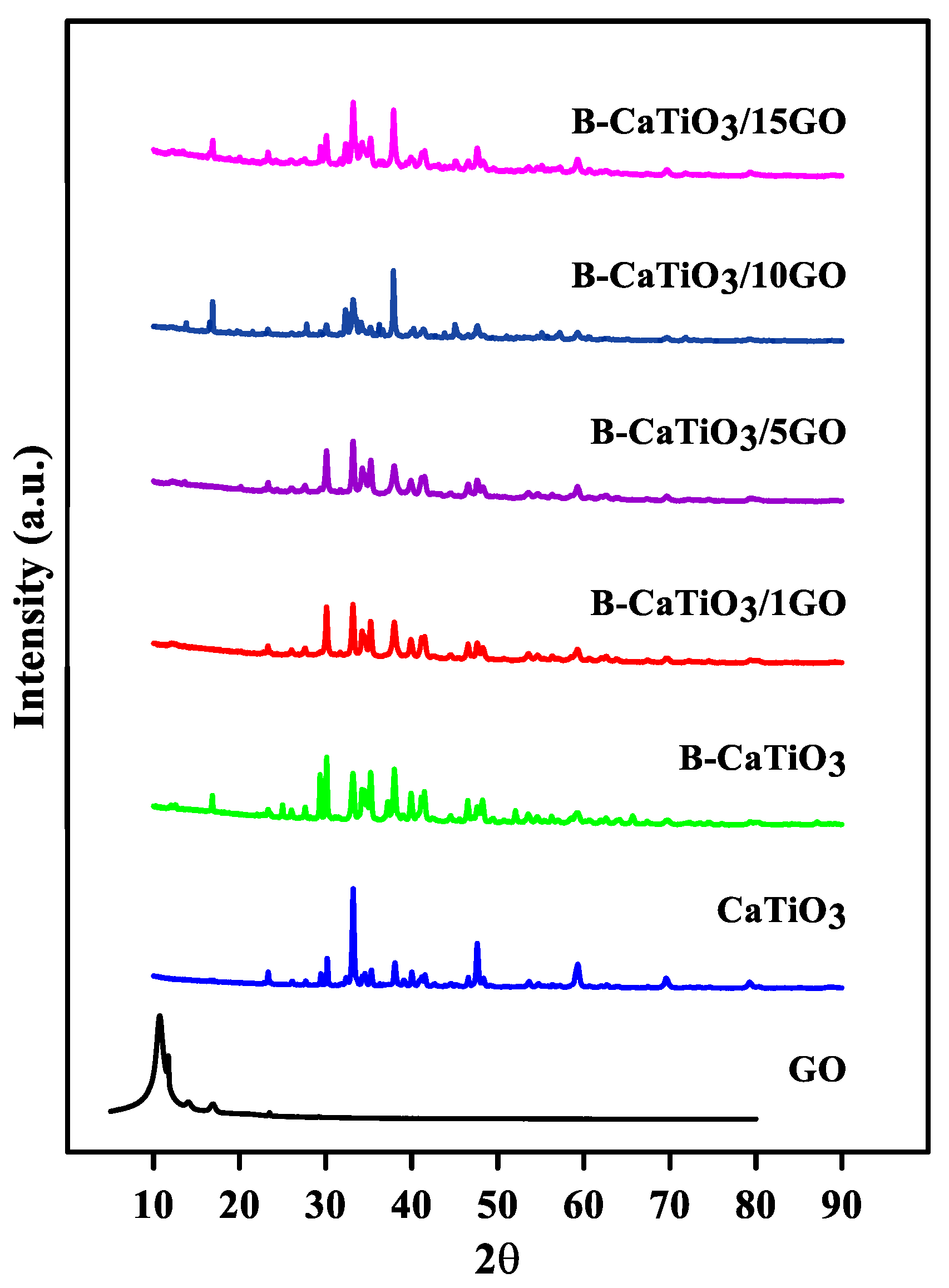
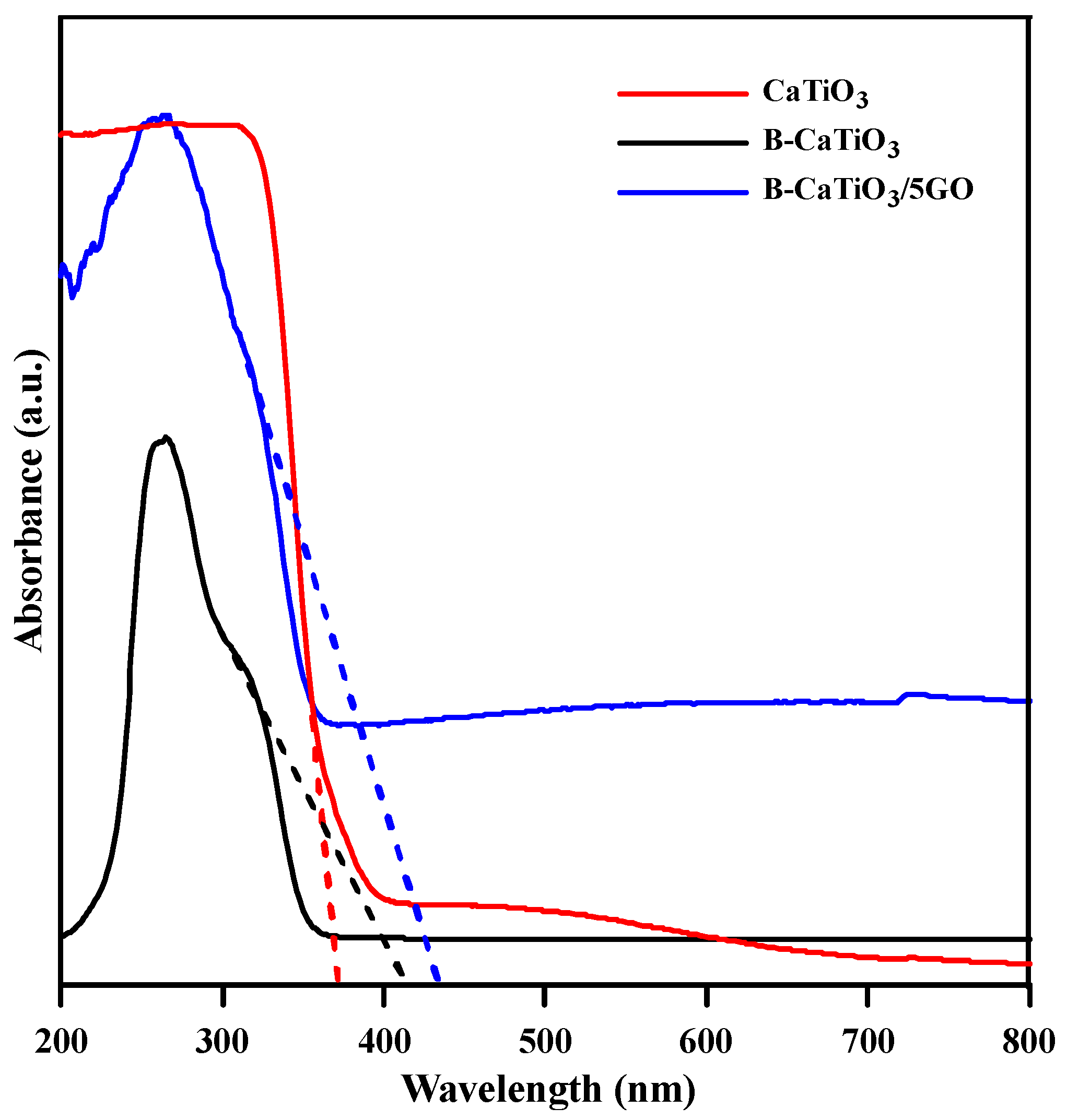
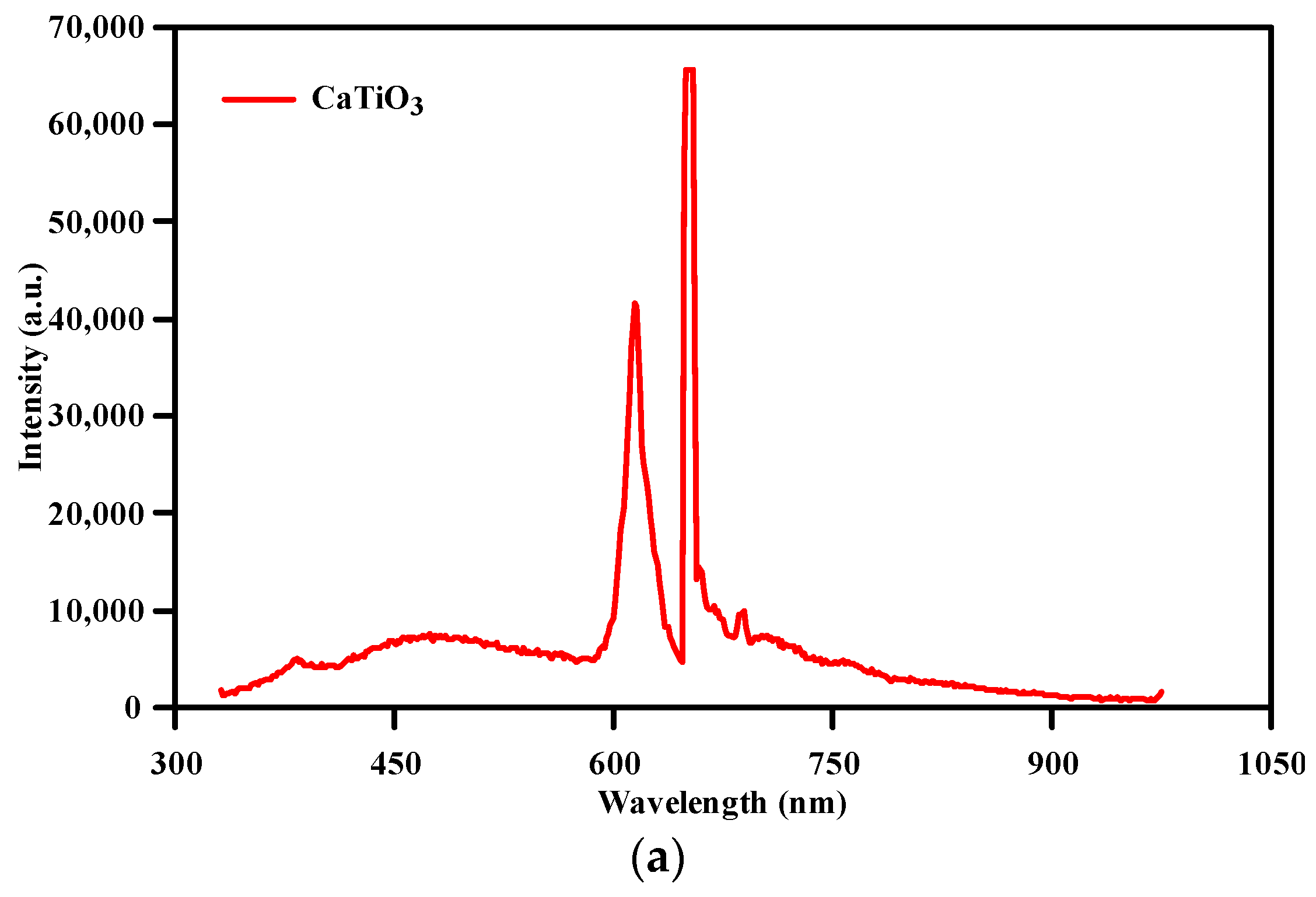
3.1. Structural and Morphological Analysis
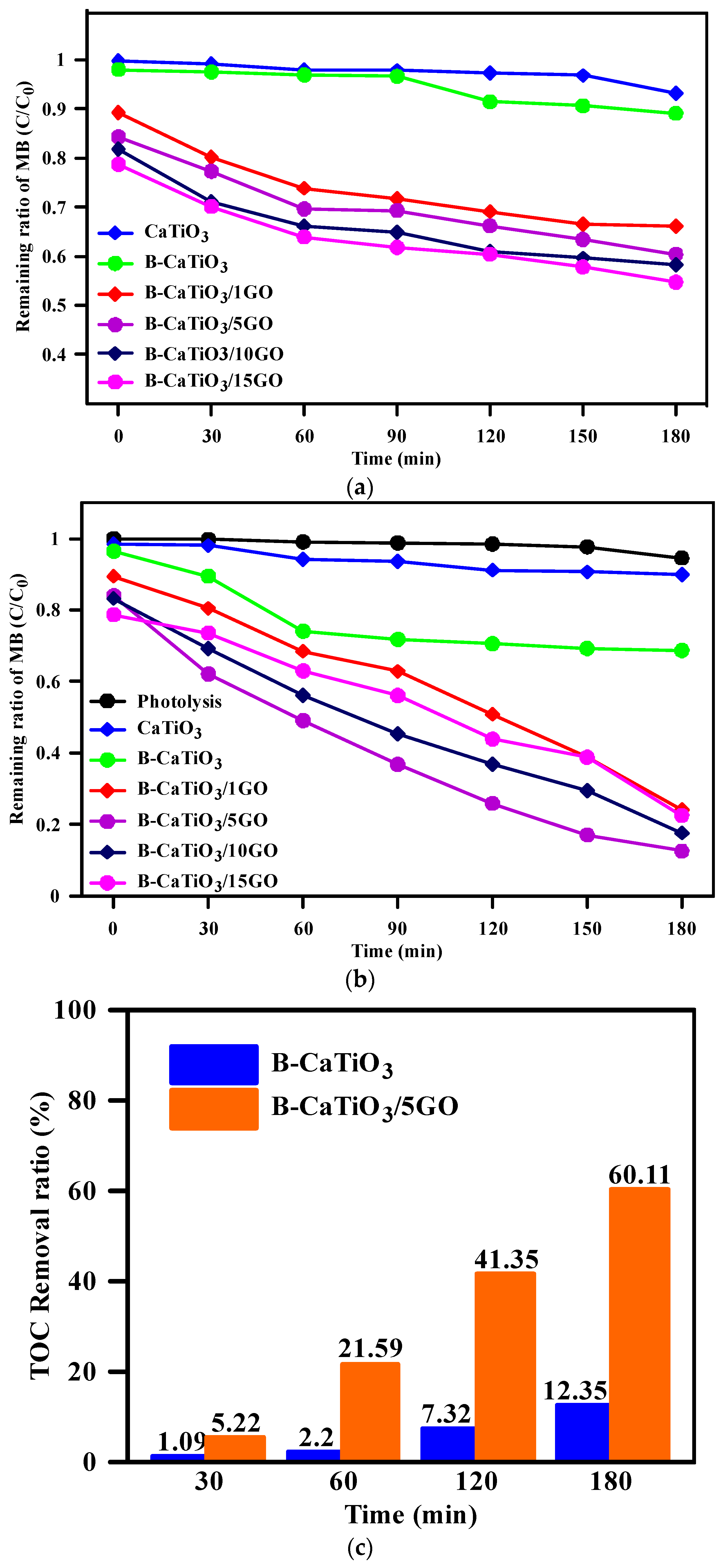
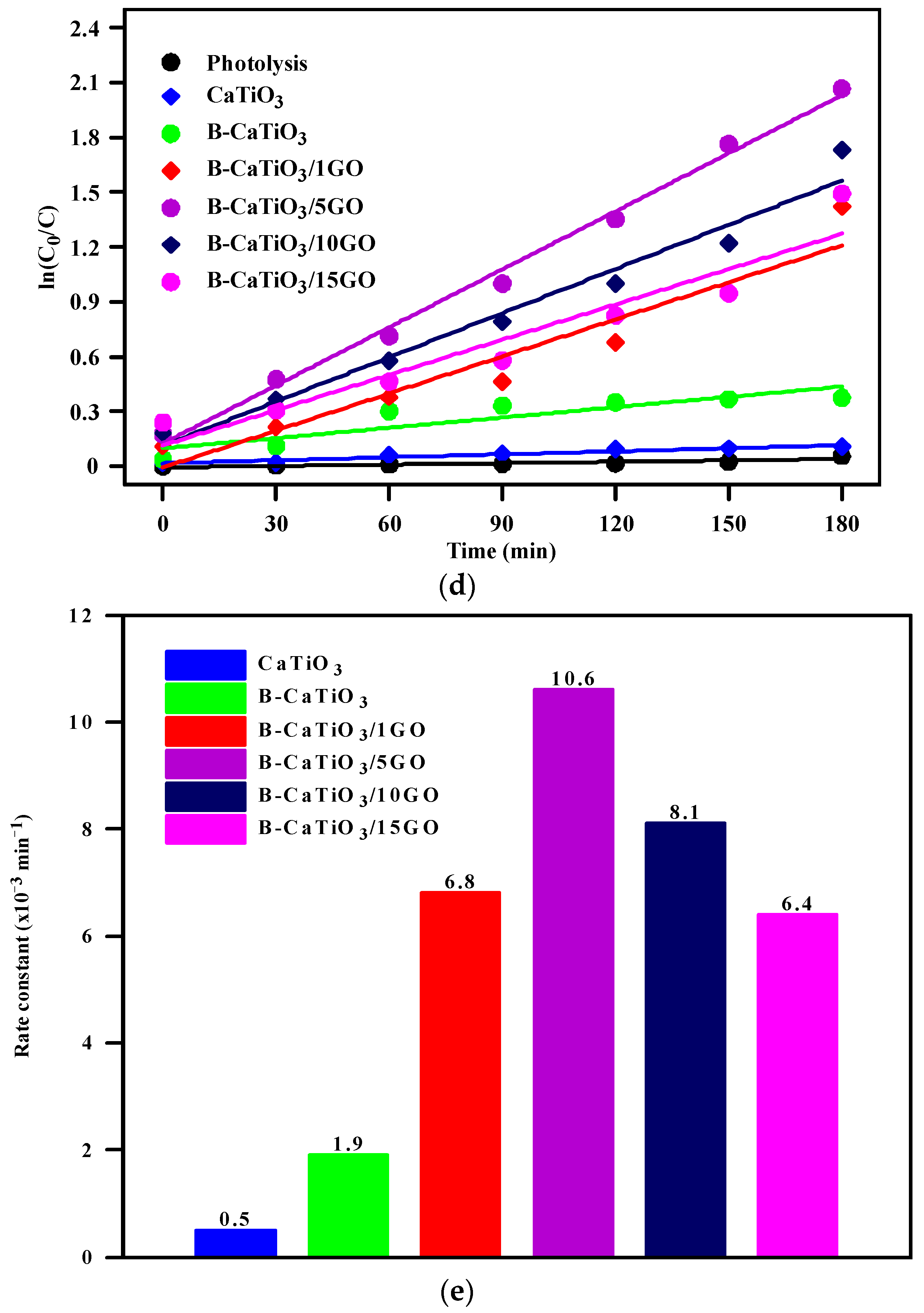
3.2. Photocatalytic Activity Studies
3.2.1. Effect of pH
3.2.2. Effect of Water Matrix
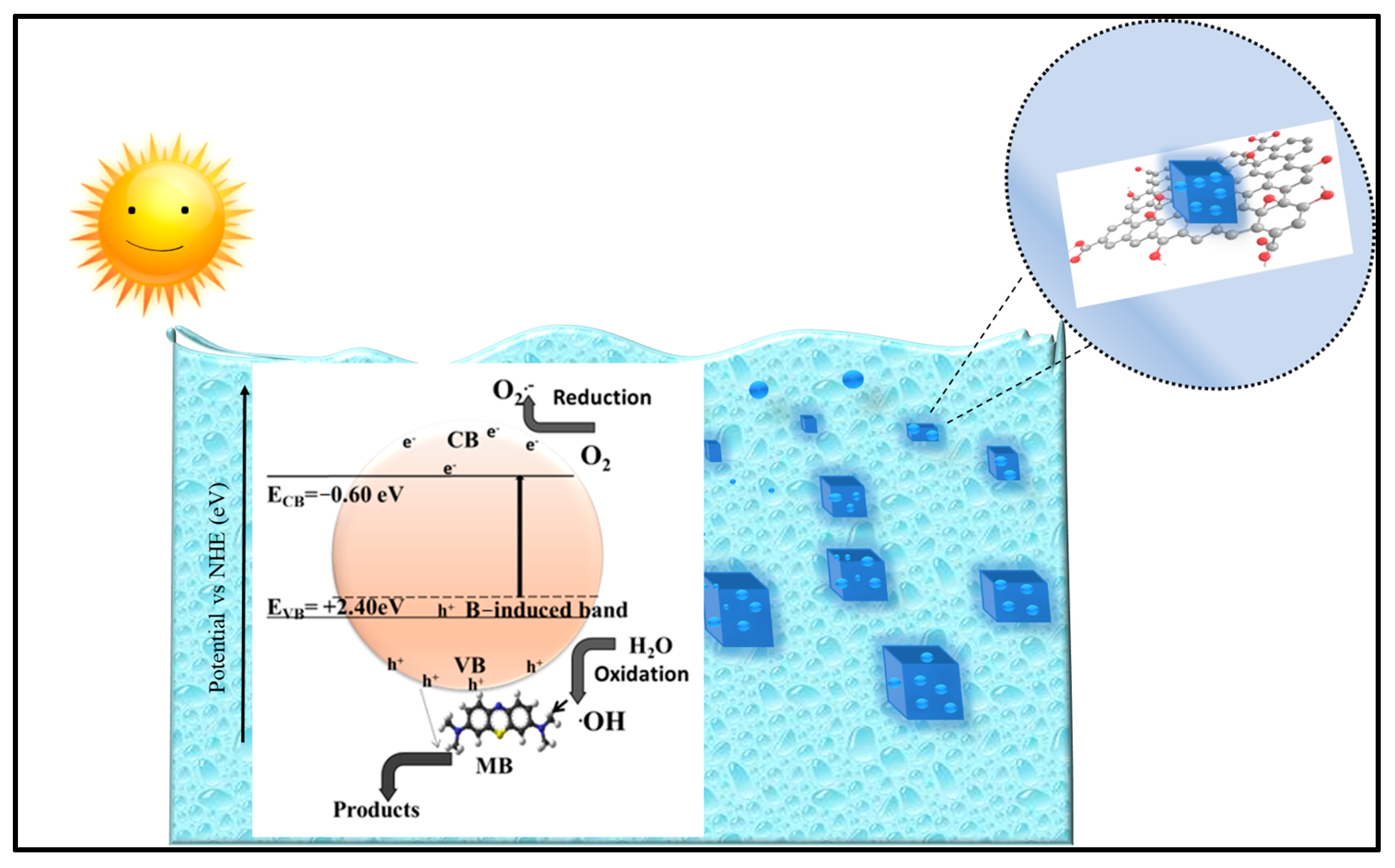
3.2.3. Mechanism of Photocatalytic Degradation
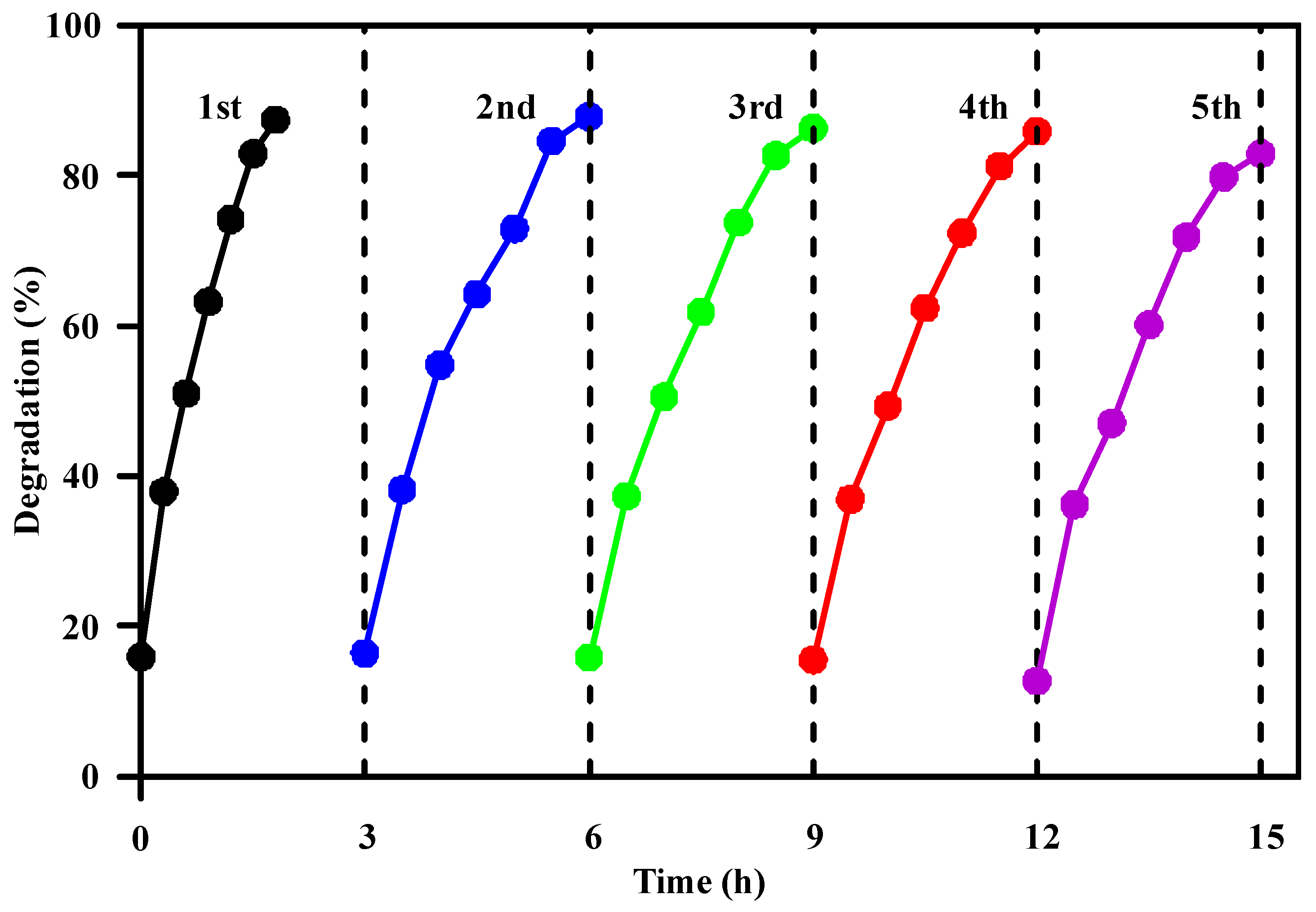
3.2.4. Photocatalyst Reusability
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Altin, I.; Sökmen, M. Buoyant photocatalyst based on ZnO immobilized on polystyrene beads for pollutants treatment. Clean Soil Air Water 2015, 43, 974–1113. [Google Scholar] [CrossRef]
- Ravichandran, K.; Nithiyadevi, K.; Sakthivel, B.; Arun, T.; Sindhuja, E.; Muruganandam, G. Synthesis of ZnO:Co/rGO nanocomposites for enhanced photocatalytic and antibacterial activities. Ceram. Int. 2016, 42, 17539–17550. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Reduced graphene oxide three-dimensionally wrapped WO3 hierarchical nanostructures as high-performance solar photocatalytic materials. Appl. Catal. A 2016, 522, 90–100. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Bo, W.; Lai, F.; Zhang, M.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A.; Long, J. Site-Sensitive Selective CO2 Photoreduction to CO over Gold Nanoparticles. Angew. Chem. Int. Ed. 2022, 61, e202204563. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behvandi, S.; Karimi, M.A.; Sohouli, E.; Karimi, M.S.; Gholipour, N.; Ahmadi, F.; Rahimi-Nasrabadi, M. Synergetic effect of graphene oxide and C3N4 as co-catalyst for enhanced photocatalytic performance of dyes on Yb2(MoO4)(3)/YbMoO4 nanocomposite. Ceram. Int. 2019, 45, 17847–17858. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behpour, M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Pourmasoud, S. New method for synthesis of BaFe12O19/Sm2Ti2O7 and BaFe12O19/Sm2Ti2O7/Ag nano-hybrid and investigation of optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2019, 30, 5854–5865. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N.; Polat, I.; Yasar, A.; Yüksel, Ö.F. Silver doped zinc stannate (Ag-ZnSnO3) for the photocatalytic degradation of caffeine under UV irradiation. Water 2021, 13, 1290. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Degirmencioglu, I.; Kucukomeroglu, T.; Yılmaz, S.; Stathopoulos, V.N. Immobilized TiO2/ZnO sensitized copper (II) phthalocyanine heterostructure for the degradation of ibuprofen under UV irradiation. Separations 2021, 8, 24. [Google Scholar]
- Altin, I.; Sökmen, M. Photocatalytic properties of silver incorporated titania nanoparticles immobilized on waste-derived polystyrene. Water Air Soil Poll. 2014, 225, 1786. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, Y.; Hu, C.; Qu, J. Enhanced Fenton degradation of Rhodamine B over nanoscaled Cu-doped LaTiO3 perovskite. Appl. Catal. B 2012, 125, 418–424. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Umirzakov, A.; Serik, A.; Baimenov, A.; Yeleuov, M.; Daulbayev, C. Multifunctional strontium titanate perovskite-based composite photocatalysts for energy conversion and other applications. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Belousov, A.S.; Parkhacheva, A.A.; Suleimanov, E.V.; Shafiq, I. Potential of Bi2WO6-based heterojunction photocatalysts for environmental remediation. Mater. Today Chem. 2023, 32, 101633. [Google Scholar] [CrossRef]
- Wei, K.; Faraj, Y.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. J. Chem. Eng. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A review of advances in multifunctional XTiO3 perovskite-type oxides as piezo-photocatalysts for environmental remediation and energy production. J. Hazard. Mater. 2022, 421, 126792. [Google Scholar] [CrossRef]
- Bradha, M.; Hussain, S.; Chakravarty, S.; Amarendra, G.; Ashok, A. Total conductivity in Sc-doped LaTiO3 perovskites. Ionics 2014, 20, 1343–1350. [Google Scholar] [CrossRef]
- Kim, Y.; Schlegl, H.; Kim, K.; Irvine, J.T.S.; Kim, J.H. X-ray photoelectron spectroscopy of Sm-doped layered perovskite for intermediate temperature-operating solid oxide fuel cell. Appl. Surf. Sci. 2014, 288, 695–701. [Google Scholar] [CrossRef]
- Hong, H.J.; Yashiro, K.; Hashimoto, S.I. Conduction properties and ionic transference behavior of CaTi1-xScxO3(x = 0.05, 0.1). ECS Trans. 2014, 61, 151. [Google Scholar] [CrossRef]
- He, Y.; Chong, H.; Qing-Wei, L.I.; Xue, X.X. Effect of baking temperature on photocatalytic properties of titanium calcium. J. Funct. Mater. 2010, 31, 1297–1299. [Google Scholar]
- Wei, Z.; Xiao, W.; Ren, Z.H.; Gang, X.U.; Ge, S.; Zan, Z.; Zhang, Y.F.; Han, G.R. Hydrothermal synthesis and photocatalytic property of CaTiO3 assisted with sodium citrate. J. Funct. Mater. 2012, 43, 50–53. [Google Scholar]
- Nishimoto, S.; Matsuda, M.; Miyake, M. Photocatalytic activities of Rh-doped CaTiO3 under visible light irradiation. Chem. Lett. 2006, 35, 308–309. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Yang, X.; Wu, Z.; Li, Y. Construction of novel 2D/1D g-C3N4/CaTiO3 heterojunction with face-to-face contact for boosting photodegradation of triphenylmethane dyes under simulated sunlight. J. Taiwan Inst. Chem. Eng. 2020, 107, 98–109. [Google Scholar] [CrossRef]
- Huang, S.; Guo, S.; Wang, Q.; Zhu, N.; Lou, Z.; Li, L.; Shan, A.; Yuan, H. CaF2-Based near infrared photocatalyst using the multifunctional CaTiO3 precursors as the calcium source. ACS Appl. Mater. Interfaces 2015, 7, 20170–20178. [Google Scholar] [CrossRef]
- Bai, L.; Xu, Q.; Cai, Z. Synthesis of Ag@AgBr/CaTiO3 composite photocatalyst with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 17580–17590. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-level matching of Fe(III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Gao, J.; Du, W.; Shangguan, L.; Zhang, X.; Lin, R.-B.; Wu, H.; Zhou, W.; Liu, X.; et al. Tunable titanium metal–organic frameworks with infinite 1D Ti-O rods for efficient visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. 2019, 7, 11928–11933. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the enhancement in photoelectrochemical properties of photocatalytically prepared TiO2-reduced graphene oxide composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Du, A.; Ng, Y.H.; Bell, N.J.; Zhu, Z.; Amal, R.; Smith, S.C. Hybrid graphene/titania nanocomposite: Interface charge transfer, hole doping, and sensitization for visible light response. J. Phys. Chem. Lett. 2011, 2, 894–899. [Google Scholar] [CrossRef]
- Altin, I. CuO-TiO2/graphene ternary nanocomposite for highly efficient visible-light-driven photocatalytic degradation of bisphenol A. J. Mol. Struct. 2022, 1252, 132199. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.; Song, W.; Wang, S.; Fan, X.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W. Synthesis of nitrogen and sulfur co-doped reduced graphene oxide as efficient metal-free cocatalyst for the photo-activity enhancement of CdS. Appl. Catal. B 2018, 236, 212–221. [Google Scholar] [CrossRef]
- Huo, Y.S.; Yang, H.; Xian, T.; Jiang, J.L.; Wei, Z.Q.; Li, R.S.; Feng, W.J. A polyacrylamide gel route to different-sized CaTiO3 nanoparticles and their photocatalytic activity for dye degradation. J. Sol Gel Sci. Technol. 2014, 71, 254–259. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Bahuguna, A.; Kumar, A.; Sharma, V.; Krishnan, V. Recyclable, bifunctional composites of perovskite type N-CaTiO3 and reduced graphene oxide as an efficient adsorptive photocatalyst for environmental remediation. Mater. Chem. Front. 2017, 1, 2391. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Huo, Y.S. Enhanced photocatalytic activity of CaTiO3-graphene nanocomposites for dye degradation. Phys. Scr. 2014, 89, 115801. [Google Scholar] [CrossRef]
- Chen, X.; Di, L.; Yang, H.; Xian, T. A magnetically recoverable CaTiO3/reduced graphene oxide/NiFe2O4 nanocomposite for the dye degradation under simulated sunlight irradiation. J. Ceram. Soc. Jpn. 2019, 127, 221–231. [Google Scholar] [CrossRef]
- Lalan, V.; Pillai, V.P.M.; Gopchandran, K.G. Enhanced electron transfer due to rGO makes Ag-CaTiO3@rGO a promising plasmonic photocatalyst. J. Sci. Adv. Mater. Dev. 2022, 7, 100468. [Google Scholar] [CrossRef]
- Luo, P.; Liu, W.; Zhu, D.; Sun, B.; Cao, Y.; Wang, Y. One-step synthesis of Ti3+ self-doped CTO/rGO composites by pulsed laser ablation in liquid with enhanced photocatalytic activity in water purification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130216. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar] [CrossRef]
- Feng, W.; Lei, Y.; Wu, X.; Yuan, J.; Chen, J.; Xu, D.; Zhang, X.; Zhang, S.; Liu, P.; Zhang, L.; et al. Tuning the interfacial electronic structure via Au clusters for boosting photocatalytic H2 evolution. J. Mater. Chem. A 2021, 9, 1759–1769. [Google Scholar] [CrossRef]
- Mu, J.; Sun, W.; Li, F.; Guan, Y.; Zhou, X.; Li, J.; Chen, L. Upconversion luminescent perovskite CaTiO3:Yb3+/Er3+ nanocubes. J. Alloys Compd. 2019, 797, 1002–1006. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Xin, Y.; Cheng, X. TiO2 nanobelts-effect of calcination temperature on optical, photoelectrochemical and photocatalytic properties. Electrochim. Acta 2013, 111, 284–291. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2-x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Reszczynska, J.; Lisowski, W.; Klimczuk, T.; Winiarski, M.; Słoma, M.; Zaleska-Medynska, A. Perovskite-type KTaO3-reduced graphene oxide hybrid with improved visible light photocatalytic activity. RSC Adv. 2015, 5, 91315–91325. [Google Scholar] [CrossRef]
- Attou, L.; Al-Shami, A.; Boujemaâ, J.; Mounkachi, O.; Ez-Zahraouy, H. Predicting the structural, optoelectronic, dynamical stability and transport properties of Boron-doped CaTiO3: DFT based study. Phys. Scr. 2022, 97, 115808. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, C.G.; Wang, L.; Wang, Y.; Zhang, X.G.; Zeng, G.M. Synthesis of fern-like Ag/AgCl/CaTiO3 plasmonic photocatalysts and their enhanced visible-light photocatalytic properties. RSC Adv. 2016, 6, 47873–47882. [Google Scholar] [CrossRef]
- Dong, G.; Xiao, X.; Zhang, L.; Ma, Z.; Bao, X.; Peng, M.; Zhang, Q.; Qiu, J. Preparation and optical properties of red, green and blue afterglow electrospun nanofibers. J. Mater. Chem. 2011, 21, 2194–2203. [Google Scholar] [CrossRef]
- Zheng, H.; Györgyfalva, G.C.; Quimby, R.; Bagshaw, H.; Ubic, R.; Reaney, I.; Yarwood, J. Raman Spectroscopy of B-Site Order-Disorder in CaTiO3-Based Microwave Ceramics. J. Eur. Ceram. Soc. 2003, 23, 2653–2659. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zhou, Y.; Gao, M.; Yang, F. One-step potentiodynamic synthesis of poly(1,5-diaminoanthraquinone)/reduced graphene oxide nanohybrid with improved electrocatalytic activity. J. Mater. Chem. A 2013, 1, 13902–13913. [Google Scholar] [CrossRef]
- Altin, I.; Ma, X.; Boffa, V.; Bacaksız, E.; Magnacca, G. Hydrothermal preparation of B-TiO2-graphene oxide ternary nanocomposite, characterization and photocatalytic degradation of bisphenol A under simulated solar irradiation. Mater. Sci. Semicond. Process 2021, 123, 105591. [Google Scholar] [CrossRef]
- Park, B.G. Photoluminescence of Eu3+-doped CaTiO3 perovskites and their photocatalytic properties with a metal ion loading. Chem. Phys. Lett. 2019, 722, 44–49. [Google Scholar] [CrossRef]
- Han, C.; Liu, J.; Yang, W.; Wu, Q.; Yang, H.; Xue, X. Enhancement of photocatalytic activity of CaTiO3 through HNO3 acidification. J. Photochem. Photobiol. A 2016, 322, 1–9. [Google Scholar] [CrossRef]
- Feng, N.; Zheng, A.; Wang, Q.; Ren, P.; Gao, X.; Liu, S.B.; Shen, Z.; Chen, T.; Deng, F. Boron environments in B-doped and (B, N) codoped TiO2 photocatalysts: A combined solid-state NMR and theoretical calculation study. J. Phys. Chem. C 2011, 115, 2709–2719. [Google Scholar] [CrossRef]
- Quinones, D.H.; Rey, A.; Alvarez, P.M.; Beltran, F.J.; Puma, G.L. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B 2015, 178, 74–81. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.-B.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.; Bhattacharyya, K.; Krishnan, V. Synergetic effect of MoS2-RGO doping to enhance the photocatalytic performance of ZnO nanoparticles. New J. Chem. 2016, 40, 5185–5197. [Google Scholar] [CrossRef]
- Jiang, E.; Song, N.; Che, G.; Liu, C.; Dong, H.; Yang, L. Construction of a Z-scheme MoS2/CaTiO3 heterostructure by the morphology-controlled strategy towards enhancing photocatalytic activity. Chem. Eng. J. 2020, 399, 125721. [Google Scholar] [CrossRef]
- Chen, D.M.; Yang, D.; Wang, Q.; Jiang, Z.Y. Effects of boron doping on photocatalytic activity and microstructure of titanium dioxide nanoparticles. Ind. Eng. Chem. Res. 2006, 45, 4110–4116. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, X.; Du, A.; Sun, D.; Leckie, J.O. Self-etching reconstruction of hierarchically mesoporous F-TiO2 hollow microspherical photocatalyst for concurrent membrane water purifications. J. Am. Chem. Soc. 2008, 130, 11256–11257. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Liu, C.; Lü, H.; Yu, C.; Ding, B.; Ye, R.; Ji, Y.; Dai, B.; Liu, W. Novel FeWO4/WO3 nanoplate with p-n heterostructure and its enhanced mechanism for organic pollutants removal under visible-light illumination. J. Environ. Chem. Eng. 2020, 8, 104044. [Google Scholar] [CrossRef]
- Coreño, J.; Coreño, O. Evaluation of calcium titanate as apatite growth promoter. J. Biomed. Mater. Res. Part A 2005, 75, 478–484. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, G.; Zhang, F. Multiple roles of graphene in heterogeneous catalysis. Chem. Soc. Rev. 2015, 44, 3023–3303. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kosel, T.H.; Kamat, P.V. Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. storing and shuttling electrons with reduced graphene oxide. Nano Lett. 2010, 10, 577–583. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Peng, W.; Luo, Y.; He, D. Effects of inorganic ions on the photocatalytic degradation of carbamazepine. J. Water Reuse Desalin. 2019, 9, 301–309. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Wei, M.; Chen, P.; Shan, G. Photolytic reaction mechanism and impacts of coexisting substances on photodegradation of bisphenol A by Bi2WO6 in water. Water Res. 2012, 46, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Skoutelis, C.G.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I.K. Probing the photolytic-photocatalytic degradation mechanism of DEET in the presence of natural or synthetic humic macromolecules using molecular-scavenging techniques and EPR spectroscopy. J. Environ. Chem. Eng. 2015, 3, 3005–3014. [Google Scholar] [CrossRef]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-structured CaTiO3 coupled with g-C3N4 as a heterojunction photocatalyst for organic pollutant degradation. Beilstein J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl. Catal. B 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Tachikawa, T.; Fujitsuka, M.; Majima, T. Mechanistic insight into the TiO2 photocatalytic reactions: Design of new photocatalysts. J. Phys. Chem. C 2007, 111, 5259–5275. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 Composite Photocatalyst under Visible and UV Light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]
- Passi, M.; Pal, B. Influence of Ag/Cu photodeposition on CaTiO3 photocatalytic activity for degradation of Rhodamine B dye. Korean J. Chem. Eng. 2022, 39, 942–953. [Google Scholar] [CrossRef]
- Andersen, T.; Haugen, H.K.; Hotop, H. Binding energies in atomic negative ions: III. J. Phys. Chem. Ref. Data 1999, 28, 1511–1533. [Google Scholar] [CrossRef]




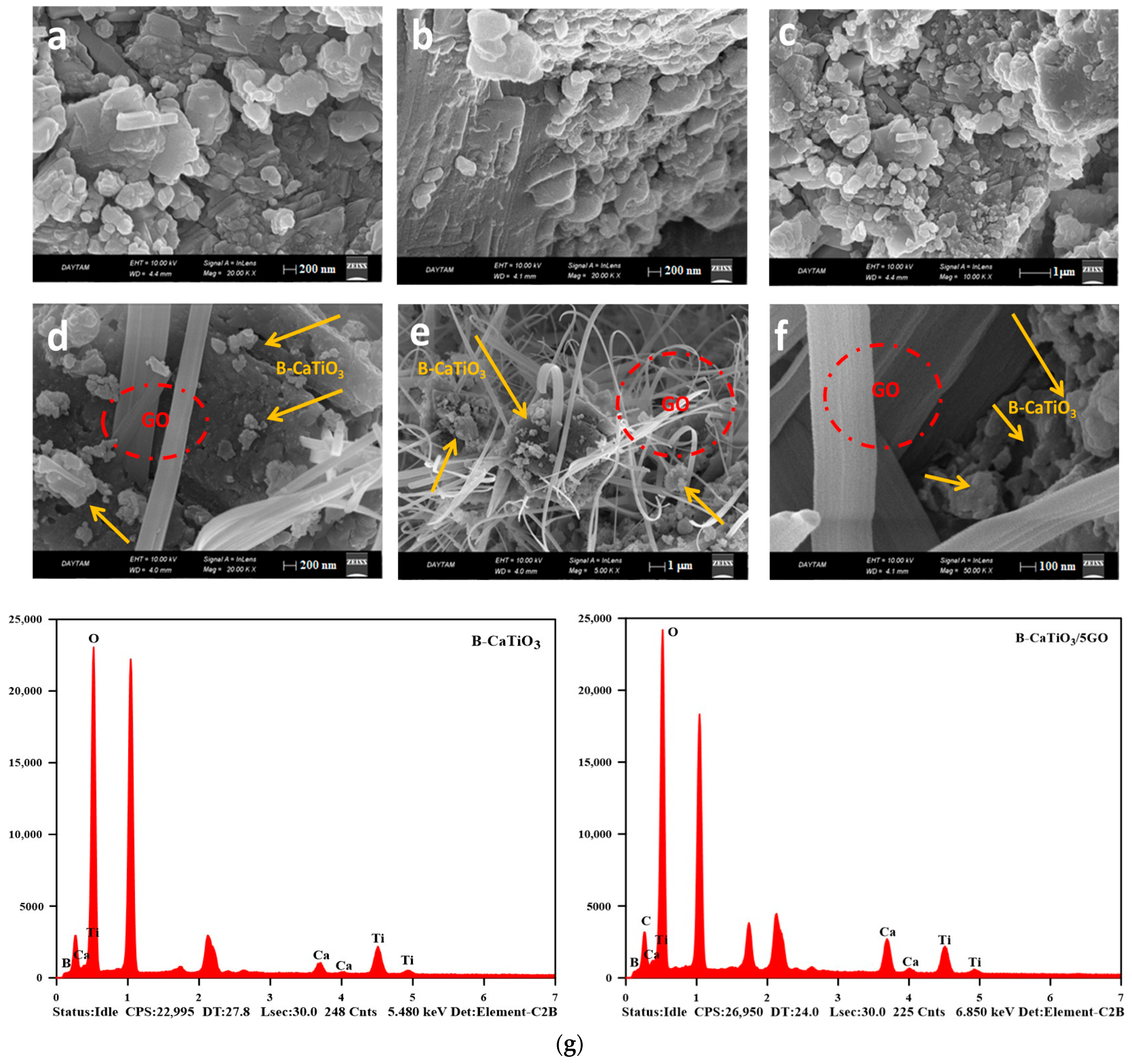
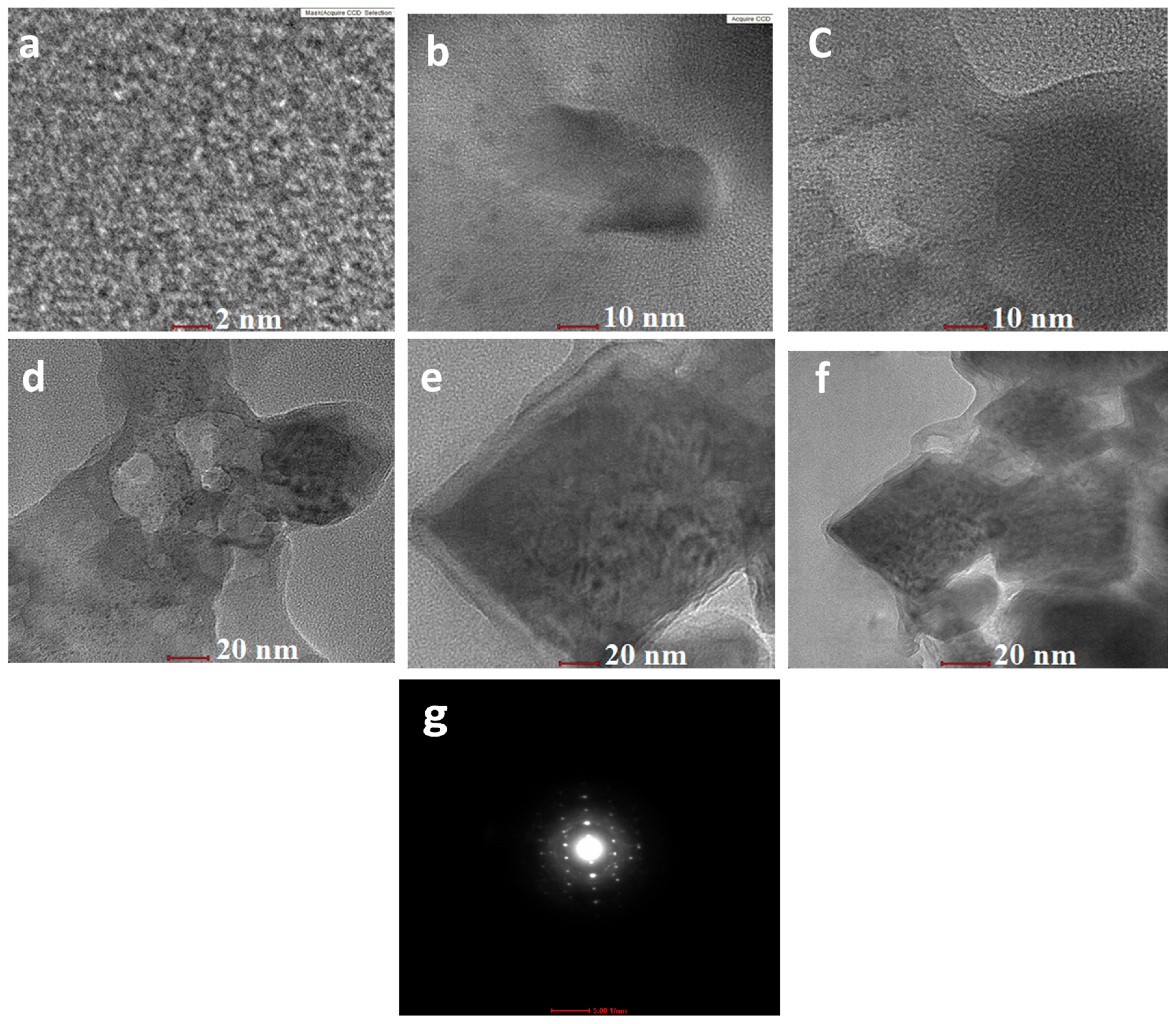





| Catalysts | SBET | a PS, Pore Size (nm) | b Vpore (cm3/g) |
|---|---|---|---|
| B-CaTiO3 | 0.0573 m2/g | 202.76 nm | 0.0012 cm3/g |
| B-CaTiO3/5GO | 3.9303 m2/g | 10.53 nm | 0.0350 cm3/g |
| Pollutant | Photocatalyst | Light Source | [Pollutant] | [Catalyst] | % Removal | Time | Refs. |
|---|---|---|---|---|---|---|---|
| MB | RGO-N-CaTiO3 | Visible light | 4 × 10−5 M | 50 mg | ~95 | 180 | [30] |
| Methyl orange | CaTiO3-graphene | UV light | 1 mg/L | 1 g/L | ~98 | 60 | [31] |
| MB | CaTiO3/rGO/NiFe2O4 | Sunlight | 5 mg/L | 0.1 g/L | ~83 | 180 | [32] |
| Methyl orange | CaTiO3/rGO | UV light | 5 mg/L | 100 mg | ~93 | 60 | [34] |
| MB | B-CaTiO3/5GO | Visible light | 10 mg/L | 1 g/L | 87.4 | 180 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altin, I. Perovskite Type B-CaTiO3 Coupled with Graphene Oxide as Efficient Bifunctional Composites for Environmental Remediation. Processes 2023, 11, 3191. https://doi.org/10.3390/pr11113191
Altin I. Perovskite Type B-CaTiO3 Coupled with Graphene Oxide as Efficient Bifunctional Composites for Environmental Remediation. Processes. 2023; 11(11):3191. https://doi.org/10.3390/pr11113191
Chicago/Turabian StyleAltin, Ilknur. 2023. "Perovskite Type B-CaTiO3 Coupled with Graphene Oxide as Efficient Bifunctional Composites for Environmental Remediation" Processes 11, no. 11: 3191. https://doi.org/10.3390/pr11113191
APA StyleAltin, I. (2023). Perovskite Type B-CaTiO3 Coupled with Graphene Oxide as Efficient Bifunctional Composites for Environmental Remediation. Processes, 11(11), 3191. https://doi.org/10.3390/pr11113191